Abstract
Study Objectives:
Although regular physical activity improves obstructive sleep apnea (OSA) in the general population, this finding has not been assessed in postmyocardial infarction (MI) patients in a rehabilitation setting (coronary artery disease, CAD). We aimed to determine whether cardiac rehabilitation may benefit post-MI patients in terms of OSA disease and associated autonomic nervous system (ANS) activity.
Methods:
Consecutive post-MI patients participating in the ambulatory cardiac rehabilitation program of St-Etienne University Hospital were included in this study. The apnea-hypopnea index calculated from electrocardiogram (ECG)-derived respiration (AHIEDR) was obtained through nocturnal Holter ECG recordings. According to AHIEDR, patients were classified as normal, mild, moderate, or severe OSA (< 5, 5–14, 15–29, ≥ 30, respectively). Physiological performance (peak VO2) was established via cardiopulmonary exercise testing. ANS activity was evaluated through spontaneous baroreflex sensibility as well as heart rate variability analysis.
Results:
Of the 105 patients with CAD and OSA included (95 men, 55.2 ± 12.4 years), 100 had at least 1 cardiovascular risk factor (98%) and 52 patients (50%) had an ANS dysfunction. Surprisingly, 68 of these patients with OSA (65%) were free of classical diurnal symptoms usually associated with sleep apnea. In response to cardiac rehabilitation, AHIEDR decreased significantly (−9.3 ± 9.5, P < .0001) only in patients with severe OSA, and the decrease was even greater when peak VO2 and baroreflex sensibility improved beyond 20% compared to basal values (−11.6 ± 9.1, P < .001).
Conclusions:
Severe OSA in patients with CAD is significantly improved after 2 months of cardiopulmonary rehabilitation. Reviving ANS activity through physical activity might be a target for complementary therapy of OSA in patients with CAD.
Citation:
Hupin D, Pichot V, Berger M, Sforza E, Raffin J, Lietar C, Poyraz E, Maudoux D, Barthelemy JC, Roche F. Obstructive sleep apnea in cardiac rehabilitation patients. J Clin Sleep Med. 2018;14(7):1119–1126.
Keywords: autonomic nervous system, cardiac rehabilitation, coronary artery disease, obstructive sleep apnea, physical activity
BRIEF SUMMARY
Current Knowledge/Study Rationale: The value of treatment with continuous positive airway pressure in patients with symptomatic coronary artery disease (daytime sleepiness and/or 2 clinical symptoms with apnea-hypopnea index ≥ 20 events/h) appears to be established; however, treatment with continuous positive airway pressure in patients with asymptomatic coronary artery disease (with apnea-hypopnea index > 30 events/h) may be too demanding. Therefore, it would be interesting to suggest other treatment modalities, and physical activity appears to be such an alternative.
Study Impact: Severe obstructive sleep apnea is improved by cardiac rehabilitation among patients with coronary artery disease. The autonomic nervous system regulation by physical activity might be a key in alternative therapy for obstructive sleep apnea.
INTRODUCTION
Almost one in two patients with coronary artery disease (CAD) may also have obstructive sleep apnea (OSA),1 and more than 1 million new myocardial infarctions occur each year in Europe and the United States combined, with a yearly incidence rate of approximately 200 cases per 100,000 people.2
OSA is not an isolated disease. On the contrary, in as many of 87% of cases it is associated with metabolic syndrome,3 hypertension, obesity, and diabetes. Interestingly, after adjustment for the five criteria of metabolic syndrome, OSA appears as the main risk factor (risk ratio = 9.1 [95% confidence interval: 2.6–31.2]) of cardiac morbidity and mortality.3
Surprisingly, although noncoronary patients most often display clear OSA symptoms, coronary patients do not present such strong clinical evidence. Therefore, it should be challenged systematically in that population despite the lack of clear symptoms such as rhonchopathy or diurnal drowsiness. This failure to express symptoms is not clearly understood. The lack of symptoms does not prevent OSA from being a strong risk factor, but it may limit the ability to convince patients to obtain long-term continuous positive airway pressure (CPAP) treatment.1,3,4–6 For those who agree, adherence is poor.7 To protect the patient, while presenting some relief for moderate obstructive OSA, mandibular advancement devices can be proposed, however, without full health benefits. Alternative therapeutic approaches to CPAP still need to be elaborated.
Physical activity (PA) appears to be such an alternative.8 It was demonstrated to be efficient in a small sample size in the general population with a decrease in apnea-hypopnea index (AHI) of almost 24% (from 32.2 to 24.6 events/h).9 However, such an approach was never evaluated in postmyocardial infarction (MI) patients.
Associated benefits may also reflect the positive effect of PA on autonomic nervous system (ANS) activity, an established strong predictor of cardiac morbidity and mortality,10,11 particularly through its parasympathetic side as assessed by heart rate variability (HRV) and baroreflex sensitivity (BRS). PA may also help equilibrate ANS activity by an antioxidant effect in decreasing reactive oxygen species (ROS).12 In this view, ROS have been demonstrated to increase sympathetic outflow from sympathetic ganglia13 as well as through an increase in central action of angiotensin at the level of the rostral ventrolateral medulla.14 Globally, PA acts in fueling a virtuous circle including clinical and biological substrates, whereas OSA induces a vicious circle by decreasing parasympathetic activity and increasing sympathetic nerve activity and associated ROS.15
METHODS
Population
The population consisted of post-MI patients referred between January 2014 and December 2015 and between 7 to 21 days following the MI event to the cardiac rehabilitation clinic run by the Clinical Physiology Exercise Department of the University Hospital.16 The local ethics committee (IRBN362016/CHUSTE) and the National Commission for Information Technology and Civil Liberties (CNIL1827279) approved the study. All patients gave their informed consent to participate in the study.
Cardiac Rehabilitation
Performance was measured through an initial and final incremental symptom-limited cardiopulmonary exercise test (CPET) performed on a cycloergometer to describe the profile of physiological variables, including maximal oxygen consumption (peak VO2), and to assess the metabolic threshold. The criteria for maximal exercise intensity were a respiratory exchange ratio of more than 1.1 and a final blood lactic acid level of more than 8 mmol/L. Between these two CPETs, the patients benefited from a 2-month multimodal supervised training session, 3 times per week, including physical training, PA teaching, and nutrition counselling.16 Physical training itself combined endurance and dynamic exercises. Endurance training was obtained through a 45-minute exercise session on a cycloergometer, preceded by a 5-minute warm-up and followed by a 5-minute recovery. If maximal CPET power output was less than 60 W, the warm-up was administered carefully without any resistance; otherwise, it was set at 30% maximal power output. Through the sessions, training intensity was progressively increased, adding 10 W every five sessions, according to clinical tolerance in order to reach the metabolic threshold. If the metabolic threshold was not reached during the initial CPET, maximal training intensity was set to 50% of the CPET maximal power output. Dynamic resistance training was performed using wrist weights, elastic bands, and a weight bench, with 10 to 15 repetitions of 10 types of movements at low intensity, and at around 40% of maximal strength. Equilibrium, flexibility, and relaxation were included in the program.
OSA Measurement Using Electrocardiogram-Derived Respiration
OSA was measured through electrocardiogram-derived respiration (EDR) computed from the analysis of an electrocardiogram (ECG) Holter recording done during the night. This was measured before entering and after leaving the rehabilitation program. EDR allows assessment of apnea and hypopnea, identifying variations in ECG morphology induced by OSA, mainly the height and width of the QRS wave.17 This did not allow classification between central or obstructive apnea, but rather it gives a combined number of central and obstructive apnea and hypopnea. The methodology was previously validated in a cohort of patients with cardiac heart failure (CHF) studied with polysomnography.17
BRS as a Measure of Cardiac Parasympathetic Activity
We measured spontaneous relative variations in blood pressure and R-R interval length to assess the response of the parasympathetic arm of the ANS by measuring simultaneously ECG and a plethysmographic value of blood pressure.10,15,18 The data were recorded over a 15-minute period on a subject lying at rest. The slope of each continuous ascending blood pressure lasting at least three beats against the change in R-R interval length gave the slope change in R-R per mmHg. The slopes were averaged to give a representative slope considered as the spontaneous baroreflex measurement.19 The number of sequences, in itself a marker of BRS activity, was also calculated. The cutoff for BRS slope (ms/mmHg) was set to reference values from the literature: ≥ 7 ms/mmHg for healthy adults and ≥ 3 ms/mmHg for patients with CHF.20
HRV Analysis
HRV indices were measured from 24-hour ECG Holter recordings (Vista, Novacor, Rueil-Malmaison, France) using HRV analysis free software (https://anslabtools.univ-st-etienne.fr/en/index.html).21 Each R-R interval was visually validated, and isolated artefacts and extrasystoles were interpolated using a spline cubic method. Indices extracted were mean R-R, mean heart rate, time domain, and frequency domain indices. Time domain indices were calculated as SDNN (ms, standard deviation of normal R-R intervals), SDANN (ms, standard deviation of the mean of all normal R-R intervals for 5-minute segments), SDNNIDX (ms, mean of the standard deviation of all normal R-R intervals for all 5-minute segments), RMSSD (ms, square root of the mean squared differences of successive R-R intervals), and pNN50 (percentage of differences between adjacent normal R-R intervals more than 50 ms).22 For Fourier analysis, the R-R signal was previously resampled at 4 Hz Fourier indices were calculated as ultra-low frequency (ULF; ms2, < 0.0033 Hz), very-low frequency (VLF; ms2, between 0.0033 and 0.04 Hz), low frequency (LF; ms2, from 0.04 to 0.15 Hz), high frequency (HF; ms2, from 0.15 to 0.40 Hz), and total power (TP; up to 0.40 Hz).
Statistical Analysis
Descriptive statistics of patients' characteristics are shown as mean ± standard deviation (mean ± SD). Chi-square was used for qualitative variables and Student the t test, or Wilcoxon test, for quantitative variables as appropriate. Statistics were performed on R (R development Core Team, 2016). Comparison of intragroup data before and after the rehabilitation period were analyzed using t test, OSA intergroup analysis was performed using Mann-Whitney U test. The significance value was set at P < .05.
RESULTS
Patient Characteristics
A cohort of consecutive 105 post-MI patients, aged 55.2 ± 12.4 (range 29–81) years and 96 males (91%), were included in the study (Table 1). They suffered from 1-vessel (n = 68; 65%), 2-vessel (n = 24; 23%), or 3-vessel (n = 13; 12%) disease. A total of 93 patients (88%) had benefited from a coronary revascularization through percutaneous transluminal coronary angioplasty and stenting. The remaining 12 patients (12%) had coronary artery bypass graft. One hundred patients (98%) had at least one cardiovascular risk factor, and 59 (56%) were over-weight (body mass index > 25 kg/m2). OSA was abnormal in all the patients (AHIEDR > 5): 16 were mild (15%), 52 were moderate (50%) and 37 were severe (35%). As many as 68 of the 105 patients (65%) with OSA were asymptomatic, having no complaints about snoring, diurnal drowsiness, nocturia, morning headache, or low libido. Weights of the three groups were not statistically different between the beginning and the end of the cardiac rehabilitation: −0.1 kg in the mild, −0.5 kg in the moderate and −0.1 kg in the severe OSA group. Peak VO2 was improved by 15.4% in patients with mild OSA (+3.3 ± 6.4 mL/min/kg, P = .01), 16.8% in patients with moderate OSA (+3.4 ± 6.4 mL/min/kg, P < .0001) and 24.9% in patients with severe OSA (+5 ± 7 mL/min/kg, P < .000001) at the end of the training cardiac rehabilitation program. There was no significant difference between groups (P > .05).
Table 1.
Description of the population.
Effects of PA on OSA
Few subjects (23%) were engaged in a regular sport and there was an inverse relationship between sport practice and OSA severity. Patients with mild and moderate OSA were more likely to practice a sport than patients with severe OSA. Sports were a factor in 25%, 17.3%, and 2.7% of the patients with mild, moderate, and severe OSA, respectively, at the inclusion in the study (Table 1).
The training rehabilitation program resulted in a significant reduction in AHIEDR (−9.26 ± 9.5, P < .00001) only for patients with severe OSA presenting with an initial AHIEDR ≥ 30 (Table 2 and Figure 1). In these 37 patients, the decrease correlated with adherence to the rehabilitation program, with a mean AHIEDR decrease reaching −11.55 ± 9.1 (P < .001) when they practiced 20 sessions. There was no significant decrease in AHIEDR for patients with mild OSA (+0.3 ± 5.4, P = .5) and patients with moderate OSA (−0.9 ± 6.9, P = .3).
Table 2.
Variables before and after cardiac rehabilitation sessions (mean and SD).
Figure 1. Evolution of AHIEDR before and after cardiac rehabilitation, according to the initial severity of OSA (mild, moderate, or severe).
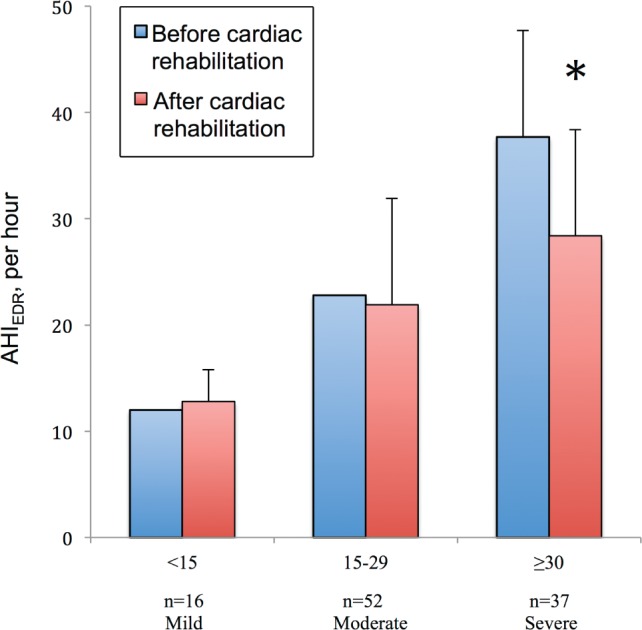
* = P < .05. AHIEDR = apnea-hypopnea index from ECG-derived respiration, OSA = obstructive sleep apnea.
Effects of PA on ANS
BRS was less than 7 ms/mmHg and 3 ms/mmHg in 52 and 13 patients (50% and 12%), respectively, at the inclusion of the study and 37 and 9 patients (35% and 9%), respectively, at the end of the study. Thirteen of 17 patients with CHD (76%) had an ANS major dysfunction (BRS < 3 ms/mmHg) before cardiac rehabilitation; there were 9 patients (53%) at the end of the rehabilitation.
The training rehabilitation program showed a significant increase in BRS slope (+3.3 ± 7.4, P = .004) only for patients with severe OSA presenting with an initial AHIEDR ≥ 30 (Table 2 and Figure 2). This increase was still correlated with adherence to the rehabilitation program, with a mean BRS slope increase reaching +3.8 ± 4.9 (P < .001) when they practiced 20 sessions. There was no significant increase of BRS for patients with mild OSA (+0.3 ± 4.6, P = .5) and patients with moderate OSA (+1 ± 7.5, P = .09). ANS indices (SDNN, RMSSD, and LF/HF) did not improve at the end of the rehabilitation, even for the severe OSA group (P > .05).
Figure 2. Evolution of baroreflex sensitivity, a representative of parasympathetic activity, before and after cardiac rehabilitation, according to the initial severity of OSA (mild, moderate, or severe).

* = P < .05. BRS = baroreflex sensitivity, OSA = obstructive sleep apnea.
DISCUSSION
In post-MI patients, 8 weeks of monitored physical training appears to be a powerful tool to decrease the sleep-related breathing disorder severity in patients with severe OSA. Improved ANS equilibrium may be the cornerstone of the obtained AHI reduction. By improving AHI, physical training may thus be appropriate to reduce the effect of sleep-related breathing disorder and its subsequent complications in patients with CAD.
Relationship Between OSA and ANS
From the Framingham23,24 and Zutphen25 cohort studies, ANS activity has been established as an important predictor of cerebrovascular and cardiovascular risk factors. Low SBR was also a predictor of post-MI mortality in a follow-up study.10 OSA is strongly related to ANS parasympathetic activity impairment as demonstrated in the PROOF cohort,26,27 with a strong association with diurnal spontaneous SBR (odds ratio: 0.94, 95% confidence interval: 0.90–0.98, P < .01).28 This means that the nocturnal excess of sympathetic activity associated with the apnea-hypopnea event measured through arterial stiffness29 or HRV disturbances during the event30 is reflected in ANS daily values.28,31 Also associated with OSA, spectral analysis of R-R variability showed an increase in both LF normalized units and in the ratio of low to high frequency.32 In this way, nocturnal ventilatory alterations may contribute to the increased incidence of cardiovascular complications in sleep disorders.
Thus, ANS rebalancing may be a mighty actor in cerebrovascular and cardiovascular improvement in OSA. As a matter of fact, parasympathetic activity is a potent regulator of established cardiovascular risk factors, such as hypertension,31,33 dyslipidemia,34 excess weight,35 insulin,36 and systemic low-grade inflammation.37 The role of exercise38 as well as of chronic subclinical inflammation39 on insulin needs should be reported. The parasympathetic increase in response to physical training is well established in sports,40,41 in the general population,42 and in animal models43 where it was shown to be a potent protector against sudden cardiac death.
In post-MI myocardial disease, an elevated BRS value, a strong indicator of parasympathetic activity and a potent predictor of survival, even compensated for the risk associated with a severely decreased left ventricular ejection fraction.12,14 Interestingly, the cardiac rehabilitation program very significantly improved BRS in our post-MI patients and ANS activity increases through training is known to definitively improve survival after MI, particularly concerning sudden cardiac death.
Exercise and OSA
Exercise has a direct effect on OSA. In patients aged 35 to 70 years who are free of known CAD, a 4-week rehabilitation program decreased AHI from 40.6 ± 19.4 to 28.0 ± 19.3 events/h (P < .001).44 As in our study, there was also an increase in peak VO2, although less pronounced, from 21.3 ± 5.6 to 22.9 ± 5.6 mL/min/kg (P < .05).
Prolonging the cardiac rehabilitation training period may bring further benefits because a 12-week training period demonstrated a beneficial AHI decrease even in those with mild to moderate OSA (from 15.2 ± 5.4 to 11.0 ± 5.3 events/h, P = .02).45 Although this last study did not concern coronary patients, it may address an interest in duration and eventually in the execution of rehabilitation programs.
Perspective: Clinical Implications of EDR as a Screening Tool for OSA in Cardiac Rehabilitation
The ease of use of EDR reinforces the yield of this study because it may be applicable as a screening tool for large CAD populations without the burden of polysomnography. EDR may be integrated into an ECG Holter system such that this screening could become part of the classic risk factor evaluation in cardiac rehabilitation.
Limitations
The gold standard for sleep analysis is polysomnography, or more commonly, polygraphy. EDR has not been systematically separately validated in patients with CAD,17 although its validation in a population of patients with CHF makes us confident in its predictive value in our setting.
Although the study was not randomized, all included patients were consecutive and underwent the same cardiac rehabilitation program. We could not exclude a spontaneous decrease in OSA severity following such MI events. Fluctuation of AHI for a long time has been frequently described in patients with CHF in response to variations in stroke volume or pulmonary capillary pressure.
EDR introduced several positive mild OSA diagnoses. It is known that some high AHIEDR values are not annotated as OSA and consequently result in false-positive detections as also reflected in the tendency for AHI overestimation. However, previous studies validated these methodologies46–48 and they showed that the computed EDRs closely resemble the real respiratory signals.49
It is of note that although the decrease in AHI is highly significant after training in the severe OSA group, most of the patients of this subgroup still experienced moderate to severe OSA according to the classic definition and the effect of a complementary CPAP treatment had to be questioned (on the ANS equilibrium as well as their peak VO2).
On the whole, an 8-week endurance training program leads to notable improvement in ANS activity, up to 30%. This alleviates the reciprocal dysautonomia associated with OSA and its clinical and biological consequences.15 Although it should be interesting to explore the continuation of AHIEDR benefits beyond the end of the rehabilitation program, this was not assessed in this study.
CONCLUSIONS
Severe OSA is significantly improved by cardiac rehabilitation in post-MI patients. PA may thus have a benefit through a direct effect on ANS regulation as well an indirect benefit by decreasing OSA severity. An extended program of PA may be needed in order to see benefits in patients with moderate to light OSA, which includes a prolongation of PA beyond usual hospital management procedures.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: DH had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. FR and DH had the idea for and designed the study. All authors had a substantial contribution to the conception and design. DH, CL, EP, and DM were responsible for collection and analysis of data. DH and VP provided statistical expertise. DH, VP, JCB, and FR were responsible for interpretation of data. DH and JCB drafted the manuscript and submitted the paper for publication. JCB, VP, MB, ES, JR, and FR critically revised the manuscript for important intellectual content. All authors approved the final version.
ABBREVIATIONS
- AHIEDR
apnea-hypopnea index calculated from ECG-derived respiration
- ANS
autonomic nervous system
- BRS
baroreflex sensitivity
- CAD
coronary artery disease
- CHF
cardiac heart failure
- CPAP
continuous positive airway pressure
- CPET
cardiopulmonary exercise test
- ECG
electrocardiogram
- EDR
ECG-derived respiration
- HRV
heart rate variability
- LF/HF
low frequency/high frequency (= sympathetic/ parasympathetic).
- MI
myocardial infarction
- OSA
obstructive sleep apnea
- PA
physical activity
- PTCA
percutaneous transluminal coronary angioplasty
- RMSSD
square root of the mean squared differences of successive R-R intervals
- ROS
reactive oxygen species
- SDNN
standard deviation of normal R-R intervals
- SRBD
sleep-related breathing disorder
REFERENCES
- 1.Mehra R, Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7(6):521–528. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Ben Ahmed H, Boussaid H, Hamdi I, Boujnah MR. Prevalence and predictors of obstructive sleep apnea in patients admitted for acute myocardial infarction. Ann Cardiol Angeiol. 2014;63(2):65–70. doi: 10.1016/j.ancard.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: A population-based study. Chest. 2002;121(2):430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 7.Roche F, Sforza E, Hupin D. CPAP for excessive sleepiness in elderly patients. Lancet Respir Med. 2014;2(10):778–779. doi: 10.1016/S2213-2600(14)70164-X. [DOI] [PubMed] [Google Scholar]
- 8.Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125(5):485–490. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline CE, Ewing GB, Burch JB, et al. Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. J Clin Sleep Med. 2012;8(4):357–365. doi: 10.5664/jcsm.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 11.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 12.Desplan M, Brun J-F, Pillard F, et al. Decreased fat oxidation during exercise in severe obstructive sleep apnoea syndrome. Diabetes Metab. 2012;38(3):236–242. doi: 10.1016/j.diabet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Zhang HJ, Whiteis CA, et al. Oxidative stress in sympathetic ganglia: a possible mechanism of increased sympathetic nerve activity and impaired baroreflex sensitivity in atherosclerosis [abstract] Hypertension. 2004;44(4):523. [Google Scholar]
- 14.Gao L, Wang W, Li Y-L, et al. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288(5):H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 15.Abboud FM. The Walter B. Cannon Memorial Award Lecture, 2009. Physiology in perspective: The wisdom of the body. In search of autonomic balance: the good, the bad, and the ugly. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1449–R1467. doi: 10.1152/ajpregu.00130.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavy B, Iliou M-C, Vergès-Patois B, et al. French Society of Cardiology guidelines for cardiac rehabilitation in adults. Arch Cardiovasc Dis. 2012;105(5):309–328. doi: 10.1016/j.acvd.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Pichot V, Chouchou F, Pepin J-L, et al. ECG-derived respiration: a promising tool for sleep-disordered breathing diagnosis in chronic heart failure patients. Int J Cardiol. 2015;186:7–9. doi: 10.1016/j.ijcard.2015.03.232. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, Dyken ME, Mark AL, et al. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnea in hypertension. Clin Auto Res. 1992;2(3):171–176. doi: 10.1007/BF01818958. [DOI] [PubMed] [Google Scholar]
- 19.Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension. 1995;25(5):1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- 20.Tank J, Baevski RM, Fender A, et al. Reference values of indices of spontaneous baroreceptor reflex sensitivity. AJH. 2000;13(3):268–275. doi: 10.1016/s0895-7061(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 21.Pichot V, Roche F, Celle S, Barthélémy JC, Chouchou F. HRV analysis: a free software for analyzing cardiac autonomic activity. Front Physiol. 2016;7:557. doi: 10.3389/fphys.2016.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 23.Tsuji H, Venditti FJ, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 25.Bijnen FC, Feskens EJ, Caspersen CJ, Nagelkerke N, Mosterd WL, Kromhout D. Baseline and previous physical in relation to mortality in elderly men: the Zutphen Elderly Study. Am J Epidemiol. 1999;150(12):1289–1296. doi: 10.1093/oxfordjournals.aje.a009960. [DOI] [PubMed] [Google Scholar]
- 26.Barthélémy J-C, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: the “PROOF” Study. Study design and population sample. Associations with sleep-related breathing disorders: the “SYNAPSE” Study. Neuroepidemiology. 2007;29(1-2):18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 27.Hupin D, Edouard P, Gremeaux V, et al. Physical activity to reduce mortality risk. Eur Heart J. 2017;38(20):1534–1537. doi: 10.1093/eurheartj/ehx236. [DOI] [PubMed] [Google Scholar]
- 28.Achour E, Roche F, Pichot V, Celle S, Barthélémy JC, Chouchou F. Sleep-related autonomic overactivity in a general elderly population and its relationship to cardiovascular regulation. Heart Vessels. 2016;31(1):46–51. doi: 10.1007/s00380-014-0573-9. [DOI] [PubMed] [Google Scholar]
- 29.Chouchou F, Pichot V, Celle S, Barthélémy JC, Gosse P, Roche F. Sleep disruptions increase arterial stiffness. Int J Cardiol. 2016;203:744–745. doi: 10.1016/j.ijcard.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Chouchou F, Pichot V, Barthélémy JC, Bastuji H, Roche F. Cardiac sympathetic modulation in response to apneas/hypopnoeas through heart rate variability analysis. PLoS One. 2014;9(1):e86434. doi: 10.1371/journal.pone.0086434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouchou F, Pichot V, Pepin JL, et al. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J. 2013;34(28):2122–2131. doi: 10.1093/eurheartj/eht208. [DOI] [PubMed] [Google Scholar]
- 32.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32(6):1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 33.Wustmann K, Kucera JP, Scheffers I, et al. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54(3):530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 34.Gil K, Bugajski A, Kurnik M, Thor P. Chronic vagus nerve stimulation reduces body fat, blood cholesterol and triglyceride levels in rats fed a high-fat diet. Folia Med Cracov. 2012;52(3-4):79–96. [PubMed] [Google Scholar]
- 35.Burneo JG, Faught R, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59(3):463–464. doi: 10.1212/wnl.59.3.463. [DOI] [PubMed] [Google Scholar]
- 36.Meyers EE, Kronemberger A, Lira V, Rahmouni K, Stauss HM. Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep. 2016;4(4):e12718. doi: 10.14814/phy2.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13(3-4):178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68(6):1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Festa A, D'Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 40.Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol. 2007;101(6):743–751. doi: 10.1007/s00421-007-0552-2. [DOI] [PubMed] [Google Scholar]
- 41.Pichot V, Bourin E, Roche F, et al. Quantification of cumulated physical fatigue at the workplace. Pflugers Arch. 2002;445(2):267–272. doi: 10.1007/s00424-002-0917-7. [DOI] [PubMed] [Google Scholar]
- 42.Pichot V, Roche F, Denis C, et al. Interval training in elderly men increases both heart rate variability and baroreflex activity. Clin Auton Res. 2005;15(2):107–115. doi: 10.1007/s10286-005-0251-1. [DOI] [PubMed] [Google Scholar]
- 43.Hull SS, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89(2):548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 44.Desplan M, Mercier J, Sabaté M, Ninot G, Prefaut C, Dauvilliers Y. A comprehensive rehabilitation program improves disease severity in patients with obstructive sleep apnea syndrome: a pilot randomized controlled study. Sleep Med. 2014;15(8):906–912. doi: 10.1016/j.sleep.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15(1):49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 46.Rachim VP, Li G, Chung W-Y. Sleep apnea classification using ECG-signal wavelet-PCA features. Biomed Mater Eng. 2014;24(6):2875–2882. doi: 10.3233/BME-141106. [DOI] [PubMed] [Google Scholar]
- 47.Varon C, Caicedo A, Testelmans D, Buyse B, Van Huffel S. A novel algorithm for the automatic detection of sleep apnea from single-lead ECG. IEEE Trans Biomed Eng. 2015;62(9):2269–2278. doi: 10.1109/TBME.2015.2422378. [DOI] [PubMed] [Google Scholar]
- 48.Maier C, Dickhaus H. Extraction of respiratory myogram interference from the ECG and its application to characterize sleep-related breathing disorders in atrial fibrillation. J Electrocardiol. 2014;47(6):826–830. doi: 10.1016/j.jelectrocard.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Roebuck A, Monasterio V, Gederi E, et al. A review of signals used in sleep analysis. Physiol Meas. 2014;35(1):R1–R57. doi: 10.1088/0967-3334/35/1/R1. [DOI] [PMC free article] [PubMed] [Google Scholar]




