Abstract
Study Objectives:
Sleep disturbances following traumatic brain injury (TBI) in Veterans are very common and often persist as chronic sequelae. In addition, sensory sensitivity, ie, discomfort upon exposure to light and noise, is common after TBI. However, the relationship between sleep disturbances and sensory sensitivity in Veterans following TBI has not yet been examined, yet both are established early markers of neurodegeneration.
Methods:
Veterans (n = 95) in the chronic phase of recovery from TBI at the VA Portland Health Care System completed an overnight polysomnography and provided self-report data on sensory (eg, light and noise) sensitivity, and sleep disturbances. Participants were categorized into four sensory sensitivity groups: (1) “neither,” neither light nor noise sensitivity (n = 36); (2) “light,” only light sensitivity (n = 12); (3) “noise,” only noise sensitivity (n = 24); and (4) “both,” light and noise sensitivity (n = 23).
Results:
Veterans with TBI reported sleep disturbances that were significantly correlated with the severity of their sensory sensitivity and associated with posttraumatic stress disorder (PTSD). Multiple linear regression revealed insomnia severity to be the strongest predictor of the relationship between sleep disturbances and sensory sensitivity. Furthermore, sensory sensitivity was associated with a higher mean heart rate during sleep, even after controlling for PTSD status.
Conclusions:
These data are the first to report the prevalence and association between sensory sensitivity and sleep disturbances in Veterans with TBI. These data also suggest that the underlying mechanism of the sleep-sensory relationship could be due in part to comorbid PTSD and autonomic nervous system hyperarousal.
Citation:
Elliott JE, Opel RA, Weymann KB, Chau AQ, Papesh MA, Callahan ML, Storzbach D, Lim MM. Sleep disturbances in traumatic brain injury: associations with sensory sensitivity. J Clin Sleep Med. 2018;14(7):1177–1186.
Keywords: autonomic hyperarousal, light sensitivity, neurodegeneration, noise sensitivity, PTSD, Veterans
BRIEF SUMMARY
Current Knowledge/Study Rationale: Traumatic brain injury (TBI) is common in Veterans and is often independently associated with persistent sleep disturbances as well as sensory (ie, light and noise) sensitivity. The current study sought to correlate sensory sensitivity with sleep disturbances in Veterans with TBI who have undergone in-laboratory polysomnography.
Study Impact: In Veterans with TBI, sensory sensitivity was strongly correlated with sleep disturbances and posttraumatic stress disorder symptom severity, and insomnia severity was the strongest predictor of this relationship. Furthermore, sensory sensitivity was associated with an increased mean heart rate during sleep, even after controlling for posttraumatic stress disorder status. These data suggest the underlying mechanism of the sleep-sensory relationship could be due in part to comorbid posttraumatic stress disorder and autonomic hyperarousal.
INTRODUCTION
Each year approximately 2.5 million Americans sustain a traumatic brain injury (TBI),1,2 with a significantly higher incidence among military personnel. Although TBI severity can range from mild, moderate, to severe, ∼80% are classified as mild,3 and can be associated with persistent and debilitating sequelae that prevent the return to normal physical, cognitive, and emotional functioning. Among the most prevalent and persistent symptoms of TBI are sleep disturbances.4–7 The pathophysiology underlying sleep disturbances following TBI remains unclear, although recent work has implicated a neuroanatomical mechanism based on an impaired orexin/hypocretin system.8–15 Additionally, sleep disturbances have been implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer disease, a process that may be accelerated by TBI.16
Light and noise sensitivity are also frequently associated sequelae of TBI.17 Although the association between light/noise sensitivity and acute TBI was established in 1967 by Jonnson et al.,18 it was objectively demonstrated in 1984 through seminal work by Waddell and Gronwall in patients with TBI at a more subacute time point (eg, 7 to 19 days after injury).19 Later, Bohnen et al., extended these findings through the objective analysis of sensory sensitivity at a chronic time point, 6 months after injury.20 Further, recent work has highlighted the importance and prevalence of sensory sensitivity in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with TBI due to blast exposure.21 However, the prevalence of self-reported sensory sensitivity in chronic TBI remains elusive, in either Veterans or civilians. For example, a civilian sample of 732 subjects with TBI and 120 control subjects (trauma exposed but without a history of TBI) found that both light and noise sensitivity were reported in subjects with TBI 1 month after injury, but only light sensitivity persisted 1 year after injury.22 Furthermore, the correlation between sensory dysfunction and sleep disturbances following TBI remains unexplored; yet both are established early markers of neurodegenerative disorders related to TBI.23–25
The chronic phase of recovery from TBI (eg, > 10 years after injury), is particularly relevant as functional outcomes can remain impaired for at least 10 years after injury.26 Nevertheless, despite the importance of studying Veterans in the chronic phase of recovery from TBI, little work has been done investigating sleep disturbances and sensory sensitivity in Veterans during this time period. Thus, the purpose of this study is to (1) assess the prevalence of light and noise sensitivity in Veterans in the chronic phase of recovery from TBI, and (2) correlate sensory sensitivity with sleep disturbances using both subjective and objective measures of sleep.
METHODS
The VA Portland Health Care System (VAPORHCS) institutional review board approved this project (MIRB #3641) and all subjects provided verbal and written informed consent prior to participation.
Overview
Veterans who presented to the VAPORHCS Sleep Clinic between May 2015 and November 2016 with a medical record-confirmed TBI were recruited for participation (n = 130). Subjects were excluded from participation if medical records did not contain information about the year of their TBI (n = 14) or if subjects returned unfinished surveys (n = 21). No subjects were excluded on the basis of their TBI recency, as nearly all subjects were > 1 year after their TBI (only 1 of the 95 was between 6–12 months after injury). Thus, a total of 95 Veterans with a medical record-confirmed TBI and complete data were included in this study. All subjects provided self-report data on sleep quality and posttraumatic stress disorder (PTSD) symptom severity, as well as completed an overnight polysomnography (PSG) study at the VAPORHCS Sleep Clinic.
Subject Grouping
Subjects' sensitivity to light and noise was assessed using two 5-point (0 to 4) Likert scales (0 = “not experienced at all,” 1 = “no more of a problem,” 2 = “a mild problem,” 3 = “a moderate problem,” and 4 = “a severe problem”) that were phrased as “light sensitivity, easily upset by bright light” and “noise sensitivity, easily upset by loud noise.” Subjects were determined to be light- or noise-sensitive if they scored ≥ 3 on the respective question (corresponding to moderate to severe sensitivity) and then stratified into four groups: (1) “neither,” no significant light or noise sensitivity (n = 36); (2) “light,” only significant light sensitivity (n = 12); (3) “noise,” only significant noise sensitivity (n = 24); and (4) “both,” significant light and noise sensitivity (n = 23).
Retrospective Medical Record Review
A thorough retrospective medical record review was conducted to assess numerous metrics related to TBI and general health. These included determining (1) the number of TBIs; (2) the recency of the subjects' TBI (determined from year of most recent TBI if > 1 TBI was identified); (3) whether or not the TBI(s) was caused by blast exposure; (4) whether the TBI caused loss of consciousness, confusion, posttraumatic amnesia, or postconcussive syndrome (PCS); (5) if subjects now suffer from tinnitus or hearing loss; (6) whether subjects were Operation Enduring Freedom/Operation Iraqi Freedom (OEF/ OIF) Veterans; (7) anxiety; (8) diabetes; (9) hypertension; (10) heart disease; (11) lung disease; and (12) medications related to pain, depression, and heart disease. Additionally, the presence of sleep apnea was extracted from subjects' overnight PSG.
Survey Instruments
Insomnia Severity Index
The Insomnia Severity Index (ISI) is a 7-item measure assessing insomnia severity (ie, difficulty initiating and staying asleep), with the total score ranging from 0–28.27 Individual items are 5-point Likert scales: 0 = “none,” 1 = “mild,” 2 = “moderate,” 3 = “severe,” 4 = “very severe.” The total scored can be subdivided into one of four evenly spaced provisional diagnosis categories: no insomnia (0–7); mild insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28). In the current study, subjects were categorized as having insomnia with an ISI score of ≥ 15 (ie, moderate to severe insomnia). Cronbach α in our sample was 0.84 (0.79–0.89), which is consistent with previously reported values.28
Functional Outcomes of Sleep Questionnaire-10
The Functional Outcomes of Sleep Questionnaire (FOSQ-10) is a 10-item measure assessing quality of life due to sleep quality.29 Individual items are 4-point Likert scales: 1 = “yes, extreme difficulty,” 2 = “yes, moderate difficulty,” 3 = “yes, a little difficulty,” 4 = “no difficulty”; however, half of the items are a 5-point Likert scale that include a rating of 0 = “I don't do this activity for other reasons.” The survey has five subscales: (1) activity level (three items), (2) vigilance (three items), (3) intimacy and sexual relationships (one item), (4) general productivity (two items), and (5) social outcomes (one item).30 The FOSQ-10 score is an average of the five subscales; thus, the FOSQ-10 has a range of 5–20 with lower values indicating worse function and higher numbers indicating better function. Cronbach α in our sample was 0.87 (0.83–0.91), which is consistent with previously reported values.29
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) is an 8-item measure assessing daytime sleepiness, with a total score ranging from 0–24.31 Individual items are 4-point Likert scales: 0 = “would never doze,” 1 = “slight chance of dozing or sleeping,” 2 = “moderate chance of dozing or sleeping,” and 3 = “high chance of dozing or sleeping.” A score ≥ 11 is indicative of abnormal daytime sleepiness. Internal consistency was 0.86 (0.82–0.90), which is consistent with previously reported values.32,33
PTSD Checklist DSM-5
The PTSD Checklist DSM-5 (PCL-5)34 is a 20-item measure used for screening and provisionally diagnosing subjects for PTSD, as well as assessing symptom severity. Individual items are 5-point Likert scales: 0 = “not at all,” 1 = “a little bit,” 2 = “moderately,” 3 = “quite a bit,” and 4 = “extremely.” Thus, the total score ranges from 0–80. The survey's 20 questions are subdivided into four subscales, or “clusters”: cluster B (intrusion; 1–5), cluster C (avoidance; 6–7), cluster D (mood and cognition; 8–14), and cluster E (arousal activity; 15–20). Cluster A, which was not administered in this study, is a structured clinical interview (eg, Clinician-Administered PTSD Scale) and is required for administering an official diagnosis of PTSD. Thus, subjects in the current study were categorized as having PTSD based on their PCL-5 cluster criteria,34 and total score (≥ 33). PCL-5 cluster criteria required subjects to rate one B item, one C item, two D items, and two E items as 2 (moderately) or higher. Both positive PCL-5 cluster criteria and total score ≥ 33 were required for PTSD determination. Cronbach α in our sample was 0.97 (0.96–0.98), which is consistent with previously reported values.35
Overnight Polysomnography
All subjects completed an in-laboratory, technician-attended overnight PSG (ie, type I sleep study). Sleep studies were recorded using Polysmith version 9.0 (Nihon Kohden, Tokyo, Japan). Sleep staging was performed by an American Academy of Sleep Medicine (AASM)-certified sleep technician and interpreted by a board-certified sleep medicine physician. Standard parameters as specified by the AASM29 were captured in the PSG recordings, including electroencephalography, electromyography of the mentalis muscle, electrooculography (left and right eyes), electrocardiography, peripheral blood-oxygen saturation, respiratory movement/effort (thorax and abdominal), airflow (nasal and oral), auditory (snoring), and body positioning (right side, left side, supine, prone).
Individual PSG data were analyzed for total sleep time (TST), time spent in each sleep stage as a percent of TST, number of sleep stage transitions, sleep efficiency, sleep latency, wake after sleep onset (WASO), and body position transitions. TST was calculated by summing the total number of 30-second epochs scored as non-rapid eye movement (NREM) or rapid eye movement (REM) sleep and converting to minutes. Sleep stage transitions were determined by counting the number of times a patient changed from one sleep stage (wake, N1, N2, N3, or R) to another stage, with the tally beginning at lights off. Sleep efficiency was calculated as the percent of time a patient spent sleeping after initially falling asleep. Sleep latency was determined by counting the number of epochs between lights off and the initial onset of NREM sleep. WASO was determined to be the length of time in minutes a patient spent awake after initially falling asleep for the night. Finally, body position transitions were calculated during sleep, while excluding transitions that occurred before sleep onset.
Heart Rate
Heart rate was extracted from overnight PSG records and an average heart rate was determined for each epoch for each subject. The mean overnight heart rate during NREM sleep was determined by a weighted average of all epochs scored as either stage N2 or N3 sleep to obtain a single average heart rate during NREM sleep. Stage N2 and N3 sleep were combined due to the very low percentages in stage N3 sleep alone. Similarly, all epochs scored as either stage R sleep or wake were averaged to determine an average heart rate during stage R sleep and wake. All epochs scored as “lights on” (ie, prior to going to sleep, or after waking) were excluded.
Statistical Analyses
All statistical analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/). For all tests, a value of P < .05 was considered statistically significant. Differences in numerical variables between sensory sensitivity groups (ie, neither, light, noise, both) were assessed using a one-way analysis of variance (ANOVA) with Tukey honestly significant difference (HSD) post hoc test if warranted. Analysis of categorical data was performed using a chi-square test with Bonferroni-corrected chi-square post hoc tests when suitable.36 When appropriate (ie, the expected value in the 2 × 2 contingency table is < 5), we used Fisher exact test with a simulated P value. The Shapiro-Wilk test was used to assess normality of our data, of which most of the survey data and all the PSG data did not meet normality assumptions. However, given that our sample size exceeded n = 30, and that nonparametric analyses agreed with parametric analyses (performed concurrently), only parametric results are reported, unless specified otherwise.
RESULTS
Sensory Sensitivity, Demographics, and General Health Parameters
Overall, of the 95 subjects in this study, the prevalence of Veterans reporting neither light nor noise sensitivity (ie, a score ≤ 2)—the “neither” group—was 38% (n = 36). The mean light and noise sensitivity scores for these subjects were 0.75 ± 0.13 and 1.28 ± 0.14, respectively. In contrast, the prevalence of Veterans reporting light and/or noise sensitivity (ie, a score of > 2) was 62% (n = 59). Within this group of participants, n = 12 (13% overall) reported only light sensitivity—the “light” group—and had a mean light and noise sensitivity score of 3.42 ± 0.15 and 1.17 ± 0.24, respectively. There were n = 24 (25% overall) participants who reported only noise sensitivity—the “noise” group—and had a mean light and noise sensitivity score of 1.17 ± 0.17 and 3.17 ± 0.08, respectively. Finally, n = 23 participants (24% overall) reported both light and noise sensitivity—the “both” group—and had a mean light and noise sensitivity score of 3.62 ± 0.11 and 3.43 ± 0.11, respectively.
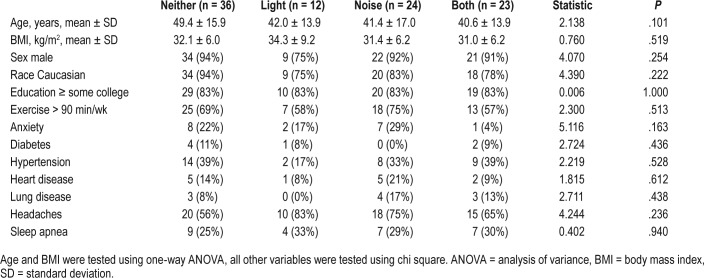
There were no differences across groups in any of the demographic and general health parameters analyzed (Table 1). Additionally, given that a common side effect of many medications is light and/or noise sensitivity, we found no differences across groups during the retrospective medical record review in the usage of prescribed opioids, benzodiazepines, bupropion, and tricyclic antidepressants (data not shown).
Table 1.
Demographic and general health parameters in Veterans with and without sensory sensitivity.
TBI Characteristics
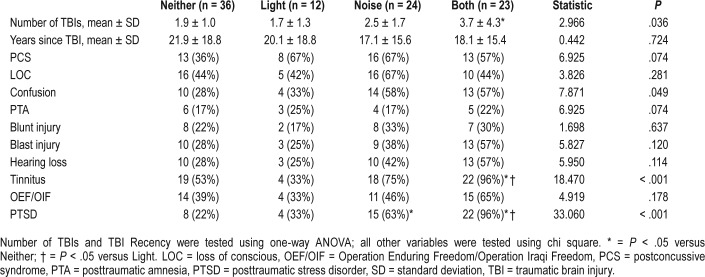
The TBI characteristics of our cohort are shown in Table 2. Notably, the mean duration elapsed following TBI was, on average, approximately 15–20 years, and all TBIs were classified as “mild” in severity. Participants with both light and noise sensitivity sustained approximately twice as many TBIs compared to participants with no sensory sensitivity (P = .040). There were no other differences among groups stratified by levels of light/noise sensitivity other than presence of tinnitus, which was reported in 96% of participants with both light and noise sensitivity compared to Veterans without sensory sensitivity (53%; P = .008). There was no difference in the proportion of subjects across groups reporting a blast exposure or blunt force-related TBI.
Table 2.
TBI characteristics and PTSD status in Veterans with and without sensory sensitivity.
Sensory Sensitivity and Sleep Disturbances
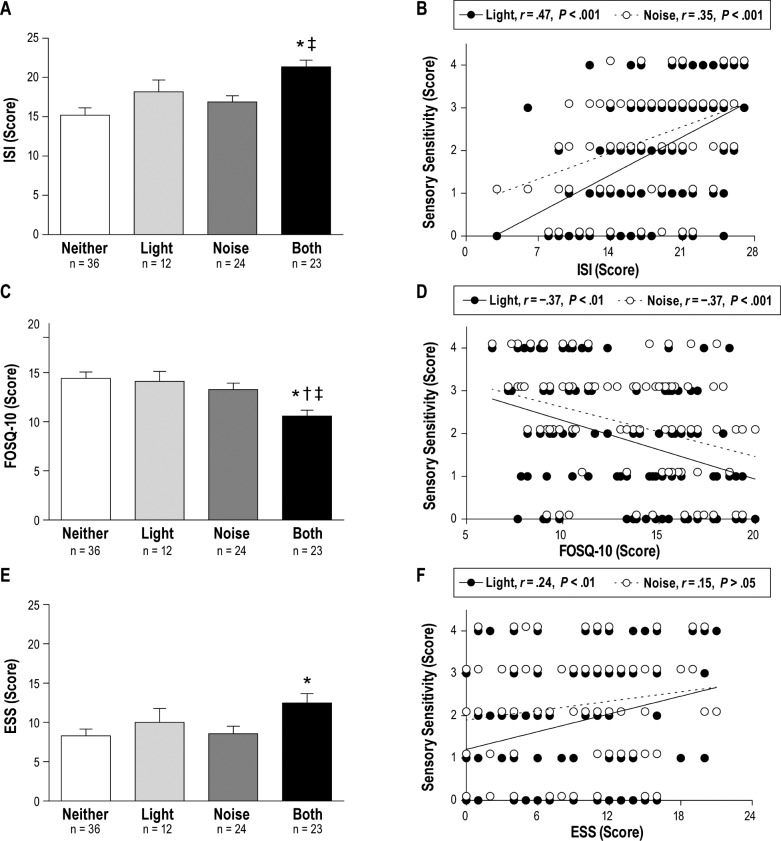
Participants with both light and noise sensitivity reported worse sleep disturbances on the ISI, FOSQ-10, and ESS than participants without sensory sensitivity. These relationships were particularly strong for the ISI and FOSQ-10. The mean ISI score (Figure 1A) was higher in subjects with both light and noise sensitivity compared to those without sensory sensitivity (P < .001) and only noise sensitivity (P = .011), but not different compared to those with only light sensitivity (P = .262). With regard to insomnia severity, 83% of subjects (10/12) with only light sensitivity, and 91% of subjects (21/23) with both light and noise sensitivity were categorized as having moderate (ISI score = 15–21) or severe (ISI score = 22–28) insomnia, compared to approximately 50% of subjects without sensory sensitivity and only noise sensitivity. Light and noise sensitivity were positively correlated with ISI score (P < .001), r = .47 and r = .39, respectively (Figure 1B).
Figure 1. Sleep disturbances are associated with sensory sensitivity in Veterans with TBI.
Symptom severity for (A) insomnia, determined by ISI score (0–28, higher = worse insomnia), (C) functional outcomes of sleep, determined by the FOSQ-10 (5–40, lower = worse outcomes), and (E) daytime sleepiness, determined by ESS score (0–24, higher = worse daytime sleepiness) is shown stratified by self-reported sensory sensitivity in Veterans with TBI: No sensory sensitivity (open bars, n = 36), only light sensitivity (light gray bars, n = 12), only noise sensitivity (dark gray bars, n = 24), and both light and noise sensitivity (filled bars, n = 23). Significantly worse scores on ISI, FOSQ-10, and ESS were seen in subjects reporting greater sensory sensitivity to light and noise (one-way ANOVA for ISI (P < .001), FOSQ-10 (P < .001) and ESS (P = .026); * = P < 0.05 versus Neither; † = P < .05 versus Light; ‡ = P < .05 versus Noise). The correlation between individual light and noise sensitivity scores and corresponding ISI (B), FOSQ-10 (D), and ESS (F) scores. Thus, there are n = 95 data points for both light sensitivity versus ISI, FOSQ-10, and ESS scores, and noise sensitivity versus ISI, FOSQ-10, and ESS scores. Data points are nudged slightly for illustrative purposes to prevent overlap between duplicate light and noise sensitivity scores. ANOVA = analysis of variance, ESS = Epworth Sleepiness Scale, FOSQ-10 = Functional Outcomes of Sleep Questionnaire-10, ISI = Insomnia Severity Index, TBI = traumatic brain injury.
Similarly, the mean FOSQ-10 score (Figure 1C) was lower (ie, worse) in subjects with both light and noise sensitivity compared to those without sensory sensitivity (P < .001), only light sensitivity (P = .024), and only noise sensitivity (P = .041). Furthermore, both light and noise sensitivity were negatively correlated (P < .001) with FOSQ-10 score (lower FOSQ-10 scores = worse outcomes), r = −.35 and r = −.35, respectively (Figure 1D).
Finally, the mean ESS score was significantly (P = .02) higher (ie, worse) in subjects with both light and noise sensitivity compared to those without sensory sensitivity (Figure 1E), and light sensitivity was positively correlated (P < .01) with ESS score, r = .26 (Figure 1F).
Predictors of Sensory Sensitivity in Veterans With TBI
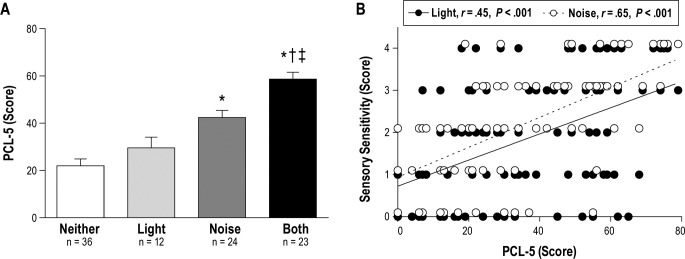
Multiple linear regression analysis indicated that ISI total score was the strongest predictor (using: age, TBI recency, the number of TBIs, ISI score, and whether or not subjects had PTSD as specific predictor variables) for both light (β = 0.106; P < .001) and noise sensitivity (β = 0.046; P = .043). In ad -dition, the number of TBIs and whether or not subjects had PTSD were additional contributing factors for noise sensitivity. This is consistent with the fact that PTSD is a known contributor to generalized sensory sensitivity, and there was a significant difference (χ23 = 33.06; P < .001) in the proportion of Veterans with comorbid PTSD across our sensory sensitivity groups (Table 2). Likewise, the mean PCL-5 score was significantly higher in Veterans with both light and noise sensitivity (Figure 2A) compared to Veterans without sensory sensitivity (P < .001), only light sensitivity (P < .001), and only noise sensitivity (P = .004). Additionally, mean PCL-5 total scores were significantly higher in Veterans with only noise sensitivity compared to those without sensory sensitivity (P < .001). Finally, both light and noise sensitivity were positively correlated (P < .001) with PCL-5 total score, r = .46 and r = .62, respectively (Figure 2B). We also performed regressions with PCL-5 cluster scores as predictor variables and there were no significant predictors on light sensitivity score, although cluster B was trending (“intrusive thoughts”; β = 0.082; P = .053). In contrast, cluster E (“arousal”) was positively associated with increased noise sensitivity (β = 0.115; P = .001), which was driven specifically by question 18, “In the past month, how much were you bothered by…Feeling jumpy or easily startled,” (β = 0.501; P < .001). Of note, the finding that ISI score was positively correlated with sensory sensitivity remained significant even after controlling for the potential contribution of PTSD.
Figure 2. PTSD symptom severity is associated with sensory sensitivity in Veterans with TBI.
(A) PTSD symptom severity determined by PCL-5 score (0–80, higher = worse PTSD) stratified by self-reported sensory sensitivity in Veterans with TBI: no sensory sensitivity (open bars, n = 36), only light sensitivity (light gray bars, n = 12), only noise sensitivity (dark gray bars, n = 24), and both light and noise sensitivity (filled bars, n = 23). Significantly worse scores on PCL-5 were seen in subjects reporting greater sensory sensitivity to light and noise. One-way ANOVA for PCL-5 (P < .001); * = P < .05 versus Neither; † = P < .05 versus Light; ‡ = P < .05 versus Noise. (B) The correlation between individual light and noise sensitivity scores and corresponding PCL-5 scores. Thus, there are n = 95 data points for both light sensitivity versus PCL-5, and noise sensitivity versus PCL-5. Data points are nudged slightly for illustrative purposes to prevent overlap between duplicate light and noise sensitivity scores. ANOVA = analysis of variance, PCL-5 = PTSD checklist for DSM-5, PTSD = posttraumatic stress disorder, TBI = traumatic brain injury.
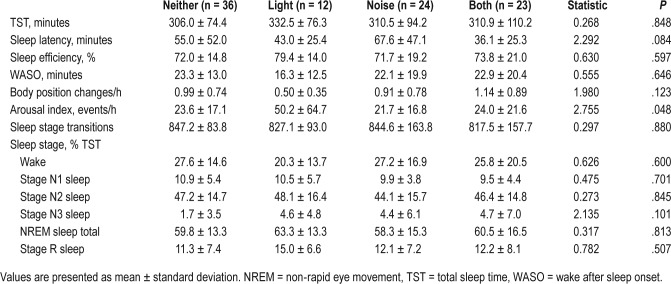
Polysomnography
There were no differences across sensory sensitivity groups in parameters related to sleep-wake staging (Table 3). The only exception here was for the arousal index, which showed a significant omnibus effect, but no significant post hoc group comparisons.
Table 3.
Polysomnography metrics in Veterans with and without sensory sensitivity.
Heart Rate
Heart rate data were extracted from each subject's overnight PSG. Mean heart rate during NREM sleep (P = .05), REM sleep (P = .004), and wake (P = .01) was significantly higher among Veterans with TBI, PTSD, and sensory sensitivity, compared to Veterans with TBI and PTSD without sensory sensitivity (Figure 3). The medical chart was examined for concurrent medication usage as a possible contributor to group differences in heart rate. We determined that the increased heart rate in Veterans with TBI, PTSD, and sensory sensitivity was not due to medications, including beta-blockers, amphetamines, and thyroid-stimulating medications (ie, medications known to have a significant effect on heart rate). Accordingly, these data suggest the presence of sensory sensitivity in Veterans with TBI is a significant contributor to an elevation in heart rate, beyond the effect of PTSD and medications.
Figure 3. Sensory sensitivity is associated with elevated heart rate during NREM and REM sleep.
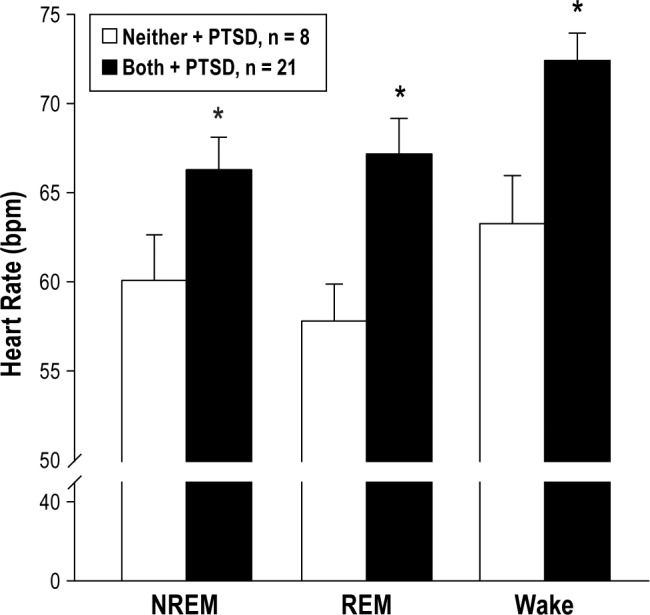
Mean heart rate recorded during subjects' overnight polysomnography, separated for NREM sleep (P = .05), REM sleep (P = .004), and Wake (P = .01), is shown comparing Veterans without sensory sensitivity and with PTSD (open bars, n = 8) compared to Veterans with both light and noise sensitivity, and PTSD (filled bars, n = 21). These data suggest sensory sensitivity is associated with elevated heart rate irrespective of PTSD status. Students two-tailed t test, * = P < .05. NREM = non-rapid eye movement, PTSD = posttraumatic stress disorder, REM = rapid eye movement.
DISCUSSION
The purpose of this study was to (1) assess the prevalence of light and noise sensitivity in Veterans with TBI, and (2) correlate sensory sensitivity with sleep disturbances using both subjective and objective measures of sleep. We found that the presence of moderate to severe light and/or noise sensitivity was present in 62% of our sample of Veterans with chronic TBI. Sensory sensitivity, particularly in Veterans with both light and noise sensitivity, was significantly associated with worse self-reported sleep (via the ISI, FOSQ-10, and ESS). Interestingly, these subjective data were in contrast to objective PSG, which showed no differences in any parameters across sensory sensitivity groups. However, there was a significantly higher mean heart rate during NREM and REM sleep in subjects with both light and noise sensitivity that remained significant after controlling for medications that affect heart rate and diagnosis of PTSD. Total ISI score was the strongest predictor of sensory sensitivity in Veterans with TBI.
Sensory Sensitivity and Traumatic Brain Injury
Although most people recover from the effects of TBI within days to weeks after injury,37,38 it is not uncommon for postconcussive symptoms, including light and noise sensitivity to persist > 6 months after injury and become a chronic ailment.20,39 However, chronic light and noise sensitivity are largely based on anecdotal reports as previous work investigating persistent light and noise sensitivity post-TBI has generally limited the time after injury to 1 year or less, and the outcome of these studies is inconclusive.40 For example, in a civilian sample of 732 subjects with TBI and 120 control subjects (traumaexposed but without a history of TBI), both light and noise sensitivity were reported in subjects with TBI 1 month after injury, but only light sensitivity persisted 1 year after injury.22 Similarly, work by Dischinger et al. showed light and noise sensitivity to be highly correlated in the acute phase of TBI, but noise sensitivity was the strongest predictor of subsequent PCS 3 months after injury.41
More recently, Callahan et al. showed significant light and noise sensitivity in a younger sample of blast-exposed OEF/ OIF Veterans (mean time post-TBI of approximately 3.5 years).21 Mean light and noise sensitivity scores (measured on a 0–4 Likert scale) in subjects with TBI were 2.00 ± 1.15 and 2.5 ± 1.19, respectively.21 These values compare very well to the current study where the mean light and noise sensitivity scores across all subjects (n = 95) was 1.85 ± 1.46 and 2.24 ± 1.23, respectively. This is particularly notable considering subjects in the current study were assessed at a significantly longer time-point following TBI (approximately 15–20 years after injury). Although on a 0–4 Likert scale, a score of 2 suggests only a mild problem, 62% of the 95 subjects in the current study (59 subjects) reported a 3 or higher for sensitivity to light and/or noise, indicating moderate to severe sensitivity.
Data from the current study also compare favorably to work by Goodrich et al. which demonstrated that 51% (44/86) of Veterans with TBI reported light sensitivity.42 Interestingly, Goodrich et al. also showed a significantly increased prevalence of light sensitivity in Veterans with a blast-related TBI (31/46 subjects; 67%) compared to Veterans without a blast-related TBI (13/40 subjects; 33%). Although there was no statistical difference in the proportion of subjects across groups in the current study that reported prior blast exposure, there was a twofold increase in the prevalence of blast exposure in those with both light and noise sensitivity compared to no sensory sensitivity. Blast-related injury has been identified as a potential confounding variable in the literature43 and despite the lack of statistical significance in the current study, it remains of interest. Additionally, the proportion of subjects with a blunt force-related injury was also not significantly different in the current study.
Sensory Sensitivity and PTSD
Although sensory sensitivity associated with PTSD is a common anecdotal occurrence, there is a surprising lack of research in the literature specifically addressing this relationship. However, the unadjusted odds ratio in a sample of 13,746 Veterans (9,998 with TBI), were 2.30 and 2.42 for TBI and PTSD, respectively, in predicting multisensory (auditory, visual, and vestibular) impairment.44 Furthermore, recent work demonstrated that PTSD is independently associated with an increased prevalence of postconcussive symptoms and that comorbid TBI and PTSD are associated with a significantly higher prevalence of postconcussive symptoms.45 Taken together, light and noise sensitivity should not be surprising findings associated with PTSD, and there is considerable support for subjects with comorbid TBI and PTSD to demonstrate significantly worse sensory impairment. In support of this is recent work by Goodrich et al. that shows the prevalence of light sensitivity is significantly higher in Veterans with comorbid TBI and PTSD (31/38 subjects; 82%), compared to those with only TBI (13/48 subjects; 27%).46 Additionally, previous work in a sample of 1,114 of civilians with TBI demonstrated that subjective light and noise sensitivity were significant independent predictors of PTSD symptomology.47 The authors speculated that the presence of light and noise sensitivity perpetuated subject's awareness of their traumatic event/injury,48 while impeding fear extinction through second-order conditioning.49 Alternatively, light and noise sensitivity might have also impeded fear extinction, and led to the exacerbation of PTSD by impairing sleep.50,51
Sensory Sensitivity and Sleep: Autonomic Nervous System Hyperarousal
Although PTSD symptom severity was the strongest predictor of sensory sensitivity in Veterans with TBI, the current study also shows that subjects with TBI-related sensory sensitivity have a higher mean heart rate during NREM and REM sleep, independent of PTSD status. These data suggest the possibility that subjects with sensory sensitivity have a higher level of basal sympathetic nervous system outflow, compared to their nonsensory sensitive counterparts. This apparent “autonomic nervous system (ANS) hyperarousal” is a well-known disruptor of sleep and thus, aligns with the finding that subjects with worse sensory sensitivity also demonstrate worse sleep disturbances.51 Other factors known to be associated with increased sympathetic nervous system activity, such as obstructive sleep apnea (OSA), body mass index, and relative fitness level were evenly distributed across groups in the current study and therefore are unlikely to explain these differences in mean heart rate during NREM and REM sleep. Although our current data only just scratch the surface of potential ANS hyperarousal mechanisms, future work could explore these phenomena using other established markers of sympathetic outflow (eg, muscle sympathetic nerve activity, heart rate variability).
Sensory Sensitivity and Sleep: Neurodegeneration
TBI is a known environmental risk factor in the development of neurodegenerative diseases including Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and frontotemporal dementia.16,53,54 Sleep disturbances may play a direct role in the pathogenesis of neurodegeneration, in light of recent data implicating sleep in facilitating the clearance of specific physiologic waste products (eg, amyloid-β) via the glymphatic system.55–57 Impaired sensory processing (ie, vision, audition, or olfaction), which may encompass hypersensitivity to these sensory inputs, may be another early marker of neurodegeneration.23–25 Thus, the relationship between sleep and sensory dysfunction following TBI may be an important early predictor of neurodegeneration.
Limitations
There are several limitations to the current study that warrant further discussion. First, the study utilizes exclusively self-reported information for light and noise sensitivity, without an objective assessment of sensory sensitivity. With the exception of two initial studies19,20 objectively documenting light and noise sensitivity following TBI, due to methodological limitations and ultimately relevance to the patient, most work in this area has relied on self-report data. Future work could seek to compare objective and self-report data in parallel. Second, our finding of elevated mean heart rate during sleep, while intriguing, is merely suggestive of ANS hyperarousal. Caveats to our heart rate finding include the small sample size, the necessity for a more detailed medical history regarding hypertension and heart disease, and the high prevalence and severity of OSA across groups. Further confirmatory measures of sympathetic outflow (ie, muscle sympathetic nerve activity, heart rate variability, etc.) in a larger sample of Veterans are warranted.
CONCLUSIONS
The current study is the first to report the high prevalence of sensory sensitivity in a cohort of Veterans in the chronic phase of recovery post-TBI (approximately 15–20 years after injury). We found that those with TBI who reported greater sensory sensitivity also showed worse self-reported sleep disturbances (via the ISI, ESS, and FOSQ-10), and a higher prevalence and severity of PTSD (via the PCL-5). Additionally, we found preliminary evidence for autonomic hyperarousal via an increase in mean heart rate during sleep even after controlling for PTSD status. Interestingly, the strongest predictor for sensory sensitivity in patients with TBI was subjects' total ISI score. Understanding the relationship between sensory sensitivity and sleep in this vulnerable population may help to drive these patients' clinical treatment plans given that both light and noise sensitivity were strongly associated with high rates of insomnia and daytime sleepiness. These findings may also help identify better early predictors of neurodegeneration as well as more multidisciplinary approaches to potential therapies.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at VA Portland Health Care System. This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, VA Career Development Award #IK2 BX002712, NIH EXITO Institutional Core, # UL1GM118964, and the Portland VA Research Foundation to M.M.L.; NIH T32 AT 002688 to J.E.E.; VA OAA Post-doctoral Nursing Research Fellowship to K.B.W.; VA OAA Advanced Research Fellowship in Polytrauma/TBI Rehabilitation and VA Career Development Award IK1RX001820 to M.A.P.. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors express their sincere appreciation and gratitude for the participation of all subjects, to the staff at the VA Portland Health Care System Sleep Disorders Clinic, and to Yvonne Barsalou and Randall Olson for assistance with data collection and database entry.
ABBREVIATIONS
- ANS
autonomic nervous system
- ESS
Epworth Sleepiness Scale
- FOSQ-10
Functional Outcomes of Sleep Questionnaire-10
- ISI
Insomnia Severity Index
- LOC
loss of conscious
- NREM
non-rapid eye movement
- OEF/OIF
Operation Enduring Freedom/Operation Iraqi Freedom
- OSA
obstructive sleep apnea
- TBI
traumatic brain injury
- PCL-5
PTSD checklist for DSM-5
- PCS
postconcussive syndrome
- PSG
polysomnography
- PTSD
posttraumatic stress disorder
- REM
rapid eye movement
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Katz DI, Cohen SI, Alexander MP. Mild traumatic brain injury. Handb Clin Neurol. 2015;127:131–156. doi: 10.1016/B978-0-444-52892-6.00009-X. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease and Control. Traumatic Brain Injury & Concussion. [Accessed June 27, 2018]. https://www.cdc.gov/traumaticbraininjury/index.html. Updated July 6, 2017.
- 3.Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352(20):2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- 4.Orff HJ, Ayalon L, Drummond SP. Traumatic brain injury and sleep disturbance: a review of current research. J Head Trauma Rehabil. 2009;24(3):155–165. doi: 10.1097/HTR.0b013e3181a0b281. [DOI] [PubMed] [Google Scholar]
- 5.Duclos C, Dumont M, Wiseman-Hakes C, et al. Sleep and wake disturbances following traumatic brain injury. Pathol Biol. 2014;62(5):252–261. doi: 10.1016/j.patbio.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3(4):349–356. [PMC free article] [PubMed] [Google Scholar]
- 7.Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep. 2017;40(5) doi: 10.1093/sleep/zsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim MM, Elkind J, Xiong G, et al. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Transl Med. 2013;5(215):215ra173. doi: 10.1126/scitranslmed.3007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66(4):555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130(Pt 7):1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 11.Baumann CR, Stocker R, Imhof HG, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65(1):147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 12.Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012;29(10):1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skopin MD, Kabadi SV, Viechweg SS, Mong J, Faden AI. Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J Neurotrauma. 2015;32(5):289–296. doi: 10.1089/neu.2014.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomasy HE, Febinger HY, Ringgold KM, Gemma C, Opp MR. Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiol Sleep Circadian Rhythm. 2017;2:71–84. doi: 10.1016/j.nbscr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to arousal-promoting brainstem neurons with traumatic brain injury. Sleep. 2016;39(6):1249–1252. doi: 10.5665/sleep.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimer JA, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 17.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson CO, Lidvall H, Malhammer G. An exploratory psychological study of the post-traumatic cerebral syndrome. Acta Neurol Scand. 1967;43(2):158–166. doi: 10.1111/j.1600-0404.1967.tb05725.x. [DOI] [PubMed] [Google Scholar]
- 19.Waddell PA, Gronwall DM. Sensitivity to light and sound following minor head injury. Acta Neurol Scand. 1984;69(5):270–276. doi: 10.1111/j.1600-0404.1984.tb07812.x. [DOI] [PubMed] [Google Scholar]
- 20.Bohnen N, Twijnstra A, Wijnen G, Jolles J. Tolerance for light and sound of patients with persistent post-concussional symptoms 6 months after mild head injury. J Neurol. 1991;238(8):443–446. doi: 10.1007/BF00314651. [DOI] [PubMed] [Google Scholar]
- 21.Callahan ML, Binder LM, O'Neil ME, et al. Sensory sensitivity in Operation Enduring Freedom/Operation Iraqi Freedom veterans with and without blast exposure and mild traumatic brain injury. Appl Neuropsychol Adult. 2018;25(2):126–136. doi: 10.1080/23279095.2016.1261867. [DOI] [PubMed] [Google Scholar]
- 22.Dikman S, Machhamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc. 2010;16(3):401–411. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- 23.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94(5):823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuben DB, Mui S, Damesyn M, Moore AA, Greendale GA. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47(8):930–935. doi: 10.1111/j.1532-5415.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 25.Gallun FG, Papesh MA, Lewis MS. Hearing complaints among veterans following traumatic brain injury. Brain Inj. 2017;31(9):1183–1187. doi: 10.1080/02699052.2016.1274781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponsford JL, Draper K, Schönberger M. Functional outcome 10 years after traumatic brain injury: Its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc. 2008;14(2):233–242. doi: 10.1017/S1355617708080272. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM. Insomnia. Psychological Assessment and Management. Guilford Press; 1993. [Google Scholar]
- 28.Smith MT, Wegener ST. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI) Arthritis Rheum. 2003;49(5S):S184–S196. [Google Scholar]
- 29.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 30.Omachi TA. Measures of sleep in rheumatologic diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI) Arthritis Care Res. 2011;63(Suppl 11):S287–S296. doi: 10.1002/acr.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 34.Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL-5) 2013. Scale available from the National Center for PTSD at www.ptsd.va.gov.
- 35.Weathers F, Litz BT, Hermann DM, Huska J, Keane TM. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX. [Google Scholar]
- 36.McDonald JH. Handbook of Biological Statistics. Sparky House Publishing; 2014. [Google Scholar]
- 37.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 38.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history and clinical management. Neurology. 1995;45(7):1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 39.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14(1):1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- 40.Greenwald BD, Kapoor N, Singh AD. Visual impairments in the first year after traumatic brain injury. Brain Inj. 2012;26(11):1338–1359. doi: 10.3109/02699052.2012.706356. [DOI] [PubMed] [Google Scholar]
- 41.Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma. 2009;66(2):289–296. doi: 10.1097/TA.0b013e3181961da2. [DOI] [PubMed] [Google Scholar]
- 42.Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL. Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci. 2013;90(2):105–112. doi: 10.1097/OPX.0b013e31827f15a1. [DOI] [PubMed] [Google Scholar]
- 43.Storzbach D, O'Neil ME, Roost SM, et al. Comparing the neuropsychological test performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress symptoms. J Int Neuropsychol Soc. 2015;21(5):353–363. doi: 10.1017/S1355617715000326. [DOI] [PubMed] [Google Scholar]
- 44.Pogoda TK, Hendricks AM, Iverson KM, et al. Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev. 2012;49(7):971–984. doi: 10.1682/jrrd.2011.06.0099. [DOI] [PubMed] [Google Scholar]
- 45.Brenner LA, Ivins BJ, Schwab K, et al. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from Iraq. J Head Trauma Rehabil. 2010;25(5):307–312. doi: 10.1097/HTR.0b013e3181cada03. [DOI] [PubMed] [Google Scholar]
- 46.Goodrich GL, Martinsen GL, Flyg HM, Kirby J, Garvert DW, Tyler CW. Visual function, traumatic brain injury, and posttraumatic stress disorder. J Rehabil Res Dev. 2014;51(4):547–558. doi: 10.1682/JRRD.2013.02.0049. [DOI] [PubMed] [Google Scholar]
- 47.Al-Ozairi A, McCullagh S, Feinstein A. Predicting posttraumatic stress symptoms following mild, moderate, and severe traumatic brain injury: the role of posttraumatic amnesia. J Head Trauma Rehabil. 2015;30(4):283–289. doi: 10.1097/HTR.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 48.Howe LL. Giving context to post-deployment post-concussive-like symptoms: blast-related potential mild traumatic brain injury and comorbidities. Clin Neuropsychol. 2009;23(8):1315–1337. doi: 10.1080/13854040903266928. [DOI] [PubMed] [Google Scholar]
- 49.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164(11):1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 50.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationship between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20(5):893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 51.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 52.Mollayeva T, D'Souza A, Mollayeva S, Colantonio A. Post-traumatic sleep-wake disorders. Curr Neurol Neurosci Rep. 2017;17(4):38. doi: 10.1007/s11910-017-0744-z. [DOI] [PubMed] [Google Scholar]
- 53.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166(7):810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987–995. doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]