Abstract
A young female vaper presented with insidious onset cough, progressive dyspnoea on exertion, fever, night sweats and was in respiratory failure when admitted to hospital. Clinical examination was unremarkable. Haematological tests revealed only thrombocytopenia, which was long standing, and her biochemical and inflammatory markers were normal. Chest radiograph and high-resolution CT showed diffuse ground-glass infiltrates with reticulation. She was initially treated with empirical steroids and there was improvement in her oxygenation, which facilitated further tests. Since the bronchoscopy and high-volume lavage was unyielding, a video-assisted thoracoscopicsurgical biopsy was done later and was suggestive of lipoid pneumonia. The only source of lipid was the vegetable glycerine found in e-cigarette (EC). Despite our advice to quit vaping, she continued to use EC with different flavours and there is not much improvement in her clinical and spirometric parameters.
Keywords: pneumonia (respiratory medicine), interstitial lung disease
Background
The popularity of e-cigarettes (ECs) with consumers has increased worldwide in recent years; however, in the UK and elsewhere, their use remains unregulated and little is known about toxic inhalational or any long-term adverse respiratory effects. We report a subacute inhalational episode and ongoing respiratory illness in a young female vaper, requiring a surgical lung biopsy for pathological diagnosis.
Case presentation
A 34-year-old British Asian woman was admitted to our hospital from a general medical take in acute type 1 respiratory failure. Prior to admission, she had experienced 3 months of progressive breathlessness on exertion and daily cough with white sputum. This was accompanied by streaky haemoptysis, reduced appetite, weight loss of 6 kg and night sweats. She was an ex-cigarette smoker of 5 years with a 10-pack-year history, having switched to ECs by vaping approximately 3 years before admission. She had a significant medical history of congenital dysmorphism with thrombocytopenia and iron deficiency anaemia under surveillance by haematologists, closure of a ventricular septal defect at age 1, gastro-oesophageal reflux disease and hypothyroidism. There were no muscle or joint pains, rash, dysphagia or sicca features. Her drug prescriptions comprised omeprazole 20 mg once daily, levothyroxine 75 µg once daily and cholecalciferol 800 U once daily. She was employed as a retail assistant in a jewellery and fashion accessories concession at Birmingham Airport, with no exposure to aircraft exhaust, gases, vapours or dust, and there were no work-related changes in presence or severity of symptoms. There were no significant inhalational exposures domestically, specifically birds or animals, moulds, damp, hot tubs or jacuzzis, compost, feather or down bedding, and no hobbies of note. On admission, she was tachypnoeic, tachycardic, saturating 96% on 4 L/min of oxygen. She exhibited long-standing clubbing. Her respiratory system examination was normal with no crackles.
Investigations
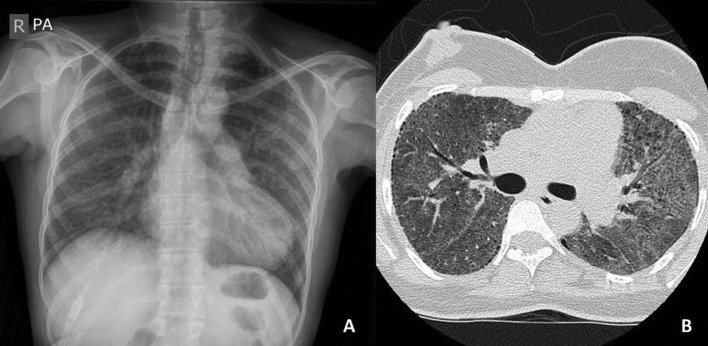
Laboratory tests revealed white blood count 9.83×109/L, neutrophils 8.08×109/L, haemoglobin 136 g/L, MCV 76 fL, platelets 56 000 cells/mm3 and C reactive protein 23 mg/L. Her renal and liver functions were normal. Chest radiograph on admission revealed bilateral infiltrates predominantly affecting mid and lower zones and high-resolution CT confirmed diffuse ground-glass opacities throughout all pulmonary lobes, apical subpleural cysts and interlobular septal thickening (figure 1).
Figure 1.
(A) Chest radiograph showing bilateral diffuse infiltrates throughout both lung fields. (B) High-resolution CT slice from upper lobes showing diffuse ground-glass opacity and subpleural cysts bilaterally.
Differential diagnosis
There is a wide differential diagnosis for subacute diffuse pulmonary infiltrates in a young woman, and initially there were few features to suggest chronicity (the clubbing was in fact congenital on closer questioning). Potential diagnoses were (1) interstitial pneumoniae: respiratory bronchiolitis interstitial lung disease or desquamative interstitial pneumonia caused by smoking, alveolar proteinosis, collagen vascular disease-related non-specific interstitial pneumonia, hypersensitivity pneumonitis or idiopathic acute interstitial pneumonia; (2) pulmonary vasculitis; (3) a cystic lung disease; or (4) infections such as atypical bacterial, viral, mycobacterial or pneumocystis, though these were thought less likely, with no clinical features of infection and normal inflammatory markers.
Treatment and further investigations
Given her acute respiratory failure, she was treated empirically with prednisolone 40 mg once daily, with improvement in her oxygenation sufficient for further investigations.
Autoimmune profile, antineutrophilic cytoplasmic antibody, HIV serology and T-spot interferon gamma release assay for Mycobacterium tuberculosis were normal, as were specific IgG to pigeon and budgerigar proteins, Mycoployspora faeni and Aspergillus fumigatus. Spirometry revealed a restrictive ventilatory defect with FEV1=1.23 L (50% predicted), FVC=1.37 L (48% predicted) and FEV1/FVC=89%, with diffusion capacity for carbon monoxide (TLCO)=1.9 mmol/min/kPa (24% predicted), KCO=1.15 mmol/min/kPa/L (59% predicted) and total lung capacity (TLC)=1.62 L (40% predicted). Fibre-optic bronchoscopy and high-volume bronchoalveolar lavage (BAL) showed a pink cloudy fluid with negative microbiology and cytology; differential cell count showed 18% lymphocytes, 2% neutrophils, 68% macrophages and 2% eosinophils (figure 2). Hypoxia during bronchoscopy and thrombocytopenia prevented transbronchial lung biopsy. Interstitial Lung Disease board discussion at that stage was unable to reach a consensus diagnosis and recommended a video-assisted thoracoscopic surgical lung biopsy of the right upper, middle and lower lobe, which revealed extensive accumulation of lipid-filled macrophages and deposition of cholesterol clefts and some inflammation representing lipoid pneumonia (figures 3 and 4). A search for sources of endogenous or exogenous inhaled lipid revealed only vegetable glycerine, found in ECs, as the likely cause.
Figure 2.
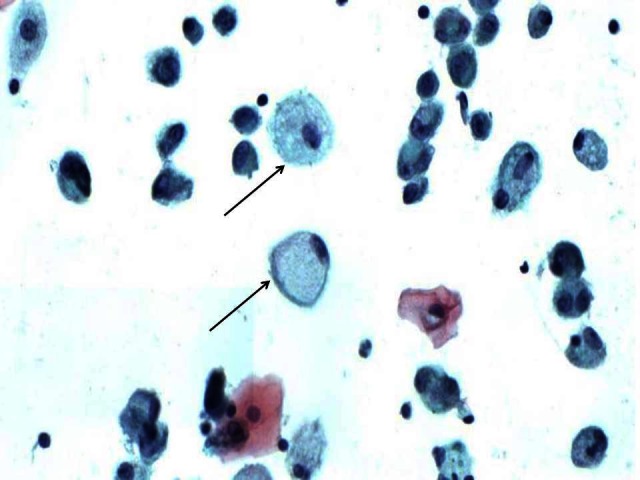
Cytology from the bronchoalveolar lavage showing an occasional alveolar macrophage with foamy cytoplasm (arrowed) consistent with phagocytosis of lipid material.
Figure 3.
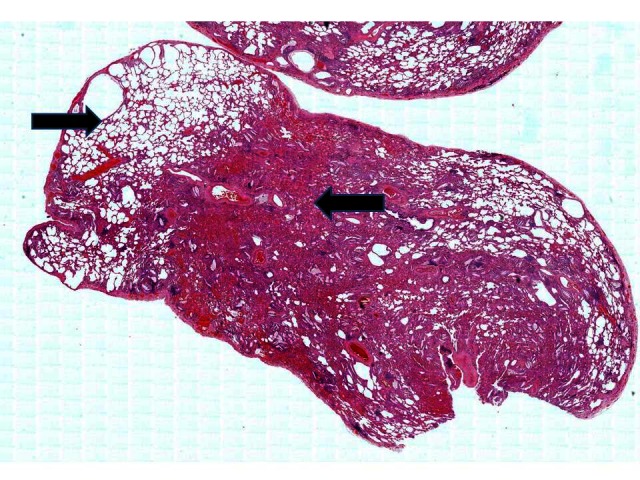
Low-power view showing uninvolved lung at 11 o’clock but a large central area of consolidation.
Figure 4.
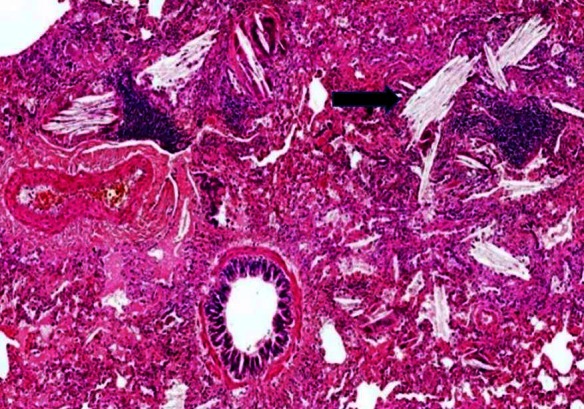
Lung biopsy (original magnification ×40) showing cholesterol clefts within the airspaces, adjacent to normal-looking bronchiole and pulmonary artery.
Outcome and follow-up
She was advised to stop vaping immediately and encouraged to use other forms of nicotine replacement, though continued to vape using ECs containing vegetable glycerine, although with a different flavouring additive. After the initial improvement, continued therapy with prednisolone 15–30 mg once daily over 18 months produced sustained improvement in her symptoms, marginal increase in spirometric indices, but no change in gas transfer (table 1).
Table 1.
Serial measures of lung function for the patient while taking 15–30 mg prednisolone
| Date | FEV1, L (%) |
FVC, L (%) |
FEV1/FVC% | DLCO, mmol/min/kPa (%) | KCO, mmol/min/kPa/L (%) | VA, L (%) |
| November 2017 | 1.38 (65) | 1.54 (62) | 89 | 2.43 (32) | 1.2 (62) | 2.04 (59) |
| June 2017 | 1.39 (58) | 1.53 (55) | 90 | 1.68 (21) | 0.87 (45) | 1.91 (50) |
| March 2016 | 1.41 (57) | 1.61 (56) | 87 | 2.54 (32) | 1.4 (72) | 1.82 (60) |
| December 2015 | 1.44 (58) | 1.57 (55) | 91 | 2.75 (35) | 1.3 (67) | 2.11 (70) |
| October 2015 | 1.23 (50) | 1.37 (48) | 89 | 1.9 (24) | 1.15 (59) | 1.65 (54) |
DLCO, carbon monoxide diffusing capacity; KCO, carbon monoxide transfer; MCV, mean corpuscular volume; VA, alveolar ventilation.
Discussion
Lipoid pneumonia results from an inflammatory response to lipids present in the alveolar space. It can be endogenous in aetiology, resulting from bronchial lipid storage disorders, bronchial obstruction or hypercholesterolaemia, or exogenous, caused by inhalation or aspiration of animal, vegetable or mineral oil. Lipid pneumonia has been described in individuals who aspirate liquid hydrocarbon through fire-eating, consume oil-based laxatives or repeatedly use petroleum-based lubricants and decongestants.1 Clinical presentation comprises a spectrum from asymptomatic chronic disease with incidental detection on chest radiograph to severe acute cases requiring ventilatory support. Symptoms are non-specific, usually cough and breathlessness on exertion, sometimes with fever, night sweats and haemoptysis, as in the current patient. Clinical examination is often unremarkable, at times there may be crackles or wheeze.2 Chest radiograph usually shows extensive bilateral consolidation or ground-glass opacities, predominant in the mid and lower zones, though lipid pneumonia can mimic lung cancers presenting as focal consolidation.3 Most lipoid pneumonia is due to aspiration and has a basal distribution. The homogeneous distribution in this case means that the lipid has been inhaled as a vapour, where most reported cases have resulted from inhaled aerosols of metal-working fluid in industry.4 Classical high-resolution CT features are of low attenuation areas (−40 to −150 HU) in the consolidated area similar to surrounding fat in the chest wall. Diagnosis is made by BAL or lung biopsy, showing lipid-laden macrophages in BAL fluid, and histology showing foreign body inflammation around lipid components.5 There are no definitive treatments for lipoid pneumonia, beyond avoidance of the causative agent; the role of steroids and whole lung lavage is unproven and usually reserved for severe cases.6
ECs contain a liquid solution mixture of nicotine, propylene glycol, water, flavouring agent and often vegetable glycerin, which is heated in a cartridge to produce aerosol (‘vapour’) containing nicotine. The liquid is heated using a battery-operated heating resistance coil housed at the base of the EC. Vegetable glycerine (‘glycerin’ or ‘glycerol’) is a sweet-tasting, colourless and odourless polyol that is extracted from palm, soy or coconut oil triglycerides by hydrolysis5; when heated, it is responsible in part for the visible ‘smoke’ element of the vapour. There are two previously reported cases of lipoid pneumonia due to vegetable glycerine in ECs.5 6 Flavouring agents are many and include di-acetyl, a chemical contained in many flavours (including fruits, butter, caramel and alcohol),7 and responsible for obliterative bronchiolitis in popcorn workers’ lung.8 We have been unable to ascertain the exact flavour constituents of our patient’s ECs due to commercial sensitivity.
ECs came to market in China in 2004, and there are now more than 500 brands worldwide. Often considered a safer alternative to smoking, some studies have shown ECs to be 95% less hazardous than the conventional cigarettes in terms of product-specific and related mortality, morbidity, dependence, economic costs and injury.9 There are 2.6 million adult users in Great Britain,10 and Public Health England endorses its use as a smoking cessation aid.11 ECs are already an established smoking cessation strategy,12 but evidence regarding their benefit in helping smokers to quit is limited.13 14 Toxicological and epidemiological research into the short-term and long-term respiratory health of EC users is urgently needed, and we advocate proper labelling of e-liquid constituent ingredients.15
Learning points.
E-cigarettes contain propylene glycol, vegetable glycerine and a number of flavouring chemical additives.
When taking an exposure history for interstitial lung disease, healthcare professionals should enquire about the use of e-cigarettes containing vegetable glycerin.
Lipoid pneumonia should be considered as a diagnosis for presentations of interstitial lung disease in vapers, and bronchoalveolar lavage may be helpful in determining the pathological diagnosis.
Further exposure to the offending agent should be avoided to prevent clinical deterioration.
Footnotes
Contributors: GW is the responsible consultant for the patient and was involved in the management of the patient all throughout and also provided guidance in preparing the article and also revising and editing the article as per BMJ format. ST and SB are members of the ILD (Interstitial Lung Disease) board and contributed significantly in crucial decision-making to reach a diagnosis. ST is a lung histopathologist who confirmed the diagnosis after considering the history of exposure. All the authors have read the article and suggested necessary amendments.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. . Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol 2010;194:103–9. 10.2214/AJR.09.3040 [DOI] [PubMed] [Google Scholar]
- 2.Baron SE, Haramati LB, Rivera VT. Radiological and clinical findings in acute and chronic exogenous lipoid pneumonia. J Thorac Imaging 2003;18:217–24. 10.1097/00005382-200310000-00002 [DOI] [PubMed] [Google Scholar]
- 3.Marchiori E, Zanetti G, Mano CM, et al. . Clinical and radiological manifestations. Respir Med 2011;105:659–66. [DOI] [PubMed] [Google Scholar]
- 4.Gondouin A, Manzoni P, Ranfaing E, et al. . Exogenous lipid pneumonia: a retrospective multicentre study of 44 cases in France. Eur Respir J 1996;9:1463–9. 10.1183/09031936.96.09071463 [DOI] [PubMed] [Google Scholar]
- 5.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest 2012;141:1110–3. 10.1378/chest.11-1334 [DOI] [PubMed] [Google Scholar]
- 6.Modi S, Sangani R, Alhajhusain A. Acute lipoid pneumonia secondary to e-cigarettes use: an unlikely replacement for cigarettes. Chest 2015;148:382A 10.1378/chest.227486025569856 [DOI] [Google Scholar]
- 7.Allen JG, Flanigan SS, LeBlanc M, et al. . Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect 2016;124:733–9. 10.1289/EHP348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreiss K, Gomaa A, Kullman G, et al. . Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med 2002;347:330–8. 10.1056/NEJMoa020300 [DOI] [PubMed] [Google Scholar]
- 9.Nutt DJ, Phillips LD, Balfour D, et al. . Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res 2014;20:218–25. 10.1159/000360220 [DOI] [PubMed] [Google Scholar]
- 10.ASH. Use of electronic cigarettes (vapourisers) among adults in Great Britain. http://ash.org.uk/information-and-resources/fact-sheets/use-of-e-cigarettes-among-adults-in-great-britain-2017/ (accessed 3 Jan 2018).
- 11.Public Health England. E-cigarettes: an evidence update. London: Public Health England, 2015. [Google Scholar]
- 12.Smoking in England. Smoking toolkit study: trends in electronic cigarette use in England. 2015. (accessed 3 Jan 2018).
- 13.Brown J, Beard E, Kotz D, et al. . Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction 2014;109:1531–40. 10.1111/add.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRobbie H, Bullen C, Hartmann-Boyce J, et al. . Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014;12:CD010216. [DOI] [PubMed] [Google Scholar]
- 15.Chun LF, Moazed F, Calfee CS, et al. . Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol 2017;313:L193–L206. 10.1152/ajplung.00071.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]