Abstract
The molecular chaperone endoplasmic reticulum protein 29 (ERp29) plays a critical role in protein folding, trafficking, and secretion. Though ubiquitously expressed, ERp29 is upregulated in response to ER stress and is found at higher levels in certain cell types such as secretory epithelial cells and neurons. As an ER resident protein, ERp29 shares many structural and functional similarities with protein disulfide-isomerases, but is not regarded as part of this family due to several key differences. The broad expression and myriad roles of ERp29 coupled with its upregulation via the unfolded protein response (UPR) upon ER stress has implicated ERp29 in a range of cellular process and diseases. We summarize the diverse activities of ERp29 in protein trafficking, cell survival and apoptosis, and ER homeostasis, and highlight a potential role of ERp29 in neuroprotection in retinal and neurodegenerative diseases.
XX.1 Introduction
Endoplasmic reticulum protein 29 (ERp29) was molecularly described via a proteomic study of rat enamel cells in the late 1990’s revealing a hydrophobic signal sequence and a variant of endoplasmic reticulum (ER)-retention motif KDEL (Lys-Asp-Glu-Leu) (Demmer et al. 1997). Further studies demonstrate that ERp29 is a reticuloplasmin, i.e. a luminal component of the ER. Although expressed ubiquitously, ERp29 is highly expressed in secretory tissues, such as pituitary, adrenal, and salivary glands, pancreas, liver and kidney (Sargsyan et al. 2002a), and in neurons throughout the central nervous system (MacLeod 2004). In addition, ERp29 was identified in the neural retina and retinal pigment epithelium (RPE) and the level of ERp29 is reduced in human retina with age-related macular degeneration (AMD) (Ethen et al. 2006).
Structurally, ERp29 contains an N-terminal domain that is homologous to thioredoxin-like domains in protein disulfide isomerase (PDI). However, unlike the PDI family members and some ER chaperones, such as glucose-regulated protein 78 (GRP78), GRP94, and calnexin, ERp29 lacks the conventional disulfide reductase/isomerase and calcium-binding activities (Demmer et al. 1997). Functionally, ERp29 acts as a putative chaperone that facilitates the processing and transport of secretory and membrane proteins from the ER to the Golgi apparatus. Apart from direct binding to its protein substrate, ERp29 interacts with other ER chaperones to promote the formation of a chaperone-substrate complex (Sargsyan et al. 2002b). In addition, ERp29 is believed to be a major chaperone in the trafficking of membrane proteins, such as Connexin43, to the plasma membrane (Das et al. 2009).
Like other PDI-like proteins, an important function of ERp29 is to prevent protein aggregation by keeping unfolded proteins in a folding-competent state and promote the protein-degrading apparatus to remove denatured proteins from the ER. This function is critical for minimizing the level of harmful proteins in ER and is vital for protein homeostasis. Additionally, recent studies have discovered some new and important roles of ERp29 in regulation of cellular stress response, gap junction formation, cell survival, and neuroprotection, though the molecular mechanisms underlying these diverse actions are not fully understood. We briefly summarize recent advances in ERp29 research in the areas related to cellular stress response, highlighting the evidence and perspective of this protein as a potential target for neuroprotection in retinal and neurodegenerative diseases.
XX.2 ERp29 and cellular stress response
The ability of a cell to respond and adapt to stress conditions is critical to maintaining its normal function and structure and ultimately determines its fate. The unfolded protein response (UPR) is a cellular stress response activated by ER stress, an accumulation of unfolded or misfolded proteins in the ER lumen, resulting from imbalances between protein synthesis, folding, trafficking, and degradation. The UPR consists of three sophisticated signaling pathways initiated by ER membrane proteins inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). In most physiological conditions, activation of these pathways restores ER homeostasis through well-orchestrated programs, including promoting protein folding and accelerating protein degradation while simultaneously slowing down protein synthesis (Zhang et al. 2014). However, in some extreme scenarios when the UPR fails to eliminate the accumulation of unfolded/misfolded protein in the ER, the UPR will switch from a cell-adaptive and cell-protective mode into a cell-destructive mode by induction of apoptotic pathways of C/EBP homologous protein (CHOP), JNK, NF-kB, and caspase-4, promoting apoptosis and inflammation and eventually leading to cell death (Zhang et al. 2014)
Although it is well established that ERp29 is critical in maintaining ER homeostasis the promoter region of the ERp29 gene lacks an ER-stress response element, the DNA motif characteristic for the UPR target genes. On the contrary, it possesses characteristics (e.g. GC-rich sequence, lack of a TATA-box, multiple transcription start sites) of a constitutively expressed housekeeping gene (Sargsyan et al. 2002a). Furthermore, recent evidence shows that ERp29 interacts with, and regulates, PERK (Farmaki et al. 2011) and ATF6 (Hirsch et al. 2014), two important branches of the UPR pathways. Overexpression of ERp29 increases total PERK protein level without altering PERK phosphorylation (Farmaki et al. 2011). Deletion of ERp29 selectively impairs the activation of ATF6 and CHOP, but has no effect on other UPR branches, such as the PERK downstream activating transcription factor 4 (ATF4) and eukaryotic initiation factor 2 alpha (eIF2α), and the X-box binding protein 1 (XBP1) pathways (Hirsch et al. 2014). Additional studies suggest that ERp29 upregulates the chaperones involved in stress response pathways, such as p58IPK, p-eIF2α, p38, or Hsp27, promoting cell survival under stress conditions. For instance, ERp29 increases the level of ER chaperone p58IPK (Gao et al. 2012), which in turn inhibits ER stress-associated pro-apoptotic cascades (Huber et al. 2013) through down-regulation of the CHOP pathway (Boriushkin et al. 2014). Additionally, ERp29 regulates MAPK activity by increasing P38 phosphorylation (Gao et al. 2012) but down-regulating ERK activity by changing the ratio of p-ERK1 to p-ERK2 (Bambang et al. 2009).
XX.3 ERp29, connexin43 trafficking, and gap junctions
Connexins (Cx) are a family of integral transmembrane proteins that form gap junctions between cells. Approximately 20 members (in higher vertebrates) of the connexin family are assembled in groups of six to form homo- and heteromeric connexons; connexons from adjacent cells form a gap junction (Laird 2006). Gap junctions are critical for the intercellular exchange of ions, small molecule metabolites, and electrical signals, though each combination has variable pore conductance, size selectivity, charge selectivity, voltage gating, and chemical gating. Gap junctions enable chemical and electrical coupling between cells throughout the organism and are found on dozens of cells types. In the retina alone gap junctions are found between every major cell type (i.e. bipolar cells, horizontal cells, amacrine cells, RGCs, photoreceptors, and glia; Danesh-Meyer et al. 2016). Though the specific functional consequences of many of these distinct couplings is unknown, the gap junctions enabling electrical coupling among bipolar cells have recently been discovered to affect the release of glutamate in the inner plexiform layer in a manner that laterally spreads bipolar cell signaling and specifically enhances RGC sensitivity to motion (Kuo et al. 2016).
Connexin43 (Cx43) is the most common gap junction protein in the organism and is present in multiple retinal cell types, including the RPE. The appropriate trafficking of Cx43 through the ER to the Golgi complex is dependent on ERp29 (Das et al. 2009). Cx43 must leave the ER as a monomer and oligomerize in the Golgi complex. To this end, ERp29 forms a complex with Cx43 in the ER which stabilizes Cx43 monomers, likely by stopping the formation of disulfide bonds through blocking cysteine oxidases in an extracellular loop domain. (Das et al. 2009).
In addition, Cx43 is down-regulated in Müller glia in high glucose conditions, which leads to decreased gap junctions with pericytes and increased apoptosis in Müller glia and retinal microvascular endothelial cells (Li and Roy 2009; Muto et al. 2014). Furthermore, retinas from diabetic or Cx43 heterozygous knockout mice develop retinal vascular lesions associated with diabetic retinopathies (Bobbie et al. 2010). Though neither ERp29 nor protein trafficking have been directly implicated in these results it is straightforward to suggest that increased ER stress under disease conditions results in the compromised ability of ERp29 to appropriately chaperone Cx43 through the ER. The continual synthesis of connexins due to their remarkably short half-life of just a few hours only magnifies the potential consequences of impaired protein trafficking in the ER and the potential that this dysfunction underlies, in part, these retinal lesions (Laird 2006).
XX.4 ERp29, neuroprotection, and neurodegenerative disease
ERp29 is a probable neuroprotectant and can potentially facilitate axonal regeneration. ERp29, normally present at high levels in the brain, is down-regulated in rat motor cortex following spinal cord injury (SCI; Liu et al. 2014). Intriguingly, lentiviral expression of ERp29 in cortex increases motor neuron survival and decreases apoptosis after SCI, while virally-mediated suppression of ERp29 increases cortical neuron death (Liu et al. 2014). Remarkably, viral-mediated overexpression of ERp29 after SCI also results in marked improvement in motor function within weeks, suggesting a potential role for ERp29 in axon regeneration as well, potentially via its regulation of ERK pathways (Liu et al. 2014).
ERp29 has been implicated in models of retinal disease where it may also act as a neuroprotectant. Proteomic and immunohistochemical analyses of human donor eyes with AMD and a mouse model of AMD reveal that ERp29 is significantly down-regulated in retina (Ethen et al. 2006; Tuo et al. 2007). The role of the ER chaperone ERp29 in AMD is intriguing given that drusen (accumulations of protein and lipid) are an early sign of AMD. Additionally, a primary risk factor for AMD is smoking and cultured RPE cells exposed long-term (three weeks) to cigarette smoke extract (CSE) have a decrease in ERp29 level (Huang et al. 2015). Furthermore, overexpression of ERp29 protects RPE cells in vitro from CSE exposure by increasing chaperones such as GRP78 and p58IPK and decreasing levels of pro-apoptotic proteins such as CHOP (Huang et al. 2015).
In parallel with the role of ERp29 in gap junction formation described above, overexpression of ERp29 in CSE-treated RPE cells in vitro attenuated the decreases in ZO-1, a tight junction protein, and cadherin levels and the corresponding damage to tight junctions (Huang et al. 2015). Thus, ERp29 may generally be critical to cell-cell contact and barrier integrity. Indeed, ERp29 has been implicated in cancer progression, in part through an effect on epithelial-mesenchymal transition (Chen and Zhang 2015).
Collectively, these results indicate a role for ERp29 in attenuating ER stress in disease and promoting retinal cell survival and cell membrane integrity in vivo and suggest a potential path to a preventative therapeutic regimen in at-risk or diseased patients.
XX.5 Conclusions/Summary
The ubiquitous ER chaperone, ERp29, is critical to protein trafficking through the ER in a variety of cells, notably secretory cells and neurons. Recent work has demonstrated a wide variety of functions for ERp29 in many different pathways (Figure 1), including a protective role for ERp29 in various diseases and injury models. These results are intriguing and correlate well with the reduction in ERp29 in AMD in human. The idea of expression of ERp29 as protective against cell death pathways in some circumstances including retinal degeneration is promising. In addition, the key role of ERp29 in coordinating the appropriate trafficking of membrane proteins such as Cx43 is intriguing but has not been investigated in the retina. Potential implications of ERp29 in maintaining protein homeostasis thus reducing or delaying the proteinopathy and its consequent catastrophic disruption of retinal neurons in various degenerative diseases are also worth pursuing.
Figure 1. Schematic summary of potential roles of ERp29 in cell survival and homeostasis.
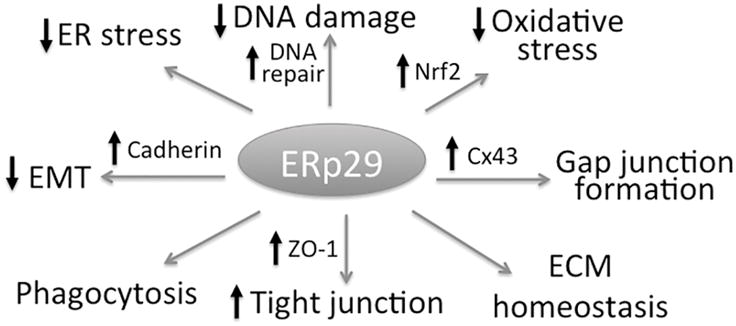
The ubiquitous ER chaperone, ERp29, has myriad functions, some of which are illustrated here. ERp29 appears to play a prominent role in protein trafficking and participate in a wide range of pathways of cell survival pertinent to ER stress, oxidative stress, and DNA repair. In addition, ERp29 is implicated critically in maintaining the integrity of cell membrane and cell-cell contact through regulation of the epithelial-mesenchymal transition (EMT), tight junction formation, gap junction formation, and extracellular matrix (ECM) homeostasis. These functions suggest a potentially strong premise of ERp29 as a therapeutic target for human diseases including retinal degeneration.
References
- Bambang IF, Xu S, Zhou J, et al. Overexpression of endoplasmic reticulum protein 29 regulates mesenchymal-epithelial transition and suppresses xenograft tumor growth of invasive breast cancer cells. Lab Invest. 2009;89:1229–42. doi: 10.1038/labinvest.2009.87. [DOI] [PubMed] [Google Scholar]
- Bobbie MW, Roy S, Trudeau K, et al. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010;51:3758–63. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriushkin E, Wang JJ, Zhang SX. Role of p58IPK in Endoplasmic Reticulum Stress-associated Apoptosis and Inflammation. J Ophthalmic Vis Res. 2014;9:134–43. [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang D. Friend or foe: Endoplasmic reticulum protein 29 (ERp29) in epithelial cancer. FEBS Open Bio. 2015;5:91–98. doi: 10.1016/j.fob.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Zhang J, Acosta ML, et al. Connexin43 in retinal injury and disease. Progress in Retinal and Eye Research. 2016;51:41–68. doi: 10.1016/j.preteyeres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Das S, Smith TD, Das Sharma J, et al. ERp29 restricts Connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer J, Zhou C, Hubbard MJ. Molecular cloning of ERp29, a novel and widely expressed resident of the endoplasmic reticulum. FEBS Letters. 1997;402:145–150. doi: 10.1016/s0014-5793(96)01513-x. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Reilly C, Feng X, et al. The Proteome of Central and Peripheral Retina with Progression of Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2006;47:2280–90. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- Farmaki E, Mkrtchian S, Papazian I, et al. ERp29 regulates response to doxorubicin by a PERK-mediated mechanism. Biochim Biophys Acta. 2011;1813:1165–71. doi: 10.1016/j.bbamcr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Gao D, Bambang IF, Putti TC, et al. ERp29 induces breast cancer cell growth arrest and survival through modulation of activation of p38 and upregulation of ER stress protein p58IPK. Lab Invest. 2012;92:200–13. doi: 10.1038/labinvest.2011.163. [DOI] [PubMed] [Google Scholar]
- Hirsch I, Weiwad M, Prell E, et al. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6–CHOP pathway of stress response. Apoptosis. 2014;19:801–15. doi: 10.1007/s10495-013-0961-0. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang JJ, Jing G, et al. ERp29 attenuates cigarette smoke extract–induced endoplasmic reticulum stress and mitigates tight junction damage in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:6196–6207. doi: 10.1167/iovs.15-16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AL, Lebeau J, Guillaumot P, et al. p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Mol Cell. 2013;49:1049–59. doi: 10.1016/j.molcel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Kuo SP, Schwartz GW, Rieke F. Nonlinear Spatiotemporal Integration by Electrical and Chemical Synapses in the Retina. Neuron. 2016;90:320–32. doi: 10.1016/j.neuron.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:1400–7. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhao W, Zhao Q, et al. Endoplasmic Reticulum Protein 29 Protects Cortical Neurons From Apoptosis and Promoting Corticospinal Tract Regeneration to Improve Neural Behavior via Caspase and Erk Signal in Rats with Spinal Cord Transection. Mol Neurobiol. 2014;250:1035–48. doi: 10.1007/s12035-014-8681-1. [DOI] [PubMed] [Google Scholar]
- MacLeod JC, Sayer RJ, Lucocq JM, et al. ERp29, a general endoplasmic reticulum marker, is highly expressed throughout the brain. J Comp Neurol. 2004;477:29–42. doi: 10.1002/cne.20222. [DOI] [PubMed] [Google Scholar]
- Muto T, Tien T, Kim D, et al. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: promotes Müller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014;55:4327–37. doi: 10.1167/iovs.14-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan E, Baryshev M, Backlund M, et al. Genomic organization and promoter characterization of the gene encoding a putative endoplasmic reticulum chaperone, ERp29. Gene. 2002a;285:127–39. doi: 10.1016/s0378-1119(02)00417-1. [DOI] [PubMed] [Google Scholar]
- Sargsyan E, Baryshev M, Szekely L, et al. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J Biol Chem. 2002b;277:17009–15. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, et al. Murine Ccl2/Cx3cr1 Deficiency Results in Retinal Lesions Mimicking Human Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–36. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Sanders E, Fliesler SJ, et al. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp Eye Res. 2014;125:30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


