Abstract
In a constantly changing environment we must adapt to both abrupt and gradual changes to incoming information. Previously, we demonstrated that a distributed network (including the anterior insula and anterior cingulate cortex) was active when participants updated their initial representations (e.g., it's a cat) in a gradually morphing picture task (e.g., now it's a rabbit; Stöttinger et al., 2015). To shed light on whether these activations reflect the proactive decisions to update or perceptual uncertainty, we introduced two additional conditions. By presenting picture morphs twice we controlled for uncertainty in perceptual decision making. Inducing an abrupt shift in a third condition allowed us to differentiate between a proactive decision in uncertainty-driven updating and a reactive decision in surprise-based updating. We replicated our earlier result, showing the robustness of the effect. In addition, we found activation in the anterior insula (bilaterally) and the mid frontal area/ACC in all three conditions, indicative of the importance of these areas in updating of all kinds. When participants were naïve as to the identity of the second object, we found higher activations in the mid-cingulate cortex and cuneus – areas typically associated with task difficulty, in addition to higher activations in the right TPJ most likely reflecting the shift to a new perspective. Activations associated with the proactive decision to update to a new interpretation were found in a network including the dorsal ACC known to be involved in exploration and the endogenous decision to switch to a new interpretation. These findings suggest a general network commonly engaged in all types of perceptual decision making supported by additional networks associated with perceptual uncertainty or updating provoked by either proactive or reactive decision making.
Keywords: Representational updating; Perceptual decision making, anterior insula; Uncertainty; Proactive vs. reactive
1. Introduction
Every day we are confronted with an enormous amount of information. Mental models compress incoming sensory information into a tractable form to optimally guide decision making (Johnson-Laird, 2004; Tenenbaum et al., 2011). We rely on such representations for a wide range of decisions. However, the world is in constant flux. In order for our mental models to be useful we must be capable of revising them in the face of environmental changes. While sometimes the decision to update to a new interpretation (e.g., Is this food edible or not?) is accompanied by a certain degree of uncertainty (e.g., When is my steak grilled to perfection?). At other times this decision is made for us and we only have to react to the changes in the environment (e.g., the steak falls from the barbecue; McGuire et al., 2014).
We previously demonstrated that a distributed network including the anterior insula, dorso-medial prefrontal cortex, and inferior parietal lobes was activated when participants updated their representations to the gradual accumulation of changing information (Stöttinger et al., 2015). Participants viewed picture sets in which one unique object (e.g., a shark) morphed slowly over fifteen iterations into a completely different unique object (e.g., a plane). Participants pressed different buttons to indicate whether they saw the first or another object. The average amount of change (in pixels) between each transition was held constant at ~ 4% with no significant difference between the individual picture positions (Stöttinger et al., 2016). Consequently, the transition from an old to a new model was internally determined by the individual as opposed to being driven by external events. The highest activations were found in the anterior insula (bilaterally) and mid frontal area including the anterior cingulate cortex (ACC). These areas were not only active at the time point of change but also immediately before, suggesting a possible causal role of these areas in updating. This finding was consistent with earlier results in patients showing that damage to the anterior insula – especially on the right – resulted in selective updating impairments in both the picture morphing task and in playing a simple competitive game (Danckert et al., 2012; Stöttinger et al., 2014; under revision), indicating a general updating impairment across different cognitive domains.
The results could be explained in three ways. First, activations may reflect proactive decisions to update, based on imprecision of the initial belief (McGuire et al., 2014): Given that differences between pictures were held constant with no abrupt deviations, it was up to the participant to decide at which point their initial representation was no longer supported by the evidence from the environment. This is similar to bistable perception where participants report which of two interpretations of an object they hold, despite no environmental change in the stimulus. The anterior insula is active when participants switch between interpretations of such stimuli (Lumer and Rees, 1999; Knapen et al., 2011; Müller et al., 2005; Weilnhammer et al., 2017). Despite the difference between spontaneous alternations in bistable perception and updating based on actual, albeit subtle, changes – a similar mechanism may be involved. In both cases the transition from the old to the new interpretation is determined internally by the participant rather than being determined by events in the environment. A second way to explain our prior results is via perceptual uncertainty: While all picture sets were based on the prerequisite that they were perceived categorically in our normative study (Stöttinger et al., 2016), we cannot fully rule out that decisions were accompanied by a certain degree of perceptual uncertainty. This would fit with research showing that a network including the anterior insula is engaged when belief updating is based on relative uncertainty in a gradually changing, noisy, uncertain, or perceptually degraded environment (Ploran et al., 2007; Heekeren et al., 2008 for review). Similarly, activation in the insula is modulated by the ambiguity of sensory information (Lamichhane et al., 2016; Sterzer and Kleinschmidt, 2010 for a review). A third potential explanation of our results would suggest that activations reflected a more general network always active whenever we update mental representations. This is in line with research on surprise-based updating which assigns a central role to the anterior insula and ACC. When observations in our environment saliently deviate from expectations, the right anterior insula initiates attentional control by activating the central executive network and deactivating the default mode network. As a consequence, cognitive resources are assigned to facilitate processing of the surprising, salient stimulus. The co-activation with the ACC allows rapid access to the motor system (Craig, 2009; Menon and Uddin, 2010; Uddin, 2015 for a review). The network found in our study might therefore be best understood more generally as a network for updating mental representations, signaled either by bottom-up salience, or internal signals.
The aim of this research was to evaluate each of these three explanations. That is, which brain areas are active regardless of the mechanism involved (proactive decision vs. perceptual uncertainty) and which brain areas are specific to each process. We presented participants with three different conditions: (1) gradual-naïve condition: In 10 separate sets pictures morphed gradually from one object to a second – replicating our initial study (Stöttinger et al., 2015). (2) A gradual-repeat condition: where all gradually morphing sets were presented twice thus diminishing perceptual uncertainty. For both series continuous changes result in the proactive decision to update a perceptual model due to the gradual accumulation of evidence. In the gradual-naïve condition the decision is accompanied by a greater degree of uncertainty, given the participant does not know what the second object will be. This uncertainty is reduced when the participant is exposed to this same set a second time (the gradual-repeat condition). As a further control for neural systems responding reactively to change we added an (3) abrupt condition where after a certain number of subtly changing pictures (akin to the gradual-naïve condition) updating was provoked by a dramatic change in the external input by switching to a new picture that was not coherent with the current pictorial set. Participants in the abrupt condition simply had to react to the abrupt change in visual input while updating in the gradual-naïve condition required them to proactively decide at which point their initial model was no longer supported by the evidence.
We first replicated our previous results. We then evaluated which activations were associated with a switch in general, irrespective of an active decision to update to a new model or perceptual uncertainty. Finally, by comparing brain activation associated with a shift in conscious percept in the gradual-naïve condition with activations for the same shift in the abrupt condition we identified areas selectively associated with proactive decisions. In a similar vein, comparing perceptual shifts in the gradual-naïve condition with shifts in the gradual repeat condition allowed us to evaluate the influence of perceptual uncertainty.
2. Methods
2.1. Participants
A total of twenty (11 female) neurologically healthy participants with normal or corrected to normal vision took part in this study for payment. Due to a technical problem, data of one participant could not be analyzed. The final sample comprised nineteen participants (10 female; mean age 24.55 years, SD=4.02). One participant reported being left-hand dominant. The individual activation pattern of this participant did not deviate from the activation pattern of the right-hand-dominant group. Given that left-handed people represent a portion of the population we decided to include this participant in the sample (see Willems et al., 2014 for that argument). None of the participants had a history of brain injury. All participants provided informed consent prior to participation. The research protocol was approved by the Office of Research Ethics at the University of Waterloo and the Tri-Hospital Research Ethics Board of the Region of Waterloo in Ontario, Canada.
2.2. The picture morphing task
2.2.1. Stimuli
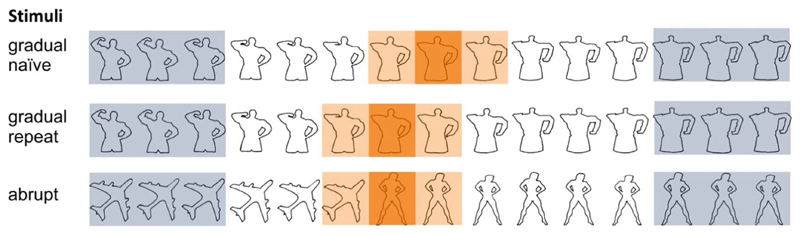
Each participant saw thirty picture sets selected from a larger set validated in an earlier study (Stöttinger et al., 2016; https://osf.io/qi2vg/). All pictures were silhouettes of line drawings and of a standard size (316 × 316 pixels) – displayed on a white background (Fig. 1). All participants saw three different types of picture sets: (1) Gradual-naïve condition: In ten picture sets line drawings of common objects morphed over fifteen iterations into a different object (a replication of Stöttinger et al., 2015). (2) Gradual-Repeat condition: all gradually morphing sets were presented a second time. (3) Abrupt condition: In ten picture sets a salient switch was induced after the 4th, 5th, 6th, 7th, or 8th position (there were two sets for each switch position and switch position was randomly assigned across the six runs with the constraint that the same switch position did not occur within the same run). This switch violated the continuous changes used in the first two conditions in that the change was to an image from an unrelated set (Fig. 1, bottom line). Please note that picture sets used in the abrupt condition were different from the sets used in the continuous conditions. In order to keep the abrupt condition as similar as possible to the gradual-naïve condition, the first three pictures of a set were repeated. This resulted in subtle changes between pictures – similar to the ones in the gradual-naïve condition. Data from a pilot study suggested that participants typically do not notice this level of repetition: No participant reported noticing the repetition and reaction times reliably ramped up before the switch in all conditions. This suggests that participants were actively looking for the second object given that they did not know whether they were in the gradual-naïve or abrupt condition. Repeating the first three pictures in the abrupt condition had the advantage that picture sets were comparable in both conditions while guaranteeing that participants did not shift to a new interpretation before the intended change point.
Fig. 1.
Examples of three different picture sets used in the gradual (naïve and repeat) and abrupt conditions. For fMRI analysis the three pictures at the beginning and end (light blue boxes) were compared with the change period (the moment of change (dark orange) plus the picture preceding and following the change picture; light orange). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Of the sixty pictures used, thirty-one depicted an animate object (e.g., animal) and twenty-nine displayed an inanimate object. In ten sets the object morphed from an animate object into a different animate object (e.g., cat – owl), in nine cases the object morphed within the inanimacy class (e.g., key – saw). In six picture sets the object morphed from an animate into an inanimate object (e.g., shark – plane) and in five cases the order was reversed, morphing from an inanimate to an animate object (e.g., tree – face). About the same number of between and within category switches were used in the gradual (6 within, 4 between) and abrupt conditions (7 within, 3 between).
2.2.2. Behavioral paradigm
The picture morphing task consisted of thirty picture sets distributed over six experimental runs. Each run included five picture sets, containing either two gradual-naïve, two gradual-repeat and one abrupt picture set or one gradual-naïve, one gradual-repeat and three abrupt picture sets. The gradual-naïve and gradual-repeat versions of one picture set were always within the same run. The order of picture sets per run was randomized with the constraint that the same picture set (i.e., gradual-naïve and gradual-repeat) was never repeated back to back. This randomized order was consistent across participants. Runs were counterbalanced between participants using a Latin square. Picture morphing in each picture set occurred over fifteen discrete steps, each corresponding with the acquisition of a whole-brain image. Each picture within a set was presented for two seconds. Pictures were randomly intermixed with eight inter-stimulus-interval periods (2, 4, 6 or 8 s) during which participants saw a fixation cross. The full presentation of one picture set took 110 s. The total duration to present all sets was 55 min. Together with the anatomical scan (about 10 min) and short breaks in between the six runs, participants spent about 70 min in the scanner. Participants provided their responses in the scanner using two buttons on a four button Cedrus fibre optic system. Participants were asked to press a button for each picture – the first button (pressed with the index finger) when they ‘saw the first object’ then changing to the second button (pressed with middle finger) when they ‘saw the second object’. Except for the repeat condition, all participants were naïve as to the identity of the second object. Although speed was not emphasized, participants were encouraged to make a button press within the 2-second time window during which the picture was presented on the screen. On a few occasions (i.e., in .56% of individual presentations of an image) participants failed to do so. Instead they made a button press in the inter stimulus interval (ISI) following the picture. Reaction times for ISI button presses were added to the presentation time of the preceding image, resulting in an RT greater than 2000 ms.
At the end of each set of 15 images the word “END” was presented for 2 s to indicate that the next picture set would begin shortly. Infrequently, a participant failed to press any button (i.e., in .63% of individual presentations of an image). Most of the time omissions were preceded and followed by the same button press, suggesting no change in the participant's conscious percept. In six individual cases the omission occurred between a switch in button presses. In those cases the moment of change was assigned to the first occasion when the second button was pressed. One participant never reported the second object in one set – both sets (gradual-naïve and gradual-repeat) were removed from further analysis. Another participant alternated between button 1 and button 2 in one set (both sets – gradual-naïve and gradual-repeat – were removed from further analysis).
To familiarize participants with the procedure and timing of the task, each participant took part in a training session a few days prior to scanning. Participants were trained with five different picture sets, none of which was used in the actual scanning period. Instructions were repeated before the start of the actual experiment.
2.3. fMRI data collection
Functional data were acquired using gradient echo-planar T2*-weighted images collected on a 1.5 T Phillips scanner located at Grand River Hospital in Waterloo, Ontario (TR = 2000 ms; TE = 40 ms; slice thickness = 5 mm with no gap; 26 slices/volume; FOV = 220 × 220 mm2; voxel size = 2.75 × 2.75 × 5 mm3; flip angle = 90°). Each experimental run consisted of 285 volumes preceded by four dummy scans to allow transient signals to diminish. At the beginning of each session, a whole brain T1-weighted anatomical image was collected for each participant (TR = 7.4 ms; TE = 3.4 ms; voxel size = 1 × 1 × 1 mm3; FOV = 240 × 240 mm2; 150 slices with no gap; flip angle = 8°). The experimental protocol was programmed using E-Prime experimental presentation software (v1.1 SP3; Psychology Software Tools, Pittsburgh, PA). Stimuli were presented on an Avotec Silent Vision™ fibre-optic presentation system using binocular projection glasses (Model SV-7021). The onset of each trial was synchronized with the onset of data collection for the appropriate functional volume using trigger pulses from the scanner.
2.4. fMRI data analysis
Functional data were analyzed using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK; Friston et al., 1997). Preprocessing included slice-time and motion correction. High-resolution T1-weighted structural images were co-registered with each subject's EPI images, and normalized to the MNI template brain (Montreal Neurological Institute, McGill, Montreal, Canada). The normalized images were resampled to isotropic 3 × 3 × 3 mm voxels and smoothed with a 6mm full width at half maximum (FWHM) Gaussian kernel. After images were smoothed, physiological noise was removed using a Functional Image Artefact Correction Heuristic (FIACH) as implemented by Tierney et al. (2016). The functional data were high-pass filtered to remove frequencies below 1/128 Hz to reduce low frequency drift. The serial correlation was taken into account using the autocorrelation AR(1) model.
We used a 2 × 3 flexible factorial design as implemented in SPM12 with the within subjects factors of Condition (gradual-naïve, gradual-repeat, abrupt) and Change (i.e., the change picture vs. stable pictures; stable pictures were the three pictures at the start and end of each set for which participants did not report a change). This analysis was calculated separately for three different time points – the change image (T0) when button presses changed from button 1 to button 2, as well as the picture preceding (− T1) and following the change (T1). Activation at T1 was analyzed for comparison purposes with our original study (Stöttinger et al., 2015).
To evaluate whether results replicated data of our previous study (Stöttinger et al., 2015) we first calculated this analysis for gradual-naïve picture sets only. Whole-brain, random-effects group analysis was conducted with contrast t maps thresholded at a family-wise-error (FWE) of = .05. Only clusters of 10 contiguous voxels or more are reported.
To identify areas jointly active in all three conditions we used a three-way conjunction null analysis for all three contrasts (1) gradual-naïve-change > gradual-naïve stable, (2) gradual-repeat-change > gradual-repeat-stable, (3) abrupt-change > abrupt-stable) of whole-brain effects. Finally, to explore selective effects in each of the three conditions we compared the change pictures between conditions. For that we applied different contrast weights at the corresponding regressors (Gläscher and Gitelman, 2008). Again, analyses were done separately for each of the three time points (− T1, T0, T1). An uncorrected threshold of p < .001 was used for both types of analyses (conjunction and selective contrasts), corrected for FWE-extended cluster threshold.
2.5. Statistical analysis of behavioral data
Data was analyzed using repeated measures ANOVAs. Statistical tests were two-tailed and an alpha-level of p < .05 was used to determine significance. To evaluate the influence of condition on response pattern the average amount of first object reports were entered into a repeated measures analysis with condition (gradual-naïve vs. gradual-repeat) as the within subjects factor. A mixed design repeated measures analysis with the between subjects factor of experiment (normative study vs. current study) and the within subjects factor of picture number (1–15) was used to determine whether response patterns (i.e., proportions of first object reports) in the fMRI study replicated behavioral data from our normative study (Stöttinger et al., 2016).
A similar analysis was done for reaction times with averaged RT entered into a repeated measures analysis with time point (− T6 to T6) and condition (gradual-naïve, gradual-repeat, abrupt) as within subjects factors. In a second step, the same analysis was calculated restricted to time points relevant to the change-point indication (− T2, − T1, T0; where T0 indicates the change report). For post-hoc analyses (e.g., comparison at T0 between gradual-naïve and gradual-repeat) a repeated measures analysis was used restricted to the two conditions of interest.
3. Results
3.1. Behavioral results
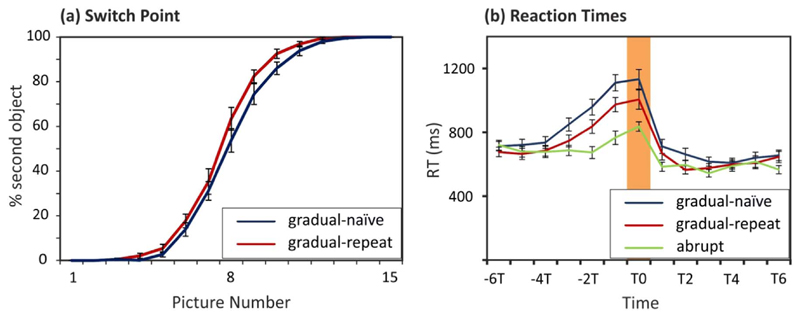
Participants reported perceiving the second object between the fourth and fourteenth pictures with the majority of reports (88.03%) between the fifth and tenth picture. Participants on average reported the second object around the 8th picture. Participants reported the second object slightly later in the gradual-naïve condition (Mean = 8.50 picture, SE = .23) compared to the gradual-repeat condition (Mean = 8.07 picture, SE = .21; F(1,18) = 7.52, p < .05, η2 = .30; Fig. 2a). Response patterns in the gradual-naïve condition replicated normative data in our earlier study (Stöttinger et al., 2016). We found neither a significant main effect for experiment [F(1,18) = .45, p > .50, η2 = .02], nor a significant interaction between image number and experiment [F(14,252) = .78, p > .45, η2 = .04]. Hence, the difference in reporting method (verbal vs. forced choice button press) did not influence response patterns in the current study.
Fig. 2.
(a) Average percentage of second object reports over the 15 pictures – displayed for the gradual conditions (naïve and repeat). (b) Average reaction times displayed for the switch point (T0), together with the six picture before (− 6T to − T1) and after the switch (T1 to T6). Error bars in both graphs reflect SE of the Mean. Blue line = gradual-naïve sets, red line = gradual-repeat sets, green line = abrupt sets. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Reaction times averaged across all 13 time points (-T6 to T6) were highest in the gradual-naïve condition (Mean = 778 ms, SE = 34 ms) and lowest in the abrupt condition (Mean = 656 ms, SE = 28 ms) [F (2,36) = 87.73, p < .001, η2 = .83] as demonstrated by a significant main effect for condition. At the time point of change (T0) reaction times were significantly higher in the gradual-naïve (Mean = 1133 ms, SE = 61 ms) compared to the gradual-repeat (Mean = 1005 ms, SE = 60 ms; F(1,18) = 6.08, p < .05, η2 = .25) and abrupt conditions (Mean = 836 ms, SE = 30 ms; F(1,18) = 45.05, p < .001, η2 = .72). Reaction times in all three conditions started to ramp up before participants reported a change (T0; Fig. 1c). A repeated measures analysis with the within subject factors of image relevant to change indication (− T2, − T1, T0; where T0 indicates the change image) and condition (gradual-naïve, gradual-repeat, abrupt) revealed a significant main effect for condition [F(2,36) = 70.36, p < .001, η2 = .80] and time [F(2,36) = 23.03, p < .001, η2 = .56] but no significant interaction between time x condition [F(4,72) = .41, p > .80, η2 = .02]. Reaction times in all three conditions reliably ramped up between − T2 and T0 [gradual-naïve: F(2,36) = 13.57, p < .01, η2 = .43; gradual-repeat: F (2,36) = 6.68, p < .01, η2 = .27; abrupt: F(2,36) = 14.71, p < .01, η2 = .45], suggesting that participants in all three conditions were actively exploring alternate interpretations of the stimuli (Fig. 2b).
3.2. Imaging results
3.2.1. Activation in the gradual-naïve condition: replicating prior results
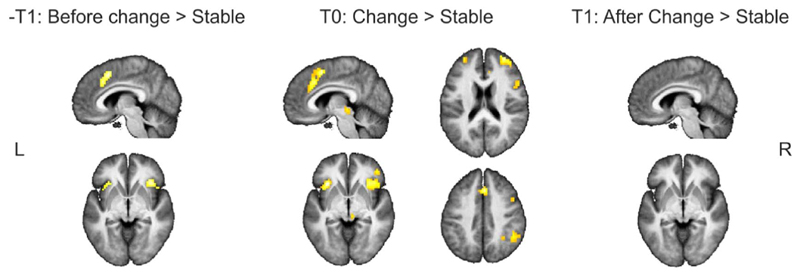
Immediately before participants reported a shift in their conscious percept (–T1) activations within a network including the anterior insula (bilateral) and mid frontal area were observed. At the time point when perceptual reports changed (T0), higher activations were observed in a network of brain regions including the anterior insula, dorsal medial frontal cortex (including the ACC), inferior frontal, and inferior parietal cortex. After a change was reported no area showed higher activation for the change picture compared to the stable pictures (Table 1; Fig. 3). These results closely resemble our initial results (Stöttinger et al., 2015) also demonstrating activations in the anterior insula (bilaterally) and mid-frontal regions (including the ACC), immediately before (~ 5 s) and at the moment of change, but not after. In addition, the lack of any activation after the switch (T1) reliably shows that the pattern of activations found in both studies cannot simply be explained by the 4% change in pixels between pictures.
Table 1.
Brain activity when perceptual reports changed (defined as the picture when button presses changed from 1 to 2 and the immediately preceding and succeeding periods), was contrasted separately against activations for stable periods (the three pictures at the start and end of each set). Only clusters are reported with a cluster size of ten or consecutive voxels.
| Neural correlates at the change period (− T1, T0) in the gradual condition | |||||
|---|---|---|---|---|---|
| Area | #voxels | T | x | y | z |
| − T1 > Stable | |||||
| R Calcarin Cortex | 33 | 7.51 | 24 | − 61 | 4 |
| L Calcarin Cortex | 34 | 7.26 | − 9 | − 73 | 13 |
| Mid Frontal/ACC | 75 | 7.06 | 6 | 14 | 49 |
| R Anterior Insula | 61 | 6.74 | 36 | 23 | − 5 |
| L Anterior Insula | 26 | 6.7 | − 30 | 23 | − 2 |
| R Inferior frontal gyrus | 32 | 6.55 | 48 | 11 | 22 |
| T0 > Stable | |||||
| Mid Frontal/ACC | 212 | 9.22 | 3 | 11 | 52 |
| L Anterior Insula | 64 | 9.12 | − 33 | 23 | − 5 |
| R Mid Frontal Gyrus | 200 | 7.96 | 33 | 50 | 19 |
| R frontal Operculum/Insula | 222 | 7.81 | 48 | 20 | 4 |
| R angular gyrus | 99 | 6.28 | 48 | − 49 | 40 |
| Brain stem/Thalamus | 13 | 6.2 | 9 | − 28 | − 8 |
| L Mid Frontal gyrus | 13 | 5.9 | − 30 | 50 | 19 |
| L Supramarginal gyrus | 10 | 5.64 | − 48 | − 40 | 46 |
Fig. 3.
Whole-brain, random-effects group analyses for the gradual-naïve condition conducted with contrast t maps thresholded at an FWE = .05. Stable pictures (the first and last three pictures of each series) were contrasted with the picture before (− T1), at (T0) and after (T1) the change. Only clusters are reported with a cluster size of ten or more consecutive voxels.
3.3. Areas commonly involved in all three conditions
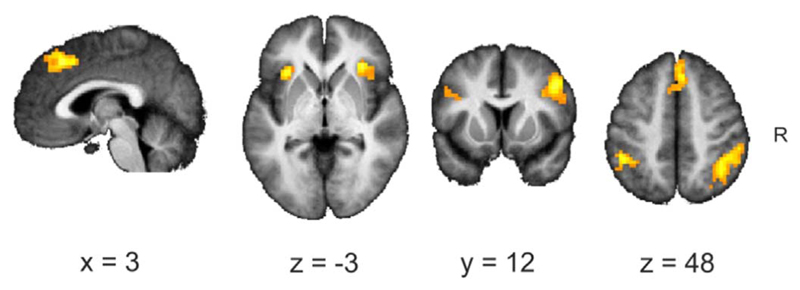
At the picture immediately preceding the switch (− T1) a conjunction analysis revealed joint overlap in all three condition in the right inferior frontal gyrus (MNI 48 8 22) only. The same conjunction analysis calculated for the time point of switch (T0) revealed joint activation in a distributed network, including the anterior insula, inferior frontal and inferior parietal regions. The strongest activations were found in the anterior insula (bilaterally; Fig. 4 and Table 2). At the picture immediately following the switch (T1) joint activations in all three conditions were found in the right angular gyrus/superior parietal lobe (MNI 42-58 49).
Fig. 4.
Conjunction analysis for gradual-naïve, gradual-repeat and abrupt at T0.
Table 2.
Regions showing significant effects at T0 in all three conditions.
| Conjunction at time T0 in gradual-naïve, gradual-repeat and abrupt | |||||
|---|---|---|---|---|---|
| Regions | #voxels | T | x | y | z |
| L anterior Insula | 74 | 6.88 | − 33 | 20 | − 8 |
| R anterior insula | 120 | 6.49 | 42 | 23 | − 8 |
| R intraparietal | 306 | 5.63 | 36 | − 58 | 58 |
| R Inferior frontal | 245 | 5.42 | 48 | 14 | 34 |
| Mid frontal area | 144 | 5.38 | 3 | 26 | 43 |
| L Intraparietal | 96 | 5.3 | − 45 | − 43 | 46 |
| R Inferior frontal | 41 | 4.62 | 48 | 41 | − 11 |
| L Inferior frontal | 39 | 4.21 | − 36 | 8 | 25 |
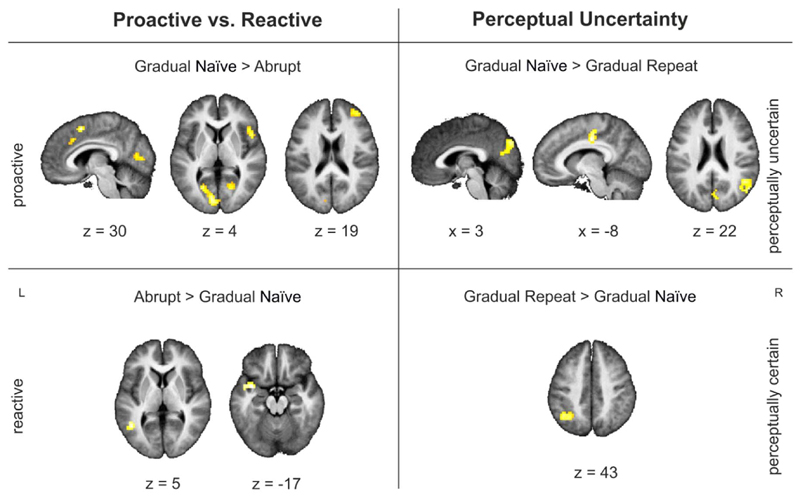
3.4. Selective involvement – perceptual uncertainty vs. proactive decision to update2
3.4.1. Perceptual uncertainty: gradual-naïve vs. gradual-repeat
Higher activations for gradual-repeat compared to gradual-naïve were found in the left angular gyrus (MNI − 36 −55 46; BA 39) at the picture immediately preceding the switch. At the actual time point when participants indicated a change in their conscious percept (T0) higher activations were found in the gradual-naïve condition compared to the gradual-repeat condition in mid-cingulate cortex, supplementary motor cortex and the cuneus, as well as in the angular gyrus/TPJ on the right. Higher activations for gradual-repeat compared to gradual-naïve were only found in the angular gyrus on the left (Table 3; Fig. 5). At the picture immediately following a switch (T1) higher activations for gradual-naïve compared to gradual-repeat were found in the left middle frontal gyrus (MNI − 36 8 28 – an area typically associated with semantic processing; Lau et al., 2008 and Whitney et al., 2010 for review). No area showed higher activation for the gradual-repeat compared to the gradual-naïve condition.
Table 3.
Selective activations in the gradual-naïve, gradual-repeat and abrupt condition at T0.
| Selective activations at T0 | |||||
|---|---|---|---|---|---|
| Regions: gradual-naïve > abrupt | # voxels | T | x | y | z |
| ACC | 55 | 5.89 | 12 | 26 | 25 |
| Supplementary motor/middle cingulate | 63 | 5.82 | 6 | 11 | 52 |
| L calcarin cortex | 283 | 5.41 | − 9 | − 79 | 7 |
| L middle cingulate cortex | 55 | 5.19 | − 15 | − 22 | 34 |
| R middle frontal gyrus | 50 | 4.83 | 33 | 50 | 22 |
| R inferior frontal gyrus/frontal operculum | 41 | 4.73 | 51 | 11 | 4 |
| Regions: abrupt > gradual-naïve | |||||
| L temporal pole | 47 | 4.65 | − 39 | 5 | − 17 |
| L middle temporal gyrus | 40 | 4.56 | − 45 | − 58 | 4 |
| Regions: gradual-naïve > gradual-repeat | |||||
| Middle cingulate cortex | 147 | 5.25 | − 6 | − 16 | 40 |
| Supplementary motor cortex | 74 | 4.88 | 15 | − 10 | 55 |
| Cuneus | 77 | 4.65 | 3 | − 79 | 34 |
| R angular gyrus/TPJ | 65 | 4.57 | 48 | − 55 | 22 |
| Regions: gradual-repeat > gradual-naïve | |||||
| L Angular gyrus | 103 | 5.46 | − 42 | − 58 | 46 |
Fig. 5.
Selective effects for gradual-naïve, gradual-repeat and abrupt condition at T0.
3.4.2. Proactive vs. reactive switch: gradual-naïve vs. abrupt
No significant differences were found at time point –T1 between gradual-naïve and abrupt pictures, suggesting that similar processes were active in both conditions before the shift. Higher activations during a gradual-naïve compared to an abrupt change at T0 were observed in the cingulate cortex, right frontal and occipital areas. Higher activations for abrupt compared to gradual-naïve switches were found the temporal cortex on the left (Table 3; Fig. 5, left panel) (Table 3; Fig. 5, left panel). At the picture immediately after the switch (T1) higher activations were found in the mid-cingulate gyrus (− 15 −19 40) for gradual-naïve compared to abrupt pictures. Areas in the middle temporal gyrus (MNI − 63 − 16 − 8) and superior frontal gyrus (MNI − 21 23 55) showed higher activations for abrupt compared to gradual-naïve pictures at time point T1.
4. Discussion
The goal of the present study was to shed light on the neural correlates engaged in updating in response to slow and subtle changes in the environment. In our previous study participants had to proactively decide in a gradually morphing picture task at which point their initial representation was no longer supported by the evidence. While our study provided interesting results it did not allow us to determine, whether these activations are specific to updating in a slowly changing environment. They also did not allow us to differentiate between different processes of perceptual uncertainty vs. proactive decision making. Participants in this study were again exposed to gradually morphing picture series. By presenting all picture morphs twice we manipulated uncertainty in perceptual decision making. Inducing an abrupt shift in a third condition allowed us to differentiate between a proactive decision in uncertainty driven updating (i.e., when an initial representation is now faced with conflicting evidence) and a reactive decision in surprise-based updating (i.e., based on the observation of unexpected external events).
Despite using fewer pictures sets (i.e., 10 instead of 20) and different analysis software (Brain Voyager in our earlier study and SPM12 here) we were able to replicate our earlier results. Again we found that the anterior insula and mid frontal/ACC region was active before participants reported a change. This demonstrates the robustness of our results – a non-trivial point given the ongoing discussion about failures to replicate in psychological science in general (Bohannon, 2015 for a review) and fMRI research in particular (Carp, 2012a, 2012b; Eklund et al., 2016).
We also tested which brain areas were active during a shift in percept regardless of the type of shift or the amount of perceptual uncertainty. At the moment of shift (T0), a distributed network – including the anterior insula (bilaterally) and the mid frontal area/ACC – was active in all conditions. This is in line with research on surprise-based updating that has typically assigned a central role to the anterior insula and ACC for updating in response to events saliently deviating from expectations (Craig, 2009; Menon and Uddin, 2010; Uddin, 2015 for a review). In a similar vein, McGuire et al. (2014) directly compared uncertainty-based and surprised based updating. They too reported joint activations in the anterior insula (bilateral), dorsomedial prefrontal cortex, and right inferior frontal junction in uncertainty and surprised based updating. Interestingly, different areas were jointly active at different time points. The right inferior frontal gyrus was active in all three conditions at the picture immediately preceding the change. This fits with studies on bistable perception suggesting a causal role of the right inferior frontal gyrus (Sterzer and Kleinschmidt, 2007; Weilnhammer et al., 2013, 2017) in perceptual alternations via mediation of activity in the visual cortex. To examine which brain networks are associated with the newly formed representation we also analyzed brain activations after the switch (T1). After participants changed their conscious percept, joint activation for all conditions was found in the right angular gyrus/superior parietal lobe. This is in line with research showing an involvement of the right angular gyrus/superior parietal lobe in inhibition of a former correct response (Seghier, 2013; Wager et al., 2005 for a review) and the maintaining of internal representations (Wolpert et al., 1998). Finally, we differentiated which brain areas are associated with perceptual uncertainty from those involved in updating due to proactive decisions. To examine the effect of uncertainty in perceptual decision making, we compared the change period for gradual-naïve picture sets with the change period for gradual-repeat picture sets. Based on the assumption that longer reaction times indicate a more difficult perceptual decision (Sterzer and Kleinschmidt, 2010), we found that a decision in the gradual-naïve condition was perceptually more difficult than a decision in the gradual repeat condition. Differences in activations for gradual-naïve compared to gradual-repeat shifts at T0 were found in the mid-cingulate cortex, supplementary motor area, cuneus, as well as in the angular gyrus/TPJ on the right. This is in line with research associating the mid-cingulate cortex and cuneus with task difficulty (Singh and Fawcett, 2008; Bush, 2009 for a review). Interestingly, no difference was found at the picture immediately preceding a shift, although reaction times before the shift were reliably higher in the gradual-naïve condition compared to the gradual repeat condition. This suggests that the activations found at the moment of shift, may not only reflect perceptual uncertainty but additional processes. The right TPJ, for example, has been reported to be involved in contextual updating of internal models (Geng and Vossel, 2013), in redirecting attention (Mitchell, 2007 for a review), and the ability to take someone else's perspective (Saxe and Wexler, 2005; Schurz et al., 2014 for a review). This activation therefore could also reflect a need to test subsequent evidence and revise the perceptual interpretation (see Filipowicz et al., 2016 for a review).
Higher activations for gradual repeat compared to gradual-naïve were found in the left angular gyrus. This was seen not only at T0 but also at the picture preceding the switch (− T1). At first glance this contradicts findings in the literature: repeated presentation of stimuli typically results in reduced activation for repeat compared to initial exposure in brain areas involved in processing these stimuli – a phenomenon known as repetition suppression (for review Henson, 2003). However, findings on repetition effects are heterogeneous and repetition can result in suppression or enhancement depending on factors particular to the paradigm (e.g., timing, task demands, attention, expectation, etc.; James and Gauthier, 2006; Segaert et al., 2013). In addition, it has been argued that enhancement effects in repetition priming might be due to an additional process like explicit memory retrieval (see Segaert et al., 2013 for this argument). This argument is plausible given that the angular gyrus is also known to be involved in episodic memory retrieval (Wagner et al., 2005 for review). In a similar vein, the left angular gyrus has also been associated with visual perspective taking (Arora et al., 2017; Schurz et al., 2013). Higher activation in the gradual-repeat compared to the gradual-naïve condition might therefore reflect repeated alternation between two different perspectives – similar to bistable perception. For our repeat conditions, uncertainty has been removed – the participant knows the muscle man will turn into a coffee pot. All that is left to do is actively switch back and forth between two perspectives (e.g., muscle man vs. coffee pot) to decide when the visual input supports one interpretation over another.
To evaluate which brain areas were more active during a proactive compared to a reactive switch we compared the change points in the gradual-naïve condition with the change points in the abrupt condition. Higher activations for proactive compared to reactive shifts were found in the mid frontal area, including the dorsal ACC, supplementary motor area and mid-cingulate cortex, right interior frontal gyrus, as well as the left calcarine cortex. The ACC – especially the dorsal part (dACC) – is typically associated with the allocation of cognitive control, error detection, outcome evaluation, conflict monitoring, and choice difficulty. In a similar vein, the dACC is also reliably active in decision making within a foraging task (i.e., striking the balance between exploration vs. exploitation) and the alteration of behavior in response to changes in the environment (Filipowicz et al., 2016; Shenhav et al., 2016 for a review). It has been argued that activation in the dorsal ACC can be more parsimoniously explained by exploration of alternative interpretations when the current representations are no longer supported by evidence from the environment (Domenech and Koechlin, 2015; McGuire and Kable, 2015; Shenhav et al., 2014; Filipowicz et a, 2016 for a review). This notion is further supported by research in non-human primates showing increased firing rates in the dACC, when a monkey chooses to move on to a new patch (i.e., at the transition between exploitation and exploration; Hayden et al., 2011). Higher activation in the dACC for gradual-naïve compared to abrupt shifts therefore most likely reflects the need to actively explore potential alternatives in the gradual-naïve condition. In the abrupt condition, the second object is “presented on a silver platter”. Consequently, there is no need for exploration of alternative interpretations of what it might be.
Higher activations in the right inferior frontal gyrus for proactive compared to reactive shifts resemble results in studies on bistable perception. Studies comparing spontaneous (active) alternations with stimulus-induced (passive) changes, typically find stronger activations in a fronto-parietal network including the inferior-frontal gyrus for spontaneous compared to stimulus induced transitions (i.e., transition are mimicked in a replay condition to create the impression of perceptual alternations; Lumer et al., 1998; Lumer and Rees, 1999; Knapen et al., 2011; Megumi et al., 2015; Müller et al., 2005; Weilnhammer et al., 2013). Effective connectivity analysis suggests a causal role of the right inferior frontal gyrus (Sterzer and Kleinschmidt, 2007; Weilnhammer et al., 2013) in perceptual transitions via mediation of activity in the visual cortex. Therefore, activation in the inferior frontal gyrus together with activation in the calcarine cortex probably reflects the proactive decision to update to a new interpretation.
Higher activations for shifts in the abrupt condition compared shifts in the gradual-naïve at T0 were found in the temporal pole and middle temporal gyrus (posterior portion) on the left – areas typically involved in semantic processing, including storage of lexical representations and semantic violation (Lau et al., 2008; Whitney et al., 2010 for a review). Higher activation for abrupt compared to gradual-naïve shifts may reflect the need to activate a new interpretation for the unexpected, abrupt change in perceptual input.
Some might argue that it is difficult to differentiate between purely perceptual and response-related processes in our tasks. Since the switch in the percept is signaled by a change in the motor response, perceptual updating and motor (re)planning seem to be inherently linked. Therefore, higher activation in frontal areas and the ACC may also reflect effects of motor inhibition and response conflict monitoring (Braver et al., 2001; Van Veen et al., 2001). However, participants in all conditions have to indicate a change in percept by a change in motor response (i.e., from pressing button one with the index finger to pressing button two with the middle finger). Any activation explicitly driven by motor planning per se would be expected to cancel out in all our contrasts.
In a similar vein, it could be argued that regions associated with a proactive decision in our study instead reflect a conflict between choices at the time of updating similar to the response conflict in a Stroop colour-word interference test. That is, while in the abrupt condition the picture clearly depicts the second object, there is a conflict between the two different interpretations in the gradual conditions. Although many of the regions we observe are also observed in response conflict paradigms (e.g., ACC, right middle frontal gyrus; Alvarez and Emory, 2006 for a review) we do not feel that updating in our task reflects a classic response conflict. In the Stroop task both stimulus properties are instantaneously present while in the gradual-naïve condition the second object is unknown to the participant. It is unlikely that an unknown (or at least uncertain) stimulus property can conflict with a known property. Also, participants are “primed” for a change. They know that change will occur and that they need to determine when the first object has become something else. This would seem to further obviate any sense of conflict.
Summary: Our main aim was to shed light on the neural correlates active in response to gradually changing environments. How do we decide at which point our steak is grilled to perfection? Which brain areas are engaged when we make decisions based on the accumulation of subtle, incremental changes in the environment? Are these activations different when the decision is taken out of our hands and made for us? Previously, we demonstrated that a network of brain regions including the anterior insula and mid-frontal cortex is active at the moment we update our conscious reports and immediately prior to making that decision (Stöttinger et al., 2015). By replicating the results here, we demonstrated the robustness of this effect. Furthermore, we found that this network of brain areas was active in all three conditions at the moment of the perceptual shift, suggestive of a generic role in updating, irrespective of perceptual uncertainty and the type of decision to be made (proactive vs. reactive). When perceptual uncertainty was a factor (i.e., contrasting gradual-naïve with gradual-repeat shifts), the brain areas active mirror those found in manipulations of task difficulty, with additional regions reflecting the shift to a new perspective. Directly contrasting proactive and reactive decisions highlighted regions known to be important for exploration of novel hypotheses and the endogenous decision to switch to new interpretations/representations. Taken together, these results are reflective of the interplay between a generic updating network and brain regions more specifically involved in distinct types of decision making.
Acknowledgments
This work was supported by the FWF Austrian Science Fund. (#V480-B27) grant (Eliese Richter Program) to E.S., the Natural Sciences and Engineering Research Council of Canada Discovery (#261628-07), Canada Research Chair grants, Heart and Stroke Foundation of Ontario #NA 6999 to J.D. and Canadian Institutes of Health Research #219972 operating grant to J.D. and B.A. The above-mentioned funding agencies had no role in the study design, data collection and analysis, decision to publish or the preparation of the manuscript. We thank Matthias G. Tholen and for assistance with flexible factorial design.
Footnotes
Differences in activation between conditions were typically a consequence of higher activation for the change image (− T1, T0, or T1) compared to the stable images in a given condition. For the comparison gradual-naïve > gradual-repeat at T0 only, the differences seen in the mid-cingulate cortex and cuneus were due to less deactivation for gradual-repeat compared to gradual-naïve.
References
- Arora A, Schurz M, Perner J. Systematic comparison of brain imaging meta-analyses of ToM with vPT. BioMed Res Int. 2017;2017 doi: 10.1155/2017/6875850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Bohannon J. Many psychology papers fail replication test. Science. 2015;349:910–911. doi: 10.1126/science.349.6251.910. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Bush G. Dorsal anterior Midcingulate Cortex: Roles in Normal Cognition and Disruption in Attention-deficit/hyperactivity Disorder. Oxford University Press; NY, USA: 2009. pp. 207–218. [Google Scholar]
- Carp J. On the plurality of (methodological) worlds: estimating the analytic flexibility of fMRI experiments. Front Neurosci. 2012a;6 doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. The secret lives of experiments: methods reporting in the fMRI literature. NeuroImage. 2012b;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Craig AB. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Danckert J, Stöttinger E, Quehl N, Anderson B. Right hemisphere brain damage impairs strategy updating. Cereb Cortex. 2012;22:2745–2760. doi: 10.1093/cercor/bhr351. [DOI] [PubMed] [Google Scholar]
- Domenech P, Koechlin E. Executive control and decision making in the prefrontal cortex. Curr Opin Behav Sci. 2015;1:101–106. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016 doi: 10.1073/pnas.1602413113. (201602413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz A, Anderson B, Danckert J. Adapting to change: the role of the right hemisphere in mental model building and updating. Can J Exp Psychol. 2016;70:201. doi: 10.1037/cep0000078. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013;37:2608–2620. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Gitelman D. In: Contrast weights in flexible factorial design with multiple groups of subjects. SPM@ JISCMAIL. AC. UK Sml, editor. 2008. pp. 1–12. [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Hum Brain Mapp. 2006;27:37–46. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Laird PN. The history of mental models. In: Manktelow K, Chung MC, editors. Psychology of Reasoning: Theoretical and Historical Perspectives. Psychology Press; Hove, England: 2004. pp. 179–212. [Google Scholar]
- Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. J Neurosci. 2011;31:10293–10301. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane B, Adhikari BM, Dhamala M. The activity in the anterior insulae is modulated by perceptual decision-making difficulty. Neuroscience. 2016;327:79–94. doi: 10.1016/j.neuroscience.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics:(de) constructing the N400. Nat Rev Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Rees G. Covariation of activity in visual and prefrontal cortex associated with subjective visual perception. Proc Natl Acad Sci USA. 1999;96:1669–1673. doi: 10.1073/pnas.96.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Kable JW. Medial prefrontal cortical activity reflects dynamic reevaluation during voluntary persistence. Nat Neurosci. 2015;18:760–766. doi: 10.1038/nn.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Nassar MR, Gold JI, Kable JW. Functionally dissociable influences on learning rate in a dynamic environment. Neuron. 2014;84:870–881. doi: 10.1016/j.neuron.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megumi F, Bahrami B, Kanai R, Rees G. Brain activity dynamics in human parietal regions during spontaneous switches in bistable perception. NeuroImage. 2015;107:190–197. doi: 10.1016/j.neuroimage.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2007;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Müller TJ, Federspiel A, Horn H, Lövblad K, Lehmann C, Dierks T, Strik WK. The neurophysiological time pattern of illusionary visual perceptual transitions: a simultaneous EEG and fMRI study. Int J Psychophysiol. 2005;55:299–312. doi: 10.1016/j.ijpsycho.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27:11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporoparietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Schurz M, Aichhorn M, Martin A, Perner J. Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P. The suppression of repetition enhancement: a review of fMRI studies. Neuropsychologia. 2013;51:59–66. doi: 10.1016/j.neuropsychologia.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–1291. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci. 2014;17:1249–1254. doi: 10.1038/nn.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. NeuroImage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proc Natl Acad Sci USA. 2007;104:323–328. doi: 10.1073/pnas.0609006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöttinger E, Guay CL, Danckert J, Anderson B. Updating impairments and the failure to explore new hypotheses following right brain damage. Exp Brain Res. doi: 10.1007/s00221-018-5259-6. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöttinger E, Filipowicz A, Marandi E, Quehl N, Danckert J, Anderson B. Statistical and perceptual updating: correlated impairments in right brain injury. Exp Brain Res. 2014;232:1971–1987. doi: 10.1007/s00221-014-3887-z. [DOI] [PubMed] [Google Scholar]
- Stöttinger E, Filipowicz A, Valadao D, Culham JC, Goodale MA, Anderson B, Danckert J. A cortical network that marks the moment when conscious representations are updated. Neuropsychologia. 2015;79:113–122. doi: 10.1016/j.neuropsychologia.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Stöttinger E, Sepahvand NM, Danckert J, Anderson B. Assessing perceptual change with an ambiguous figures task: normative data for 40 standard picture sets. Behav Res Methods. 2016;48:201–222. doi: 10.3758/s13428-015-0564-5. [DOI] [PubMed] [Google Scholar]
- Tenenbaum JB, Kemp C, Griffiths TL, Goodman ND. How to grow a mind: statistics, structure, and abstraction. Science. 2011;331:1279–1285. doi: 10.1126/science.1192788. [DOI] [PubMed] [Google Scholar]
- Tierney TM, Weiss-Croft LJ, Centeno M, Shamshiri EA, Perani S, Baldeweg T, Carmichael DW. FIACH: a biophysical model for automatic retrospective noise control in fMRI. NeuroImage. 2016;124:1009–1020. doi: 10.1016/j.neuroimage.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CYC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weilnhammer VA, Ludwig K, Hesselmann G, Sterzer P. Frontoparietal cortex mediates perceptual transitions in bistable perception. J Neurosci. 2013;33:16009–16015. doi: 10.1523/JNEUROSCI.1418-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilnhammer V, Stuke H, Hesselmann G, Sterzer P, Schmack K. A predictive coding account of bistable perception-a model-based fMRI study. PLoS Comput Biol. 2017;13(5):e1005536. doi: 10.1371/journal.pcbi.1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci. 2014;15:193–201. doi: 10.1038/nrn3679. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O'sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex. 2010;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci. 1998;1:529. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]