Abstract
Aphasia is a language disorder characterised by loss of ability to produce or comprehend written or spoken language. In majority of the cases, it is due to stroke. Aphasia may also present as an ictal or postictal state of temporal or frontal lobe seizures. Nevertheless, its isolated occurrence in individuals without a clear-cut history of seizures raises diagnostic difficulties with important therapeutic implications.
A case of epileptic aphasia is reported in which the diagnosis was confirmed by electroencephalogram with a dramatic therapeutic response to an antiepileptic drug.
Keywords: neurology (drugs and medicines), epilepsy and seizures
Background
Aphasia is a language disorder characterised by loss of ability to produce or comprehend written or spoken language. Virtually any pathological process or insult that results in language network damage can cause aphasia. The most frequent cause of aphasia is stroke, although innumerous other pathologies can cause this language disorder.1 More rarely, aphasia may present as a sole manifestation of temporal or frontal lobe seizures. Its manifestation in individuals without a previous history of epilepsy raises diagnostic difficulties and thus maintaining a high clinical suspicion in such cases remains vital for early diagnosis and suitable management of patients. The authors present a rare condition in a middle-aged patient, with a late diagnose of epileptic aphasia that evolves through several years before a clear diagnosis. Our case underscores the importance of including epilepsy in the differential diagnosis of patients presenting with isolated and unexplained aphasia, even in the absence of previous epilepsy history.
Case presentation
The authors present a case of a 68-year-old, right-handed woman, with a medical history of chronic liver disease. She had many previous hospitalisations, 14 years earlier, due to isolated language disorder and diagnosed initially as hepatic encephalopathy and stroke. According to her family, she experienced for many years repeated episodes of language disorder that lasted for several seconds to minutes, in which she stayed confused and aphasic but with preserved awareness. Usually, symptoms ceased spontaneously, which is why they were initially devalued by her family. On admission day, she presented to the emergency department with history of a sudden-onset speech disorder that, contrary to previous episodes, lasted for several hours. No other signs or symptoms were reported, such as fever, other neurological deficits or loss of consciousness. There was no personal or family history of seizures, head trauma, brain surgery, infection or migraine. General physical examination, including vital signs, was unremarkable. A complete neurological examination was performed and revealed global aphasia. She was awake although not able to comprehend simple instructions, and had a severe expressive deficit, with marked naming deficit and paraphasic errors. There were no motor or sensitive deficits, and examination of cranial nerves was normal. Tendon reflexes were within normal limits and symmetrical with flexor plantar response.
Investigations
A routine blood panel revealed mild thrombocytopenia (114x109/L, normal range 150x109–300x109/L) but no other abnormal laboratory results were observed, including electrolytes and serum ammonia. Additional testing with metabolic panel, inflammatory markers, autoantibodies and urinalysis were unremarkable.
Thoracoabdominopelvic CT scan did not reveal any suspicious malignant lesions. A cerebral CT scan was performed and did not reveal any acute lesions. Carotid Doppler ultrasound did not show evidence of carotid stenosis or atheroma, and transthoracic echocardiogram was unremarkable. Lumbar puncture yielded acellular fluid with a slight hyperproteinorraquia of 50.6 (normal range: 45 mg/dL) and normal glucose levels. Bacteriological and mycobacterial liquor analyses were negative.
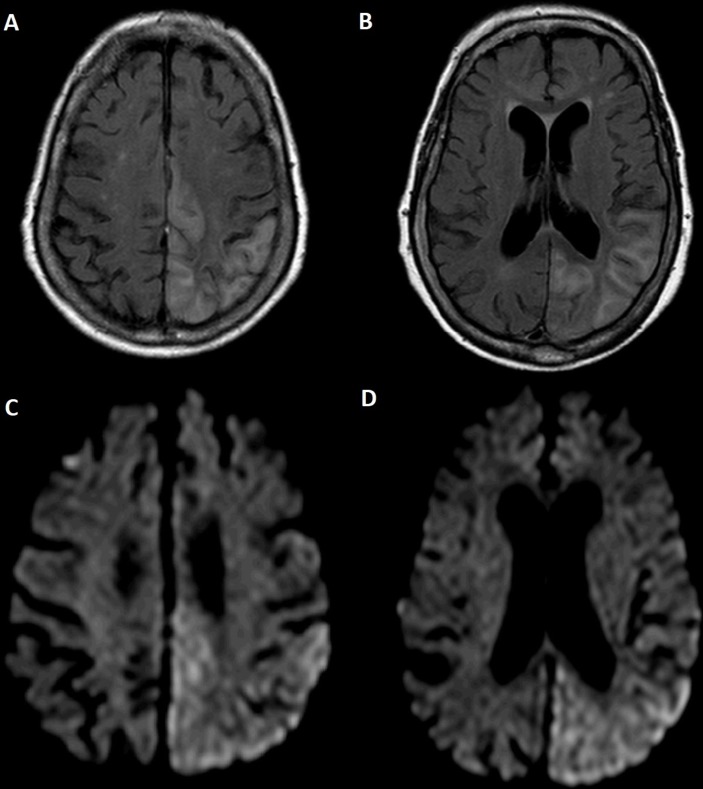
Due to persistence of language disorder, a cerebral MRI was performed and revealed extensive alteration of the cerebral cortex signal at the level of the left cerebral hemisphere, covering almost all the temporal, parietal and occipital lobes, also extending to the posterior portion of the frontal lobe; the cortex of these regions were expanded and had hyperintensity in T2/Fluid Acquisition Inversion Recovery (FLAIR) - oedema and in contiguous subcortical substance (figure 1A,B). However, the signal of the deep white matter was preserved. Diffusion-weighted imaging showed hyperintensity foci in the cortex of these regions, some of which appear to have translation on the Apparent diffusion coefficient (ADC) map as abnormal restriction diffusion areas (figure 1C,D). MR angiography (MRA) excluded changes in the contour, position and calibre of intracranial arteries. Scalp electroencephalogram (EEG) was performed only 8 days after admission due to schedule restrictions and revealed epileptiform activity in left temporal region.
Figure 1.
(A and B) Cerebral MRI showed extensive alteration of the cerebral cortex signal at the level of the left cerebral hemisphere; the cortex of these regions was expanded and had hyperintensity in FLAIR (oedema) and in contiguous subcortical substance. (C and D) Diffusion-weighted imaging showed hyperintensity foci in the cortex of these regions, some of which appear to have translation on the ADC map as abnormal restriction diffusion areas.
Differential diagnosis
In the face of the acute onset of symptoms, the first diagnostic hypothesis was an acute stroke which was excluded after cerebral imaging. Hepatic encephalopathy was also probable, although no altered consciousness was observed. In fact, due to the prevalence of neurological complications in patients with liver cirrhosis, status epilepticus in such patients can be a clinical challenge to diagnose.2 Brain tumour or cerebral involvement of a paraneoplastic disorder was also considered, but no other signs or symptoms of neoplastic conditions were found in the diagnostic work-up performed. No history of trauma or migraine with aura were known. Additionally, other possible aetiologies such as infections, neurodegenerative and metabolic were extensively studied and excluded in the patient. Autoimmune encephalitis was another potential cause for this clinical picture, but the history of previous self-limited episodes for several years do not favour that hypothesis.
Treatment
Treatment was initiated on the ninth day of hospitalisation due to delayed EEG execution. Antiepileptic drug therapy with levetiracetam 1000 mg daily was finally initiated, with clinical improvement and complete reversal of symptoms in a 48-hour period.
Outcome and follow-up
Follow-up cerebral imaging obtained 1 month after the diagnosis revealed almost complete resolution of the abnormalities observed previously, except for a small hyperintense focus on T2/FLAIR, in parietal cortical position, suggesting sequelae area (figure 2). EEG showed disappearance of epileptiform activity. No further seizures were reported at 3 years of follow-up.
Figure 2.
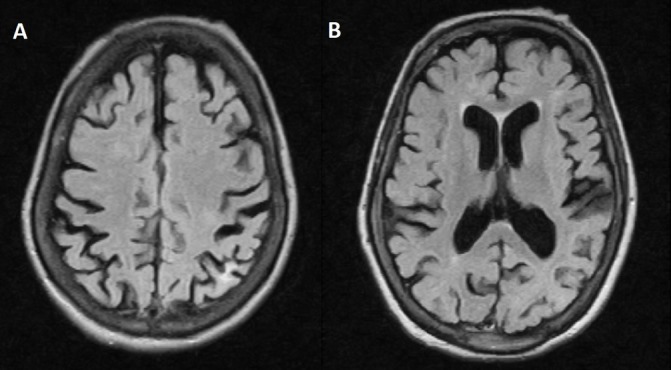
Follow-up MRI obtained 1 month after diagnosis revealed almost complete resolution of the abnormalities observed previously, except for a small hyperintense focus on T2/FLAIR, in parietal cortical position, suggesting sequelae area.
Discussion
Aphasia is a common manifestation of seizure and may be presented as an ictal phenomenon or a postictal manifestation, usually accompanied by other symptoms, such as altered consciousness or convulsive movements.1 Nevertheless, isolated aphasia as manifestation of a seizure, as proven in this case report, is a rarely described syndrome. Various types of aphasia (motor, sensory and mixed) have been reported as ictal symptoms, although the relationship between the types of aphasia and the location of epileptic discharges is not straightforward.3 4
Usually, aphasic status epilepticus is associated with structural brain lesions, including brain tumours, trauma and stroke. Other aetiologies can be associated with epileptic aphasia, including central nervous system infection and metabolic disorders.1 3 Interestingly, in our patient, no clear cause underlying epileptic aphasia was identified.
Epileptic aphasia can be difficult to diagnose due to low clinical suspicion and non-specific findings in complementary tests. Diagnostic criteria for epileptic aphasia were defined by Rosenbaum et al5 in 1986 and posteriorly adapted by Grimes and Guberman1 in 1997. These criteria comprise preservation of language production during seizures followed by a postictal aphasic phase with preservation of consciousness. Additionally, aphasia must be correlated with epileptic activity registered by EEG monitoring, and should improve with successful treatment of seizures. Our patient successfully fulfilled all the criteria of epileptic aphasia. Although an EEG should be performed in every patient with isolated and unexplained aphasia, as it is the gold-standard examination to diagnose epilepsy, the detection of epileptic activity in prolonged aphasia can be challenging. EEG recording might show abnormalities only intermittently and/or existence of electrical activity might not be detected by surface electrodes, and for that matter electroclinical correlation is not always obvious,6 postponing the diagnosis and aggravating long-term prognosis. In fact, our patient had for many years innumerous and self-limited episodes of isolated aphasia, previously misdiagnosed as stroke or metabolic encephalopathies, aetiologies more frequently attributed to isolated language disorder which delayed appropriate treatment for several years.
To overcome these difficulties, Ericson et al stated that continuous EEG is necessary to make a definite diagnosis and monitor subsequent treatment.6 Other authors have also recommended cerebral PET scan as an alternative examination to diagnose a case of suspected epileptic aphasia with a normal scalp EEG.7
CT and MRI can be useful in diagnosing ictal changes especially due to its easier access than EEG in urgent setting. It can be useful in excluding other causes of isolated aphasia. MRI changes associated with status epilepticus include increased signal intensity on T2-weighted or diffusion-weighted images in the affected cortex or underlying white matter.8 However, MRI changes do not necessarily correlate to the exact localisation of the epileptic focus and abnormalities can be seen distant from the primary language areas.9
Aphasia can either be an ictal or postictal phenomenon, and discerning between these states can be extremely difficult due to the variability of seizure duration of aphasia, that can extend from several days to weeks.10–12
In our report, the patient had isolated aphasia that lasted for 9 days during hospitalisation, up until appropriate treatment was initiated. Therefore, Quintas et al state13 that initiating empirical treatment with antiepileptic drugs is reasonable, especially if EEG is not available and/or delayed, and other structural, infectious or inflammatory causes of isolated aphasia have been excluded, as proper treatment can dramatically improve long-term prognosis.
Treatment include antiepileptic drugs, and as defined by Rosenbaum’s criteria, should improve clinical manifestations and eliminate seizure activity in EEG recording. In the reported case, clinical and EEG abnormalities were eliminated, and no recurrent symptoms were observed at follow-up. As for MRI abnormalities, although significantly improved, no complete resolution of cerebral lesions were observed.
This case underscores the importance of including epilepsy in the differential diagnosis of patients presenting with isolated and unexplained aphasia, even in the absence of previous epilepsy history. An EEG should be performed in such cases in order to identify this rare but treatable cause of aphasia, to avoid delays in diagnosis and treatment for this reversible cause of aphasia.
Learning points.
Aphasia is a language disorder characterised by loss of ability to produce or comprehend written or spoken language. Usually, it is associated with structural brain lesions, including brain tumour, trauma and stroke.
Isolated epileptic aphasia is a rare entity, and maintaining high clinical suspicion in unexplained aphasia is mandatory for early diagnosis and suitable management of patients.
Electroencephalogram (EEG) is the gold-standard test, although brain imaging (including CT scan and MRI) can help distinguish an aphasic seizure from other causes of aphasia.
Treatment includes antiepileptic drugs and should improve both clinical and EEG abnormalities.
Footnotes
Contributors: EMM directly attended the patient and was responsible for drafting the article and revising it critically. AM and CR directly attended the patient and contributed to the article conception and design and article reviewing. DG contributed to the article reviewing and final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Grimes DA, Guberman A. De novo aphasic status epilepticus. Epilepsia 1997;38:945–9. 10.1111/j.1528-1157.1997.tb01262.x [DOI] [PubMed] [Google Scholar]
- 2.Rudler M, Marois C, Weiss N, et al. Status epilepticus in patients with cirrhosis: How to avoid misdiagnosis in patients with hepatic encephalopathy. Seizure 2017;45:192–7. 10.1016/j.seizure.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 3.Benatar M. Ictal aphasia. Epilepsy Behav 2002;3:413–9. 10.1016/S1525-5050(02)00519-X [DOI] [PubMed] [Google Scholar]
- 4.Loesch AM, Steger H, Losher C, et al. Seizure-associated aphasia has good lateralizing but poor localizing significance. Epilepsia 2017;58:1551–5. 10.1111/epi.13835 [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum DH, Siegel M, Barr WB, et al. Epileptic aphasia. Neurology 1986;36:822–5. 10.1212/WNL.36.6.822 [DOI] [PubMed] [Google Scholar]
- 6.Ericson EJ, Gerard EE, Macken MP, et al. Aphasic status epilepticus: electroclinical correlation. Epilepsia 2011;52:1452–8. 10.1111/j.1528-1167.2011.03084.x [DOI] [PubMed] [Google Scholar]
- 7.Dong C, Sriram S, Delbeke D, et al. Aphasic or amnesic status epilepticus detected on PET but not EEG. Epilepsia 2009;50:251–5. 10.1111/j.1528-1167.2008.01782.x [DOI] [PubMed] [Google Scholar]
- 8.Lansberg MG, O’Brien MW, Norbash AM, et al. MRI abnormalities associated with partial status epilepticus. Neurology 1999;52:1021–7. 10.1212/WNL.52.5.1021 [DOI] [PubMed] [Google Scholar]
- 9.Toledo M, Munuera J, Sueiras M, et al. MRI findings in aphasic status epilepticus. Epilepsia 2008;49:1465–9. 10.1111/j.1528-1167.2008.01620.x [DOI] [PubMed] [Google Scholar]
- 10.DeToledo JC, Minagar A, Lowe MR. Persisting aphasia as the sole manifestation of partial status epilepticus. Clin Neurol Neurosurg 2000;102:144–8. 10.1016/S0303-8467(00)00091-3 [DOI] [PubMed] [Google Scholar]
- 11.Herskovitz M, Schiller Y. Prolonged ictal aphasia: a diagnosis to consider. J Clin Neurosci 2012;19:1605–6. 10.1016/j.jocn.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Chung PW, Seo DW, Kwon JC, et al. Nonconvulsive status epilepticus presenting as a subacute progressive aphasia. Seizure 2002;11:449–54. 10.1053/seiz.2002.0678 [DOI] [PubMed] [Google Scholar]
- 13.Quintas S, Ródriguez-Carrillo JC, Toledano R, et al. When aphasia is due to aphasic status epilepticus: a diagnostic challenge. Neurol Sci 2018;39 10.1007/s10072-017-3218-9 [DOI] [PubMed] [Google Scholar]