Abstract
Purpose
We have previously shown that the expression of the thiamine transporter THTR2 is decreased sevenfold in breast cancer, which may leave breast cancer cells vulnerable to acute thiamine starvation. This concept was supported by the observation that MDA231 breast cancer xenografts demonstrated growth inhibition in mice fed a thiamine-free diet.
Methods
We purified recombinant Bacillus thiaminolyticus thiaminase I enzyme, which digests thiamine, to study acute thiamine starvation in breast cancer.
Results
Thiaminase I enzyme was cytotoxic in six breast cancer cell lines with IC50s ranging from 0.012 to 0.022 U/ml. The growth inhibitory effects of the combination of thiaminase I with either doxorubicin or paclitaxel were also examined. Over a wide range of drug concentrations, thiaminase 1 was consistently synergistic or additive with doxorubicin and paclitaxel in MCF-7, ZR75, HS578T and T47D cell lines, with most combinations having a calculated combination index (CI) of less than 0.8, indicating synergy. Although thiaminase I exposure did not stimulate the energy-sensing signaling kinases AKT, AMPK and GSK-3β in MCF-7, ZR75, HS578T and T47D cell lines, thiaminase I exposure did stimulate expression of the ER stress response protein GRP78. In summary, thiaminase I is cytotoxic in breast cancer cell lines and triggers the unfolded protein response.
Conclusion
These findings suggest that THTR2 down-regulation in breast tumors may present a nutritional vulnerability that could be exploited by thiaminase I enzyme therapy.
Keywords: THTR2, Doxorubicin, Paclitaxel, Unfolded protein response
Introduction
Cancer cells have altered energy metabolism involving thiamine-dependent pathways that preferentially shunt glucose into anaerobic glycolysis under aerobic conditions. Instead of entering the tricarboxylic acid cycle through the thiamine-dependent pyruvate dehydrogenase pathway, pyruvate is converted to lactate and exported unused from the cell. This observation, known as the Warburg effect, may reflect the untethering of malignant cells from growth regulation signals and may represent an important step in transformation [3, 7].
Thiamine cannot readily diffuse into cells, and cells require two specific transport proteins, THTR1 and THTR2, for thiamine uptake. We have previously shown that RNA levels of THTR1 and THTR2 are down-regulated in breast and lung cancer relative to the levels seen in adjacent nonmalignant tissue [11, 12]. While it is possible that the characteristic alterations in energy metabolism in cancer cells may lessen the requirement for thiamine, an alternative view is that THTR1 and THTR2 down-regulation may create a partial thiamine-starvation adapted state that contributes to the malignant process.
We have hypothesized that the down-regulation of thiamine uptake may make tumor cells more sensitive to thiamine starvation, a nutritional vulnerability that could be exploited clinically. An analogous situation is found in acute lymphoblastic leukemia cells, which have down-regulated asparagine synthase, making asparagine an essential nutrient for tumor cells. Treatment with asparaginase exploits this vulnerability, which digests and subsequently depletes asparagine. Adapting this concept for breast cancer, down-regulation of the thiamine transporter gene can be exploited by further depleting the essential nutrient thiamine in breast cancer cells.
We first tested the idea that thiamine starvation, achieved through dietary restriction, would result in growth delay in a breast tumor xenograft. We then tested a potential therapeutic agent, the bacterial enzyme thiaminase I, in breast cancer cell lines alone and in combination with doxorubicin and paclitaxel, chemotherapies that are frequently used in breast cancer treatment. By immunoblot analysis, we also explored the underlying cellular response of breast cancer cells to thiaminase I.
Methods
Xenograft experiments
Studies were performed according to the University of Kentucky Institutional Animal Care and Use Committee guidelines. Female athymic nude mice (nu/nu) were obtained from Harlan at 8–10 weeks of age. MDA231 cells grown were harvested in exponential growth phase, and resuspended in serum-free RPMI 1640. The mixture of 50 μl of cell suspension (5 × 106 cells) and 50 μl of Matrigel™ Matrix (BD, Franklin Lakes, NJ) were subcutaneously (SC) injected along one leg of animals. Following implantation the mice were placed in their cages and allowed to recover. Animals were fed either normal chow or the same chow without thiamine (TestDiet Richmond, IN) in cohorts of three to four mice per cohort. Tumor size was measured using calipers and tumor volumes were calculated using the following formula: tumor volume = 0.5 × L × W 2, where L and W represent the largest diameter and the smallest diameter, respectively. Animal weights and tumor size (length and width) were measured every 2 days.
Thiaminase I production
The E. coli BL21 (DE3) thiaminase I overexpression stain was constructed by the Begley laboratory [5]. The expression vector pET22b(+) is IPTG-inducible and has an N-terminal polyhistidine tag that allows detection and efficient purification of the expressed recombinant enzyme. After IPTG-induction, cells were collected and lysed, and the recombinant enzyme was purified from cytosol using a HisTrap FF column (GE Healthcare, NJ) according to the manufacturer’s protocol. Thiaminase I enzyme activity was determined with a spectrophotometric assay based on a method developed by Lienhard and modified by Costello et al. [5]. The assay is based on a change of absorbance at 252 nm resulting from the reaction of thiamine with secondary nucleophiles.
Cell culture conditions
Human breast cancer cells MDA231, Hs578T, ZR75, MCF7 and T47D were obtained from the American Type Culture Collection (ATCC, Rockville MD). Hs578T was maintained in DMEM, and the other cell lines were maintained in RPMI 1640, respectively. Both media were supplemented with 5% fetal calf serum and 1% penicillin/ streptomycin in a humidified atmosphere of 5% CO2.
Cytotoxicity assays
Breast cancer cells were plated in triplicate in 96-well microtiter plates in medium containing 5% fetal bovine serum at densities of 1,000 cells/well. After 24 h, medium containing thiaminase I was added to the cells. After 4–5 days, the cells were fixed in 10% trichloracetic acid, rinsed with water, and dried. The cells were stained with 0.4% sulforhodamine in 1% acetic acid, washed in 1% acetic acid, and dried as previously described [13]. The stained cells were solubilized in 200 μl of 10 mM Tris base pH 10.5, and the absorbance at 570 nm was determined on a microplate reader. The experiments were repeated three times in triplicate. The IC50 was calculated from the dose response curve as the concentration of drug that produced a 50% decrease in the mean absorbance compared to the untreated wells using Prism GraphPad software. For synergy experiments, cells were plated at three concentrations of thiaminase I representing the approximate IC40, IC60 and IC80 concentrations for each cell line, and then co-incubated in increasing concentrations of doxorubicin or paclitaxel. The experiments were repeated three times in triplicate and the results analyzed with Calcusyn software.
Determination of cellular ATP levels
ATP was measured by a luciferin-luciferase assay using Bioluminescent Somatic Cell Assay kit (Sigma, MO). According to manufacturer’s protocol, 100 μl of cell lysate was added to 100 μl of ATP assay mix solution and light emission was measured immediately with a luminometer (MonolightTM 3010, PharMingen). ATP concentrations were calculated from a calibration curve constructed for each experiment using standard ATP dissolved in the appropriate buffer in which the experiment was performed.
Immunoblot analysis
Cells were treated with thiaminase or drugs for the indicated times and washed with ice-cold PBS. Cells were lysed with a triple-detergent lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 0.02% sodium azide and protease inhibitors). Equal amounts of protein were loaded into each well and separated by 10% SDS-PAGE gel, followed by transfer onto nitrocellulose membranes. The membranes were blocked, incubated with the indicated primary antibodies at 4°C overnight, and the appropriate horseradish peroxidase-conjugated secondary antibody was added for 1 h at room temperature. Immunoblots were developed by use of the enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL) according to the manufacturer’s protocol and autoradiography. All of the primary antibodies were purchased from Cell Signaling Technologies (Danvers, MA). The secondary antibodies were purchased from Sigma (St. Louis, MO). An anti-β-actin antibody was used as a control for protein loading.
Results
To determine whether thiamine starvation could cause tumor growth delay in a breast cancer xenograft, we implanted MDA231 cells subcutaneously in the flanks of nude mice and fed mice either a control diet or a diet in which thiamine was absent. As seen in Fig. 1a, the xenografts in the thiamine-starved mice showed modest tumor growth delay. As predicted, thiamine starvation was toxic over time, and animals began losing weight in the third week (Fig. 1b). Although thiamine starvation through a dietary approach would not be tolerable, this study demonstrated the potential for a therapeutic response of breast cancer cells to acute thiamine deprivation by administration of the thiaminase I enzyme.
Fig. 1.
a Shows growth of an MDA231 xenograft in female nu/nu mice fed either normal chow or identical chow deficient in thiamine. b Shows weight of the mice bearing the xenografts in a
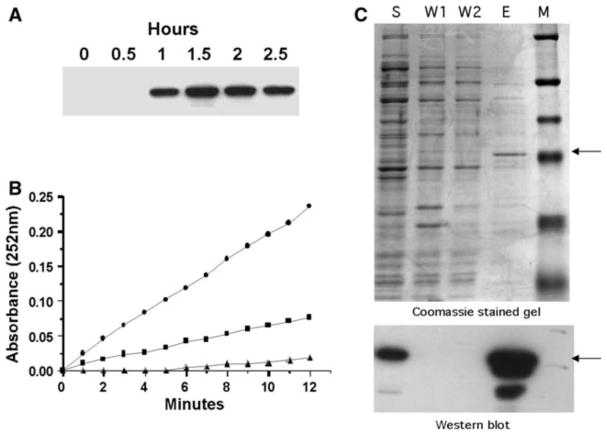
To explore acute thiamine starvation of breast cancer cells through a pharmacologic approach, we produced recombinant thiaminase I enzyme. Figure 2a demonstrates detection of recombinant thiaminase I in crude bacterial lysate with the antibody against the polyhistidine tag linker. The activity of the recombinant enzyme was confirmed by a spectrophotometric assay (Fig. 2b). Figure 2c shows that one of the elution fractions from the purification column, marked lane E, contains a purified protein that is of the expected size (approximately 48 kDa) and identified by the anti-polyhistidine tag antibody.
Fig. 2.
Expression and activity of thiaminase 1 from Bacillus thiaminolyticus. a Recombinant thiaminase I expression in BL21(DE3) cells following induction with 2 mM IPTG. Immunoblots were performed against the polyhistidine tag linker using the monoclonal Anti-Xpress™ antibody (Invitrogen). b Thiaminase 1 activity determined spectrophotometrically at 252 nm. (σ) Buffer alone. (ν) 20μl of bacterial lysate prior to IPTG induction (0 h). (λ) 20μl of bacterial lysate 1.5 h after IPTG induction. c Thiaminase I purification using a nickel-charged sepharose resin column (HisTrapFF). Western blot and Coomassie stained gels of thiaminase I-expressing BL21(DE3) bacteria induced with IPTG. The top gel is a Coomassie stained gel showing size fractionated proteins from the purification steps. The bottom gel is a Western blot of a replicate gel probed with a monoclonal antibody against the polyhistidine tag. S bacterial supernatant before applying to column, W1 and W2 column washes, E eluate of the column with the fraction containing the recombinant thiaminase I protein, M marker (the fourth marker band from the top is 47.5 kDa)
The cytotoxicity of thiaminase I in breast cancer cell lines is shown in Fig. 3. Thiaminase I IC50s were spread over an 18-fold range, from 0.012 to 0.22 U/ml. Of interest, the MDA231 cell line transfected with the thiamine transporter THTR2 (MDA231/THTR2) (3) showed a 67% increase in resistance compared to empty vector control MDA231 cells (0.020 ± 0.003 vs. 0.012 ± 0.001 U/ml). IC50 values for the other cell lines were MCF-7 0.06 ± 0.012 U/ml; T47D 0.22 ± 0.09 U/ml; ZR-75 0.12 ± 0.04 U/ ml; and HS578T 0.05 ± 0.005 U/ml.
Fig. 3.
Cytotoxicity assays of breast cancer cell lines exposed to increasing concentrations of recombinant thiaminase I. MDA231/THTR2 are MDA231 cells transfected with the THTR2 thiamine transporter (4) and MDA231/pcDNA are MDA231 cells transfected with the empty expression vector
Since thiamine depletion affects ATP production, we determined whether thiaminase I treatment decreased cellular ATP levels. As shown in Fig. 4a, ATP depletion after incubation in 0.5 U/ml thiaminase I after 1 day (upper panel) and after 4 days (lower panel) was variable in different cell lines. After 4 days, the ATP depletion effect was most pronounced in MDA231/pcDNA cells (26% of control) and Hs578T cells (29% of control), and least pronounced in T47D cells (84% of control). The correlation between the IC50s in Fig. 3 and ATP depletion after 4 days in Fig. 4a is shown in Fig. 4b. Of interest, the breast cancer cell lines showed a linear relation between thiaminase I-induced ATP depletion and thiaminase I cytotoxicity (diamonds) (r2 = 0.91), with the exception of the MDA231 cells transfected with the THTR2 transporter (square), The increased expression of the THTR2 transporter in MDA 231 cells prevented thiaminase I-induced ATP depletion, but did not change sensitivity of the MDA231 cells to the cytotoxic effects of thiaminase I, indicating that thiaminase I cytotoxicity is not dependent on THTR2 expression.
Fig. 4.
a ATP levels in cell lines treated with 0.5 U/ml thiaminase 1 for 1 day (top) or 4 days (bottom). ATP levels were determined as described in “Methods”. The results represent three independent experiments performed in duplicate. b ATP depletion after 4 days as a function of thiaminase I IC50. The red square represents the THTR2-transfected cell line MDA231/THTR2
To determine whether thiaminase I treatment would have additive or synergistic activity with two drugs commonly used in breast cancer treatment, we examined doxorubicin and paclitaxel cytotoxicity in breast cancer cell lines during co-incubation with thiaminase I. Dose response curves were analyzed using the median-dose effect model (Calcusyn), and multiple combination indices (CIs; y axis) were calculated over a range of concentrations of each drug and thiaminase I combination that produced a range of cytotoxic effects (Fa; x axis). The CI was less than 0.8 for most drug-thiaminase I combinations for four breast cancer cell lines for both doxorubicin and paclitaxel, indicating a synergistic interaction, and most of the remaining CIs were in the 0.8–1.2 range, indicating additive cytotoxicity. These data suggest that acute thiamine starvation may improve the effectiveness of breast cancer chemotherapy. Interestingly, colony forming assays undertaken to assess the potential sensitizing effect of thiaminase I on radiation sensitivity showed that thiaminase I did not sensitize T47D, MCF-7 or ZR-75 cells to radiation (data not shown).
Since thiaminase I decreases ATP levels in breast cancer cells and could be expected to alter cellular energy regulation, we examined energy pathways in breast cancer cells after thiaminase I treatment. There was no consistent change in Akt, AMPK or GSK-3β expression or phosphorylation among the breast cancer cell lines under the conditions studied (Fig. 6). The only change observed was an increase in GRP78 expression in T47D and HS578T cells of 64 and 37%, respectively as determined by densitometry, suggesting activation of the unfolded protein response (UPR).
Fig. 6.
Immunoblot analysis of Akt, p-Akt, AMPK, p-AMPK, GSK3β, p-GSK3β, β-catenin, cyclin D1, PTEN, caspase 3 and GRP78 in four breast cancer cell lines plus MDA231 breast cancer cells transfected with THTR2 or empty vector pcDNA as a control after exposure to thiaminase I. Cytosolic protein (30 μg) was probed using standard immunoblot procedures with antibodies as described in “Methods”
To further examine the effect of thiaminase I on the UPR, four breast cancer cell lines were exposed to thapsigargin and tunicamycin as positive controls, as well as to thiaminase I, and then analyzed for UPR activation by immunoblot analysis. All four breast cancer cell lines showed a marked induction in GRP78 after thapsigargin and tunicamycin exposure (Fig. 7), with variable activation of downstream effectors CHOP, p-EIF2α and p-PERK. The three breast cancer cell lines most sensitive to thiaminase I, ZR-75, MCF-7 and HS578T, all showed induction of GRP78 with increases measured by densitometry and normalized to actin of 67, 139 and 154%, respectively. MCF-7 and ZR-75 cells also showed downstream activation of p-PERK (76 and 53% at 6 h, respectively) and p-EIF2α (156 and 37% at 6 h, respectively) after thiaminase I exposure.
Fig. 7.
Immunoblot analysis of UPR pathway proteins GRP78, CHOP, p-PERK and p-EIF2α after exposure to thiaminase I in the four breast cancer cell lines MCF-7, ZR-75, T47D and HS578T. Tunicamycin (5 μg/ ml) and thapsigargin (1 μM) were used as positive controls for stimulating the UPR
Discussion
Chronic thiamine starvation is toxic, leading to the neurological and cardiovascular symptoms of beriberi and Wernicke–Korsakov syndrome, and could not realistically be considered as an anticancer therapeutic approach. However, acute short-lived thiamine starvation may have less toxicity, and could potentially be achieved by administration of an enzyme such as thiaminase I. Thiaminase catabolizes thiamine and can cause acute thiamine deficiency. Several forms of the enzyme thiaminase exist in nature, including plant, animal and bacterial forms of the enzyme [4, 14, 15]. Since thiamine is an essential vitamin and since thiamine in excess is not known to be toxic, the physiologic role of thiaminase is not known [5].
Although the down-regulation of thiamine transporter gene THTR2 expression may represent a nutritional vulnerability, it was not clear whether thiamine was relevant to tumor growth. In Fig. 1 we showed that an MDA231 breast cancer xenograft showed growth delay in mice fed a thiamine-free diet, even though this nutritional deprivation had expected toxicities after a 3 week period.
Previous studies of thiamine starvation have utilized thiamine analogs, such as pyrithiamine and oxythiamine and amprolium, which all compete for uptake and may inhibit thiamine dependent enzymes [1, 2]. Bettendorff et al. have previously reported that adaptation to physiologic (6 nM) extracellular thiamine concentration still required addition of the thiamine analog amprolium to observe a decrease ATP levels in cultured neuroblastoma cells [1]. However, uncertainty about the extent of both thiamine uptake inhibition and extent of thiamine-dependent enzyme inhibition by thiamine analogs led us to conclude that these analogs could not definitively mimic acute thiamine starvation.
Instead of thiamine antagonists we explored the potential therapeutic efficacy of the bacterial enzyme thiaminase I. As shown in Fig. 3, thiaminase I had cytotoxic activity against all breast cancer cell lines tested, indicating that acute thiamine deprivation does have toxic consequences in breast cell lines. In addition, there was a 1.8-fold difference in the thiaminase I IC50 between MDA231 cells transfected with the THTR2 transporter versus control MDA231 cells. In previous studies we have explored the cellular effects of altered THTR2 expression by transfecting cancer cells with THTR2 [12]. RNA microarray studies identified genes that were up- and down-regulated with increased THTR2 expression. In comparing tumor cells to non-malignant tissues, the pattern of gene expression predicted by the micro-array experiments was reflected in subsequent studies of RNA levels in tumors compared to non-malignant adjacent tissue, and in THTR2 knock-down experiments [12]. The studies here indicate that the down regulation of THTR2 observed in breast cancer cells may increase their sensitivity to acute thiamine starvation.
We also explored the possibility that thiamine deprivation might also enhance chemotherapy sensitivity in breast cancer cells. Thiamine starvation could sensitize cancer cells to chemotherapy for several reasons. Thiamine deprivation might augment the toxicity of drugs that are substrates for ATP-dependent drug efflux pumps (ABC transporters) because of less efficient ATP production. These drugs include doxorubicin and paclitaxel, both of which are pumped out of cells by the MDR1 drug efflux pump, and both of which are active drugs frequently used in breast cancer therapy. As shown in Fig. 5, at almost all concentration combinations, thiaminase I was additive or synergistic with paclitaxel and doxorubicin in all four breast cancer cell lines in which these synergy studies were performed.
Fig. 5.
Analysis of combined cytotoxicity of thiaminase I and doxorubicin (a) or paclitaxel (b) in four breast cancer cell lines MCF-7, ZR-75, T47D and Hs578T. The cells were incubated for 4–5 days in increasing concentrations of combinations of drugs and surviving cells were determined as described in “Methods”. The combination index (CI) was calculated for each combination of thiaminase I and drug using Calcusyn software, and the CIs plotted against the combined cytotoxicity (Fa) to demonstrate additivity or synergy over a range of drug combinations and cytotoxic effects
Surprisingly, given the decrease in ATP levels in all breast cancer cell lines after thiaminase I exposure, there was no consistent increase in proteins involved in energy sensing and regulation, including phosphorylation of Akt, AMPK or GSK3β after thiaminase I treatment (Fig. 6). However, we also examined GRP78 protein expression because of the possibility that inhibition of thiamine-dependent pathways might result in protein damage that could create endoplasmic reticulum stress and trigger the UPR. The sensing chaperone GRP78 sequesters three proteins in the endoplasmic reticulum (PERK, IRE1 and ATF6) resulting in UPR activation [6, 9].
All of the breast cancer cell lines showed a marked increase in GRP78 expression in response to thapsigargin and tunicamycin (Fig. 7). There was considerable variation, however, in downstream effects of GRP78 activation by these agents among the four breast cancer cell lines. Similarly thiaminase I, while not as potent a GRP78 inducer as thapsigargin or tunicamycin, also showed variable response downstream of GRP78. UPR can activate both pro-survival and pro-apoptotic pathways, and the variability in downstream activation among breast cancer cell lines is apparent in Fig. 7, making the effect of UPR activation on cell survival difficult to predict. GRP78 overexpression confers anticancer drug resistance against several classes of agents in cell lines from varied tumors of origin [10], while knocked down expression of GRP78 increased sensitivity to cells to anticancer drugs [8]. Thus, it is not clear whether UPR activation by thiaminase I directly results in apoptosis activation, or contributes to the observed synergy with chemotherapeutic agents, in breast cancer cells.
In summary, these studies demonstrate that recombinant thiaminase I has activity in vitro against breast cancer cell lines both alone and in combination paclitaxel and doxorubicin. The mechanism of action may involve decreased ATP levels or creation of ER stress and activation of the UPR. Further studies are needed to determine whether the thiaminase I enzyme can be developed as a novel therapeutic agent for breast cancer.
Acknowledgments
This work was supported by the Susan G. Komen for the Cure Foundation.
Footnotes
Conflict of interest statement None.
Contributor Information
Shuqian Liu, Division of Pediatric Hematology-Oncology, Department of Pediatrics, University of Kentucky College of Medicine, 740 S. Limestone Room J457, Lexington, KY 40536, USA.
Noel R. Monks, Division of Pediatric Hematology-Oncology, Department of Pediatrics, University of Kentucky College of Medicine, 740 S. Limestone Room J457, Lexington, KY 40536, USA
Jeremiah W. Hanes, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA
Tadhg P. Begley, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA
Hui Yu, Division of Pediatric Hematology-Oncology, Department of Pediatrics, University of Kentucky College of Medicine, 740 S. Limestone Room J457, Lexington, KY 40536, USA.
Jeffrey A. Moscow, Division of Pediatric Hematology-Oncology, Department of Pediatrics, University of Kentucky College of Medicine, 740 S. Limestone Room J457, Lexington, KY 40536, USA
References
- 1.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem. 1995;64:2013–2021. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 2.Bettendorff L, Wins P. Mechanism of thiamine transport in neuroblastoma cells. J Biol Chem. 1994;269:14379–14385. [PubMed] [Google Scholar]
- 3.Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24(26):4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 4.Campobasso N, Costello CA, Kinsland C, Begley TP, Ealick SE. Crystal structure of thiaminase-I from Bacillus thiaminolyticus at 2.0 A resolution. Biochemistry. 1998;37:15981–15989. doi: 10.1021/bi981673l. [DOI] [PubMed] [Google Scholar]
- 5.Costello CA, Kelleher NL, Abe M, McLafferty FW, Begley TP. Mechanistic studies on thiaminase I overexpression and identification of the active site nucleophile. J Biol Chem. 1996;271:3445–3452. doi: 10.1074/jbc.271.7.3445. [DOI] [PubMed] [Google Scholar]
- 6.Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008;7:865–869. doi: 10.4161/cc.7.7.5615. [DOI] [PubMed] [Google Scholar]
- 7.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 8.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Huang H, Lu X, Golinski M, Comesse S, Watt D, Grossman RB, Moscow JA. Down-regulation of thiamine transporter THTR2 gene expression in breast cancer and its association with resistance to apoptosis. Mol Cancer Res. 2003;1:665–673. [PubMed] [Google Scholar]
- 12.Liu S, Stromberg A, Tai HH, Moscow JA. Thiamine transporter gene expression and exogenous thiamine modulate the expression of genes involved in drug and prostaglandin metabolism in breast cancer cells. Mol Cancer Res. 2004;2:477–487. [PubMed] [Google Scholar]
- 13.Monks N, Liu S, Xu Y, Yu H, Bendelow A, Moscow J. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther. 2007;6:587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- 14.Nishimune T, Watanabe Y, Okazaki H. Studies on the polymorphism of thiaminase I in seawater fish. J Nutr Sci Vitaminol (Tokyo) 2008;54:339–346. doi: 10.3177/jnsv.54.339. [DOI] [PubMed] [Google Scholar]
- 15.Thomas KW. The effect of thiaminase-induced subclinical thiamine deficiency on growth of weaner sheep. Vet Res Commun. 1986;10:125–141. doi: 10.1007/BF02213975. [DOI] [PubMed] [Google Scholar]