Abstract
Adverse remodeling after myocardial infarction (MI) is strongly influenced by T cells. Stem cell therapy after MI, using mesenchymal stem cells (MSC) or cardiomyocyte progenitor cells (CMPC), improved cardiac function, despite low cell retention and limited differentiation. As MSC secrete many factors affecting T cell proliferation and function, we hypothesized the immune response could be affected as one of the targets of stem cell therapy. Therefore, we studied the immunosuppressive properties of human BM-MSC and CMPC and their extracellular vesicles (EVs) in co-culture with activated T cells. Proliferation of T cells, measured by carboxyfluorescein succinimidyl ester dilution, was significantly reduced in the presence of BM-MSC and CMPC. The inflammatory cytokine panel of the T cells in co-culture, measured by Luminex assay, changed, with strong downregulation of IFN-gamma and TNF-alpha. The effect on proliferation was observed in both direct cell contact and transwell co-culture systems. Transfer of conditioned medium to unrelated T cells abrogated proliferation in these cells. EVs isolated from the conditioned medium of BM-MSC and CMPC prevented T cell proliferation in a dose-dependent fashion. Progenitor cells presence induces up- and downregulation of multiple previously unreported pathways in T cells. In conclusion, both BM-MSC and CMPC have a strong capacity for in vitro immunosuppression. This effect is mediated by paracrine factors, such as extracellular vesicles. Besides proliferation, many additional pathways are influenced by both BM-MSC and CMPC.
Keywords: Immunology, Stem cell research
1. Introduction
In Europe ischemic heart disease remains the most common cause of death, responsible for the death of 19% of males and 20% of females [1]. After ischemia and the subsequent reperfusion, a strong immune response ensues [2, 3, 4]. Many types of circulating immune cells, such as neutrophils, monocytes, and dendritic cells are recruited to the heart [4, 5, 6, 7]. Some of these cells pick up cardiac actin and myosin from the post-infarct debris and present them to T cells, which can then become auto-reactive for these cardiac antigens [6, 8, 9, 10]. The auto-reactive T cells can attack cardiac cells displaying these antigens for a long time after the initial event, leading to adverse remodeling and a gradually decreasing heart function [11, 12]. In fact, experimental models show the transfer of immune cells, including T cells, from donors with cardiac disease will lead to decreased heart function in healthy recipients [12, 13]. Currently, none of the heart failure therapies are aimed at modulating this inflammatory process. The available immunosuppressive drugs suppress indiscriminately and can cause severe side effects, such as cardiac rupture [14, 15, 16].
Recently a lot of focus has been on the use of stem cell therapy to regenerate the damaged heart. Studies using different kind of progenitor cells, such as mesenchymal stem cells (MSC) or cardiomyocyte progenitor cells (CMPC), show a reduction in the decrease of cardiac function after MI [17, 18, 19, 20, 21], which is maintained up to at least three months after cell administration [17]. However, poor engraftment has been observed for both MSC and CPMC [17, 18, 19, 22] and, in the case of MSC, cardiac-differentiated cells have rarely been found [18]. This strengthens the hypothesis that most beneficial effects of cardiac stem cell injections arise from the secretion of paracrine factors [17, 22, 23]. Paracrine factors produced by stem cells can direct many processes, including stimulation of cardiomyocyte survival and angiogenesis, which could improve outcome after MI [18, 22]. An often overlooked area of interest could be cardiac inflammation, as MSC are well known for their immunomodulating actions [14, 24, 25, 26]. MSC are able to reduce inflammation by suppressing the different cells of the immune system or force them into anti-inflammatory or even regulatory subtypes [14, 25, 26]. In addition, MSC are known to secrete extracellular vesicles (EVs) [27, 28], which are small lipid bi-layered vesicles with a diameter between 30-100nm, containing a specific mixture of proteins, peptides, lipids and genetic material [28]. These EVs function as a form of intracellular communication and are able to influence many processes in the body, including inflammation [21, 27, 28, 29, 30]. For this reason, we compared the effect both BM-MSC, CMPC and their EVs have on the activated immune system and in specific, how they alter allogeneic T cell responses in vitro.
2. Materials and methods
2.1. Cell culture
After approval of the Ethics Committee and obtaining informed consent from the donors, human bone marrow-derived BM-MSC and human CMPC, both adult and fetal, were obtained and characterized in our lab as described previously [23, 31, 32]. The adult, patient-derived progenitor cells were obtained from patients with severe heart failure due to ischemia. Of both BM-MSC and CMPC four different donors were used between passage 6 and passage 17. Cells were cultured in plastic culture flasks coated with 0.1% gelatin. BM-MSC were cultured in MEM-alpha (Gibco, 22561) supplemented with 10% fetal bovine serum (FBS; Gibco, 10099-141), 100U/ml Penicillin and 100 μg/ml Streptomycin (Lonza, 17-602E), 1 ng/ml bFGF (Sigma F0291) and 0.2 mM L-ascorbic acid-2-phosphate (Sigma A4034), as described before [19]. CMPC were cultured in CMPC culture medium (1 part endothelial basal medium (EGM-2; Lonza CC-3156) and 3 parts M199 (Lonza BE12-119F) supplemented with 10% FBS, 100 U/ml Penicillin and 100 μg/ml Streptomycin and 1% Non-essential Amino Acids (Lonza 13-114), as described before [31, 33]. Both BM-MSC and CMPC were passaged when reaching 80–90% confluence by trypsin digestion (0.25% Trypsin; Lonza, CC-5012) at 37 °C for maximally two minutes.
Endothelial colony forming cells (ECFCs) were isolated from human umbilical cord blood and subsequently characterized as previously described [34]. Briefly, the mononuclear cell (MNC) fraction was isolated from whole blood using Ficoll-paque density gradient centrifugation (GE life sciences, 17-1440-02). MNCs were plated on rat-tail collagen type I (BD Biosciences, 354236) coated six-well culture plates in a final concentration of 2 × 107 cells per well in endothelial growth medium, consisting of EGM-2 supplemented with 10% FBS, 1% GlutaMax (Gibco, 35050038), 100U/ml Penicillin and 100 μg/ml Streptomycin. Medium was refreshed daily for the first four days. After day seven the cells were trypsinized and plated on fresh collagen type I coated wells until colonies appeared. ECFC colonies were isolated and passaged at 90% confluency.
To generate conditioned medium (CM), cell cultures were maintained at 37 °C for at least 3 days in a humidified atmosphere (5% CO2 and 20% O2). The CM was directly used in experiments, used for isolation of EVs, or stored in −20 °C for later use.
2.2. T cell isolation
Human blood of healthy volunteers was collected via the in-hospital donor-service after obtaining written consent. This donor service has been approved by the Ethics Committee of the University Medical Centre Utrecht and complies with the Declaration of Helsinki and the Good Clinical Practice guidelines. T cells were freshly isolated from the blood using the subsequent steps: Peripheral blood mononuclear cells (PBMC) were purified by a Ficoll-Paque density gradient (1.077 g/mL, GE healthcare, 17-1440-02), according to the manufacturer's protocol [23, 35]. Using anti-CD3 magnetic beads (BD Biosciences, 552593) and the BD IMagnet (BD Bioscience, 552311), T cells were isolated from the PBMCs according to manufacturer's protocol. T cells were labeled with 1.5μM carboxyfluorescein succinimidyl ester (CFSE; Sigma, 21888), as described previously [23]. CFSE was diluted stepwise to the desired concentration and incubated with the cells for 10 minutes at 37 °C in a dark, shaking water bath. Afterwards 5% FBS was added to block further uptake and cells were washed twice to remove excess CFSE.
2.3. Proliferation assay
T cell proliferation was determined in the presence of BM-MSC, CMPC, their conditioned medium or EVs. For this, stem cells were plated at a concentration of 5.0*104 cells per well in a 48-wells plate. In case of the pre-conditioning experiments, 20 ng/mL IFN-gamma (Sigma I3265) was added. After 24 hours, the medium was removed and 5.0*104 freshly isolated, CFSE-labeled T cells were added in RPMI-1640 (Lonza, BE12-702F) supplemented with 10% autologous human serum, 100U/ml Penicillin and 100 μg/ml Streptomycin.
T cells were activated using phorbol 12-myristate 13-acetate (PMA 0.123 ng/ml; Sigma, P8139) and IL-2 (120 IU/ml, BD Pharmingen, 554563) as described before [23]. After a six-day culture, cells were collected and Sytox blue (1μM; Invitrogen, S34857) was added to determine viability. Cell viability and proliferation was measured by flow cytometry (Gallios, Beckmann Coulter) and analyzed using Kaluza Analysis Software (Beckman Coulter, version 1.3) as followed: The fluorescent CFSE signal upon each cell division allowed us to count the number of cells present in each division. From this, we could calculate what percentage of the initial population had divided at the time of measurement (Suppl. Fig. 1). To allow comparisons between different donors, proliferation of the stimulated T cell sample was used for normalization. Where indicated 1-MT (Sigma 447439), Indomethacin (Sigma I7378) and Alk5 (Sigma S4696) were added at the start of the co-culture.
2.4. Cytokine analysis
Conditioned medium (CM) was collected from CMPC, BM-MSC and T cells or from CMPC and BM-MSC co-culture with T cells after 6 days. Conditioned medium was centrifuged at 500× g for 10 minutes and supernatant was collected. After filtering through a 0.2 μm filter (Corning, 431219), the CM was stored in −20 °C for analysis and further experiments.
Cytokines were measured using the multiplex immunoassay system (BioPlex, 200; Bio-Rad Laboratories) combined with the BioPlex Precision Pro Human Cytokine 10-Plex Panel (Bio Rad 171-A1001P), according to the manufacturer's protocol. This multiplex assay detects IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), IL-13, IFN-gamma and TNF-alpha. For analysis, the data was normalized to stimulated T cells to allow optimal comparisons between the different donors and groups.
2.5. Conditioned medium experiments
In the experiments where CM was used for T cell suppression, 2.0*104 freshly isolated, CFSE-labeled T cells were added per well in a 96 wells plate and CM was added 1:1 with fresh medium. T cells were collected for analysis by flow cytometry after 4 days of culture.
2.6. Extracellular vesicle (EV) isolation and purification
For the isolation of EVs, CMPC and BM-MSC were cultured in EV-free medium. Hereto all serum components were centrifuged 60 minutes at 150,000 x g. EVs were isolated and validated from the CM by differential centrifugation as described before [36]. The CM was subsequently centrifuged for 15–30 minutes at 2,000 g, for 30 minutes at 10,000 g at 4 °C (Beckman, Optima LE-80K Ultracentrifuge), and finally pelleted in a final centrifugation step (60 minutes at 100,000 g at 4 °C). The EV pellet was washed with PBS and pelleted by another centrifugation step for 60 minutes at 100,000 g at 4 °C. Finally, the washed pellets were resuspended in PBS and before their use in functional experiments total EV protein concentration was determined with BCA protein assay (ThermoScientific). Further characterization of the obtained EVs in our lab was performed as described previously [27, 36, 37]. To determine the effect of CMPC and BM-MSC derived EVs on activated T cells, these exosomes were added immediately after PMA and IL-2 activation. After six days, the cells were collected and analyzed by flow cytometry. All functional tests were performed with unlabeled EVs.
To visualize uptake, the EV pellet was stained with PKH-26 (Sigma, PKH26GL), and after labeling excess PKH-26 was inactivated with EV-free FBS. PKH-26-labeled EVs and excess PKH-26 were separated by sucrose gradient purification and subsequently pelleted again by centrifugation at 100,000 g. The labeled EVs were added to CFSE-labeled T cells. After incubation of various durations, T cells were trapped on glass slides, the nucleus was stained with Hoechst 1:10,000 (Invitrogen H3570), fixed with 4% paraformaldehyde, and analyzed with fluorescence microscopy.
2.7. RNA sequencing
Samples for the RNAseq were isolated and put in co-culture as described above. We included T cells from three different donors and progenitor cells from two different donors. After 3 days, the non-adherent T cells were collected, washed twice with PBS and stored at −80 °C. In the end, 16 samples were selected based on quality, including both unstimulated (n = 3) and stimulated (n = 3) T cells, as well as stimulated T cells which had been in contact with BM-MSC (n = 5) or CMPC (n = 5). RNA was isolated and libraries were created using the TruSeq Stranded Total RNA Sample Preparation LS according to manufacturer's protocol. An Illumina NextSeq500 and read-count analysis was performed by the Utrecht DNA Sequencing Facility (Utrecht, the Netherlands). RNA-seq reads were aligned to the human reference genome GRCh37 using STAR version2.4.2a [http://bioinformatics.oxfordjournals.org/content/29/1/15]. Read groups were added to the BAM files with Picard's AddOrReplaceReadGroups (v1.98). The BAM files are sorted with Sambamba v0.4.5 and transcript abundances are quantified with HTSeq-count version 0.6.1p1 [https://doi.org/10.1093/bioinformatics/btu638] using the union mode. Subsequently, RPKM's are calculated with edgeR's rpkm() function [https://doi.org/10.1093%2Fbioinformatics%2Fbtp616].
The resulting read counts per mRNA were subsequently analyzed according to the DESeq2 pipeline, to calculate differential expression (padj < 0.05) between the 4 different groups of samples [38]. PCA analysis was performed using DESeq2 command plotPCA() with “ntop = 5000” parameter.
2.8. Data analysis
All data are reported as mean ± SEM. Analysis was performed with IBM SPSS 20.0. For group comparison, parametric (one-way ANOVA) or non-parametric (Kruskal-Wallis) analysis was performed followed by a LSD and Mann-Whitney post-hoc analysis with a Bonferroni correction for significance respectively. A p-value <0.05 was considered significant.
3. Results
3.1. BM-MSC and CMPC suppress proliferation of allogeneic T cells
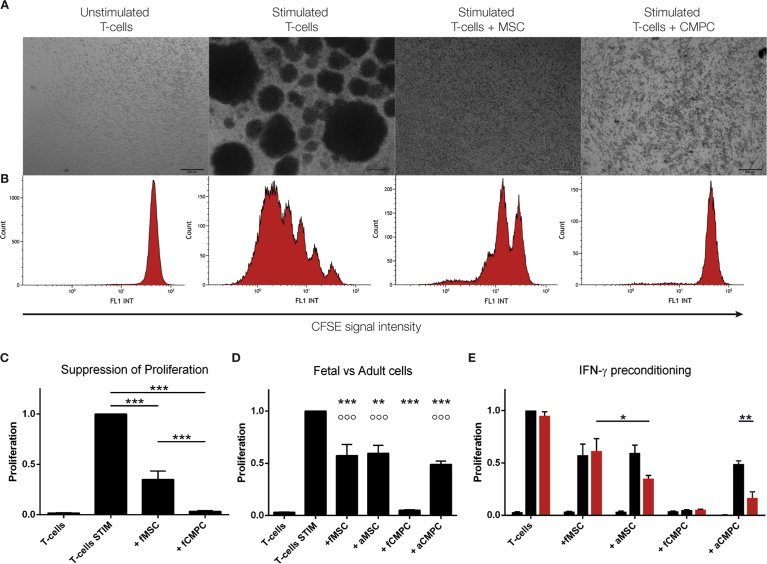
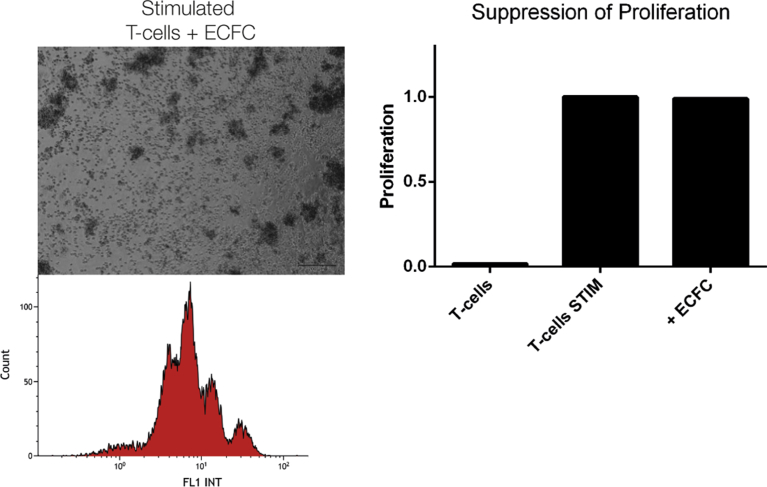
Several forms of T-cell stimulation were tested, including IL-2/PHA, CD3/CD28 beads, and mixed lymphocyte reactions, yet we decided to work with IL-2/PMA as it gave the strongest proliferative response. Upon stimulation T cells form proliferation clusters (Fig. 1A). In the presence of BM-MSC and CMPC this cluster formation was strongly reduced or even absent, whereas in the presence of endothelial colony forming cells (ECFC) proliferating T cell colonies still formed (Fig. 2). After checking for viability, proliferation of the T cells was measured and subsequently calculated based on CFSE-signal intensity using flow cytometry (Fig. 1B+C). No differences in viability were observed between the groups. In the non-stimulated conditions no proliferation could be observed, indicating the isolation process itself did not activate the T cells. In IL-2/PMA-stimulated cultures, the percentage of proliferating T cells was significantly lower in the presence of BM-MSC (65% ± 8%; p < 0.001) or CMPC (97% ± 0.6%; p < 0.001; Fig. 1C). ECFC, on the other hand, did not influence the proliferation of T cells (Fig. 2; 0% ± 0.2%). Comparing the two types of progenitor cells, the CMPC perform significantly better than the BM-MSC (Fig. 1C; p < 0.001). As there evidence that the age or health of the donor can affect the function of stem cells [39, 40], we tested whether there is a difference in potency when comparing the fetal-derived progenitor to adult, patient-derived progenitor cells (Fig. 1D). With respect to the BM-MSC, the age or health status of the donor did not impact the suppressive capacities of the cells. This is not the case for the CMPC, where the fetal CMPC performed significantly better than the diseased adult CMPC (p < 0.001). Even so, the adult CMPC still maintain immunosuppressive abilities comparable to fetal and adult BM-MSC.
Fig. 1.
BM-MSC and CMPC co-cultures with T cells after 6 days of stimulation. A. Light microscopical representation of T cell co-cultures after 6 days. In the non-stimulated samples, small individual cells are spread throughout the well. Upon stimulation, T cells form proliferating colonies. In the presence of BM-MSC and CMPC, the formation of these colonies is strongly reduced or even absent. Bar is 200 μm. B. Proliferation of T cells as measured by flow cytometry. Non-stimulated T cells have a single FL1 peak at a high fluorescent intensity. Upon stimulation, lower intensity peaks form, halving the fluorescent signal upon each cell division. C. Quantification of proliferation of T cells in different co-cultures. The first bar represents the unstimulated control, while the second bar is the stimulated T cells. Proliferation of T cells is significantly reduced in the presence of BM-MSC (65% ± 8) and CMPC (97% ± 0.6) (BM-MSC and CMPC: n = 12). D. Differences in suppressive capacity based on donor age. Both fetal and adult cells suppress T cell proliferation significantly. Adult BM-MSC suppress similar to fetal BM-MSC (40% ± 7.5 and 43% ± 11, resp.), while adults CMPC perform worse than fetal CMPC (51% ± 3.1 and 95% ± 0.4, resp.). E. The additional effect on T cell suppression due to ‘licensing’ was investigated. Progenitor cells were preconditioned with 20 ng/mL IFN-gamma (red bars) and compared to the unconditioned cells (black bars). Preconditioning had no effect on fetal stem cells. Adult BM-MSC improved to 65% ± 3 suppression, while adult CMPC improved to 83% ± 5.8 suppression (p = 0.006). ***p < 0.001 and **p < 0.01 compared to stimulated T cells. °°°p < 0.001 compared to T cells + fCMPC.
Fig. 2.
ECFC do not suppress number or proliferating T cells. A similar co-culture was performed with several endothelial colony forming cell (ECFC) donors. Unlike the mesenchymal stem cell and the cardiomyocyte progenitor cell, the ECFC were not able to prevent the formation of the T cell clusters or prevent proliferation (0 ± 0.2 suppression).
Several publications have shown that progenitor cells need to be ‘licensed’ before they show any suppressive capacities [41, 42, 43]. We investigated whether pre-conditioning affected the suppressive potential of these cells. Incubation with IFN-gamma for 24 hours had no effect on the progenitor cells of fetal origin (Fig. 1E). However, the adult progenitor cells perform better after the IFN-gamma treatment, which is especially clear in the case of the adult CMPC (p = 0.006).
3.2. BM-MSC and CMPC alter the inflammatory environment of stimulated T cells
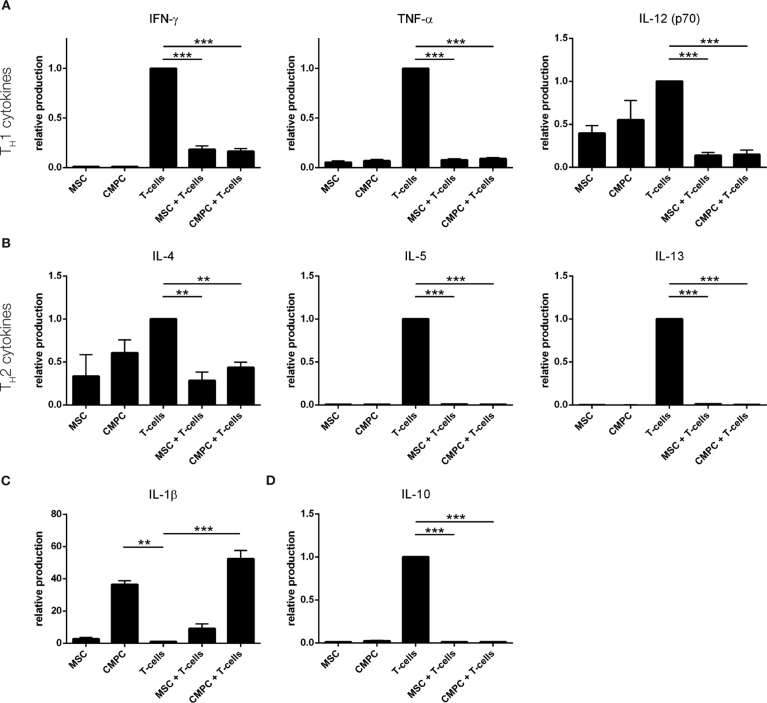
To examine the production and release of cytokines, conditioned medium (CM) was collected from individual BM-MSC, CMPC, and T cell cultures and from co-cultures of BM-MSC or CMPC with T cells. Cytokines indicative of both TH1 and TH2 were significantly reduced in the presence of BM-MSC and CMPC (Fig. 3A; Table 1). Interleukin-10 (IL-10), usually suggested to be anti-inflammatory and an inducer of Treg, was produced in high levels by active and proliferating T cells, whereas its release was strongly diminished in the presence of BM-MSC or CMPC (p < 0.001 for both; Fig. 3B). The cytokine IL-1beta was produced by BM-MSC and in high levels by CMPC compared to stimulated T cells (p < 0.01). In the co-culture experiments, these levels were increased even further, to 13-fold for BM-MSC and 52-fold for CMPC (p < 0.001).
Fig. 3.
Alteration of inflammatory environment. The cytokine production by BM-MSC and CMPC only, by T cells only, and upon co-culture of stem cells and T cells was measured by Luminex assays. A. Pro-inflammatory TH1 cytokines cytokines IFN-gamma, TNF-alpha and IL-12 are produced by stimulated T cells but down-regulated upon co-culturing with both BM-MSC and CMPC. A significant reduction in these cytokines in the conditioned medium indicated an environment that does not support the development of TH1 cells. B. The development of TH2 cells is determined by the presence of IL-4, IL-5 and IL-13. These cytokines are all upregulated in response to T cell activation and are significantly suppressed in the presence of progenitor cells. C. The release of IL-1b, which is produced by BM-MSC and CMPC yet hardly by T cells, is further increased upon co-culture with T cells. D. Release of IL-10, a supposedly anti-inflammatory cytokine, is strongly suppressed when progenitor cells are present in the co-culture, yet highly produced by stimulated T cells. For values see also Table 1. ***p < 0.001 and **p < 0.01.
Table 1.
Alteration of inflammatory environment.
| Cytokine release | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | IL-12-p70 | IL-13 | IFN-gamma | TNF-alpha | ||
| T-cells | unstimulated | 3.64 ± 2.51 p = 1.000 |
0.01 ± 0.01 p = 1.000 |
0.08 ± 0.02 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.26 ± 0.14 p < 0.001 |
0.01 ± 0.01 p < 0.001 |
0.04 ± 0.03 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.02 ± 0.01 p < 0.001 |
| stimulated | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | |
| BM-MSC | unstimulated | 0.43 ± 0.06 p = 1.000 |
0.24 ± 0.17 p = 1.000 |
0.33 ± 0.26 p = 0.002 |
0.01 ± 0.00 p < 0.001 |
1.25 ± 0.08 p = 0.495 |
0.01 ± 0.00 p < 0.001 |
0.37 ± 0.04 p = 0.001 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.04 ± 0.00 p < 0.001 |
| stimulated | 2.74 ± 0.85 p = 1.000 |
27.24 ± 18.15 p = 0.070 |
0.33 ± 0.25 p = 0.002 |
0.01 ± 0.00 p < 0.001 |
1.25 ± 0.11 p = 0.476 |
0.01 ± 0.00 p < 0.001 |
0.40 ± 0.09 p = 0.002 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.05 ± 0.01 p < 0.001 |
|
| CMPC | unstimulated | 5.86 ± 2.34 p = 0.996 |
0.01 ± 0.00 p = 1.000 |
0.50 ± 0.12 p = 0.051 |
0.01 ± 0.00 p < 0.001 |
1.09 ± 0.03 p = 0.999 |
0.02 ± 0.01 p < 0.001 |
0.50 ± 0.19 p = 0.017 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.06 ± 0.01 p < 0.001 |
| stimulated | 36.49 ± 2.32 p = 0.005 |
1.03 ± 0.22 p = 1.000 |
0.61 ± 0.15 p = 0.233 |
0.01 ± 0.00 p < 0.001 |
1.09 ± 0.04 p = 0.999 |
0.03 ± 0.00 p < 0.001 |
0.55 ± 0.23 p = 0.047 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.07 ± 0.01 p < 0.001 |
|
| BM-MSC + T-cells | unstimulated | 0.51 ± 0.12 p = 1.000 |
0.22 ± 0.07 p = 1.000 |
0.21 ± 0.08 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
1.26 ± 0.07 p = 0.123 |
0.01 ± 0.00 p < 0.001 |
0.17 ± 0.13 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.05 ± 0.02 p < 0.001 |
| stimulated | 9.13 ± 2.93 p = 0.689 |
31.71 ± 10.07 p = 0.001 |
0.28 ± 0.10 p = 0.005 |
0.01 ± 0.00 p < 0.001 |
1.29 ± 0.07 p = 0.065 |
0.01 ± 0.00 p < 0.001 |
0.14 ± 0.03 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.18 ± 0.04 p < 0.001 |
0.08 ± 0.01 p < 0.001 |
|
| CMPC + T-cells | unstimulated | 8.10 ± 1.84 p = 0.777 |
0.01 ± 0.00 p = 1.000 |
0.25 ± 0.03 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
1.09 ± 0.02 p = 0.983 |
0.06 ± 0.02 p < 0.001 |
0.12 ± 0.04 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.01 ± 0.00 p < 0.001 |
0.06 ± 0.01 p < 0.001 |
| stimulated | 52.41 ± 5.21 p < 0.001 |
1.17 ± 0.07 p = 1.000 |
0.44 ± 0.06 p = 0.005 |
0.01 ± 0.00 p < 0.001 |
1.07 ± 0.02 p = 0.997 |
0.01 ± 0.00 p < 0.001 |
0.15 ± 0.049 p < 0.001 |
0.00 ± 0.00 p < 0.001 |
0.16 ± 0.03 p < 0.001 |
0.09 ± 0.01 p < 0.001 |
|
Table showing the fold-increase or decrease in cytokine production compared to the stimulated T-cells. P-values are shown where applicable.
IL = Interleukin, IFN-gamma = interferon-gamma, TNF-alpha = tumor necrosis factor-alpha, MSC = mesenchymal stem cells, CMPC = cardiomyocyte progenitor cells.
3.3. Paracrine factors are responsible for T cell suppression
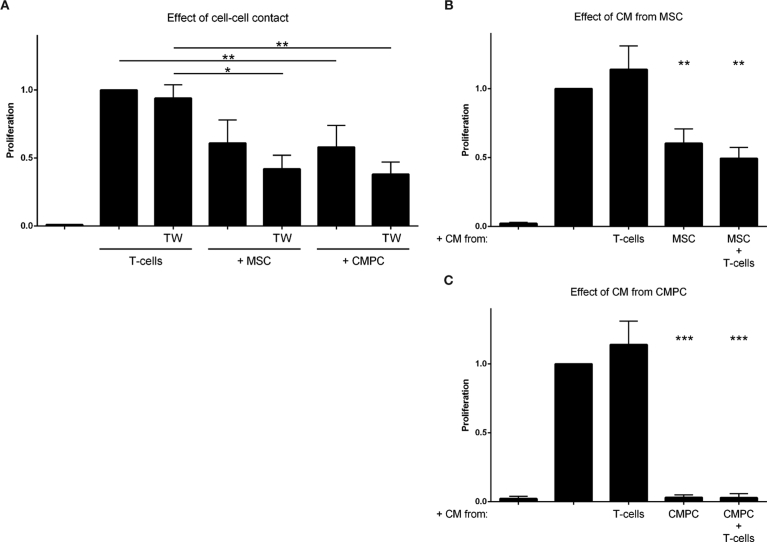
To investigate the dependence on direct cell-cell contact, we used an insert during culture to separate the different cell types from each other. Inhibitions of cell-contact did not reduce the immunomodulative effects (BM-MSC: 58 ± 10%, CMPC: 62 ± 9%, p < 0.05 for both; Fig. 4A). No statistically significant differences were observed between the groups with or without transwell filter.
Fig. 4.
Paracrine effect. A. Transwell experiment (TW) were performed where BM-MSC or CMPC are located in the bottom part and activated T cells on top of the 0.4 μm TW-filter. In control groups the cells were allowed cell-cell contact. A. Stimulated T cell co-cultures with BM-MSC and CMPC (TW −) and without (TW +) direct cell-cell contact. Suppression of proliferation occurs in cell contact groups (BM-MSC: 39 ± 17%, n.s., CMPC: 42 ± 16%, p < 0.05). Reduction of proliferation still occurs in the absence of direct cell-cell interactions (BM-MSC: 58 ± 10%, CMPC: 62 ± 9%, p < 0.05 for both) (n = 5). A. Stimulated T cells grown in the presence of CM from BM-MSC or the BM-MSC-T cell co-culture have a significantly reduced proliferation (40 ± 10% (n = 3) and 51 ± 10% (n = 7), respectively), whereas CM from stimulated T cell has no effect (14 ± 17% increase). B. Stimulated T cells proliferate significantly less after addition of CM from CMPC (97 ± 0.2%, p < 0.0001) or the CMPC-T cell co-culture (97 ± 0.3%). ***p < 0.001, **p < 0.01 and *p < 0.05.
Subsequently, we collected conditioned medium (CM) after six days of single- or co-culture, to investigate if a soluble, stable compound was present in cellular secretions. The obtained CM was added 1:1 with fresh medium to newly isolated and stimulated T cells. A significant suppression of T cell proliferation occurred in the presence of CM obtained from BM-MSC (40 ± 10%, p < 0.01), while the CM from the co-culture of BM-MSC with T cells performed even better (51 ± 8%, p < 0.01; Fig. 4B). This difference was, however, not significant. Even more pronounced effects were observed with the CMPC, where the CM from both the CMPC and the co-culture completely suppressed T cell proliferation (p < 0.0001) (Fig. 4C). No significant effect, neither suppressive nor stimulatory, was seen after addition of CM derived from stimulated T cells only (1.14 ± 17%, Fig. 4B and C).
3.4. Stem cell derived extracellular vesicles can inhibit T cell proliferation
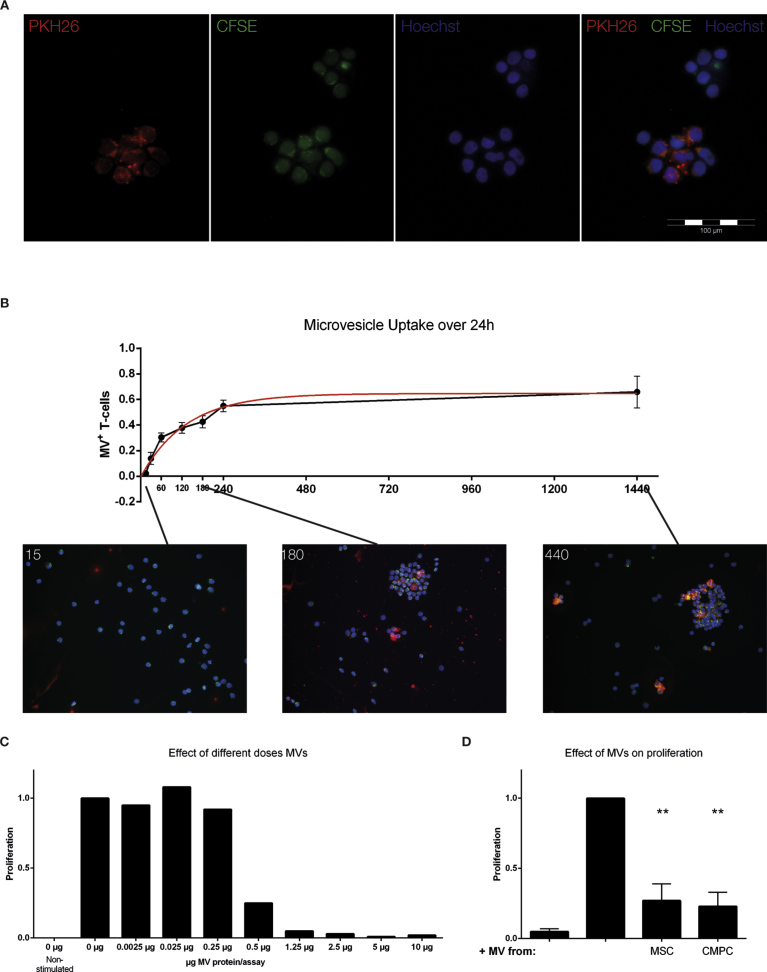
To investigate whether extracellular vesicles (EVs) secreted by stem cells can influence the proliferation of T cells, we isolated EVs from conditioned medium of BM-MSC and CMPC. To demonstrate uptake of EVs by stimulated T cells, we added purified PKH-26 (red) labeled EVs to CFSE-labeled (green) T cells. After overnight incubation, we observed by fluorescence microscopy a clear co-localization of the exosome-, T cell- and nuclear labels (Fig. 5A). We then quantified the uptake of these EVs by T cells over time (Fig. 5B) and noticed most EV uptake occurred in the first hour after addition. Around 35% of the T cells is positive at this time-point, which rises to approximately 60% after 24 hours. Control stainings were performed that suggest that free PKH-26 dye, obtained after ultracentrifugation, did not lead to signal uptake.
Fig. 5.
Effect of stem cell derived EVs on T cell proliferation. EVs were isolated from the conditioned medium of CMPC and BM-MSC to investigate their immunomodulative potential. A. Fluorescence microscope visualization of the EV uptake by T cells. Red: EVs (PKH-26). Green: T cells (CFSE). Blue: nucleus (Hoechst). B Titration curve of CMPC derived EV protein concentration and the effect on T cell proliferation after stimulation. A cut-off point is reached around 1 μg of EV protein. C BM-MSC- and CMPC-derived EVs significantly reduce T cell proliferation (BM-MSC-EV: 73 ± 12%, CMPC-EV: 77 ± 10%). **p < 0.01 compared to stimulated T cell.
Next, we examined the effect of EVs on T cell proliferation in a dose-response experiment using 0.0025μg–10μg of EVs (Fig. 5C). A clear dose-dependent effect on T cell proliferation was visible, as shown in Fig. 5C. We subsequently added 1.5μg of BM-MSC- or CMPC-derived EVs to stimulated T cells. The addition of BM-MSC- or CMPC-derived EVs resulted in a strong decrease of proliferation (Fig. 5D; 73 ± 12%, p < 0.01 and 77 ± 10%, p < 0.01, respectively).
3.5. Pathway inhibitors
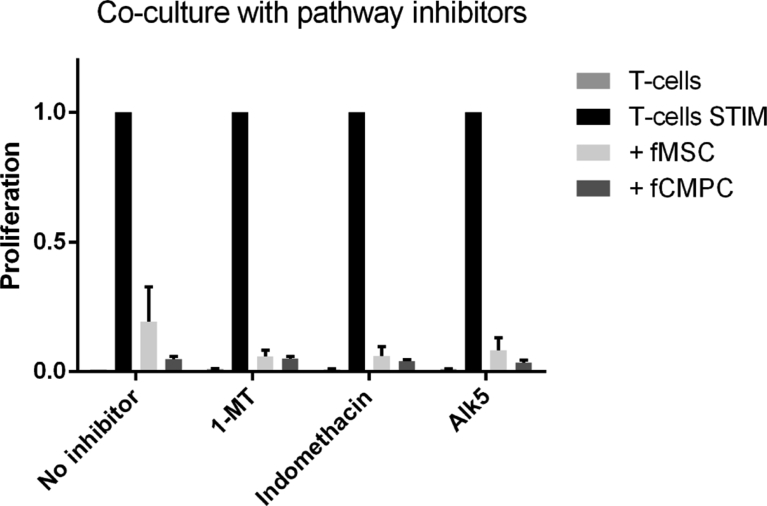
Several studies have reported a number of pathways to be involved in the immunosuppressive responses, amongst which indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) [14,41,44,45]. However, we studied the proteomics of our EVs and did not find any components of the IDO or PGE2 pathways to be present (data not shown). Even so, we performed the co-culture assay in the presence of several inhibitors: 1-methyl-L-tryptophan (against IDO), indomethacine (against PGE2) and Alk5 (against TGF-beta). Upon co-culture we did not see any change in the T cell suppression (Fig. 6). In addition to the inhibitor doses used by other research groups [46, 47, 48], we have performed a dose titration, as well as preconditioning and repetitive administration of the inhibitors to block the immunosuppression. However, we still did not find any effect of these inhibitors.
Fig. 6.
Effect of inhibitors on immunosuppression. Co-culture was performed as described, in the presence or absence of progenitor cells and inhibitors for the immunosuppressive pathways: 1-MT (1 mM; blocks IDO), Indomethacin (10 μM; block PGE2) and Alk5 (10 μM: competes with TGF-beta signaling). None of these inhibitors showed an effect compared to the control group. This is one representative set of many experiments, which all showed no effect on T cell proliferation.
3.6. RNAseq shows differentially regulated genes
In order to get an unbiased view on the changes induced in the T cells, we performed an RNA sequence on several individual T cell donors that were exposed to different progenitor cell donors.
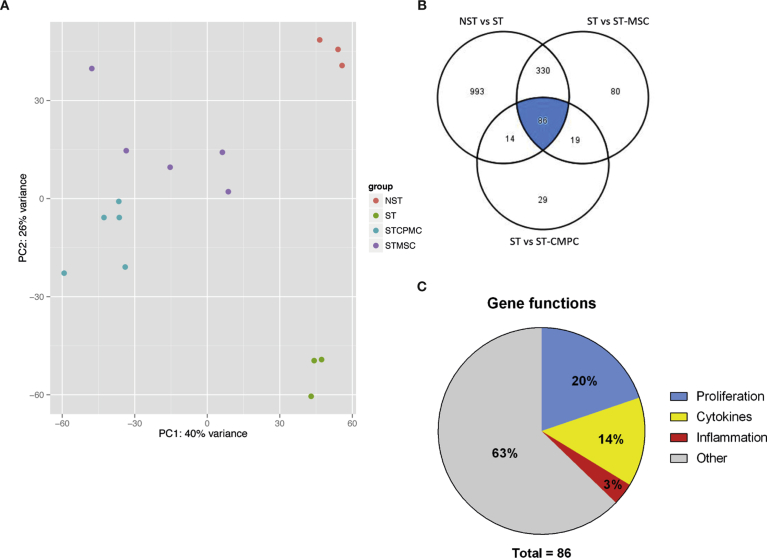
Unsupervised principal component analysis in the sixteen T cell samples, color-coded by group (Fig. 7A) based on 5000 most variable transcripts (ref DESeq2), illustrated a close proximity of samples per group. Additionally, principal component analysis showed that 66% of the variation in the dataset can be explained by either the presence of the progenitor cells (PC1; 40%) and the T cell activation (PC2; 26%).
Fig. 7.
RNAseq of T cell modulation. A. Principal component analysis shows a division of samples in four groups, matching our experimental conditions. Most variation between the samples is explained by the presence of stem cells in the culture (PC1: 40%) and the activation of T cells (PC2: 26%) (NST – non stimulated, ST stimulated etc) B. Venn-diagram showing the overlap between genes that are >2-fold upregulated upon activation (left-upper circle), and >2-fold downregulated in the vicinity of BM-MSC (right-upper circle) or CMPC (lower circle). C. Pie-chart showing the functions of the 86 genes found.
Upon activation of T cells, almost 1500 genes were significantly upregulated (>2-fold, padj < 0.05). Of these genes, 416 were suppressed (>2-fold) in the presence of BM-MSC, compared to only 100 genes in the presence of CMPC (Fig. 7B). 86 T cell genes were upregulated upon activation and suppressed in the presence of either BM-MSC or CMPC, as shown in the Venn diagram (see also Table 2). Subsequently, we entered these genes in IPA, which showed pathways concerning inflammation and immune cell proliferation. However, we noticed all these pathways depended largely on a small number of the 86 genes. Therefore, we used the NBCI Gene database (http://www.ncbi.nlm.nih.gov/gene/) and the GeneCards database (http://www.genecards.org/) to investigate the function of these genes in T cells. As depicted in the pie-chart in Fig. 7C, 20% of these genes (17 genes) has a known role in cell proliferation. Another 14% (12 genes) is related to the production and release of cytokines and/or their receptors. Lastly, only 3 genes (∼3%) are associated with inflammation. This leaves 54 genes (63%) that have no clear role in proliferation/inflammation or no known function.
Table 2.
86 altered genes.
| Symbol | Entrez Gene Name | Location | Type(s) | |
|---|---|---|---|---|
| 1 | ASB2 | ankyrin repeat and SOCS box containing 2 | Nucleus | transcription regulator |
| 2 | ASB9 | ankyrin repeat and SOCS box containing 9 | Nucleus | transcription regulator |
| 3 | DDX4 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 | Nucleus | enzyme |
| 4 | DEPDC1 | DEP domain containing 1 | Nucleus | transcription regulator |
| 5 | DHRS2 | dehydrogenase/reductase (SDR family) member 2 | Nucleus | enzyme |
| 6 | EBNA1BP2 | EBNA1 binding protein 2 | Nucleus | other |
| 7 | GINS2 | GINS complex subunit 2 (Psf2 homolog) | Nucleus | other |
| 8 | HIST1H1A | histone cluster 1, H1a | Nucleus | other |
| 9 | HIST1H2AI | histone cluster 1, H2ai | Nucleus | other |
| 10 | HIST1H2BC | histone cluster 1, H2bc | Nucleus | other |
| 11 | HIST1H2BL | histone cluster 1, H2bl | Nucleus | other |
| 12 | HIST1H3J | histone cluster 1, H3j | Nucleus | other |
| 13 | HIST2H2AB | histone cluster 2, H2ab | Nucleus | other |
| 14 | HIST2H2BF | histone cluster 2, H2bf | Nucleus | other |
| 15 | HIST3H2BB | histone cluster 3, H2bb | Nucleus | other |
| 16 | MCM2 | minichromosome maintenance complex component 2 | Nucleus | enzyme |
| 17 | POLR3G | polymerase (RNA) III (DNA directed) polypeptide G (32kD) | Nucleus | enzyme |
| 18 | RANBP1 | RAN binding protein 1 | Nucleus | other |
| 19 | S100A2 | S100 calcium binding protein A2 | Nucleus | other |
| 20 | TERT | telomerase reverse transcriptase | Nucleus | enzyme |
| 21 | APOBEC3B | apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3B | Cytoplasm | enzyme |
| 22 | BSPRY | B-box and SPRY domain containing | Cytoplasm | other |
| 23 | CAMK1 | calcium/calmodulin-dependent protein kinase I | Cytoplasm | kinase |
| 24 | CCNB1 | cyclin B1 | Cytoplasm | kinase |
| 25 | DAPP1 | dual adaptor of phosphotyrosine and 3-phosphoinositides | Cytoplasm | other |
| 26 | GAD1 | glutamate decarboxylase 1 (brain, 67kDa) | Cytoplasm | enzyme |
| 27 | GALNT18 | polypeptide N-acetylgalactosaminyltransferase 18 | Cytoplasm | enzyme |
| 28 | GLDC | glycine dehydrogenase (decarboxylating) | Cytoplasm | enzyme |
| 29 | KLK1 | kallikrein 1 | Cytoplasm | peptidase |
| 30 | MB | myoglobin | Cytoplasm | transporter |
| 31 | NCF2 | neutrophil cytosolic factor 2 | Cytoplasm | enzyme |
| 32 | NME1 | NME/NM23 nucleoside diphosphate kinase 1 | Cytoplasm | kinase |
| 33 | ODF1 | outer dense fiber of sperm tails 1 | Cytoplasm | other |
| 34 | PAICS | phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoimidazole succinocarboxamide synthetase | Cytoplasm | enzyme |
| 35 | PLCG2 | phospholipase C, gamma 2 (phosphatidylinositol-specific) | Cytoplasm | enzyme |
| 36 | PTPN3 | protein tyrosine phosphatase, non-receptor type 3 | Cytoplasm | phosphatase |
| 37 | SERPINB10 | serpin peptidase inhibitor, clade B (ovalbumin), member 10 | Cytoplasm | other |
| 38 | STON2 | stonin 2 | Cytoplasm | other |
| 39 | TPRG1 | tumor protein p63 regulated 1 | Cytoplasm | other |
| 40 | ART3 | ADP-ribosyltransferase 3 | Plasma Membrane | enzyme |
| 41 | CCR2 | chemokine (C-C motif) receptor 2 | Plasma Membrane | G-protein coupled receptor |
| 42 | CHRNA6 | cholinergic receptor, nicotinic, alpha 6 (neuronal) | Plasma Membrane | transmembrane receptor |
| 43 | CLECL1 | C-type lectin-like 1 | Plasma Membrane | other |
| 44 | CPNE5 | copine V | Plasma Membrane | other |
| 45 | ENPP2 | ectonucleotide pyrophosphatase/phosphodiesterase 2 | Plasma Membrane | enzyme |
| 46 | GAP43 | growth associated protein 43 | Plasma Membrane | other |
| 47 | GJB2 | gap junction protein, beta 2, 26kDa | Plasma Membrane | transporter |
| 48 | GNA14 | guanine nucleotide binding protein (G protein), alpha 14 | Plasma Membrane | enzyme |
| 49 | HCAR1 | hydroxycarboxylic acid receptor 1 | Plasma Membrane | G-protein coupled receptor |
| 50 | IGHM | immunoglobulin heavy constant mu | Plasma Membrane | transmembrane receptor |
| 51 | IL17RB | interleukin 17 receptor B | Plasma Membrane | transmembrane receptor |
| 52 | IL23R | interleukin 23 receptor | Plasma Membrane | transmembrane receptor |
| 53 | MYO3B | myosin IIIB | Plasma Membrane | kinase |
| 54 | NINJ2 | ninjurin 2 | Plasma Membrane | other |
| 55 | TNFRSF8 | tumor necrosis factor receptor superfamily, member 8 | Plasma Membrane | transmembrane receptor |
| 56 | CGREF1 | cell growth regulator with EF-hand domain 1 | Extracellular Space | other |
| 57 | EBI3 | Epstein-Barr virus induced 3 | Extracellular Space | cytokine |
| 58 | FBLN5 | fibulin 5 | Extracellular Space | other |
| 59 | IFNG | interferon, gamma | Extracellular Space | cytokine |
| 60 | IL17A | interleukin 17A | Extracellular Space | cytokine |
| 61 | IL17F | interleukin 17F | Extracellular Space | cytokine |
| 62 | IL5 | interleukin 5 | Extracellular Space | cytokine |
| 63 | IL9 | interleukin 9 | Extracellular Space | cytokine |
| 64 | LTA | lymphotoxin alpha | Extracellular Space | cytokine |
| 65 | NAPSA | napsin A aspartic peptidase | Extracellular Space | peptidase |
| 66 | PRG4 | proteoglycan 4 | Extracellular Space | other |
| 67 | TNFSF15 | tumor necrosis factor (ligand) superfamily, member 15 | Extracellular Space | cytokine |
| 68 | C4orf26 | chromosome 4 open reading frame 26 | Other | other |
| 69 | CDC20P1 | cell division cycle 20 pseudogene 1 | Other | other |
| 70 | CKS2 | CDC28 protein kinase regulatory subunit 2 | Other | kinase |
| 71 | COX17P1 | COX17 cytochrome c oxidase copper chaperone pseudogene 1 | Other | other |
| 72 | HMSD | histocompatibility (minor) serpin domain containing | Other | other |
| 73 | HPDL | 4-hydroxyphenylpyruvate dioxygenase-like | Other | other |
| 74 | HSPE1P2 | heat shock 10kDa protein 1 pseudogene 2 | Other | other |
| 75 | LINC00158 | long intergenic non-protein coding RNA 158 | Other | other |
| 76 | LINC00877 | long intergenic non-protein coding RNA 877 | Other | other |
| 77 | LINC00892 | long intergenic non-protein coding RNA 892 | Other | other |
| 78 | LINC01132 | long intergenic non-protein coding RNA 1132 | Other | other |
| 79 | LINC01281 | long intergenic non-protein coding RNA 1281 | Other | other |
| 80 | NAPSB | napsin B aspartic peptidase, pseudogene | Other | other |
| 81 | PAICSP4 | phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoimidazole succinocarboxamide synthetase pseudogene 4 | Other | other |
| 82 | PHBP3 | prohibitin pseudogene 3 | Other | other |
| 83 | PIK3CD-AS2 | PIK3CD antisense RNA 2 | Other | other |
| 84 | RCAN2 | regulator of calcineurin 2 | Other | other |
| 85 | RNU5A-8P | RNA, U5A small nuclear 8, pseudogene | Other | other |
| 86 | SLC16A9 | solute carrier family 16, member 9 | Other | other |
This table contains the 86 genes significantly upregulated by activation of T cells, and significantly downregulated in the presence of BM-MSC or CMPC. It shows the symbol, name, location and the type of the end-product.
4. Discussion
In this study, we have demonstrated that both BM-MSC and CMPC inhibit T cell proliferation in vitro, with CMPC having a significantly stronger suppressive effect than BM-MSC. There was no difference in low and high passage progenitor cells on suppressing T cell proliferation (data not shown) but CMPC showed differences based on donor age. In addition to suppressing proliferation, both BM-MSC and CMPC also suppressed the release of pro-inflammatory cytokines from T cells. Although a shift towards a specific T cell phenotype is occasionally reported [35, 49], we found cytokines of both TH1, TH2 and Treg subtypes to be suppressed, suggesting that the T cells remain in a naïve, inactivated state.
It was interesting to notice both BM-MSC and CMPC produce high levels of IL-1beta, when compared to the stimulated T-cells. Although it is known cardiomyocytes can produce IL-1 under ischemic stress, this has not yet been observed for these progenitors [50]. It could be hypothesized that inflammatory stress might trigger CMPC to produce IL-1, thereby potentially stimulating inflammation. However, a recent study showed IL-1 actually primes MSC to maintain an anti-inflammatory phenotype [51]. Therefore, one could also argue that progenitor cells produce this cytokine to control their own function in inflammatory conditions. More studies on this will be needed to further elicit the processes at play here.
Suppression of T cell proliferation by BM-MSC has been shown in previous studies [24, 25, 26, 46, 52], albeit with strong variation in suppression between different donors [52]. With our discovery that CMPC are strong modulators of the immune system and the reports of immunomodulative effects of neural stem cells [52, 53], we wondered whether immunomodulation is a more general stem cell trait. For this reason, we included ECFC, a circulatory endothelial colony forming cell, which proved unable to alter the number of proliferating cells.
Both BM-MSC and CMPC mediated immunomodulation by production of paracrine factors, which was readily demonstrated in our transwell experiments. This finding is in agreement with DiNicola et al. [24] who also found that cell-cell contact was dispensable for immunosuppression by BM-MSC, while others [41, 46, 54] found reduced suppression in absence of cell-contact. A possible explanation for this is that cell-cell contact is not necessary for the suppression itself, but for induction of the T-regulatory phenotype [41]. Another explanation for these observed differences could be the different origins, isolation methods and (co-)culture methods in the different studies, which makes it hard to compare an already heterogenous group of progenitor cells [52].
The immunomodulative paracrine factors were already present during normal expansion cultures without any immune cells being present (Figs. 4 and 5). This is in contrast with studies that claim the progenitor cells need to be ‘licensed’ by immune cells to release these suppressive factor [41, 42, 43]. Again, it is quite likely that the different origins of progenitor cells in different studies could be responsible there. Our study shows that adult progenitor cells appear to diminish in immunosuppressive capacity, but can be re-activated by exposing them to IFN-gamma, while on the fetal progenitor cells this preconditioning has no effect.
Immunosuppression by EVs derived from (modified) dendritic cells [55, 56, 57] or cancer stem cells [58] has been reported in the past. Meanwhile, more recent studies have shown effects of BM-MSC-derived EVs on inflammation in different organ tissues and proliferation of lymphocytes [21, 29, 30, 59, 60, 61, 62]. In many of these studies either an animal model or the whole peripheral blood mononuclear cell (PBMC) or splenocyte fraction was used, leaving it ambiguous whether observed effects were caused by direct interference with the T cells or indirectly via another cell type, such as macrophages [29, 30, 61, 63]. Only few other studies directly look at a suppressive effect on T-cells [21, 64]. We examined the immunomodulating capacity of not only BM-MSC-derived exosomes, but also the CMPC-derived EVs on pure CD3+ T cells and observed a strong inhibition of proliferation in vitro when either EVs was added to stimulated T cells. Our CMPC-EV titration experiment indicated this effect is dose-dependent, as was also observed by others for MSC-EVs [21]. However, the amount of suppression differs between the different studies, likely due to different culture and isolation methods, as well as subtle differences in amounts of EVs added. We do believe that, although BM-MSC- and CMPC-derived EVs are important mediators of immunomodulation, they do not cover the complete suppressive effect, and will most likely function optimally in combination with several growth factors or cytokines produced by the progenitor cells.
Several potential mediators have been investigated for their involvement in the immunomodulatory effects, including interleukin-10 (IL-10), inducible nitric oxide synthase (iNOS), transforming growth factor-beta (TGF-β), prostaglandin E2, and indoleamine 2,3-dioxygenase (IDO) [14, 25, 41, 44, 46, 65]. Of these, the last two have been most investigated in different settings. Several studies have attempted to block these pathways, often resulting in a variable decrease of the immune suppressive effect of BM-MSC. However, these experiments had variable outcomes and until now the exact mechanisms of immune suppression remain controversial [14, 41, 46, 65, 66]. In our hands, addition of inhibitors for these pathways did not show any effect on the immunosuppressive effects of the progenitor cells. We did not include an inhibitor against iNOS in these experiments, as our CM experiments already demonstrated the mediator is a stable compound, which nitric oxide (NO) is not.
An explanation for our observed ineffectiveness of pathway inhibition is suggested by our RNA sequencing. We found 86 genes which are upregulated during T cell activation and are suppressed in the presence of progenitor cells. Less than half of these genes is directly linked to proliferation or inflammation; the majority has either completely different or unknown functions. We believe these genes to play an important role in the modulation of T cells and warrant further investigation.
We recognize some limitations of this study. The first is inherent in the study of the immune system in vitro. We used a non-physiological method of T cell activation, as the complex activation seen after MI is impossible to simulate in vitro [14, 67]. This leads directly to the second limitation in our study, which applies to all in vitro immune research: the immune system is a complex and interactive system in which all components strongly influence each other and excluding a specific cell type could unbalance this system and possibly influence the interactions with BM-MSC or CMPC. Thirdly, the exact murine counterpart of the human cardiac-derived CMPC has not yet been identified. Therefore, in vivo research using human CMPC is exclusively performed in immunodeficient mice to reduce immediate stem cell rejection. Unfortunately, this also prevents the investigation of T-cell responses upon stem cell injections after MI, as these animals have no adaptive immune system. A humanized mouse model would be necessary to confirm the in vivo potential of these cells is as strong as observed here in vitro.
We demonstrated that both mesenchymal stem cells (BM-MSC) and cardiomyocyte progenitor cells (CMPC) strongly modulate the immune system by attenuating T cell proliferation in vitro and reducing release of pro-inflammatory cytokines. This suppression is not dependent on ‘licencing’ nor on cell-cell contact. It is mediated via paracrine factors, which are already produced during regular culture. EVs isolated from the conditioned medium were shown to be dose-dependently capable of suppressing T cell proliferation and should be further studied as a possible new treatment for post-MI inflammation, to reduce damage to the heart in both short and long term. Lastly, despite earlier publication on pathways involved, we found a pallet of unstudied genes expected to play a major role in the activation and suppression of T cells which need further investigation.
Declarations
Author contribution statement
Frederieke van den Akker: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Krijn Vrijsen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Janine Deddens: Performed the experiments; Analyzed and interpreted the data.
Jan-Willem Buikema, Linda Van Laake: Analyzed and interpreted the data; Wrote the paper.
Michal Mokry, Pieter Doevendans: Analyzed and interpreted the data.
Joost Sluijter: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by a grant from the Alexandre Suerman program for MD/PhD students of the University Medical Center Utrecht, the Netherlands. This work was further supported by the ZonMw-TAS program (#116002016), the European Research Council (ERC) consolidator grants Evicare (#725229) and the CVON2011-12 HUSTCare grant from the Netherlands CardioVascular Research Initiative (CVON) (a collaboration between the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors also thank dr. D. Muylaert for providing ECFC and A. Schoneveld for his technical help.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig. 1.
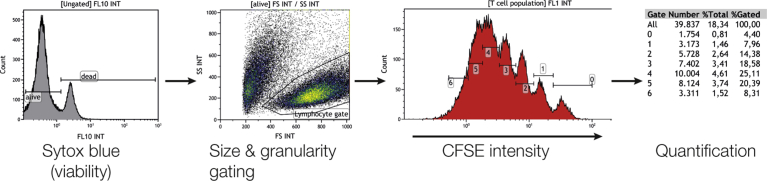
Flow cytometric gating and analysis. The non-adherent T cells were collected from the culture plates and labeled with Sytox blue as a viability marker. These cells were then checked for viability and only the living fraction was plotted in a forward-sideward scatter. Subsequently, we created a lymphocyte gate based on size and granularity, and in these cells we visualized the CFSE signal. Clear peaks were visible, allowing us to set gates per division. Taking into account the symmetrical division of T cells, this allowed us to calculate the number of original T cells that proliferated.
References
- 1.Townsend N., Nichols M., Scarborough P. Cardiovascular disease in Europe – epidemiological update 2015. Eur. Heart J. 2015;36:2696–2705. doi: 10.1093/eurheartj/ehv428. [DOI] [PubMed] [Google Scholar]
- 2.Yokota N., Burne-Taney M., Racusen L. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2003;285:F319–F325. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z., Sharma A.K., Linden J. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalheiro T., Velada I., Valado A. Phenotypic and functional alterations on inflammatory peripheral blood cells after acute myocardial infarction. J. Cardiovasc. Transl. Res. 2012;5:309–320. doi: 10.1007/s12265-012-9365-8. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis N.G., Smith C.W., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 6.Fukui D., Yasukawa H., Sugi Y. Transient reduction and activation of circulating dendritic cells in patients with acute myocardial infarction. Int. J. Cardiol. 2012;160:216–219. doi: 10.1016/j.ijcard.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz A., Dietel B., Cicha I. Emergence of dendritic cells in the myocardium after acute myocardial infarction – implications for inflammatory myocardial damage. Int. J. Biomed. Sci. 2010;6:27–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Moraru M., Roth A., Keren G. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. Int. J. Cardiol. 2006;107:61–66. doi: 10.1016/j.ijcard.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 9.De Scheerder I., Vandekerckhove J., Robbrecht J. Post-cardiac injury syndrome and an increased humoral immune response against the major contractile proteins (actin and myosin) Am. J. Cardiol. 1985;56:631–633. doi: 10.1016/0002-9149(85)91024-0. [DOI] [PubMed] [Google Scholar]
- 10.Shmilovich H., Danon A., Binah O. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int. J. Cardiol. 2007;117:198–203. doi: 10.1016/j.ijcard.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 11.Varda-Bloom N., Leor J., Ohad D.G. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J. Mol. Cell. Cardiol. 2000;32:2141–2149. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- 12.Maisel A., Cesario D., Baird S. Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circ. Res. 1998;82:458–463. doi: 10.1161/01.res.82.4.458. [DOI] [PubMed] [Google Scholar]
- 13.Ismahil M.A., Hamid T., Bansal S.S. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 2013 doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Akker F., Deddens J.C., Doevendans P.A. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim. Biophys. Acta. 2013;1830:2449–2458. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano G.R., Giugliano R.P., Gibson C.M. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am. J. Cardiol. 2003;91:1055–1059. doi: 10.1016/s0002-9149(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 16.Sholter D.E., Armstrong P.W. Adverse effects of corticosteroids on the cardiovascular system. Can. J. Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 17.Smits A.M., van Laake L.W., den Ouden K. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc. Res. 2009;83:527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 18.Noort W.A., Feye D., Van Den Akker F. Mesenchymal stromal cells to treat cardiovascular disease: strategies to improve survival and therapeutic results. Panminerva Med. 2010;52:27–40. [PubMed] [Google Scholar]
- 19.Noort W.A., Sluijter J.P., Goumans M.J. Stem cells from in- or outside of the heart: isolation, characterization, and potential for myocardial tissue regeneration. Pediatr. Cardiol. 2009;30:699–709. doi: 10.1007/s00246-008-9370-5. [DOI] [PubMed] [Google Scholar]
- 20.Dimmeler S., Zeiher A.M., Schneider M.D. Unchain my heart: the scientific foundations of cardiac repair. J. Clin. Investig. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monguio-Tortajada M., Roura S., Galvez-Monton C. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: implications for nanomedicine. Theranostics. 2017;7:270–284. doi: 10.7150/thno.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnecchi M., Zhang Z., Ni A. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noort W.A., Oerlemans M.I., Rozemuller H. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J. Cell Mol. Med. 2011 doi: 10.1111/j.1582-4934.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Nicola M., Carlo-Stella C., Magni M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 25.Hoogduijn M.J., Popp F., Verbeek R. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int. Immunopharm. 2010 doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 27.Vrijsen K.R., Maring J.A., Chamuleau S.A. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv. Healthcare Mater. 2016;5:2555–2565. doi: 10.1002/adhm.201600308. [DOI] [PubMed] [Google Scholar]
- 28.Sluijter J.P.G., Verhage V., Deddens J.C. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 29.Shigemoto-Kuroda T., Oh J.Y., Kim D.K. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8:1214–1225. doi: 10.1016/j.stemcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doeppner T.R., Herz J., Gorgens A. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits A.M., van Vliet P., Metz C.H. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 32.Noort W.A., Kruisselbrink A.B., in't Anker P.S. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp. Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 33.Goumans M.J., de Boer T.P., Smits A.M. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 34.de Jonge N., Muylaert D.E., Fioretta E.S. Matrix production and organization by endothelial colony forming cells in mechanically strained engineered tissue constructs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy M.M., Ritter T., Ceredig R. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrijsen K.R., Sluijter J.P.G., Schuchardt M.W.L. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J. Cell Mol. Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mol E.A., Goumans M.J., Doevendans P.A. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017 doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Wu Q., Wang Y. Senescence of mesenchymal stem cells (Review) Int. J. Mol. Med. 2017;39:775–782. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 40.Charif N., Li Y.Y., Targa L. Aging of bone marrow mesenchymal stromal/stem cells: implications on autologous regenerative medicine. Bio Med. Mater. Eng. 2017;28:S57–S63. doi: 10.3233/BME-171624. [DOI] [PubMed] [Google Scholar]
- 41.English K., Ryan J.M., Tobin L. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krampera M., Cosmi L., Angeli R. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells (Dayton Ohio) 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 43.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25:1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 44.Meisel R., Zibert A., Laryea M. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 45.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 46.Ren G., Su J., Zhang L. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 47.Roemeling-van Rhijn M., Mensah F.K., Korevaar S.S. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front. Immunol. 2013;4:203. doi: 10.3389/fimmu.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.English K., Barry F.P., Field-Corbett C.P. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Mándi Y., Vécsei L. The kynurenine system and immunoregulation. J. Neural Transm. (Vienna Austria 1996) 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 50.Taqueti V.R., Mitchell R.N., Lichtman A.H. Protecting the pump: controlling myocardial inflammatory responses. Annu. Rev. Physiol. 2006;68:67–95. doi: 10.1146/annurev.physiol.68.040104.124611. [DOI] [PubMed] [Google Scholar]
- 51.Redondo-Castro E., Cunningham C., Miller J. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017;8:79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Akker F., de Jager S.C.A., Sluijter J.P.G. Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors. Med Inflam. 2013;2013 doi: 10.1155/2013/181020. 181020- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ben-Hur T. Immunomodulation by neural stem cells. J. Neurol. Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Ren G., Zhao X., Zhang L. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S.H., Lechman E.R., Bianco N. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 56.Kim S.H., Bianco N., Menon R. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.H., Bianco N.R., Shufesky W.J. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J. Immunol. 2007;179:2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 58.Teng H., Hu M., Yuan L.X. Suppression of inflammation by tumor-derived exosomes: a kind of natural liposome packaged with multifunctional proteins. J. Liposome Res. 2012;22:346–352. doi: 10.3109/08982104.2012.710911. [DOI] [PubMed] [Google Scholar]
- 59.Conforti A., Scarsella M., Starc N. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23:2591–2599. doi: 10.1089/scd.2014.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman M.J., Regn D., Bashratyan R. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes. 2014;63:1008–1020. doi: 10.2337/db13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B., Yin Y., Lai R.C. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 62.Teng X., Chen L., Chen W. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. : Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 63.Del Fattore A., Luciano R., Pascucci L. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24:2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 64.Matula Z., Nemeth A., Lorincz P. The role of extracellular vesicle and tunneling nanotube-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cells. Stem Cells Dev. 2016;25:1818–1832. doi: 10.1089/scd.2016.0086. [DOI] [PubMed] [Google Scholar]
- 65.Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 66.Ren G., Zhang L., Zhao X. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Frangogiannis N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]