Abstract
Purpose of review
Significant advances have been made in the study of ubiquitination-mediated regulation of androgen receptor (AR). This review will highlight the latest developments in the mechanisms by which E3 ubiquitin ligases control AR activity, with implications in castration-resistant prostate cancer (CRPC).
Recent findings
Several ubiquitin ligases have been identified to interact with and ubiquitinate AR, and consequently regulate positively or negatively e the AR transcriptional program.. Different ubiquitin ligases can use distinct mechanisms to modulate the expression of AR target genes, including local turnover of AR chromatin complex, recruitment of AR co-activators, and global AR stability. The expression or activity of ubiquitin ligases can be altered in prostate cancer and thus contribute to the growth of androgen-insensitive prostate cancer cells by modulating the AR transcriptional activity.
Summary
Understanding the regulation of AR transcriptional activity by ubiquitin ligases will contribute to the elucidation of mechanisms underlying AR re-activation that is believed to drive the development of CPPC. Ubiquitin ligases could potentially serve as promising targets for developing therapeutics in the treatment of advanced prostate cancers.
Keywords: Ubiquitination, ubiquitin ligase, androgen receptor, castration-resistant prostate cancer, transcriptional regulation
Introduction
Androgen deprivation therapy (ADT) is the first-line of treatment for metastatic or advanced prostate cancer. Although ADT can cause regression of prostate cancer, the disease invariably becomes resistant to ADT over time and progresses to a state termed castration-resistant prostate cancer (CRPC). Considerable progress has been made in understanding the underlying molecular pathways that contribute to disease progression in prostate cancer, which has led to several clinically useful treatments that can palliate and improve overall survival among men with CRPC. Nevertheless, CRPC essentially remains an incurable disease state. Thus, significant challenges still remain in understanding the various mechanisms that contribute to the CRPC phenotype, which will be critical for the development of more effective treatments for this disease.
It is currently believed that in most patients undergoing ADT, resistance to castration occurs due to the reactivation of the androgen-androgen receptor (AR) axis. AR belongs to the steroid receptor superfamily, and is activated to regulate gene expression upon binding of ligand (dihydrotestosterone (DHT) or testosterone (T)). The reactivation of AR in CRPC can result from changes in AR and/or its ligand. Alterations in AR include AR over expression (1), AR mutations that alter ligand binding specificities (2), and generation of constitutively active AR variants, among others (3). Intra-tumoral androgen production via de novo steroidogenesis or conversion of adrenal androgen precursors can help maintain intracellular androgen levels in CRPC cells (4, 5). Several AR-independent mechanisms that may contribute to the development of CRPC have also been proposed, such as the compensation of androgen signaling by other signaling pathways (6), neuroendocrine differentiation (NED) of prostate cancer cells (7), and involvement of prostate cancer stem-like cells (8). Nonetheless, alterations in serum PSA (a well-characterized AR target) during disease progression, and in response to the newer-generation ‘hormonal’ therapies enzalutamide and abiraterone support a central role for the reactivation of AR in the development of CRPC.
Ubiquitination is a process whereby ubiquitin is covalently attached to substrate lysines via an isopeptide bond. It is an important post-translational modification that regulates a vast array of cellular processes (9). Ubiquitin can be attached to substrates as single ubiquitin(s) (mono-ubiquitination) or as ubiquitin chains (polyubiquitination). The polyubiquitin chains can adopt different topologies, and are named according to which of the 7 lysines (K) within ubiquitin are used to link the chains. K48-linked chains and K63-linked chains are well studied. The former leads to the degradation of substrates by 26S proteasome, while the latter can alter the protein’s activity, interaction or localization.
Ubiquitination is carried out by the sequential action of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3), respectively. E3 ubiquitin ligases determine the substrate specificity and promote the transfer of ubiquitin to the substrates (10). The E3 ligases can be classified into three families: really interesting new gene (RING), homologous to E6AP carboxyl terminus (HECT), and UFD2 homology (U-box). The RING family is the largest family of E3 ubiquitin ligases. They can function as individual proteins or as components of multi-subunit complexes.
AR activity is regulated by a variety of post-translational modifications such as phosphorylation, acetylation, methylation, sumoylation, and ubiquitination (11). This review will highlight the role of ubiquitination and E3 ubiquitin ligases in the regulation of AR activity in CRPC.
Siah2 regulates AR turnover for selective AR target genes
Siah2 is a RING-finger E3 ubiquitin ligase, which has an N-terminal domain, a central RING finger/Zinc finger domain, and a C-terminal substrate-binding domain. Like other RING-finger E3 ubiquitin ligases, Siah2 simultaneously binds to substrate and E2 ubiquitin-conjugating enzyme, facilitating the transfer of ubiquitin from E2 to substrate. Siah2 regulates a number of biological processes by ubiquitinating and degrading substrate proteins (12). Inhibition of Siah2 blocks the development of several types of cancers, supporting a tumor-promoting role for Siah2 (12).
More recently, Siah2 was found to play an important role in CRPC (13). Higher levels of Siah2 staining were detected in high-grade prostate cancer and CRPC samples on a tissue microarray (TMA) (13). Consistent with this, another study identified Siah2 as one of the top biomarkers for predicting biochemical recurrence among prostate cancer patients who underwent radical prostatectomy (14). Inhibition of Siah2 blocks the proliferation and survival of androgen-insensitive prostate cancer cell lines in vitro as well as orthotopic tumor formation in castrated mice (13). In a transgenic adenocarcinoma of the mouse prostate (TRAMP) model, knockout of Siah2 enhances tumor regression in surgically castrated mice (13). Taken together, these data suggest that Siah2 contributes to progression of prostate cancer.
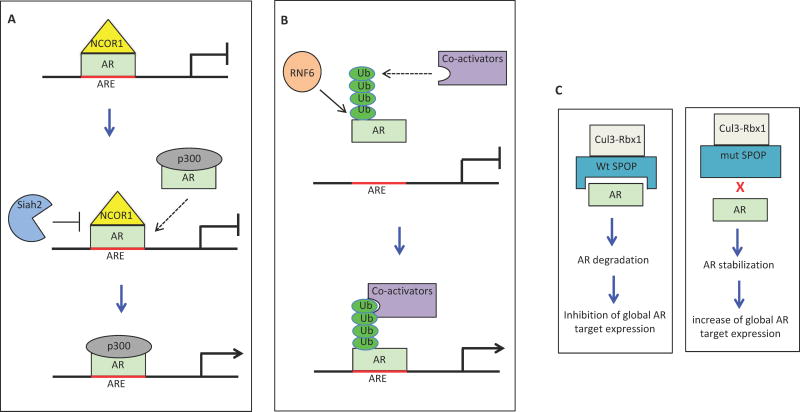
Knockdown of Siah2 in prostate cancer cells reveals that the AR transcriptional network is among the most significantly affected signaling networks in these cells (13). Siah2 regulates selective AR target genes, including those involved in lipid metabolism and steroid biosynthesis. Similarly, Siah2 is required for the expression of selective AR targets in prostate tumors in TRAMP mice, and in castrate resistant tumor xenografts. Given a central role of AR in prostate cancer, these results suggest that Siah2 may contribute to progression and perhaps also development of castration resistance via regulation of AR activity. Biochemical analyses demonstrate an interaction between Siah2 and AR, and over expression of Siah2 results in the K48-linked polyubiquitination and proteasome-dependent degradation of AR, suggesting that AR may be a substrate of Siah2 (13). However, interestingly, knockdown of Siah2 has no effect on the global levels of AR or nuclear receptor co-repressor NCOR1 (a known Siah2 substrate). Rather, ChIP-PCR reveals that increased amounts of AR and NCOR1 become bound to the enhancers of selective AR target genes. Therefore, endogenous Siah2 does not control the stability of global AR or NCOR1, but instead targets the degradation of AR/NCOR1 complexes associated with a small number of AR target genes. NCOR1 is known to repress AR transcriptional activity; thus, removal of the transcriptionally inactive AR/NCOR1 complex will allow the subsequent recruitment of AR/co-activator complexes to increase the transcriptional output of selective AR target genes (Fig. 1a). The idea that Siah2 regulates the stability of a small pool of transcriptional complexes to promote the transcription of a select number of genes is corroborated in a recent elegant ChIP-seq study performed on 3T3-L1 cells (15). In this study, Siah2 did not affect the global NCOR1 levels but degraded NCOR1 within the CREB/NCOR1 complex bound on the promoters of nuclear mitochondrial genes, thus increasing the CREB-dependent expression of the respective genes (15). Also, post-translational modifications (PTMs) such as phosphorylation have been shown to modulate the interaction of Siah2 with its substrate (16, 17), but it remains to be determined which PTMs of Siah2, AR and/or NCOR1 enhance or alter the recognition of AR/NOCR1 by Siah2. Of note, the E3 ubiquitin ligase MDM2 can regulate AR turnover on the PSA promoter and induce cyclic transcription of PSA mRNA in human fibroblast cells or Hela cells expressing an ectopic AR (18). It will be of interest to know whether MDM2 can use the same mechanism to regulate the expression of AR target genes in prostate cancer.
FIGURE 1.
A. Siah2 ubiquitinates and degrades the AR-NCOR1 complex on AREs of select AR targets. This allows the subsequent recruitment of AR-p300 to activate the transcription of these AR targets. B. RNF6 induces the atypical ubiquitination of AR, and this causes the recruitment of co-activators that have the ubiquitin-binding domain. The AR/co-activator complex binds to AREs of selective AR targets and increases the transcription of these AR targets. C. wild-type (wt) SPOP interacts with AR, leading to the ubiquitination and degradation of AR by the Cul3-Rbx1 ubiquitin ligase complex. Mutant (mut) SPOP cannot interact with AR, resulting in the stabilization of AR and increase of global AR target expression.
RNF6-induced ubiquitination of AR promotes recruitment of AR co-activators
RNF6 is a RING-finger E3 ubiquitin ligase. It is up regulated in androgen-insensitive prostate cancer cell lines and in clinical CRPC specimens in comparison to hormone-naïve PCa samples (19). Knockdown of RNF6 inhibits the growth of prostate cancer cell lines under androgen-deprived conditions and growth of xenograft tumors in castrated mice (19). Biochemical and mass spectrometry analysis have demonstrated interactions between RNF6 and AR, suggesting that RNF6 may regulate AR activity and function. RNF6 induces polyubiquitination of AR without effect on AR stability. The RNF6-induced ubiquitin chains in AR are non-canonical K6- and K27-linked, and ubiquitination occurs primarily on the K845 amino acid residue of AR. Mutation of AR K845 abolishes RNF6-induced activation of AR mediated luciferase reporter activity, and attenuates the recruitment of AR to selective AR target genes (19). The RNF6-induced non-canonical polyubiquitination of AR can promote the binding of AR co-activators such as ARA54 that have ubiquitin-binding domains (Fig. 1b). Profiling array analyses reveal that RNF6 does not affect the global expression of AR target genes, but is required for the expression of a subset of AR target genes. This suggests that certain PTMs of AR may be required for the recruitment of RNF6 to such AR target genes. Consistent with this notion, the kinase PIM-1 induces Thr-850 phosphorylation of AR, which facilitates the binding of RNF6 to AR (20).
It is worth noting that RNF6 and Siah2 are required for the expression of certain subsets of AR target genes. However, largely different sets of AR target genes are regulated by RNF6 and Siah2. This highlights the fact that the two ubiquitin ligases use different mechanisms in regulating AR transcriptional activity, and that distinct sets of AR target genes can contribute to the CRPC phenotype.
SPOP regulates transcriptional output of AR by controlling global AR stability
The Cullin-RING ubiquitin ligases (CRLs) are multi-subunit complexes that include a cullin scaffold protein, a RING domain protein (Rbx1 or Rbx2) that interacts with E2, and a substrate adaptor protein that determines substrate specificity. Speckle-type POZ protein (SPOP) is the substrate adaptor protein for the Cullin3/Rbx1 CRL, and it is mutated in up to 15% of prostate cancers (21, 22). The mutations of SPOP are confined to specific amino acid residues within the substrate-binding pocket, and are expected to attenuate substrate binding. A profiling array analysis of prostate cancer cells expressing ectopic wild-type or mutant SPOP revealed that the AR transcriptional network was among the most affected gene sets (23). Wild-type SPOP represses the expression of AR target genes, whereas mutant SPOPs increase the expression of these genes. The gene signature of mutant SPOPs identified in prostate cancer cell lines are positively correlated with the androgen-induced gene signature in published datasets from human prostate cancer specimens (23). AR harbors a SPOP-binding motif (ASSTT) in its hinge region, and this motif is required for the interaction between AR and SPOP (23, 24). Overexpression of wild-type SPOP induces the ubiquitination and degradation of AR. Conversely, knockdown of SPOP in prostate cancer cells or hemizygous knockout of SPOP in mouse prostate tumor xenografts increases AR protein levels (23, 24). These results demonstrate that wild-type SPOP controls the stability and transcriptional activity of AR via ubiquitination and degradation of AR (Fig. 1c). In contrast, mutant SPOP cannot interact with and degrade AR and thus increases AR stability and transcriptional activity (Fig C). As heterozygous mutant alleles of SPOP affect AR, mutant SPOPs may have a dominant-negative effect in modulating AR degradation mediated by wild-type SPOP. This possibility is supported by another study, which shows that prostate cancer SPOP mutations exert a dominant-negative effect on wild-type SPOP activity through formation of heteromeric complexes (25). Furthermore, expression of mutant SPOPs in prostate cancer cells promotes cell proliferation in vitro and tumor growth in immune-deficient mice (23). SPOP has also been found to induce the ubiquitination and degradation of SRC-3 (26), a key co-activator of AR. Thus, elevation of SRC-3 by a mutant SPOP may also contribute to the transcriptional activation of AR. In addition to mutations, SPOP can be downregulated in prostate cancer (27, 28), and this may provide another mechanism to increase AR stability and transcriptional activity. Additional studies will help clarify the role of SPOP in CRPC.
Other ubiquitin ligases
Several other E3 ubiquitin ligases for AR have been reported, including MDM2 (29), CHIP (30), NEDD4 (31) and SKP2 (32). Common to these ubiquitin ligases is the induction of ubiquitination and degradation of AR upon their over expression. It remains to be determined whether these ubiquitin ligases are altered in CRPC, and whether they are involved in the regulation of AR transcriptional output in CRPC. Profiling array analyses and knockdown studies will be important to establish their role in regulating AR transcriptional output.
Conclusion and Future Directions
AR transcriptional activity is subject to regulation by ubiquitination. The ubiquitination of AR induced by different E3 ubiquitin ligases may have different consequences on the fate of AR, including 1) local turnover of AR (e.g. Siah2) or recruitment of AR co-activators (e.g. RNF6) in certain AR target genes, or 2) global degradation of AR (e.g. Cullin3/Rbx1/SPOP CRL). E3 ubiquitin ligases using the first two mechanisms promote the expression of selected AR targets, while those using the latter mechanism are expected to repress the global expression of AR target genes. These ubiquitin ligases are either up regulated (Siah2, RNF6) or mutated (SPOP) in prostate cancer. In addition, the E3 ubiquitin ligases may promote progression of prostate cancer by targeting the degradation of substrates other than AR. For example, Siah2 can contribute to NED phenotypes by increasing HIF activity (33). Mutant SPOPs may also increase the levels of several other substrates (e.g. DEK, TRIM24, SRC-3), which could in turn contribute to the progression of prostate cancer (25). Thus, in summary, accumulating evidence provides a rationale for targeting ubiquitin ligases for potential therapeutic effect in prostate cancer.
Ubiquitin ligases can contribute to CRPC via regulation of AR levels or activities.
Ubiquitin ligase can enhance AR activity by local degradation of AR on certain target genes
Ubiquitin ligase can promote the recruitment of co-activators by atypical ubiquitination of AR
Ubiquitin ligase can repress AR activity by global degradation of AR
Acknowledgments
This work was supported by NCI grant CA154888 (to J.Q.), and in part by a Merit Review Award, Dept of Veterans Affairs (to A.H.)
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES and RECOMMENDED READING
- 1.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nature medicine. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 2.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer research. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 3.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer research. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocrine-related cancer. 2013;20:R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 7.Komiya A, Yasuda K, Watanabe A, Fujiuchi Y, Tsuzuki T, Fuse H. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Molecular and clinical oncology. 2013;1:257–262. doi: 10.3892/mco.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, Calhoun-Davis T, Li H, Palapattu GS, Pang S, et al. The PSA(−/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell stem cell. 2012;10:556–569. doi: 10.1016/j.stem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger MB, Hristova VA, Weissman AM. HECT and RING finger families of E3 ubiquitin ligases at a glance. Journal of cell science. 2012;125:531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nature structural & molecular biology. 2014;21:301–307. doi: 10.1038/nsmb.2780. [DOI] [PubMed] [Google Scholar]
- 11.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. The Journal of endocrinology. 2012;215:221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 12.Qi J, Kim H, Scortegagna M, Ronai ZA. Regulators and effectors of Siah ubiquitin ligases. Cell biochemistry and biophysics. 2013;67:15–24. doi: 10.1007/s12013-013-9636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi J, Tripathi M, Mishra R, Sahgal N, Fazli L, Ettinger S, Placzek WJ, Claps G, Chung LW, Bowtell D, et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer cell. 2013;23:332–346. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzardi AE, Rosener NK, Koopmeiners JS, Isaksson Vogel R, Metzger GJ, Forster CL, Marston LO, Tiffany JR, McCarthy JB, Turley EA, et al. Evaluation of protein biomarkers of prostate cancer aggressiveness. BMC cancer. 2014;14:244. doi: 10.1186/1471-2407-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catic A, Suh CY, Hill CT, Daheron L, Henkel T, Orford KW, Dombkowski DM, Liu T, Liu XS, Scadden DT. Genome-wide map of nuclear protein degradation shows NCoR1 turnover as a key to mitochondrial gene regulation. Cell. 2013;155:1380–1395. doi: 10.1016/j.cell.2013.11.016. This study uses genome-wide mapping with ChIP-seq to show the ubiqutination-mediated degradtion of chromatin-asssoictated proteins is associated with the promoters/enhancers of actively expressed genes such as the nuclear-encoded mitochondrial genes. Ubiquitin ligase Siah2 is found to ubiqutinate and degrade a small pool of NCOR1 associated with transcription factor CREB on the promoters of the nucear-encoded mitochondrial genes, and thus enhance the transcripiton of these genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calzado MA, de la Vega L, Moller A, Bowtell DD, Schmitz ML. An inducible autoregulatory loop between HIPK2 and Siah2 at the apex of the hypoxic response. Nature cell biology. 2009;11:85–91. doi: 10.1038/ncb1816. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar TR, Sharan S, Wang J, Pawar SA, Cantwell CA, Johnson PF, Morrison DK, Wang JM, Sterneck E. Identification of a Src tyrosine kinase/SIAH2 E3 ubiquitin ligase pathway that regulates C/EBPdelta expression and contributes to transformation of breast tumor cells. Molecular and cellular biology. 2012;32:320–332. doi: 10.1128/MCB.05790-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chymkowitch P, Le May N, Charneau P, Compe E, Egly JM. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. The EMBO journal. 2011;30:468–479. doi: 10.1038/emboj.2010.337. This study shows that cdk7 kinase phosphorylates AR, and this phosphorylation promotes the recuritment of ubiquitin ligase MDM2 to induce the ubiquitination and degradation of AR at the promoter of the gene encoding PSA. The MDM2-mediated cyclic degradtion of AR on the PSA promotor is correlated with the cyclic recruitment of the transcriptional machinery to the PSA promoter and cyclic expression of PSA transcript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F, Guo Z, Chen H, Li W, Kong X, et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer cell. 2009;15:270–282. doi: 10.1016/j.ccr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linn DE, Yang X, Xie Y, Alfano A, Deshmukh D, Wang X, Shimelis H, Chen H, Li W, Xu K, et al. Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases. The Journal of biological chemistry. 2012;287:22959–22968. doi: 10.1074/jbc.M111.338350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blattner M, Lee DJ, O’Reilly C, Park K, MacDonald TY, Khani F, Turner KR, Chiu YL, Wild PJ, Dolgalev I, et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia. 2014;16:14–20. doi: 10.1593/neo.131704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, Fiskus W, Rajendran M, Chew SA, Zimmermann M, et al. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer research. 2014;74:5631–5643. doi: 10.1158/0008-5472.CAN-14-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An J, Wang C, Deng Y, Yu L, Huang H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell reports. 2014;6:657–669. doi: 10.1016/j.celrep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, Wild PJ, Blattner M, Groner AC, Rubin MA, et al. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. This study uses SILAC-based mass-spectrometry to determine the global alterations in ubiquitinated proteins in prostate cells expressing wild-type or mutant SPOPs. This approach has allowed the identification of several SPOP targets such as DEK, TRIM24 and SRC3, which are upregulated in prosate cancer tissues harboring SPOP mutations. This study demonstrates a dominnat-negative effect of mutant SPOP on the degradtion of substrates mediated by wild-type SPOP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2013;121:626–633. doi: 10.1111/apm.12030. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Flores M, Casanova-Salas I, Rubio-Briones J, Calatrava A, Dominguez-Escrig J, Rubio L, Ramirez-Backhaus M, Fernandez-Serra A, Garcia-Casado Z, Lopez-Guerrero JA. Clinico-pathological significance of the molecular alterations of the SPOP gene in prostate cancer. European journal of cancer. 2014;50:2994–3002. doi: 10.1016/j.ejca.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. The EMBO journal. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar S, Brautigan DL, Parsons SJ, Larner JM. Androgen receptor degradation by the E3 ligase CHIP modulates mitotic arrest in prostate cancer cells. Oncogene. 2014;33:26–33. doi: 10.1038/onc.2012.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Xu LL, Masuda K, Raymundo E, McLeod DG, Dobi A, Srivastava S. A feedback loop between the androgen receptor and a NEDD4-binding protein, PMEPA1, in prostate cancer cells. The Journal of biological chemistry. 2008;283:28988–28995. doi: 10.1074/jbc.M710528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Lu W, Yang Q, Yu X, Matusik RJ, Chen Z. Skp2 regulates androgen receptor through ubiquitin-mediated degradation independent of Akt/mTOR pathways in prostate cancer. The Prostate. 2014;74:421–432. doi: 10.1002/pros.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, Krajewski S, Mercola D, Carpenter PM, Bowtell D, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]