Abstract
Study Objectives:
Our study aims were to examine (1) the association between fear of sleep and posttraumatic stress disorder (PTSD) symptoms, (2) the association between fear of sleep and subjective and objective insomnia symptoms and disruptive behaviors during sleep, and (3) whether fear of sleep decreases following cognitive behavioral therapy for insomnia (CBT-I).
Methods:
Forty-five adults with PTSD and insomnia participated in the study. Fear of sleep was assessed using the Fear of Sleep Inventory; PTSD symptoms were assessed using the Clinician Administered PTSD Scale; and sleep disturbance symptoms were assessed using the Insomnia Severity Index, polysomnography, sleep diaries, and the Pittsburgh Sleep Quality Index Addendum for PTSD. Participants were randomly assigned to 8 weeks of CBT-I (n = 29) or a waitlist control condition (n = 16).
Results:
Greater fear of sleep was associated with greater PTSD symptom severity, greater nightmare frequency, and greater hypervigilance intensity. Greater fear of sleep was associated with decreased wake after sleep onset (WASO), reduced total sleep time, and greater disruptive nocturnal behaviors. Following CBT-I, there was a significant reduction in fear of sleep compared to the waitlist condition. These improvements persisted 6 months later.
Conclusions:
Fear of sleep was related to sleep disturbances specific to trauma rather than “classic” insomnia symptoms. Unexpectedly, greater fear of sleep was associated with reduced WASO. These results may be related to having a truncated sleep period and thus more consolidated sleep. Fear of sleep deceased following CBT-I despite not being a permissible target for this research protocol and not being related to insomnia symptoms.
Clinical Trial Registration:
Registry: CinicalTrials.gov; Name: Treating People with Post-traumatic Stress Disorder with Cognitive Behavioral Therapy for Insomnia; Identifier: NCT00881647; URL: https://clinicaltrials.gov/ct2/show/NCT00881647
Citation:
Kanady JC, Talbot LS, Maguen S, Straus LD, Richards A, Ruoff L, Metzler TJ, Neylan TC. Cognitive behavioral therapy for insomnia reduces fear of sleep in individuals with posttraumatic stress disorder. J Clin Sleep Med. 2018;14(7):1193–1203.
Keywords: cognitive behavioral therapy, fear of sleep, insomnia, sleep disturbance, posttraumatic stress disorder, PTSD
BRIEF SUMMARY
Current Knowledge/Study Rationale: Fear of sleep may be contributing to the bidirectional relationship between sleep disturbance and posttraumatic stress disorder (PTSD) symptoms. This is the first study to examine fear of sleep in individuals with PTSD and insomnia using standard objective and subjective measures and a therapeutic manipulation of sleep.
Study Impact: Results from our study demonstrate that fear of sleep is related to sleep disturbance symptoms characteristic of PTSD (eg, nightmares, disruptive behaviors during sleep), rather than classic insomnia symptoms (eg, difficulty staying asleep). Despite not being a treatment target and not being related to insomnia symptoms, fear of sleep decreased following cognitive behavioral therapy for insomnia (CBT-I), further demonstrating the usefulness of CBT-I in a PTSD sample.
INTRODUCTION
Sleep disturbance is a highly prevalent component of posttraumatic stress disorder (PTSD) and is often considered a “hallmark feature” of PTSD.1,2 Sleep disturbance often precedes a PTSD diagnosis,3,4 exacerbates daytime PTSD symptoms,5,6 and is one of the most commonly endorsed residual symptoms following PTSD treatment.7–9 Despite this, little research has been done examining mechanisms that may be contributing to the bidirectional relationship between sleep disturbance and PTSD symptoms. Fear of sleep may be one possible pathway underlying this relationship.8,10,11 The purpose of the current study is to examine the role of fear of sleep in individuals with PTSD treated for insomnia.
Fear of sleep is a common feature of PTSD and appears to have a linear relationship with PTSD symptom severity; greater fear of sleep is associated with more severe PTSD symptoms.10 Individuals with PTSD may experience fear of sleep for a variety of reasons. One possibility is that fear of sleep is driven by the frequency and intensity of nightmares common in PTSD.10–12 Indeed, previous studies have found that individuals with nightmares report greater fear of sleep than individuals without nightmares,13 that nightmares with trauma-related content are associated with greater fear of sleep,14 and that fear of sleep decreases when nightmares are treated.15 Interestingly, a recent study also found that greater fear of sleep predicted greater subsequent nightmare frequency in individuals with PTSD,16 suggesting that there may be a bidirectional relationship between fear of sleep and nightmares in this population. Another possible explanation for why individuals experience fear of sleep may be the inability to be vigilant while sleeping,10,17 as hypervigilance is a prominent feature of PTSD. Supporting this idea, findings from one study demonstrated that fear of lack of vigilance was associated with poor sleep quality in a sample of Operation Enduring Freedom and Operation Iraqi Freedom veterans with PTSD.18 The association between fear of sleep and hypervigilance may be particularly salient for individuals whose trauma took place in bed or in darkness (eg, sexual assault). To our knowledge, only one study has examined this idea, finding that fear of sleep and PTSD symptomatology differentially contribute to the association between trauma type and risk for insomnia in a sample of young African-American adults.19 More research is needed to further our understanding of how fear of sleep is related to PTSD symptomatology in individuals with PTSD and insomnia.
Fear of sleep may play a role in the onset and maintenance of insomnia symptoms in individuals with PTSD.8,10,11 Insomnia—a sleep disorder characterized by subjective difficulties with sleep onset and/or maintenance—occurs in approximately 60% to 90% of individuals with PTSD11,20 and is associated with a worse course of illness and poorer quality of life.5,6,21 “Classic” insomnia symptoms include prolonged sleep onset latency (SOL) and wake after sleep onset (WASO), early morning awakenings, and reduced sleep efficiency. Previous research has suggested that fear of sleep may be involved in the pathogenesis of insomnia in this population. For example, Pruiksma and colleagues and Hall Brown and Mellman found that greater fear of sleep was associated with greater insomnia symptom severity.10,22 Short and colleagues found that greater fear of sleep was associated with poorer subsequent sleep quality and reduced sleep efficiency.16 Based on this literature and the literature demonstrating an association between fear of sleep and PTSD symptoms, one possible model for the role of fear of sleep in individuals with PTSD and insomnia is that greater PTSD symptomatology (eg, more nightmares, greater hypervigilance) leads to greater fear of sleep, which in turn leads to insomnia-related sleep disruptions (eg, longer SOL, reduced sleep efficiency). However, this theorized model is complicated by research demonstrating that insomnia symptoms persist despite a reduction in hypervigilance and nightmares following PTSD treatment. If fear of sleep is being driven by nightmares and hypervigilance, and if fear of sleep is contributing to insomnia symptoms, then one would expect that following an improvement in nightmares and hypervigilance, insomnia symptoms would also improve. Yet, this is frequently not the case and several studies have demonstrated that following successful PTSD treatment, insomnia symptoms often persist in a large percentage of individuals.7–9 Therefore, more research is needed to further parse out the association between fear of sleep and insomnia in individuals with PTSD.
The association between fear of sleep and insomnia in PTSD is further complicated by the fact that insomnia is often comorbid with other sleep disturbances and that objective measures of sleep yield mixed results. For instance, individuals with PTSD also may experience nocturnal panic attacks, awakening with a startle or panic, bad dreams unrelated to trauma events, hot flashes at bedtime or during sleep, acting out dreams, and thrashing movements during sleep.23–27 Further, objective measures of sleep in this population have provided conflicting reports. Results from several studies have demonstrated objective difficulties with sleep initiation and/or sleep maintenance and reduced sleep efficiency,26,28–31 whereas other studies have failed to find objective markers of sleep disturbance.32–35 One possibility for these mixed findings is that differences in fear of sleep may be contributing to the interindividual variation of sleep disturbance in this population.
The Fear of Sleep Inventory (FoSI)10,36 was developed to assess fear of sleep resulting from nightmares and nighttime vigilance associated with previous trauma exposure and has been used to examine fear of sleep in undergraduate, trauma-exposed, and PTSD samples.10,16,19 The goal of the current study was to build on this literature by conducting a preliminary study examining fear of sleep in individuals with PTSD and insomnia using standard objective and subjective measures and a therapeutic manipulation of sleep. We consider this study to be preliminary given that this is the first examination of how fear of sleep relates to symptom presentation in individuals with PTSD and insomnia, which is an important first step for better understanding how to conceptualize and treat these complex cases. The first aim was to replicate and expand on previous studies examining the association between fear of sleep and characteristics of PTSD. We hypothesized that greater fear of sleep would be associated with greater PTSD symptom severity, increased hypervigilance intensity, and increased nightmare frequency and that fear of sleep would be more severe in individuals whose trauma may have been associated with the bed/bedroom (ie, sexual assault) as assessed by the Clinician-Administered PTSD Scale for DSM-IV (CAPS). The second aim was to examine the association between fear of sleep and subjective and objective insomnia symptoms and disruptive behaviors during sleep. Specifically, we hypothesized that greater fear of sleep would be associated with greater insomnia symptom severity as assessed by the Insomnia Severity Index (ISI),37 longer SOL, greater WASO, reduced total sleep time (TST), and reduced time in bed (TIB) as measured by daily sleep diaries and polysomnography (PSG), and greater disruptive nocturnal behaviors as assessed by the Pittsburgh Sleep Quality Index – Addendum (PSQI-A).23 The final aim was to determine whether fear of sleep decreases following cognitive behavioral therapy for insomnia (CBT-I). We hypothesized that fear of sleep would decrease following CBT-I, compared to a waitlist control condition, and that results would persist at 6-month follow-up. Secondary analyses for the final aim were to examine whether a reduction in fear of sleep was associated with a reduction in PTSD and/or sleep disturbance symptoms following CBT-I. These data were collected as part of a previously reported-on NIMH-funded study (5R34MH077667-03) examining the efficacy of CBT-I for the treatment of insomnia in PTSD.31
METHODS
Participants
Forty-five adults between the ages of 18 to 65 years with PTSD and an insomnia diagnosis participated in the study. Participants were eligible if (1) they had chronic PTSD lasting at least 3 months based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic criteria (DSM-IV)38 or partial PTSD operationalized as a past diagnosis of PTSD plus at least one current B symptom and either the C cluster criteria or the D cluster criteria (40 participants met full criteria for PTSD and 5 participants met partial criteria); (2) were currently undergoing treatment for PTSD including medication therapy, specialized PTSD programs, and/or individual psychotherapy with a licensed clinician for a duration of at least three months prior to study enrollment; (3) medications, when present, were at a stable dose (ie, no changes in the dosage or frequency of medication use) for at least 4 weeks prior to enrollment in the study; and (4) they had persistent insomnia as defined by meeting research diagnostic criteria for insomnia.39
Exclusion criteria for all participants included: (1) a lifetime history of any psychiatric disorder with psychotic features and/ or a diagnosis of bipolar disorder; (2) an alcohol and/or substance use diagnosis within the past year; (3) current exposure to a recent trauma or exposure to a traumatic event in the past 3 months; (4) a diagnosis of sleep apnea, a neurologic disorder, a systemic illness affecting the central nervous system, and/ or anemia; (5) current suicidal risk and/or homicidal risk; (6) pregnancy and/or breast-feeding; (7) reports that insomnia began or worsened after starting selective serotonin reuptake inhibitor therapy; (8) a history of sleep restriction therapy or cognitive restructuring therapies for beliefs related to sleep; (9) a current prescription for benzodiazepine, benzodiazepine receptor agonists, opiates, or trazodone, and/or the use of over-the-counter sleep aids; (10) termination of benzodiazepine or benzodiazepine receptor agonists, anticonvulsants, atypical antipsychotic medication, and/or antidepressant medications in the past two months; (11) overnight shift work; (12) unstable housing; and (13) nonclinically significant or subthreshold insomnia as indicated by a score of 0–14 on the ISI.37
Procedure
The Committee on Human Research at the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center approved all study procedures. Participants in in this study were recruited through Internet postings and contact with relevant clinicians and community resources in the San Francisco Bay area as part of the randomized controlled trial. This procedure was previously reported; see Talbot et al.31
Participants first completed a phone screen to establish preliminary eligibility. After preliminary eligibility was determined, participants were then invited for an in-person, pretreatment assessment at the San Francisco VA Medical Center. During the pretreatment assessment written informed consent was obtained and clinician-administered and self-report measures were collected. PTSD diagnoses were confirmed using the CAPS40 and insomnia diagnoses were confirmed using the Duke Structured Interview for Sleep Disorders (DSISD). A comprehensive medical history interview and the Structured Clinical Interview for Axis I Disorders (SCID) were used to assess current and past medical and/or mental health conditions and also to establish potential exclusionary criteria (eg, bipolar disorder or a neurological disorder). During the pretreatment assessment, participants completed several self-report measures including the ISI,37 the PSQI-A,23 and the FoSI.10,36 Following the pretreatment assessment, eligible participants were provided with sleep diaries and were scheduled for 2 consecutive nights of PSG sleep assessment, which was used to screen for possible sleep apnea. All PSG sleep assessments took place in the participants' home environments and were scheduled prior to the start of treatment.
Participants were randomized to one of two conditions: CBT-I (n = 29) or a waitlist, monitor-only control condition (n = 16). CBT-I is a structured intervention with core behavioral components of sleep restriction and stimulus control, along with sleep hygiene guidelines, a cognitive intervention focused on catastrophic beliefs, and relapse prevention. Of note, cognitive interventions that were focused on PTSD, including concerns about safety, were prohibited in this trial. The monitor-only waitlist condition consisted of continuous monitoring of sleep using a sleep diary and actigraphy. Please refer to Talbot et al. for more specifics about randomization processes, retention, treatment conditions, and adherence monitoring.31 Following 8 weekly sessions of CBT-I or monitoring only, participants returned to the laboratory for a posttreatment assessment. Posttreatment assessment procedures were identical to the pretreatment assessment and also included 2 consecutive overnight PSG sleep recordings. Participants in both treatment conditions completed sleep diaries for the duration of the study (pretreatment, 8-week treatment, or monitor-only period, and posttreatment). Participants in the CBT-I condition also had repeat assessments and procedures (with the exception of PSG) 6 months following the posttreatment assessment. We did not include a 6-month follow-up assessment for the waitlist group because we wanted to give these participants the option of participating in CBT-I immediately following their posttreatment assessment.
Measures
Fear of Sleep Inventory
The Fear of Sleep Inventory (FoSI)10,36 is a 23-item self-report measure designed to assess fear of sleep related to PTSD. Specifically, the FoSI was developed to measure avoidance and dread of nightmares or re-experiencing, fear of loss of vigilance, and nighttime vigilant behaviors. Each item is rated using a 5-point Likert scale (0 = Not at all to 4 = Nearly every night) and total scores range from 0–92, with higher scores indicating greater fear of sleep severity. Previous research has demonstrated that the FoSI possesses excellent internal consistency as well as temporal stability.10,36 FoSI total score from pretreatment, posttreatment, and 6-month follow-up were used as outcome measures for all study aims.
Clinician-Administered PTSD Scale for DSM-IV
The CAPS was used to assess current PTSD. The CAPS measures both frequency and intensity of PTSD-related symptoms using a 5-point Likert scale (0 = Absent to 4 = Extreme/in -capacitating). Possible total CAPS scores range from 0–136, with higher scores indicating greater PTSD symptom severity. The psychometric properties of the CAPS have been studied extensively. The CAPS has demonstrated excellent reliability and validity and the factor structure of the CAPS corresponds well with symptom conceptualizations of PTSD; see Weathers et al. for a review.40 The CAPS was used to derive outcome measures for the first aim. More specifically, the outcome measures used were: (1) the pretreatment CAPS total score with the insomnia, hypervigilance, and nightmare items removed; (2) the pretreatment CAPS nightmare frequency score; (3) the pretreatment CAPS hypervigilance intensity score; and (4) index trauma type (ie, sexual assault versus other types of trauma).
Insomnia Severity Index
The ISI is a well-validated, 7-item measure of insomnia severity in the past week.37 Total scores range from 0–28, with a higher score indicative of greater insomnia severity. The ISI has excellent internal consistency and temporal stability, has been validated with both sleep diaries and PSG, and is sensitive to treatment response.37,41 The pretreatment ISI total score was used as an outcome variable for the second aim.
Sleep Diary
Sleep diaries are a gold-standard daily self-report measure of sleep.42 Average SOL, WASO, TST, and TIB were derived from sleep diaries. Participants were asked to complete the sleep diary immediately upon awakening every morning. More specifically, they were asked to estimate when they got into bed (bedtime), when they got out of bed (rise time), the total amount of time it took them to fall asleep (SOL), and how long they were awake in total in the middle of the night (WASO). TIB was calculated by determining the time spent in bed from bedtime to rise time. TST for each night was calculated by subtracting SOL, WASO, and early morning awakenings from total TIB. SOL, WASO, TST, and TIB were averaged across the week. Pretreatment averages for SOL, WASO, TST, and TIB were outcome variables for the second aim.
Pittsburgh Sleep Quality Index – Addendum
The Pittsburgh Sleep Quality Index – Addendum (PSQI-A)23 was used to assess disruptive nocturnal behaviors related to PTSD, including sleep disturbance related to hot flashes, nervousness, traumatic memories, anxiety or panic, bad dreams, terror or screaming, and acting out dreams. The total score ranges from 0–21, with higher scores indicating more severe disruptive behaviors. The PSQI-A has demonstrated good internal consistency and convergent validity.23,43 The pretreatment PSQI-A total score was used as an outcome variable for the second aim.
Polysomnography
PSG recordings were obtained at pretreatment and posttreatment in the participants' home environments. Each timepoint included two overnight recordings, with the first night serving as a standard adaptation night. Registered PSG technicians completed the PSG electrode attachments, but did not stay for the duration of the night. Notably, previous studies have demonstrated that unattended home PSG is comparable to attended laboratory settings.44,45 PSG was acquired using an ambulatory recorder (Trackit; Lifelines Ltd., Stockbridge, United Kingdom), which is a research-grade device. These recorders filter and amplify raw electroencephalogram (EEG) signals, then digitize the signals at 256 Hz and record to a removable hard disk in the EDF file format. The Trackit and recording software contain internal calibration routine to ensure that the values recorded in the EDF files truly represent the EEG amplitude. The parameters recorded included EEG at leads C3, C4, O1, and O2, left and right electrooculograms (EOG), submental electromyogram (EMG), bilateral anterior tibialis EMGs, and electrocardiogram in accordance with standardized guidelines. The EEG and EOG leads were referenced to linked mastoids. Participants were screened for obstructive sleep apnea by measuring reductions in oronasal airflow with a thermistor, pulse oximetry for detection of oxygen desaturation events, and two channels of respiratory effort using strain gauges to measure chest and abdominal movement during breathing. No participants had an apnea-hypopnea index of 10 events/h or greater. Digitized PSG data were imported in Twin software version 4.5.2 (Grass Technologies, Middleton, Wisconsin, United States) for visual scoring and the data were scored in 30-second epochs by an experienced registered PSG technician using standard scoring criteria.46 The results were used to generate the following outcome variables for the second aim: SOL, WASO, TST, and TIB derived from the second night of pretreatment recording.
Analysis Plan
Means and standard deviations were calculated for sociodemographic characteristics and PTSD and sleep variables not previously reported previously in Talbot et al.31 Independent samples t tests and chi-square tests assessed pretreatment differences in these features across the CBT-I and waitlist treatment groups.
To examine the first aim, Pearson correlations were performed to establish associations among pretreatment FoSI total score and pretreatment CAPS total score, pretreatment CAPS hypervigilance intensity, and pretreatment CAPS nightmare frequency. To assess whether fear of sleep differed depending on trauma type, we conducted an independent samples t test to compare FoSI means across sexual assault traumas compared to all other trauma types as assessed by the CAPS index trauma.
To assess the association between pretreatment FoSI total score and other sleep disturbance symptoms for the second aim, we once again performed Pearson correlations. Specifically, we included the following sleep variables in our analyses: pretreatment ISI total score, pretreatment sleep diary and PSG measurements of SOL, WASO, TST, TIB, and pretreatment PSQI-A total score.
For the third aim, we excluded three participants from the analyses because they did not complete the treatment or waitlist period and thus did not have posttreatment data (two dropped from CBT-I: n = 1 dropped out after session 2 citing work stress and n = 1 dropped out after session 3 due to a change in psychiatric treatment; and 1 dropped from the waitlist control due to a family emergency). To examine whether fear of sleep improved following CBT-I compared to the waitlist control condition, an analysis of covariance, covarying for pretreatment FoSI total score, was conducted to assess posttreatment group differences in fear of sleep. Paired t tests were used to compare pretreatment FoSI data to 6-month follow-up FoSI data in the CBT-I group.
We also conducted secondary analyses to determine whether a reduction in fear of sleep was associated with a reduction in PTSD and/or sleep disturbance symptoms following CBT-I. To examine these associations, hierarchical linear regressions were performed for variables from the first two aims that demonstrated a statistically significant association with FoSI. We calculated pretreatment to posttreatment change scores for FoSI and significant sleep/PTSD variables by subtracting posttreatment variables from pretreatment variables. Change scores were then mean-centered. Treatment group status was dummy coded (Waitlist = 0; CBT-I = 1) and treatment group by sleep/
PTSD interaction terms were calculated by multiplying dummy coded treatment group status by mean-centered continuous change scores. Hierarchical linear regressions were conducted using a stepwise approach; treatment group status and mean-centered continuous change variables were introduced in the first level and interaction terms were introduced in the second level. Effect size estimates were calculated for all analyses.
RESULTS
Means and standard deviations for sociodemographic, PTSD, and sleep variables are presented in Table 1. There were no significant differences for any sociodemographic or clinical characteristic across treatment groups, with the exception of veteran status. There were significantly more veterans in the waitlist control condition (n = 6, 37.5%) than the CBT-I treatment condition (n = 3, 10.3%).
Table 1.
Sociodemographic and clinical characteristics of participants.
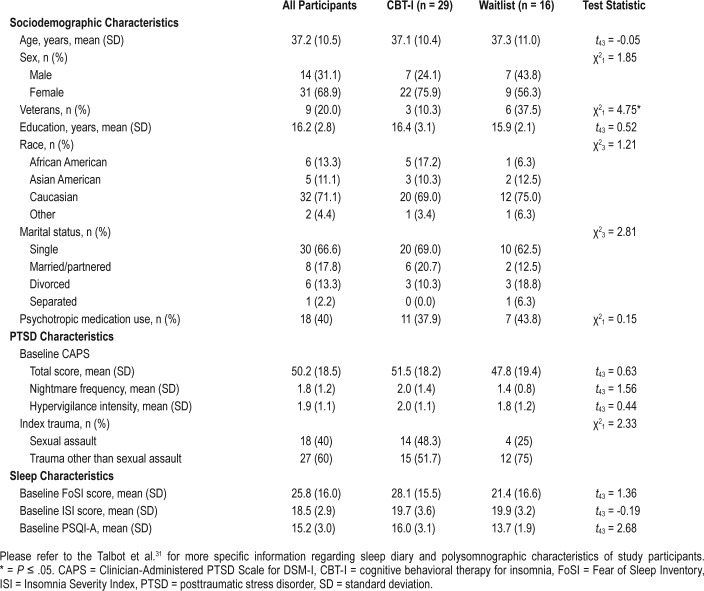
Results from the first aim are presented in Figure 1. Greater pretreatment FoSI total score was associated with greater CAPS total score (r = .40, P < .01, 95% confidence interval [CI] [0.12 to 0.62]), greater CAPS hypervigilance intensity (r = .30, P = .04, 95% CI [0.01 to 0.55]), and greater CAPS nightmare frequency (r = .34, P = .02, 95% CI [0.05 to 0.56]). Fear of sleep did not significantly differ depending on trauma type (ie, sexual assault versus other types of trauma; results nonsignificant and not displayed in Figure 1).
Figure 1. Association between fear of sleep and PTSD symptoms.
CAPS total score: r = .40, P < .01, 95% CI (0.12 to 0.62). CAPS hypervigilance intensity: r = .30, P = .04, 95% CI (0.01 to 0.55). CAPS nightmare frequency: r = .34, P = .02, 95% CI (0.05 to 0.56). CAPS = Clinician Administered PTSD Scale for DSM-IV, CI = confidence interval, PTSD = posttraumatic stress disorder.
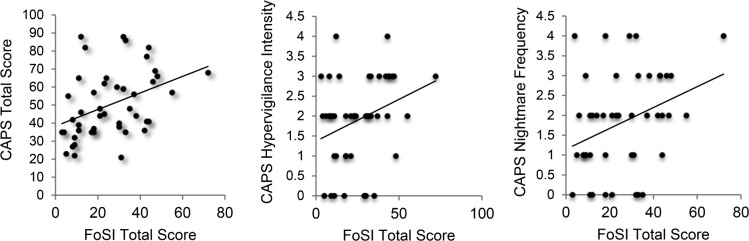
Results from the second aim are presented in Figure 2. Pearson correlations demonstrated that greater pretreatment FoSI total score was associated with less WASO as assessed by sleep diaries (r = −.43, P < .01, 95% CI [-0.64 to -0.15]), less TST as assessed by PSG (r = −.30, P = .05, 95% CI [-0.55 to -0.01], and greater disruptive behaviors during sleep as assessed by the PSQI-A total score (r = .42, P < .01, 95% CI [0.12 to 0.62]). Pretreatment FoSI was not associated with any other subjective or objective sleep variable.
Figure 2. Association between fear of sleep and objective and subjective measures of sleep disturbance.
WASO (sleep diaries): r = −.43, P < .01, 95% CI (-0.64 to -0.15). TST (PSG): r = −.30, P = .05, 95% CI (-0.55 to -0.01). PSQI-A: r = .42, P < .01, 95% CI (0.12 to 0.62). CI = confidence interval, PSG = polysomnography, PSQI-A = Pittsburgh Sleep Quality Index, Addendum for PTSD, TST = total sleep time, WASO = wake after sleep onset.
Results from the third aim are presented in Figure 3. Following CBT-I, there was a significant reduction in fear of sleep (F1,42 = 7.90, P < .01, partial η2 = 0.15, 95% CI [0.01 to 0.35]). Reductions in fear of sleep were maintained at the 6-month follow-up assessment (t22 = 5.95, P < .01, Cohen d = 1.20). Regression models for the secondary analyses for the third aim are presented in Table 2. There were two significant interactions; treatment group by reduction in PTSD symptom severity (β = 0.50, P = .02, partial η2 = 0.10, 95% CI [0.01 to 0.32]) and treatment group by reduction in hypervigilance both predicted a reduction in fear of sleep (β = 0.58, P < .01, η2 = 0.14, 95% CI [0.01 to 0.37]). Interaction plots and associated statistics are presented in Figure 4. Follow-up bivariate correlation analyses for each treatment group demonstrated that in the CBT-I group, a reduction in PTSD symptom severity (r = .57, P < .01, 95% CI [0.24 to 0.78) and hypervigilance (r = .43, P = .02, 95% CI [0.06 to 0.67]) were associated with a reduction in fear of sleep. Though the interaction was not significant, Pearson correlations demonstrated that a reduction in disruptive behaviors during sleep was associated with a reduction in fear of sleep in the CBT-I group (PSQI-A: r = .45, P = .02, 95% CI [0.09 to 0.71].
Figure 3. Change in fear of sleep following insomnia treatment.
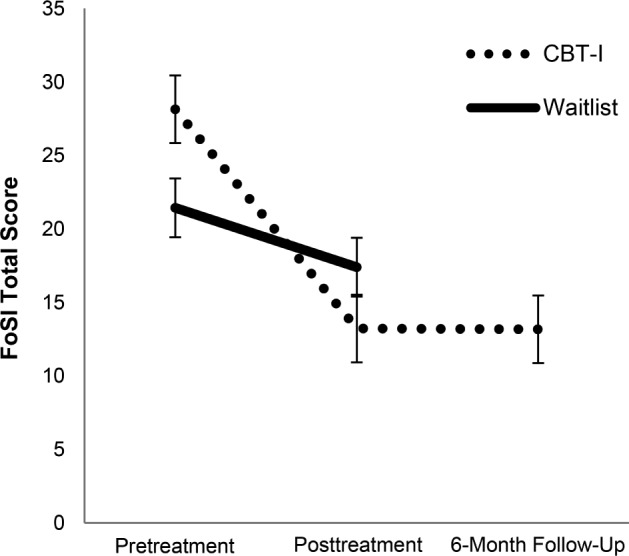
CBT-I: pretreatment FoSI = 28.43 ± 15.45; posttreatment FoSI = 14.31 ± 15.26; 6-month FoSI = 13.17 ± 16.61; pre to post t28 = 6.26, P < .01, Cohen d = 1.16; pre to 6-month follow-up t22 = 5.95, P < .01, Cohen d = 1.20. Waitlist: pretreatment FoSI = 21.43 ± 16.57; posttreatment FoSI = 19.31 ± 14.67; pre to post t15 = 0.79, P = NS, Cohen d = 0.20. CBT-I = cognitive behavioral therapy for insomnia, FoSI = Fear of Sleep Inventory, NS = nonsignificant.
Table 2.
Reduction in fear of sleep predicted by treatment group, reductions in PTSD and sleep symptoms, and treatment group by symptom interactions.
Figure 4. Reduction in fear of sleep predicted by treatment condition by reduction in PTSD symptoms interactions.
Reduction in CAPS total score: CBT-I: r = .57, P < .01, 95% CI (0.24 to 0.78); waitlist: r = −.14, P = NS. Reduction in hypervigilance intensity: CBT-I: r = .43, P = .02, 95% CI (0.06 to 0.67); waitlist: r = −.45, P = NS. CAPS = Clinician Administered PTSD Scale for DSM-IV, CBT-I = cognitive behavioral therapy for insomnia, CI = confidence interval, NS = nonsignificant, PTSD = posttraumatic stress disorder.
DISCUSSION
The overarching goal of the current study was to better understand the role of fear of sleep in individuals with PTSD and insomnia. The first aim was to examine the association between fear of sleep and PTSD symptoms. Consistent with our hypotheses, results demonstrated a moderate association between fear of sleep and PTSD symptoms; greater pretreatment fear of sleep was associated with greater pretreatment PTSD symptom severity, greater pretreatment hypervigilance intensity, and greater pretreatment nightmare frequency. These findings add to the current literature demonstrating parallel associations between fear of sleep and PTSD characteristics in undergraduate, trauma-exposed, and PTSD samples.10,16,19 Surprisingly, fear of sleep was not associated with trauma type. Notably, in our study, trauma type was defined dichotomously (sexual assault versus other) and derived from the CAPS index trauma and thus, information about where/when the trauma took place (eg, bed/bedroom and/or in darkness) was not available. Further, it is possible that participants may have experienced additional traumas that may be associated with fear of sleep that were not captured by the CAPS. Future studies should consider examining the association between fear of sleep and trauma type using a measure specifically designed to assess trauma history.
The second aim was to examine the association between fear of sleep and subjective and objective insomnia symptoms and disruptive behaviors during sleep. Results from these analyses yielded three significant findings. First, greater pretreatment fear of sleep was moderately associated with reduced pretreatment objective TST. This is consistent with a previous finding demonstrating an association between fear of sleep and short sleep duration in urban-dwelling, African-American young adults.22 One possible explanation for the association between fear of sleep and reduced TST is that fear of sleep is contributing to avoidance of the bed. Although not significant at the P ≤ .05 level, there was a moderate association between fear of sleep and reduced TIB as assessed by PSG (r = −.28, P = .07). As such, it is possible that individuals with greater fear of sleep are spending less time in bed, and thus, have less opportunity to sleep. This TST finding may also be driving the second significant finding related to pretreatment WASO. Contrary to our hypothesis, greater pretreatment fear of sleep was moderately associated with reduced pretreatment subjective WASO. In line with our TST finding, it is possible that individuals with greater fear of sleep have a truncated sleep period (ie, reduced TST), which may contribute to a more consolidated sleep and thus, reduced WASO. Finally, as hypothesized, greater pretreatment fear of sleep was moderately associated with increased pretreatment disruptive behaviors during sleep as assessed by the PSQI-A. Therefore, in individuals with PTSD and insomnia, fear of sleep appears to be related to nocturnal panic attacks, awakening with a startle or panic, bad dreams unrelated to trauma events, hot flashes at bedtime or during sleep, acting out dreams, and/or thrashing movements during sleep.
Notably, fear of sleep was not associated with insomnia severity, SOL, or any other objective or subjective insomnia symptom. Previous studies have demonstrated a significant association between fear of sleep and insomnia-related sleep disruptions in individuals with PTSD10,16 and fear of sleep has been hypothesized to play a role in insomnia pathogenesis. Instead, in our sample, fear of sleep was associated with sleep disturbance symptoms characteristic of PTSD (eg, nightmares, disruptive nocturnal behaviors) rather than “classic” insomnia symptoms. These results contradict the hypothesized model that PTSD symptomatology is associated with fear of sleep, which in turn, leads to insomnia-related sleep disruptions. Rather, the combined results from our first two aims suggest that greater PTSD symptom severity (eg, greater hypervigilance and nightmare frequency) is associated with greater fear of sleep, which in turn is associated with greater sleep disruptions specific to PTSD (eg, disruptive nocturnal behaviors). Importantly, the direction of these associations remains unclear. Future studies should try to ascertain directionality by using standard prospective measures and longitudinal assessments.
Results from the final aim demonstrated a moderate reduction in fear of sleep following CBT-I, which persisted at the 6-month follow-up assessment. This improvement in fear of sleep following CBT-I is particularly noteworthy for two reasons. The first is that trial therapists intentionally did not address any beliefs about the safety of the bed/bedroom in order to avoid introducing elements of cognitive behavioral therapy for PTSD. Thus, fear of sleep was not a protocol-permissive target for intervention. Despite this, fear of sleep significantly improved following CBT-I treatment. The second is that fear of sleep was not related to pretreatment insomnia symptoms and thus, a reduction in fear of sleep following CBT-I is not likely due to a reduction in insomnia symptoms. Instead, follow-up analyses demonstrated that a reduction in fear of sleep following CBT-I was associated with a reduction in PTSD symptom severity and a reduction in hypervigilance intensity. These results complement the literature suggesting that the treatment of insomnia across mental health conditions is associated with an improvement in both sleep disturbance symptoms and non-sleep symptoms (eg, symptoms associated with anxiety and/ or mood disorders47–49). In this case, the treatment of insomnia was associated with an improvement in PTSD symptoms and relatedly, fear of sleep.
Results from this study should be interpreted in light of several limitations. First, this was a preliminary study. Therefore, we did not adjust for multiple comparisons. Effect sizes for our findings were in the medium range, suggesting that fear of sleep plays a moderate role in individuals with PTSD and insomnia. Ideally, results from this study will be used to inform future studies that are sufficiently powered. The second is that we had a limited number of nights of PSG and our results may have been strengthened by additional adaptation nights. Third, future studies should consider including prospective measurements of alcohol, nicotine, and caffeine use as use of these substances is common in PTSD, have been shown to influence sleep, and thus may influence the associations among fear of sleep, PTSD, and sleep disturbance symptoms.50 Finally, the study sample may not have been completely representative of the general PTSD population given our inclusion/exclusion criteria. Further, as this was a CBT-I trial, we specifically recruited for insomnia disorder. Future studies should consider adopting more of a research domain criteria (RDoC) approach by looking at the association between fear of sleep and insomnia symptoms, regardless of an insomnia diagnosis.
In summary, this was the first study to examine fear of sleep in individuals with PTSD and insomnia using standard objective and subjective measures and a therapeutic manipulation of sleep. Results from our study demonstrate that fear of sleep is strongly related to symptoms specific to PTSD rather than classic insomnia symptoms. Interestingly, fear of sleep decreased following CBT-I, which appears to be related to a decrease in PTSD symptomatology. This suggests that CBTI not only improves insomnia symptoms in individuals with PTSD, but also may improve PTSD symptoms, including fear of sleep. Given the limited studies and mixed findings of the current literature, more research is needed to better understand the role of fear of sleep in insomnia pathogenesis in individuals with PTSD.
DISCLOSURE STATEMENT
Work for this study was performed at San Francisco VA Health Care System. This project was supported by grants from the National Institute for Mental Health (TCN: 5R01MH073978-04, 5R34MH077667-03) and the Mental Illness Research and Education Clinical Center of the US Veterans Health Administration. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center, San Francisco, CA. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: All authors contributed to the design, analysis, and interpretation of the manuscript and have approved the final version of the manuscript being submitted.
ABBREVIATIONS
- CAPS
Clinician Administered PTSD Scale for DSM-IV
- CBT-I
cognitive behavioral therapy for insomnia
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- FoSI
Fear of Sleep Inventory
- ISI
Insomnia Severity Index
- PSG
polysomnography
- PSQI-A
Pittsburgh Sleep Quality Index, Addendum for PTSD
- PTSD
posttraumatic stress disorder
- RDoC
research domain criteria
- SCID
Structured Clinical Interview for Axis I Disorders
- SOL
sleep onset latency
- TIB
time in bed
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the Hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 3.Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1–15. doi: 10.1016/j.janxdis.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009–1018. doi: 10.5665/sleep.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short NA, Allan NP, Schmidt NB. Sleep disturbance as a predictor of affective functioning and symptom severity among individuals with PTSD: an ecological momentary assessment study. Behav Res Ther. 2017;97:146–153. doi: 10.1016/j.brat.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70(4):318–327. doi: 10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Zayfert C, De Viva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17(1):69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]
- 9.Pruiksma KE, Taylor DJ, Wachen JS, et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol Trauma. 2016;8(6):697–701. doi: 10.1037/tra0000150. [DOI] [PubMed] [Google Scholar]
- 10.Pruiksma KE, Taylor DJ, Ruggero C, et al. A psychometric study of the Fear of Sleep Inventory-Short Form (FoSI-SF) J Clin Sleep Med. 2014;10(5):551–558. doi: 10.5664/jcsm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: Findings from a nationally representative sample of male Vietnam Veterans. Am J Psychiatry. 1998;155(7):929–933. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 12.Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449–452. doi: 10.1016/s0022-3956(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 13.Krakow B, Tandberg D, Scriggins L, Barey M. A controlled comparison of self-rated sleep complaints in acute and chronic nightmare sufferers. J Nerv Ment Dis. 1995;183(10):623–627. doi: 10.1097/00005053-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Davis JL, Byrd P, Rhudy JL, Wright DC. Characteristics of chronic nightmares in a trauma-exposed treatment-seeking sample. Dreaming. 2007;17(4):187–198. [Google Scholar]
- 15.Davis JL, Rhudy JL, Pruiksma KE, et al. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J Clin Sleep Med. 2011;7(6):622–631. doi: 10.5664/jcsm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Short NA, Allan NP, Stentz L, Portero AK, Schmidt NB. Predictors of insomnia symptoms and nightmares among individuals with post-traumatic stress disorder: an ecological momentary assessment study. J Sleep Res. 2018;27(1):64–72. doi: 10.1111/jsr.12589. [DOI] [PubMed] [Google Scholar]
- 17.Haynes PL, Epstein DR. The ambivalent sleeper. J Clin Sleep Med. 2010;6(5):511–512. [PMC free article] [PubMed] [Google Scholar]
- 18.Pietrzak RH, Morgan CA, Southwick SM. Sleep quality in treatment-seeking veterans of Operations Enduring Freedom and Iraqi Freedom: the role of cognitive coping strategies and unit cohesion. J Psychosom Res. 2010;69(5):441–448. doi: 10.1016/j.jpsychores.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Hall Brown TS, Akeeb A, Mellman TA. The role of trauma type in the risk for insomnia. J Clin Sleep Med. 2015;11(7):735–739. doi: 10.5664/jcsm.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–478. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- 21.Moul DE, Nofzinger EA, Pilkonis PA, Houck PR, Miewald JM, Buysse DJ. Symptom reports in severe chronic insomnia. Sleep. 2002;25(5):553–563. [PubMed] [Google Scholar]
- 22.Hall Brown T, Mellman TA. The influence of PTSD, sleep fears, and neighborhood stress on insomnia and short sleep duration in urban, young adult, African Americans. Behav Sleep Med. 2014;12(3):198–206. doi: 10.1080/15402002.2013.784704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Germain A, Hall M, Krakow B, Katherine Shear M, Buysse DJ. A brief Sleep Scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Krakow B, Germain A, Tandberg D, et al. Sleep breathing and sleep movement disorders masquerading as insomnia in sexual-assault survivors. Compr Psychiatry. 2000;41(1):49–56. doi: 10.1016/s0010-440x(00)90131-7. [DOI] [PubMed] [Google Scholar]
- 25.Freed S, Craske MG, Greher MR. Nocturnal panic and trauma. Depress Anxiety. 1999;9(3):141–145. doi: 10.1002/(sici)1520-6394(1999)9:3<141::aid-da8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 27.Sheikh JI, Woodward SH, Leskin GA. Sleep in post-traumatic stress disorder and panic: Convergence and divergence. Depress Anxiety. 2003;18(4):187–197. doi: 10.1002/da.10066. [DOI] [PubMed] [Google Scholar]
- 28.Hefez A, Metz L, Lavie P. Long-term effects of extreme situational stress on sleep and dreaming. Am J Psychiatry. 1987;144(3):344–347. doi: 10.1176/ajp.144.3.344. [DOI] [PubMed] [Google Scholar]
- 29.Ross RJ, Ball WA, Dinges DF, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 30.Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20(1):46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- 31.Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/ daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38(3):174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz TD, Mahowald MW, Kuskowski M, Engdahl BE. Polysomnographic sleep is not clinically impaired in Vietnam combat veterans with chronic posttraumatic stress disorder. Biol Psychiatry. 1998;44(10):1066–1073. doi: 10.1016/s0006-3223(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 34.Klein E, Koren D, Arnon I, Lavie P. Sleep complaints are not corroborated by objective sleep measures in post-traumatic stress disorder: A 1-year prospective study in survivors of motor vehicle crashes. J Sleep Res. 2003;12(1):35–41. doi: 10.1046/j.1365-2869.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Li Y, Zhu H, et al. Characteristics of objective daytime sleep among individuals with earthquake-related posttraumatic stress disorder: a pilot community-based polysomnographic and multiple sleep latency test study. Psychiatry Res. 2017;247:43–50. doi: 10.1016/j.psychres.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Huntley ED, Hall Brown TS, Kobayashi I, Mellman TA. Validation of the Fear of Sleep Inventory (FOSI) in an urban young adult African American sample. J Trauma Stress. 2014;27(1):103–107. doi: 10.1002/jts.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 39.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 40.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 41.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buysse D, Ancoli-Israel S, Edinger J, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 43.Insana SP, Hall M, Buysse DJ, Germain A. Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in U.S. Male Military Veterans. J Trauma Stress. 2013;26(2):192–200. doi: 10.1002/jts.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iber C, Redline S, Kaplan Gilpin AM, et al. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–540. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 45.Bruyneel M, Libert W, Ameye L, Ninane V. Comparison between home and hospital set-up for unattended home-based polysomnography: a prospective randomized study. Sleep Med. 2015;16(11):1434–1438. doi: 10.1016/j.sleep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 47.Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev. 2011;31(4):638–652. doi: 10.1016/j.cpr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychol. 2015;83(3):564–577. doi: 10.1037/a0038655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237–247. doi: 10.3109/09540261.2014.901300. [DOI] [PMC free article] [PubMed] [Google Scholar]