Abstract
Purpose of Review
Respiratory syncytial virus (RSV) is a global human pathogen responsible for lower respiratory tract infections (LRTI). While RSV infection is innocuous in healthy adults, it is the leading cause of infant hospitalization for respiratory tract infection. Nearly everyone shows evidence of an RSV infection by the age of 3. However, there is still not a vaccine commercially available. This review will provide an update on the clinical and preclinical vaccine studies and different approaches to prevent RSV infection.
Recent Findings
Novel vaccine approaches that induce protection against RSV without enhancement of respiratory tract disease.
Summary
Recent technological approaches have led to generation of different strategies to prevent RSV infection, including live attenuated, chimeric, and subunit vaccines, virus-like particles, and nanoparticles. These vaccine approaches represent promising candidates towards an efficient RSV vaccine that effectively protects infants, children, and adults.
Keywords: Respiratory syncytial virus, Paramyxovirus, Vaccine
Introduction
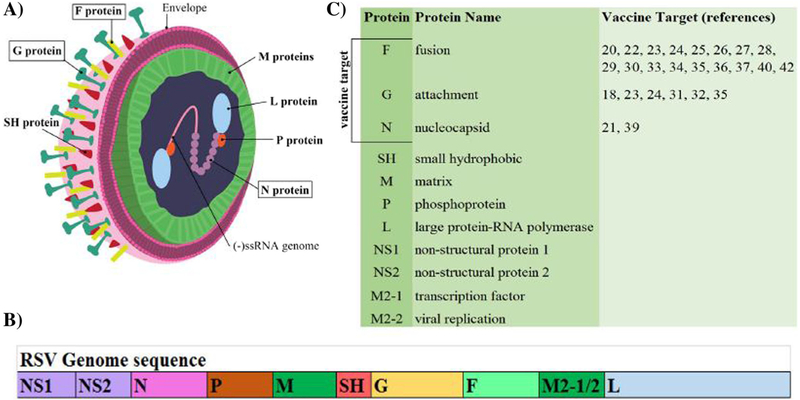
Human respiratory syncytial virus (RSV) is an enveloped, negative sense, single stranded RNA virus as part of the Paramyxoviridae family [1–3]. Its genome of 15.2 kb, encodes 11 proteins including the attachment (G), small hydrophobic (SH), fusion (F), nucleoprotein (N), phosphoprotein (P), polymerase (L), matrix proteins (M1, M2-1, M2-2), and two non-structural proteins (NS1, NS2) [2, 3] (Fig. 1a, b). RSV was originally isolated in 1956 as chimpanzee coryza agent [4] and it was further identified in infants 1 year later [5]. RSV has been identified as the leading cause of epidemic lower respiratory tract infections (LRTI) in infants and children. RSV is a major cause of bronchiolitis and pneumonia estimated to cause more than 30 million new cases of lower respiratory tract illness in children younger than 5 years, with 3–4 million hospitalizations, and 66,000–200,000 fatal outcomes with more than 95% of these deaths occurring in developing countries [6–8]. By the age of 3, virtually all children have been infected by RSV [9]. Other high-risk populations for RSV infection include the elderly and immunecompromised individuals [7, 10]. Reinfection by the same and different strains of RSV are common throughout the lifetime of adults, but usually asymptomatic [1]. Failure of RSV to stimulate a lasting immune response is still a popular puzzle for basic research, and it makes designing an efficacious vaccine very challenging.
Fig. 1.
RSV proteins, structure, and genome sequence. a A representation of RSV structure. Boxes indicate those major vaccine targets. b RSV genome sequence. c Summary of RSV proteins, their names, and the corresponding references
Since its identification, 60 years ago, RSV has been a priority for vaccine research in order to reduce the lower respiratory tract morbidity in the risk populations. The first RSV vaccine tested in a clinical trial, back in 1966, consisted in a formalin inactivated virus (FI-RSV) from the Bernett strain, which was administered in 3 doses to 23 infants between 2 and 7 months of age [11]. Previous potency and safety tests executed in guinea pigs, cynomologous monkeys, rabbits, and mice did produce satisfactory results. However, the FI-RSV vaccine had a disastrous conclusion since it failed to induce an adequate response of neutralizing antibodies and resistance to infection. Furthermore, immunized children developed a more severe pulmonary disease and peribronchiolar infiltration of eosinophils that led to the death of two infants aged 14 and 16 months, following natural infection with RSV [11].
The most common vaccine target for RSV is F protein (Fig. 1a), which is a highly conserved envelope protein across different RSV subgroups A and B [12] (Fig. 1c). Furthermore, F protein has been identified as the primary antigen target of neutralizing antibodies with multiple specific peptide sequences having been identified [13–16]. More recently the structural conformation of F protein has been examined for antigenic peptides. McLellan et al. identified pre-fusion F protein as having the majority of epitopes for neutralizing antibodies [15]. Structural conformation of F protein has started to take a contribution to modern vaccine design, hopefully leading to more efficacious vaccines.
The inability of the virus to trigger a protective memory has also made several vaccines ineffective at preventing disease. Therefore, following the failure of the FI-RSV vaccine trial, numerous new RSV vaccine candidates have been designed. This brief overview highlights the current advances in the different approaches including live attenuated vaccines, chimeric vaccines, subunit vaccines, virus-like particles vaccines, and nanoparticles vaccines, which show promise towards protecting against RSV infection.
Live Attenuated Virus Vaccines
Initial development of RSV live attenuated vaccines included in vitro serial passages at a temperature of 26 °C [17]. Among the advantages found in the live attenuated vaccines is that unlike FI-RSV, they do not cause an enhanced disease severity after exposure to natural RSV infection. In these vaccine candidates, it is critical to have a balance between an attenuated viral replication and an induction of an effective immunity. A recent example of live attenuated vaccine demonstrated that removing the CX3C motif of G protein can improve vaccine safety by polarizing the T helper response towards a Th1 response and away from a Th2 response associated with enhanced respiratory disease (ERD) [18]. However, there are some concerns about how the genetic stability of genetic attenuation of live vaccines will hold up against strong selective pressure. To test this, phenotypical analysis has been performed in serial passages of codon-deoptimized live attenuated viruses through Vero cells. The results indicate that specific open reading frames (ORFs) are less stable than others [19]. This effect raises concerns about whether the ORF conserves the sequence responsible for attenuation without other random and potentially compensatory mutations emphasizing another aspect of safety testing necessary for live attenuated vaccine candidates. As long as the virus is replication proficient, it is subject to selective pressures. Safety is an especially serious concern in vaccines that target vulnerable populations, including infants. Special consideration was given to this concern in a recently designed severely attenuated live vaccine candidate, OE5. It used a strategy of codon deoptimization of NS1, NS2, and G proteins and completely removed SH protein [20]. This strategy severely limits the replication of the virus while maintaining the F protein intact, the strongest target protein for neutralizing antibodies. The OE5 vaccine stimulated a protective antibody response up to 100 days post infection as demonstrated in BALB/c mice and cotton rats without triggering ERD [20]. Thus, OE5 represents a promising safe and effective vaccine candidate.
Chimeric Vaccines
One of the challenges of a live attenuated vaccine is the inability of natural RSV infection to create a lasting protective memory response. There are multiple epitopes particularly on the F and G proteins capable of being recognized by the adaptive immune response, but protection wanes after viral clearance as infants can be sequentially infected by the same subgroup during a single RSV season. To surmount this obstacle, several research groups created recombinant chimeras expressing immunogenic RSV proteins. A recent example of this is the RSV N gene expressing Bacillus Calmette-Guerin (BCG) vaccine [21]. The BCG vaccine is a live bacteria adapted from Mycobacterium bovis to prevent tuberculosis. Unlike RSV, BCG is capable of triggering a lasting immunity, particularly a T cell response, opposed to a B cell response. The study demonstrates that attaching RSV N gene to BCG was effective at stimulating a lasting CD4+ and CD8+ T cell response and was protective against RSV infection in mice [21]. Concerns exist because BCG is a live vaccine and not approved for immunecompromised individuals, one of the main populations at serious risk of major disease from RSV infection. Another recent example of a chimeric RSV vaccine is a Sendai virus (SeV) chimera. SeV is a member of the paramyxoviridae family that primarily infects mice and other rodents. Wiegand et al. used a replication deficient SeV as the backbone of their vaccine. Then they removed the F protein of SeV and replaced it with the F protein of RSV [22]. The replication deficient SeV provides a safe vector that carries RSV F protein as an essential structural component. The vaccine stimulates a T cell and antibody response through both an intranasal and an intramuscular immunization [22].
Potentially, the most promising chimeric vaccines are parainfluenza 5 virus (PIV5) and RSV chimeras. Parainfluenza virus (PIV) is the second most common cause of respiratory tract infection hospitalization in children. Two PIV5 chimeras expressing RSV F or RSV G protein were used to inoculate cotton rats and African green monkeys [23]. Although both chimeras fully protected naive animals from RSV infection, the RSV F protein chimera (PIV5/F) stimulated a higher antibody titer in African Green monkeys than RSV G protein (PIV5/G) [23]. Also, they both boosted antibody levels in RSV pre-exposed African Green monkeys, suggesting that the PIV5/F vaccine could be helpful to naïve babies and pre-exposed elderly people [23]. Furthermore, genetic stability tests demonstrated that while random mutations were present in both in vivo (African green monkeys) and in vitro (Vero cells) passages, none of those mutations abolished antigen expression [24]. While genetic instability is possibly the biggest concern in using RNA viruses as chimeras, this insertion of RSV F protein in between PIV5 HN and L genes appears to be stable. A further improved chimeric RSV vaccine candidate included a pre-fusion F protein in a PIV5 backbone. The vaccine was protective against RSV in mice and cotton rats [25]. Using a similar approach, a parainfluenza 1 (PIV1) vectored vaccine was designed using F protein stabilized in its pre-conformation structure either with RSV F protein or with RSV F protein with the transmembrane and cytoplasmic tail domains replaced by PIV1 analogs [26]. This vaccine was able to elicit neutralizing antibodies similar to wild-type RSV infection and conferred protection against RSV challenge [26]. Similarly, a chimeric vaccine developed from recombinant human and bovine parainfluenza 3 (PIV3) with RSV F protein stabilized in its pre-fusion conformation was codon optimized to increase immunogenicity in hamsters and in vivo replication to increase neutralizing antibody titers [27].
Subunit Vaccines
Subunit vaccines are inoculations that include only a single protein, protein complex, or even an isolated peptide with or without an adjuvant. The goal of this vaccine approach is to promote a memory response to the viral protein without the danger of introducing a live pathogen or the risk of enhanced respiratory disease, most commonly associated with inactivated virus (FI-RSV). Using a cotton rat experimental model, a recent study found that at high doses, the RSV F protein subunit vaccine did not cause ERD. However, low doses caused ERD with and without an adjuvant [28], raising concerns about the dose and safety in vaccinated individuals.
Because of safety concerns using the whole protein in this type of immunization, subunit vaccines targeting specific peptides have become a new area of development with mixed response. An F protein peptide adjuvanted with poly I:C, a synthetic analog of double stranded RNA, was successful at conferring complete long-term (5 months) protection in mice after a single i.n. vaccination [29]. In comparison with that success, a lipid core peptide vaccine created a very strong antibody response, but those antibodies failed to bind natural F proteins or offer any protection against RSV challenge [30]. These two cases highlight the importance of testing vaccines against challenge protection rather than measuring antibody titers, particularly in the case of peptide subunit vaccines.
The RSV attachment glycoprotein (G) is another typical candidate to induce protective immunity against RSV. A successful preclinical study demonstrated that intramuscular administration of unglycosylated G protein was not associated with ERD and created a protective immune response to RSV [31]. However, another concern with developing a RSV vaccine is strain differences. Most laboratories use RSV A2 laboratory-adapted strain, but infants can be exposed to more than one RSV strain. Thus, an effective vaccine will need to provide protection against a broad spectrum of RSV strains across both A and B subtypes. By creating a recombinant G protein to combine A and B RSV subtypes (GcfAB), Lee J-Y and colleagues show that mice vaccinated via intranasal and sublingual routes induced a strong humoral response against both RSV subtypes but enhanced lung pathology in the RSV-infected animals [32].
Virus-Like Particles (VLP)
Virus-like particles (VLP) are small replication deficient groups of assembled viral proteins without any genetic material. A recent example of this approach is a construction of recombinant matrix protein (M) and fusion glycoprotein (F) [33, 34]. Cai et al. generated VLPs with influenza virus (IFV) M1 matrix protein and RSV F or RSV G protein. Both the F and G vaccines were effective at creating specific antibodies against RSV and protected mice against RSV challenge [35]. There is some debate over using either pre- or post-fusion F protein conformation. Several recent VLP approaches included the matrix (M) protein from RSV [33], HMPV [34], and NDV [36] and both pre- and post-fusion RSV F protein [33, 34, 36]. In all cases, the VLPs were immunogenic and triggered a desirable Th1 adaptive immune response [33, 34, 36]. Although pre-fusion RSV F protein had a higher neutralizing antibody response than post-fusion RSV F protein, a combination of pre- and post-fusion F proteins produced the strongest Th1 response with the highest proportion of IgG2 antibodies [34].
VLPs are also able to polarize the immune response towards a Th1 response after a booster vaccination with formalin inactivated RSV, suggesting that VLPs can be effective vaccines, despite previous damaging vaccination history [37]. During a challenge with live RSV (A2 strain), the VLP vaccinated mice were more protected than unvaccinated mice, but not more than mice previously infected with live RSV [37]. Future work will need to explore improving the level of protection provided by the VLP vaccine so that exceeds the inadequate protection of natural RSV infection.
Micro and Nanoparticles
The size of a micro or nanoparticle impacts how the antigen is transported to the draining lymph node. Small nanoparticles (2–200 nm) can freely drain to the lymph nodes. Larger nanoparticles (> 500 nm) will need to be phagocytosed inside a dendritic cell. The benefit of larger nanoparticles is that they stay in the lymph nodes longer. Being phagocytosed inside an antigen presenting cell allows the larger nanoparticles to remain in the lymph nodes longer than the smaller nanoparticles (reviewed in [38]). A recent preclinical trial targeted the N protein of RSV, loaded with nanorings and delivered by a transdermal vaccine delivery approach [39]. The patches were loaded with RSV N-nanorings (Viaskin®-N). The epicutaneous delivery of the RSV N-nanorings was successful at stimulating a protective CD4+ T cell response in both mice and piglets [39]. RSV N gene is an uncommon target protein, but a potential target depending on the epitope variability with other RSV subtypes and if it can cause ERD.
Another example of RSV nanoparticle vaccine (RSV F vaccine) has progressed to a phase II clinical trial when given to healthy adult women at different dosages, number of doses, and with variable amounts of adjuvant [40]. The vaccinated women were 50% more protected from RSV infections than placebos [40]. The vaccine looks very promising; however, it still needs to be tested on the target population. It is intended for women in their third trimester of pregnancy to bolster antibody levels to cross the placenta to the fetus. Potentially vaccinating mother can protect the infants postpartum for the first month of life. Additionally, mothers who contract RSV have a 57% chance of passing it on to the fetus [41]. Vaccinating expectant mothers can help protect their infants through maternal antibodies. A phase III clinical trial for women in the third trimester of pregnancy is currently ongoing.
One of the particularly interesting aspects of RSV is its inability to create a lasting immunity causing frequent reinfection even by the same strain within a single season. This means that the same RSV vaccine might still be effective across age groups and naiveté. The aforementioned RSV F nanoparticle vaccine for pregnant women [40] has been tested in a phase I clinical trial in elderly people. The trial did not reveal any safety concerns [42]. Subjects also had significantly higher antibody titer 12 months post inoculation, suggesting potential protection [42]. A phase II trial is necessary to discern if the antibody response is protective in elderly people.
Conclusion
The search for an RSV vaccine has been high on the agenda for several decades, due to the high rate of infections in infants, but also other vulnerable populations like immunocompromised adults and elderly people, where RSV poses a significant health risk. Previous vaccine attempts have been hindered by the inability of natural RSV infection to create a lasting memory protection against conserved immunogen epitopes in addition to extremely cautious efforts towards clinical trial after the first disastrous attempt and enhanced respiratory disease after inoculations with some vaccine candidates.
Recent technological advancements have opened doors to new vaccine approaches that will hopefully revolutionize RSV vaccine development (Fig. 1c). Improvements to the precision of gene editing have allowed for more specific live attenuated vaccines, more possible recombinant proteins for subunit vaccines, and an ample variety of possible chimeric vaccines. The development of micro and nanoparticles open up more doors to specific manipulation of the type of adaptive immune response desired. Even varied inoculation routes allow researchers to tailor the desired response to their vaccine [43]. These new vaccine approaches will contribute to finding an effective solution to prevent RSV infection in infants and other target populations.
Acknowledgements
The authors wish to thank Haley Hatfield with the LSU Office of Research and Economic Development for the illustration included in Fig. 1.
Funding information Preparation of this article was supported in part by grants from the Flight Attendant Medical Research Institute Clinical Innovator Award (CIA) and from the U.S. National Institute of Health (P30GM1107060) to A.G.P. C.M.C. was supported by a Fellowship from the Louisiana State University Board of Regents Graduate Fellowship Program.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–28. [DOI] [PubMed] [Google Scholar]
- 2.Collins PL, Crowe J. Respiratory syncytial virus and metapneumovirus In: Knipe DM, Howley PM, editors. Fields virology. 2 5th ed. Philadelphia: Wolters Kluwer; 2007. p. 1601–46. [Google Scholar]
- 3.Jha A, Jarvis H, Fraser C, Openshaw PJM. Respiratory syncytial virus In: Hui DS, Rossi GA, Johnston SL, editors. SARS, MERS and other viral lung infections. Sheffield: Wellcome Trust–Funded Monographs and Book Chapters; 2016. [Google Scholar]
- 4.Blount RE Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92(3):544–9. [DOI] [PubMed] [Google Scholar]
- 5.Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg. 1957;66(3):291–300. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12(2):92–7. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson FW, Collier AM, Clyde WA Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10):530–4. [DOI] [PubMed] [Google Scholar]
- 10.Madhi SA, Venter M, Madhi A, Petersen MK, Klugman KP. Differing manifestations of respiratory syncytial virus-associated severe lower respiratory tract infections in human immunodeficiency virus type 1-infected and uninfected children. Pediatr Infect Dis J. 2001;20(2):164–70. [DOI] [PubMed] [Google Scholar]
- 11.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PR, Collins PL. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol. 1988;69(Pt 10):2623–8. [DOI] [PubMed] [Google Scholar]
- 13.Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73(Pt 9): 2225–34. [DOI] [PubMed] [Google Scholar]
- 14.Lopez JA, Bustos R, Orvell C, Berois M, Arbiza J, Garcia-Barreno B, et al. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol. 1998;72(8):6922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109(8):3089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald WT, Forsyth BR, Smith CB, Gharpure MA, Chanock RM. Low-temperature-grown RS virus in adult volunteers. JAMA. 1968;204(8):690–4. [PubMed] [Google Scholar]
- 18.Boyoglu-Barnum S, Todd SO, Meng J, Barnum TR, Chirkova T, Haynes LM, et al. Mutating the CX3C motif in the G protein should make a live respiratory syncytial virus vaccine safer and more effective. J Virol. 2017;91(10). 10.1128/JVI.02059-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Nouen C, McCarty T, Brown M, Smith ML, Lleras R, Dolan MA, et al. Genetic stability of genome-scale deoptimized RNA virus vaccine candidates under selective pressure. Proc Natl Acad Sci U S A. 2017;114(3):E386–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stobart CC, Rostad CA, Ke Z, Dillard RS, Hampton CM, Strauss JD, et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun. 2016;7:13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cespedes PF, Rey-Jurado E, Espinoza JA, Rivera CA, Canedo-Marroquin G, Bueno SM, et al. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35(5):757–66. [DOI] [PubMed] [Google Scholar]
- 22.Wiegand MA, Gori-Savellini G, Gandolfo C, Papa G, Kaufmann C, Felder E, et al. A respiratory syncytial virus vaccine vectored by a stable chimeric and replication-deficient Sendai virus protects mice without inducing enhanced disease. J Virol. 2017;91(10). 10.1128/JVI.02298-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Phan S, DiStefano DJ, Citron MP, Callahan CL, Indrawati L, et al. A single-dose recombinant parainfluenza virus 5-vectored vaccine expressing respiratory syncytial virus (RSV) F or G protein protected cotton rats and African green monkeys from RSV challenge. J Virol. 2017;91(11). 10.1128/JVI.00066-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan SI, Adam CM, Chen Z, Citron M, Liang X, Espeseth AS, et al. Genetic stability of parainfluenza virus 5-vectored human respiratory syncytial virus vaccine candidates after in vitro and in vivo passage. J Virol. 2017;91(19). 10.1128/JVI.00559-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan SI, Zengel JR, Wei H, Li Z, Wang D, He B. Parainfluenza virus 5 expressing wild-type or prefusion respiratory syncytial virus (RSV) fusion protein protects mice and cotton rats from RSV challenge. J Virol. 2017; 91 (19). 10.1128/JVI.00560-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Liang B, Ngwuta J, Liu X, Surman S, Lingemann M, et al. Attenuated human parainfluenza virus type 1 (HPIV1) expressing the respiratory syncytial virus (RSV) fusion F glycoprotein from an added gene: effects of pre-fusion stabilization and packaging of RSV F. J Virol. 2017. 10.1128/JVI.01101-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang B, Ngwuta JO, Surman S, Kabatova B, Liu X, Lingemann M, et al. Improved prefusion stability, optimized codon usage, and augmented virion packaging enhance the immunogenicity of respiratory syncytial virus fusion protein in a vectored-vaccine candidate. J Virol. 2017;91(15). 10.1128/JVI.00189-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider-Ohrum K, Cayatte C, Bennett AS, Rajani GM, McTamney P, Nacel K, et al. Immunization with low doses of recombinant postfusion or prefusion respiratory syncytial virus F primes for vaccine-enhanced disease in the cotton rat model independently of the presence of a Th1-biasing (GLA-SE) or Th2-biasing (alum) adjuvant. J Virol. 2017;91(8). 10.1128/JVI.02180-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg R, Latimer L, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Intranasal immunization with a single dose of the fusion protein formulated with a combination adjuvant induces long-term protective immunity against respiratory syncytial virus. Hum Vaccin Immunother 2017:0 10.1080/21645515.2017.1349584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaberolansar N, Chappell KJ, Watterson D, Bermingham IM, Toth I, Young PR, et al. Induction of high titred, non-neutralising antibodies by self-adjuvanting peptide epitopes derived from the respiratory syncytial virus fusion protein. Sci Rep. 2017;7(1):11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuentes S, Klenow L, Golding H, Khurana S. Preclinical evaluation of bacterially produced RSV-G protein vaccine: strong protection against RSV challenge in cotton rat model. Sci Rep. 2017;7:42428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JY, Chang J. Universal vaccine against respiratory syncytial virus A and B subtypes. PLoS One. 2017;12(4):e0175384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao YY, YH F, Yan YF, Hua Y, Ma Y, Zhang XJ, et al. A single intranasal administration of virus-like particle vaccine induces an efficient protection for mice against human respiratory syncytial virus. Antivir Res. 2017;144:57–69. [DOI] [PubMed] [Google Scholar]
- 34.Cimica V, Boigard H, Bhatia B, Fallon JT, Alimova A, Gottlieb P, et al. Novel respiratory syncytial virus-like particle vaccine composed of the postfusion and prefusion conformations of the F glycoprotein. Clin Vaccine Immunol. 2016;23(6):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai M, Wang C, Li Y, Gu H, Sun S, Duan Y, et al. Virus-like particle vaccine by intranasal vaccination elicits protective immunity against respiratory syncytial viral infection in mice. Acta Biochim Biophys Sin Shanghai. 2017;49(1):74–82. [DOI] [PubMed] [Google Scholar]
- 36.Cullen LM, Schmidt MR, Morrison TG. The importance of RSV F protein conformation in VLPs in stimulation of neutralizing antibody titers in mice previously infected with RSV. Hum Vaccin Immunother. 2017:1–10. 10.1080/21645515.2017.1329069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang HS, Lee YT, Kim KH, Ko EJ, Lee Y, Kwon YM, et al. Virus-like particle vaccine primes immune responses preventing inactivated-virus vaccine-enhanced disease against respiratory syncytial virus. Virology. 2017;511:142–51. [DOI] [PubMed] [Google Scholar]
- 38.Jorquera PA, Tripp RA. Synthetic biodegradable microparticle and nanoparticle vaccines against the respiratory syncytial virus. Vaccines (Basel) 2016;4(4). 10.3390/vaccines4040045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herve PL, Descamps D, Deloizy C, Dhelft V, Laubreton D, Bouguyon E, et al. Non-invasive epicutaneous vaccine against respiratory syncytial virus: preclinical proof of concept. J Control Release. 2016;243:146–59. [DOI] [PubMed] [Google Scholar]
- 40.Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis. 2016;213(3):411–22. [DOI] [PubMed] [Google Scholar]
- 41.Chu HY, Katz J, Tielsch J, Khatry SK, Shrestha L, LeClerq SC, et al. Clinical presentation and birth outcomes associated with respiratory syncytial virus infection in pregnancy. PLoS One. 2016;11(3):e0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fries L, Shinde V, Stoddard JJ, Thomas DN, Kpamegan E, Lu H, et al. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing. 2017;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–58. [DOI] [PubMed] [Google Scholar]