Abstract
Purpose:
Long-term occlusion of coiled aneurysms frequently fails, likely due to poor intrasaccular healing and inadequate endothelialization along the aneurysm neck. The purpose of this study was to determine if seeding coils with autologous stem cells could improve the healing response of experimental aneurysms.
Material and Methods:
With institutional animal care and use committee approval, aneurysms were created in rabbits and embolized with control platinum coils (Axium, Medtronic) (n=6) or coils seeded ex vivo with autologous adipose-tissue mesenchymal stem cells (MSC) (n=7). Stability of aneurysmal occlusion after embolization was evaluated at one month with DSA. Histological samples were analyzed with gross imaging and graded based on neck and dome healing on hematoxylin-eosin staining. Fibrosis was evaluated using a ratio of the total area presenting collagen. Endothelialization of the neck was quantitatively analyzed using CD31 immunohistochemistry. Chi-squared and student’s t-test were used to compare groups.
Results:
Healing score (11.5 versus 8.0, p=.019), fibrosis ratio (10.3 versus 0.13, p= 0.006.), endothelialization (902,262 μm2 versus 14,106 μm2, p= 0.04113) were significantly greater in the MSC group compared with controls. The MSC group showed marked cellular proliferation and thrombus organization, with continuous membrane bridging the neck. Angiographic stable or progressive occlusion rate was significantly lower in the MSCs group (0.00, 95%CI: 0.00-0.41) compared to controls (0.67, 95%CI: 0.22-0.96) (p=.02).
Conclusions:
MSCs significantly improved histological healing of coiled aneurysms, but angiographic outcomes were not improved. This indicates the need for larger experiments to evaluate the safety and long-term outcomes of stem-cell therapy for intracranial aneurysms.
INTRODUCTION
Endovascular therapy of intracranial aneurysms is the gold standard and has supplanted the surgical approach in many types of aneurysms(1). However, numerous clinical studies indicate that up to 30% of aneurysms recur within one year after coiling(2, 3), leading not only to frequent re-treatment and its associated perioperative risks but also to risk of hemorrhage if untreated(4–6). Thus, complete and durable aneurysm occlusion following coiling remains an important unmet clinical need. The recanalization phenomenon attributable to coil compaction is particularly observed in wide-necked or large aneurysms(7–13). The frequent recurrence is most likely due to the inert nature of the platinum(14, 15), which fails to induce homing and proliferation of either circulating or resident cells that might reconstruct the deficient segment of the vessel wall. Lack of cells, rare endothelialization at the entrance zone of the aneurysm and deficient deposition of extracellular matrix proteins within aneurysm cavities represent the main biological reasons for recurrence of aneurysms after endovascular therapy(9, 13, 16–18). Thus, the aneurysm cavity remains unable to withstand the ongoing mechanical stress from continued arterial pulsation, leading to aneurysm recurrence and possible rupture.
Many different strategies have been proposed to improve aneurysm occlusion rates, including modification of coils with addition of polymers, growth factors, beta emitters, and cells(14, 19–28). To date, clinical trials have been disappointing regarding the efficacy of these second-generation, modified coil technologies(2, 29–40). Other studies have used cell therapy with mature and differentiated cells to promote aneurysm healing(41–52). However, terminally differentiated, implanted cells do not differentiate into other cell types normally present with in the blood vessel wall. Ideally, the cells lining the implanted coils would differentiate into arterial endothelial cells and those residing deep to the endothelial lining would become medial smooth muscle cells(13, 53, 54). From a biological perspective, mesenchymal stem cells (MSCs) represent an ideal cell type to populate saccular aneurysms(26, 41, 53, 55–58).
To study the effect of autologous MSCs on aneurysm healing, We conducted a case-control study comparing the healing effects of MSCs on coils compared to bare platinum coils in treatment of elastase-induced aneurysm model in rabbits. The purpose of this study was to determine if seeding platinum coils with autologous MSCs could improve the healing response of experimental aneurysms.
MATERIAL AND METHODS
The animal care and use committee at our institution approved the animal procedures.
Aneurysm creation
Elastase-induced saccular aneurysms were induced in 13 rabbits as previously described(59). Briefly, under general anesthesia, the right common carotid artery (RCCA) was exposed and ligated distally. A 5 F vascular sheath was advanced retrogradely into the RCCA, and a 3 F Fogarty balloon inflated with iodinated contrast medium was advanced through the sheath to the level of the origin of the RCCA under fluoroscopic guidance. Porcine elastase was incubated within the lumen of the RCCA above the inflated balloon for 20 min, after which the catheter, balloon and sheath were removed. The RCCA was then ligated below the arteriotomy. No morbidity or mortality was observed with aneurysm creation.
Autologous MSCs isolation and culture
Adipose tissue was harvested during the aneurysm creation procedure from the neck of the rabbit. Autologous MSCs were isolated from adipose tissue extracted from the fat of the rabbit neck. Briefly, adipose tissue was digested with collagenase, then passed through a 70μm cell strainer (BD Biosciences, San Diego, California, USA) and incubated in red blood cell lysis buffer (Sigma-Aldrich). Harvested cells were cultured in a medium consisting of Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum and 2 mM l-glutamine. Adherent MSCs were cultured until 5×106 cells had been obtained from each rabbit with about four cell passages for each rabbit. MSC membranes were labeled with fluorescent Dil (Sigma/Aldrich Chemical Co).
Coil preparation
Axium detachable coils (Medtronic, Irvine, California, USA) were placed in 15mL Falcon tubes. An MSC suspension (1×106 cells/mL) was poured into the Falcon tubes containing the coils. The tubes were agitated at 300 rpm for 1 hour in an orbital shaker, and then the coils were cultured in the same culture medium as previously described and stored in the cell incubator for 24 hours at 37°C. Cell-seeded coils were then thoroughly washed with phosphate-buffered saline to exclude non-adherent cells, and resheathed in the delivery sheath. Axium coils incubated with culture medium without MSCs served as controls. Figure 1 illustrates MSCs growing on the coil 24 hours after seeding viewed by scanning electronic microscopy (figure 1A) and by fluorescence microscopy after nuclear staining (Syto16; Thermo Fisher Scientific, Waltham, Massachusetts, USA) (figure 1B).
Figure 1. Platinum coils seeded with adipose-derived autologous mesenchymal stem cells (MSCs).
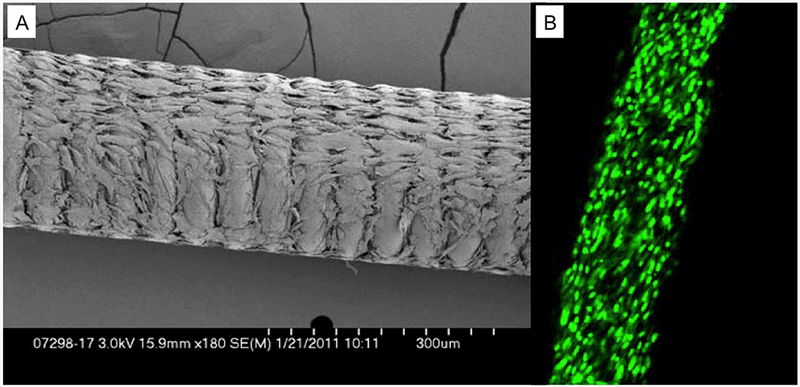
A) Scanning electron microscopic image showing coil seeded with MSCs. B) Confocal microscopic image of MSCs seeded on the coil surface, stained with nuclear stain Syto16 (green).
To confirm that the resheathing and delivery of the coil through the microcatheter had not stripped a large proportion of cells off the coils, we pushed a cell-seeded coil through an Excel (Target Therapeutics) delivery microcatheter ex vivo and then flushed the catheter with saline. We measured the number of non-adherent cells afterwards, which was less than 5% of the initial number of cells seeded on the coil. The non-adherent cells were cultured in the culture medium to confirm that the cells were alive after the resheathing and delivery steps.
Coiling of the experimental aneurysms
Aneurysms were treated at least 3 weeks after aneurysm creation(60). Under general anesthesia, a 5 F catheter was advanced from the right common femoral artery into the brachiocephalic artery. Using coaxial technique, an Excel (Target Therapeutics) microcatheter was advanced into the aneurysm. Aneurysms were embolized with Axium coils without MSCs (control group, n=6) or Axium coils carrying autologous MSCs (test group, n=7). Following the embolization, a final control DSA was performed. A blinded reader with 20 years of experience estimated aneurysm occlusion and packing density with the Angiocalc tool (http://www.angiocalc.com).
Follow-up
Control DSA was performed 4 weeks after coiling via the left auricularis rostralis artery under general anesthesia. All angiograms at death were compared with immediate post-treatment angiograms and assessed for any increase or decrease in contrast filling of the aneurysms. Aneurysms were assigned to one of three result groups: stable occlusion (no modification between immediate post-treatment and 4-week angiograms), progressive occlusion (decrease in aneurysm opacification between immediate post-treatment and 4-week angiograms), or coil compaction (increase in aneurysm opacification between immediate post-treatment and 4-week angiograms). Angiography results are expressed as the proportion of aneurysms demonstrating stable or progressive occlusion comparing immediate post-coiling and follow-up results. For this evaluation, the reader was blinded to the treatment group.
Tissue harvest and processing
After follow-up DSA, animals were killed by lethal injection of pentobarbital. Median sternotomy and pericardiotomy were performed. The animals were then perfusion-fixed with 10% buffered formalin for 10 min followed by flushing with heparinized saline for 5 min. The coiled aneurysm was then harvested and immersed in Tris-buffered saline (TBS). Under a dissection microscope (Leica MZ 125), the parent artery was cut longitudinally to expose the neck orifice for gross inspection to evaluate tissue growth at the neck; the sample was then photographed using the MicroPublisher 5.0 RTV camera attached to the dissection microscope. After photography, the sample was fixed in 10% formalin for 2 hours for further whole tissue mount staining.
Whole-mount en face immunostaining
Details of the procedure are described in a previous study (61). Briefly, after the macrophotographs were taken, the aneurysms were fixed in 10% neutral buffered formalin and then washed with TBS; they were then incubated with 5% normal donkey serum in 0.3% Tween in TBS. The samples were then incubated with primary antibodies against CD31 (1:30; Dako, Carpentaria, California, USA) or smooth muscle actin (1:200; Dako) at 4°C. Specific binding was visualized using a secondary antibody Cy3 or fluorescein isothiocyanate-conjugated IgG (1:200; Jackson Immuno Research, West Grove, Pennsylvania, USA). Sytox green (1:1000; Life Technologies, Grand Island, New York, New York, USA) served as a nuclear counterstain to identify inflammatory cells.
Histologic analysis
Tissues were embedded in paraffin and then sectioned at 1000 microns. Metallic coils were gently removed under a dissecting microscope. Paraffin sections were then re-embedded in paraffin blocks and sectioned at 4 μm intervals. Mounted sections were stained with either Hematoxylin and Eosin (H&E) for conventional histopathological evaluation or Masson Trichrome stain for collagen deposition. Sections were viewed by 2 blinded and independent experienced reviewers paying particular attention to the thickness of the cellular layer across the neck of aneurysms and the cellularity within the aneurysm dome. An ordinal grading system was used to evaluate histological healing (62). Briefly, neck healing score was calculated based on tissue coverage, coil microcompaction at the neck was based on the shape of the coil mass across the neck, and the healing characteristics in the dome was categorized based on the density of cellular infiltration and area of organized tissue. These scores, neck average, microcompaction and healing, were added together to obtain a total score representative of the aneurysm’s healing. The degree of inflammation was defined and scored as 0) no inflammatory cell infiltration; 1) mild, scant, scattered inflammatory cells infiltration; 2) moderate, patchy inflammatory cells infiltration; and 3) marked, attenuated, diffuse inflammatory cells infiltration(47).
Neck endothelialization at the level of the aneurysm neck analysis
For endothelialization analysis, we used whole-mount en face immunostaining with primary antibodies against CD31. We quantitatively analyzed the endothelialization of the neck area with measurement of the CD31 positive field viewed from neck orifice.
Collagen deposition analysis
We quantitatively analyzed collagen deposition or fibrosis identified with Masson Trichrome staiing using an image analysis technique based on Photoshop software (Adobe Systems, San Jose, CA)(47). A fibrosis ratio, the total area of fibrosis within the aneurysmal cavity and neck divided by the total area of the aneurysmal cavity and neck, was calculated for each aneurysm.
Immunohistochemistry (IHC)
Sections were immunostained for RAM-11(63)(1:50, macrophage-specific monoclonal antibody for rabbit, Dako, Carpentaria, California, USA). RAM-11 is a macrophage-specific monoclonal antibody(64) used to detect inflammation in the aneurysm dome.
Statistical Analyses
Wilcoxon rank-sum tests were performed to compare geometries and packing density between groups. Differences between groups for histological finding were assessed with between-group Wilcoxon rank-sum test. Results are expressed as median and interquartiles (25th & 75th). For DSA outcomes, groups were compared using Fisher’s exact test and reported with exact Klopper-Pearson 95% confidence intervals. Statistical significance was set at p<.05.
RESULTS
Seeding of autologous MSCs on Axium coils
Diffuse, confluent cells growth on the coil’s surface, and between coil loop was obtained after 24 hours seeding, which was confirmed by scanning electronic microscopy and fluorescence microscopy (Figure 1).
Angiographic findings
There were no significant differences in aneurysm neck size (p= .69), aneurysm width (p= .37), and aneurysm height (p=.64) between groups. Packing density was significantly different between groups, with median (IQR) of 22.5% (18.8-24.8) for the control group and 30.0% (25.0-34.0) for the MSCs group (p=.01).
All the aneurysms in the MSCs group presented coils compaction at follow-up with neck recurrences, whereas there were 2 cases of coil compaction, 3 stable total occlusions and one progressive total occlusion in the control group. Stable or progressive occlusion rate was significantly lower in the MSCs group (0.00, 95%CI: 0.00-0.41) as compared to controls (0.67, 95%CI: 0.22-0.96) (p=.02).
Qualitative histology
Gross microscopy showed the coil loops at the neck orifice were bare without membranous tissue covering (Figure 2b) in the control group; endothelialization on the coil surface was not noticed with CD-31 immunostaining (Figure 2c). In the test group, the coil loops which crossed over the neck orifice were covered with transparent and translucent membranous tissue (Figure 3b); this membranous tissue was lined with a layer of CD31 positive cells (Figure 3c), indicating endothelializaiton on the coil surface.
Figure 2. A set of representative images of a single aneurysm packed with Axium coils without MSC (control group).
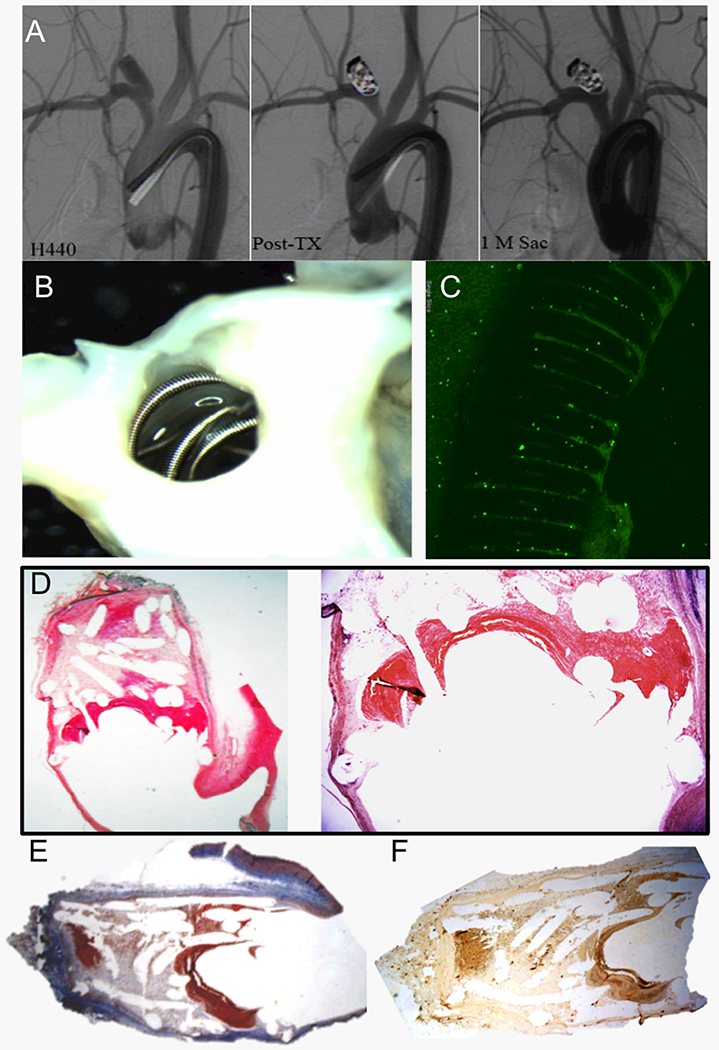
(A) Angiograms show stability of the aneurysm occlusion between the immediate post-treatment and 1-month follow-up angiograms with Raymond I occlusion of the aneurysm. (B) Macrophotography taken from the neck orifice demonstrates the coil loops crossing over the neck orifice are bare (original magnification, 3.2×). (C) Whole tissue mount staining (antibody for CD31) shows there are no CD31+ cells detected on the coil surface at the neck orifice (water lens, original magnification 20×). (D) Microphotograph shows poor organized thrombus at the neck interface; loose connective tissue with thin extra-cellular matrix within the dome (H&E), original magnification 12.5× and 40×) (E) Masson Trichrome staining shows the aneurysm barely has collagen deposit within the cavity (Masson Trichrome, original magnification 12.5×) (F) Immunohistochemistry ( IHC) for RAM-11 shows a few, scattered macrophages within the dome tissue (IHC, antibody for RAM11, original magnification 25×).
Figure 3. A set of representative images of a single aneurysm packed with Axium coils with seeded autologous MSCs (MSCs group).
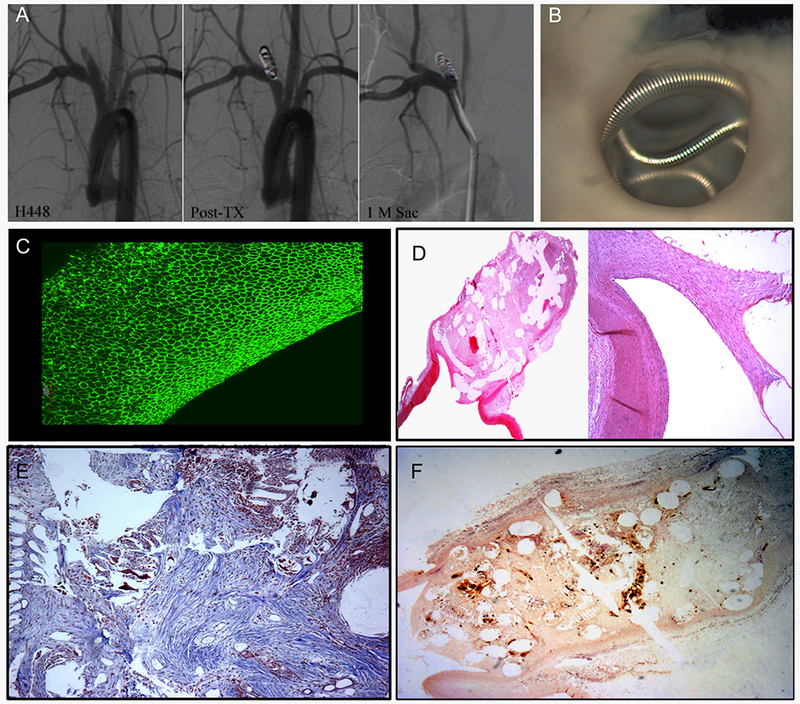
(A) Angiograms show stability of the aneurysm occlusion between the immediate post-treatment and 1-month follow-up angiograms with Raymond I occlusion of the aneurysm. (B) Macrophotograph taken from the neck orifice shows the coil loops at the neck orifice are almost covered with white, translucent and transparent membranous tissue (original magnification 6.3×). (C) Whole tissue mount staining (antibody for CD31) shows single layer, confluent, CD31 positive cells on the coil loop at neck orifice (water lens, original magnification 20×). (D) Microphotograph shows the dome is completely filled with dense connective tissue; endothelium lined thin and thick neointima completely transverses the entire neck interface. Local, dense inflammation is also shown at the top of the dome (H&E, original magnification 12.5×). (E) Masson Trichrome shows a dense packing of collagen deposition in an organized fashion in the aneurysm sac (Masson Trichrome, original magnification 100×). (F) RAM-11 immuno-staining shows local or and diffuse, dense macrophages within the dome surrounding the coil struts (IHC, original magnification 25×).
Microscopy showed six of six aneurysms in control group demonstrated large neck remnant with poor organized thrombus crossing over neck interface (Figure 2d); loose connective tissue associated with scattered macrophages and thin ECM filing in the aneurysm dome; collagen deposit was few to be detected (Figure 2d-f); whereas endothelium-lined, thin and thick neointima completely covered neck interface in the test group (Figure 3d). Denser connective tissue associated with diffuse macrophages infiltration, thick ECM and collagen deposit within the aneurysmal cavity was also observed in the test group (Figures 3d-f).
Histologic scoring
The MSCs group did have a significantly higher inflammation score than the control group (3.0, 3.0-3.0 vs 2.0, 2.0-2.0, p<.01) indicating a larger amount of multinuclear giant cells and macrophages. In total, there was a significantly greater total histologic healing score in MSCs (11.5, 11.0-12.0) versus control (8.0, 6.25-9.0) groups (p=.019).
Quantitative analysis of aneurysm fibrosis
The quantitative analysis of aneurysm fibrosis based on the evaluation of collagen within the aneurysm cavity, demonstrated that the MSCs group (10.3, IQR: 7.98-28.3) had a significantly higher fibrosis ratio compared to the control group (0.13, IQR: 0.055-0.272), p=0.006.
Quantitative analysis of coils endothelialization at the level of the aneurysm neck
We noted a significant higher amount of endothelialization of the aneurysm necks in the MSCs group (902,262 μm2, IQR: 608,991-179,9849) than in the control group (31,810 μm2, IQR: 14,106 - 397,315), p= 0.041.
DISCUSSION
This study demonstrated that autologous MSCs attached to platinum coils improved endothelial cell growth across the neck of the aneurysm and increased fibrosis within the aneurysm sac. Thus, ex vivo loading of standard coils can achieve improved biological healing of aneurysm cavities.
Notably, however, angiographic outcomes were not improved over controls with MSCs. It remains possible that the induced fibrosis from the implanted cells actually caused retraction of coils, rather than stabilization, at the aneurysm neck. Prior to clinical translation, therefore, substantial additional preclinical evaluation of ex vivo autologous MSCs implantation is warranted.
Previous studies have used cell therapy with mature and differentiated cells to promote aneurysm healing(41–52). However, none of these studies demonstrated the improvements in healing responses in both aneurysm neck and aneurysm sac seen in our study. Some authors showed that fibroblasts could improve intra-saccular fibrosis without positive effect on the endothelium and neo-intima formation(47, 48, 50, 51). Furthermore, fibroblasts have an important procoagulant activity due to intense expression of tissue factor (53). As opposed to fibroblasts, mesenchymal stem cells (MSCs), either from adipose tissue of bone-marrow, poorly express TF and are devoid of procoagulant activity, limiting thrombus formation(53). Others have used smooth muscle cells delivered into the aneurysm. Raymond et al.(45), as well as Marbacher et al.(42), observed thicker neointima but without significant impact on fibrosis and endothelium reconstruction through implantation of smooth muscle cells. Use of endothelial progenitor cells in preclinical aneurysm models led to development of confluent monolayers of endothelial cells with underlying neointima but without effect on intra-saccular healing(42, 49).
Those previous studies confirm that terminally differentiated implanted cells do not differentiate into other cell types normally present within the blood vessel wall. Ideally, the cells lining the implanted coils would differentiate into arterial endothelial cells and those residing deep to the endothelial lining would become medial smooth muscle cells(13, 53, 54). From a biological perspective, MSCs represent an ideal cell type to populate saccular aneurysms because of their potential to differentiate into various cell types based on their specific environment(26, 41, 53, 55–57). While our current methodology did not allow specific histopathologic identification of implanted versus recruited cells, previous studies have demonstrated that transplanted MSCs can differentiate into specific phenotypes of damaged cells in vivo under the influence of local host factors(65–67). Under conditions of laminar flow, shear stress induces MSCs to differentiate into endothelial cells, so the implanted MSCs lining the neck of the aneurysm preferentially differentiate into cells lining the arterial wall(68, 69). This was also observed in the present study using autologous stem cells with continuous endothelium at the aneurysms necks and thick neo-intima. Furthermore, the embolized aneurysm cavity is rich in platelets stimulating MSCs to differentiate toward smooth muscle cell phenotypes(70); this was observed in our study with smooth muscle cells largely present in the aneurysms sacs with production of collagen responsible for fibrosis and wound healing.
Recently published studies demonstrate the benefits of MSCs in promoting intracranial aneurysm healing(53, 58, 71). Rouchaud et al. used autologous MSCs from bone marrow directly injected into the aneurysm by endovascular route through a catheter with no scaffold. They observed good results regarding the reduction of the aneurysms size and histological healing but there was a low yield of cells effectively attached into the aneurysm sac because of the absence of scaffold to hold them inside the aneurysm(53). This diminished the beneficial effect of the MSCs therapy and also increased the risk of cells trafficking and subsequent ischemic stroke. Recently, Adibi et al. published a series of 3 rabbits treated with combined endovascular coiling and intra-aneurysmal allogeneic MSCs from bone marrow injected into the aneurysm just after the framing coil and observed a significant improvement in histological scores(58). The authors used allogeneic MSCs with no deleterious impact in rabbits but this technique is likely to induce an immune response against transplanted cells in other animals or in humans(72). Another limitation of this study is the fact that the authors relied on framing coil to promote intra-aneurysmal stasis, thus containing the implanted cells in the aneurysm sac and avoiding cell migration and to evaluate the risk of ischemic lesions.
Limitations
Our study is limited by its small sample size. Another limitation is that the rabbits were killed at only one time point, making it impossible to evaluate if the effects were permanent; also it is possible that the beneficial effects on histological healing observed with MSCs could appear earlier than 1 month. Furthermore, only a dose of 5×106 cells was evaluated, which might not be the appropriate dose. Also, MSCs were not labeled for in vivo tracking, therefore we cannot determine if the implanted cells were viable at the time of death or whether the cells were responsible for the tissue changes noted. Furthermore, the packing density was significantly different between the groups, being higher in the MSC group, which may have biased some of the histological results.
To the best of our knowledge, this study is the largest to use autologous stem cells for the treatment of intracranial aneurysms in an experimental study. Furthermore, despite the small sample size, the effect was statistically significant in favor of MSCs for histological healing, encouraging larger experiments to evaluate safety and long-term outcomes. Further studies are required to evaluate outcomes at different time points to determine the effect of MSCs over time and evaluate the stability or progression of neck remnants. Also, an autologous approach with MSCs does not seem to be appropriate for acute treatment of ruptured aneurysms, since time is needed to produce sufficient cells for each patient.
We used a cell dose of 5×106 cells used in the previous studies by Adibi et al.(58) and Rouchaud et al.(53), but further studies with different amount of cells would be needed to determine the appropriate dose-response efficiency.
CONCLUSION
Autologous MSCs attached to platinum coils significantly improve histological healing compared with standard coils, with more endothelial cells across the neck of the aneurysm and collagen matrix within the aneurysm sac. However, these findings did not correlate with the angiographic findings.
ACKNOWLEDGMENTS
The Axium coils used for this study were provided by Medtronic (Irvine, CA). We thank Medtronic (Irvine, Calif) for generously providing the coils for our study.
FUNDING
This work was supported by research grant NS0767491 from the National Institute of Health and Medtronic.
This work was partially funded by the SNIS Foundation Fellow Research Grant.
Aymeric Rouchaud was supported by a research grants from the French Society of Radiology and Therese Planiol Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
Guarantors of integrity of entire study, R.K., D.D.; study concepts/study design or data acquisition or data analysis/ interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, A.R., W.B., D.F.K.; experimental studies, A.R., Y.H.D., D.D., D.S., R.K., D.F.K.; statistical analysis, T.G.; and manuscript editing, A.R., W.B, R.K., D.D., D.F.K.
DISCLOSURES OF CONFLICTS OF INTEREST
A.R. No relevant conflicts of interest to disclose. D.D. No relevant conflicts of interest to disclose. Y.H.D. No relevant conflicts of interest to disclose. W.B. No relevant conflicts of interest to disclose. D.S No relevant conflicts of interest to disclose. T.G. No relevant conflicts of interest to disclose. R.K. No relevant conflicts of interest to disclose. D.F.K. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received consulting fees for clinical trials planning and implementation from eV3/Covidien and Codman; institution received funds for preclinical research from MicroVention, Sequent, and Codman; institution received funds for clinical trials from MicroVention and Codman; receives royalties for patent from UVA Patent Foundation. Other relationships: none to disclose.
REFERENCES
- 1.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–74. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.White PM, Lewis SC, Gholkar A, Sellar RJ, Nahser H, Cognard C, et al. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet. 2011;377(9778):1655–62. doi: 10.1016/S0140-6736(11)60408-X PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Ogilvy CS, Chua MH, Fusco MR, Griessenauer CJ, Harrigan MR, Sonig A, et al. Validation of a System to Predict Recanalization After Endovascular Treatment of Intracranial Aneurysms. Neurosurgery. 2015;77(2):168–73; discussion 73-4. doi: 10.1227/NEU.0000000000000744 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Ausman JI. The future of neurovascular surgery. Part I: Intracranial aneurysms. Surg Neurol 1997;48(1):98–100. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Murayama Y, Vinuela F, Suzuki Y, Do HM, Massoud TF, Guglielmi G, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery. 1997;40(6):1233–43; discussion 43-4 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Raymond J, Roy D, Bojanowski M, Moumdjian R, L’Esperance G. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg 1997;86(2):211–9. doi: 10.3171/jns.1997.86.2.0211 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34(6):1398–403. doi: 10.1161/01.STR.0000073841.88563.E9 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Vinuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 2000;93(4):561–8. Epub 2000/10/03. doi: 10.3171/jns.2000.93.4.0561 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. The New England journal of medicine. 2006;355(9):928–39. doi: 10.1056/NEJMra052760 PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 10.Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44(1):29–37. doi: 10.1161/STROKEAHA.112.663559 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann TJ, Huston J 3rd, Cloft HJ,Mandrekar J,Gray L, Bernstein MA, et al. A prospective trial of 3T and 1.5T time-of-flight and contrast-enhanced MR angiography in the follow-up of coiled intracranial aneurysms. AJNR Am J Neuroradiol 2010;31(5):912–8. doi: 10.3174/ajnr.A1932 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinjikji W, Yong Hong D, Dai D, Schroeder DJ, Kallmes DF, Kadirvel R. Statins are not associated with short-term improved aneurysm healing in a rabbit model of unruptured aneurysms. J Neurointerv Surg 2016. doi: 10.1136/neurintsurg-2016-012265 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 13.Brinjikji W, Kallmes DF, Kadirvel R. Mechanisms of Healing in Coiled Intracranial Aneurysms: A Review of the Literature. AJNR Am J Neuroradiol 2015;36(7):1216–22. doi: 10.3174/ajnr.A4175 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribourtout E, Raymond J. Gene therapy and endovascular treatment of intracranial aneurysms. Stroke. 2004;35(3):786–93. doi: 10.1161/01.STR.0000117577.94345.CC PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Murayama Y, Vinuela F, Tateshima S, Gonzalez NR, Song JK, Mahdavieh H, et al. Cellular responses of bioabsorbable polymeric material and Guglielmi detachable coil in experimental aneurysms. Stroke. 2002;33(4):1120–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Khan SH, Nichols C, Depowell JJ, Abruzzo TA, Ringer AJ. Comparison of coil types in aneurysm recurrence. Clin Neurol Neurosurg 2012;114(1):12–6. doi: 10.1016/j.clineuro.2011.07.017 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 17.Hoppe AL, Raghavan ML, Hasan DM. Comparison of the association of sac growth and coil compaction with recurrence in coil embolized cerebral aneurysms. PLoS One. 2015;10(4):e0123017. doi: 10.1371/journal.pone.0123017 PubMed PMID: ; PubMed Central PMCID: PMCPMC4404091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659–76. doi: 10.1038/jcbfm.2012.84 PubMed PMID: ; PubMed Central PMCID: PMCPMC3434628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond J, Leblanc P, Morel F, Salazkin I, Gevry G, Roorda S. Beta radiation and inhibition of recanalization after coil embolization of canine arteries and experimental aneurysms: how should radiation be delivered? Stroke. 2003;34(5):1262–8. doi: 10.1161/01.STR.0000069014.84151.85 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Raymond J, Metcalfe A, Desfaits AC, Ribourtout E, Salazkin I, Gilmartin K, et al. Alginate for endovascular treatment of aneurysms and local growth factor delivery. AJNR Am J Neuroradiol 2003;24(6):1214–21. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto H, Terada T, Tsuura M, Itakura T, Ogawa A. Basic fibroblast growth factor released from a platinum coil with a polyvinyl alcohol core enhances cellular proliferation and vascular wall thickness: an in vitro and in vivo study. Neurosurgery. 2003;53(2):402–7; discussion 7-8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Reinges MH, Krings T, Drexler AY, Ludolph A, Sellhaus B, Bovi M, et al. Bare, bio-active and hydrogel-coated coils for endovascular treatment of experimentally induced aneurysms. Long-term histological and scanning electron microscopy results. Interv Neuroradiol 2010;16(2):139–50. PubMed PMID: ; PubMed Central PMCID: PMCPMC3277983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toma N, Imanaka-Yoshida K, Takeuchi T, Matsushima S, Iwata H, Yoshida T, et al. Tenascin-C-coated platinum coils for acceleration of organization of cavities and reduction of lumen size in a rat aneurysm model. J Neurosurg 2005;103(4):681–6. doi: 10.3171/jns.2005.103.4.0681 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Tsumoto T, Matsumoto H, Terada T, Tsuura M, Itakura T, Hamamoto T. A polyvinyl alcohol core coil containing basic fibroblast growth factor evaluated in rabbits with aneurysms induced by elastase. Neurosurgery. 2007;61(1):160–6; discussion 6. doi: 10.1227/01.neu.0000279737.07683.57 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Kallmes DF, Fujiwara NH, Yuen D, Dai D, Li ST. A collagen-based coil for embolization of saccular aneurysms in a New Zealand White rabbit model. AJNR Am J Neuroradiol. 2003;24(4):591–6. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Lu Z, Chen C, Cui X, Liu Y, Zheng T, et al. Mesenchymal stem cells and endothelial progenitor cells accelerate intra-aneurysmal tissue organization after treatment with SDF-1alpha-coated coils. Neurol Res 2016;38(4):333–41. doi: 10.1080/01616412.2016.1164433 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Wang Q, Cui X, Liu Y, Zheng T, Chen C, et al. Controlled release of stromal cell-derived factor-1alpha from silk fibroin-coated coils accelerates intra-aneurysmal organization and occlusion of neck remnant by recruiting endothelial progenitor cells. Int J Clin Exp Pathol 2014;7(12):8366–80. PubMed PMID: ; PubMed Central PMCID: PMCPMC4314014. [PMC free article] [PubMed] [Google Scholar]
- 28.Raymond J, Mounayer C, Salazkin I, Metcalfe A, Gevry G, Janicki C, et al. Safety and effectiveness of radioactive coil embolization of aneurysms: effects of radiation on recanalization, clot organization, neointima formation, and surrounding nerves in experimental models. Stroke. 2006;37(8):2147–52. doi: 10.1161/01.STR.0000231724.18357.68 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 29.Smith MJ, Mascitelli J, Santillan A, Brennan JS, Tsiouris AJ, Riina HA, et al. Bare platinum vs matrix detachable coils for the endovascular treatment of intracranial aneurysms: a multivariate logistic regression analysis and review of the literature. Neurosurgery. 2011;69(3):557–64; discussion 65. doi: 10.1227/NEU.0b013e31821a86da PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Rivet DJ, Moran CJ, Mazumdar A, Pilgram TK, Derdeyn CP, Cross DT. Single-institution experience with matrix coils in the treatment of intracranial aneurysms: comparison with same-center outcomes with the use of platinum coils. AJNR Am J Neuroradiol 2007;28(9):1736–42. doi: 10.3174/ajnr.A0633 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piotin M, Spelle L, Mounayer C, Loureiros C, Ghorbani A, Moret J. Intracranial aneurysms coiling with matrix: immediate results in 152 patients and midterm anatomic follow-up from 115 patients. Stroke. 2009;40(1):321–3. doi: 10.1161/STROKEAHA.108.520866 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.O’Hare AM, Fanning NF, Ti JP, Dunne R, Brennan PR, Thornton JM. HydroCoils, occlusion rates, and outcomes: a large single-center study. AJNR Am J Neuroradiol 2010;31(10):1917–22. doi: 10.3174/ajnr.A2210 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linfante I, DeLeo MJ 3rd, Gounis MJ, Brooks CS, Wakhloo AK. Cerecyte versus platinum coils in the treatment of intracranial aneurysms: packing attenuation and clinical and angiographic midterm results. AJNR Am J Neuroradiol 2009;30(8):1496–501. doi: 10.3174/ajnr.A1617 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii A, Murayama Y, Nien YL, Yuki I, Adapon PH, Kim R, et al. Immediate and midterm outcomes of patients with cerebral aneurysms treated with Matrix1 and Matrix2 coils: a comparative analysis based on a single-center experience in 250 consecutive cases. Neurosurgery. 2008;63(6):1071–7; discussion 7-9. doi: 10.1227/01.NEU.0000334047.30589.13 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Gunnarsson T, Tong FC, Klurfan P, Cawley CM, Dion JE. Angiographic and clinical outcomes in 200 consecutive patients with cerebral aneurysm treated with hydrogel-coated coils. AJNR Am J Neuroradiol 2009;30(9):1657–64. doi: 10.3174/ajnr.A1691 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaba RC, Ansari SA, Roy SS, Marden FA, Viana MA, Malisch TW. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay, and durability of therapy. Stroke. 2006;37(6):1443–50. doi: 10.1161/01.STR.0000221314.55144.0b PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with matrix detachable coils. Neurosurgery. 2006;58(1):51–9; discussion −9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Cloft HJ. HydroCoil for Endovascular Aneurysm Occlusion (HEAL) study: 3-6 month angiographic follow-up results. AJNR Am J Neuroradiol 2007;28(1):152–4. Epub 2007/01/11. doi: 28/1/152 [pii]. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 39.Kimchi TJ, Willinsky RA, Spears J, Lee SK, ter Brugge K. Endovascular treatment of intracranial aneurysms with matrix coils: immediate posttreatment results, clinical outcome and follow-up. Neuroradiology. 2007;49(3):223–9. Epub 2007/01/04. doi: 10.1007/s00234-006-0173-1 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Niimi Y, Song J, Madrid M, Berenstein A. Endosaccular treatment of intracranial aneurysms using matrix coils: early experience and midterm follow-up. Stroke. 2006;37(4):1028–32. Epub 2006/03/04. doi: 01.STR.0000206459.73897.a3 [pii] 10.1161/01.STR.0000206459.73897.a3 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Adibi A, Sen A, Mitha AP. Cell Therapy for Intracranial Aneurysms: A Review. World Neurosurg 2016;86:390–8. doi: 10.1016/j.wneu.2015.10.082 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 42.Marbacher S, Frosen J, Marjamaa J, Anisimov A, Honkanen P, von Gunten M, et al. Intraluminal cell transplantation prevents growth and rupture in a model of rupture-prone saccular aneurysms. Stroke. 2014;45(12):3684–90. doi: 10.1161/STROKEAHA.114.006600 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 43.Allaire E, Muscatelli-Groux B, Guinault AM,Pages C,Goussard A,Mandet C, et al. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann Surg 2004;239(3):417–27. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond J, Venne D, Allas S, Roy D, Oliva VL, Denbow N, et al. Healing mechanisms in experimental aneurysms. I. Vascular smooth muscle cells and neointima formation. J Neuroradiol 1999;26(1):7–20. Epub 1999/06/11. doi: MDOI-JNR-04-1999-26-1-0150-9861-101019-ART1 [pii]. PubMed PMID: . [PubMed] [Google Scholar]
- 45.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke. 1999;30(8):1657–64. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 46.Kwon OK, Han MH, Oh CW, Park IA, Choe G, Lee KH, et al. Embolization with autologous fibroblast-attached platinum coils in canine carotid artery aneurysms: histopathological differences from plain coil embolization. Invest Radiol 2003;38(5):281–7. doi: 10.1097/01.RLI.0000064698.12359.79 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 47.Dai D, Ding YH, Danielson MA, Kadirvel R, Helm GA, Lewis DA, et al. Endovascular treatment of experimental aneurysms with use of fibroblast transfected with replication-deficient adenovirus containing bone morphogenetic protein-13 gene. AJNR Am J Neuroradiol 2008;29(4):739–44. doi: 10.3174/ajnr.A0892 PubMed PMID: ; PubMed Central PMCID: PMCPMC2605703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai D, Ding YH, Danielson MA, Kadirvel R, Hunter LW, Zhan WZ, et al. Endovascular treatment of experimental aneurysms by use of fibroblast-coated platinum coils: an angiographic and histopathologic study. Stroke. 2007;38(1):170–6. doi: 10.1161/01.STR.0000252128.83405.71 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 49.Aronson JP, Mitha AP, Hoh BL, Auluck PK, Pomerantseva I, Vacanti JP, et al. A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms. J Neurosurg 2012;117(3):546–54. doi: 10.3171/2012.5.JNS091308 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 50.Marx WE, Cloft HJ, Helm GA, Short JG, Do HM, Jensen ME, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001;22(2):323–33. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 51.Kawakami O, Miyamoto S, Hatano T, Yamada K, Hashimoto N, Tabata Y. Accelerated embolization healing of aneurysms by polyethylene terephthalate coils seeded with autologous fibroblasts. Neurosurgery. 2005;56(5):1075–81; discussion −81. PubMed PMID: . [PubMed] [Google Scholar]
- 52.Li S, Tian Y, Huang X, Zhang Y, Wang D, Wei H, et al. Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain Res 2014;1593:65–75. doi: 10.1016/j.brainres.2014.09.077 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 53.Rouchaud A, Journe C, Louedec L, Ollivier V, Derkaoui M, Michel JB, et al. Autologous mesenchymal stem cell endografting in experimental cerebrovascular aneurysms. Neuroradiology. 2013;55(6):741–9. doi: 10.1007/s00234-013-1167-4 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 54.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19(1):35–42. doi: 10.1038/nm.3028 PubMed PMID: ; PubMed Central PMCID: PMCPMC3998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki T, Nozaki K. Preemptive Medicine for Cerebral Aneurysms. Neurol Med Chir (Tokyo). 2016. doi: 10.2176/nmc.st.2016-0063 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esfahani DR, Viswanathan V, Alaraj A. Nanoparticles and stem cells - has targeted therapy for aneurysms finally arrived? Neurol Res 2015;37(3):269–77. doi: 10.1179/1743132814Y.0000000435 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 57.Yamawaki-Ogata A, Hashizume R, Fu XM, Usui A, Narita Y. Mesenchymal stem cells for treatment of aortic aneurysms. World J Stem Cells. 2014;6(3):278–87. doi: 10.4252/wjsc.v6.i3.278 PubMed PMID: ; PubMed Central PMCID: PMCPMC4109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adibi A, Eesa M, Wong JH, Sen A, Mitha AP. Combined endovascular coiling and intra-aneurysmal allogeneic mesenchymal stromal cell therapy for intracranial aneurysms in a rabbit model: a proof-of-concept study. J Neurointerv Surg 2016. doi: 10.1136/neurintsurg-2016-012520 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 59.Altes TA, Cloft HJ, Short JG, DeGast A, Do HM, Helm GA, et al. 1999 ARRS Executive Council Award Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol 2000;174(2):349–54. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara NH, Cloft HJ, Marx WF, Short JG, Jensen ME, Kallmes DF. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR American journal of neuroradiology. 2001;22(4):698–703. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 61.Dai D, Ding YH, Rezek I, Kallmes DF, Kadirvel R. Characterizing patterns of endothelialization following coil embolization: a whole-mount, dual immunostaining approach. J Neurointerv Surg 2016;8(4):402–6. doi: 10.1136/neurintsurg-2014-011513 PubMed PMID: ; PubMed Central PMCID: PMCPMC4924626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai D, Ding YH, Lewis DA, Kallmes DF. A proposed ordinal scale for grading histology in elastase-induced, saccular aneurysms. AJNR Am J Neuroradiol 2006;27(1):132–8. Epub 2006/01/19. doi: 27/1/132 [pii]. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 63.Dai D, Ding YH, Kadirvel R, Danielson MA, Lewis DA, Cloft HJ, et al. A longitudinal immunohistochemical study of the healing of experimental aneurysms after embolization with platinum coils. AJNR Am J Neuroradiol 2006;27(4):736–41. PubMed PMID: . [PMC free article] [PubMed] [Google Scholar]
- 64.Tsukada T, Rosenfeld M, Ross R, Gown AM. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986;6(6):601–13. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 65.Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23(1):109–19. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 66.Fang D, Seo BM, Liu Y, Sonoyama W, Yamaza T, Zhang C, et al. Transplantation of mesenchymal stem cells is an optimal approach for plastic surgery. Stem Cells. 2007;25(4):1021–8. doi: 10.1634/stemcells.2006-0576 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 67.Zhao Q, Gong P, Tan Z, Yang X. Differentiation control of transplanted mesenchymal stem cells (MSCs): a new possible strategy to promote periodontal regeneration. Med Hypotheses. 2008;70(5):944–7. doi: 10.1016/j.mehy.2007.09.013 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 68.Dong JD, Gu YQ, Li CM, Wang CR, Feng ZG, Qiu RX, et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol Sin. 2009;30(5):530–6. doi: 10.1038/aps.2009.40 PubMed PMID: ; PubMed Central PMCID: PMCPMC4002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Cearbhaill ED, Punchard MA, Murphy M, Barry FP, McHugh PE, Barron V. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials. 2008;29(11):1610–9. doi: 10.1016/j.biomaterials.2007.11.042 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 70.Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: implications for neovascularization. Expert Opin Biol Ther 2010;10(1):57–71. doi: 10.1517/14712590903379510 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 71.Zhang S, An Q, Li Q, Huang J, Chen X, Chen X, et al. Therapeutic benefit of bone marrow-derived endothelial progenitor cell transplantation after experimental aneurysm embolization with coil in rats. PLoS One. 2014;9(2):e90069. doi: 10.1371/journal.pone.0090069 PubMed PMID: ; PubMed Central PMCID: PMCPMC3938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plissonnier D, Henaff M, Poncet P, Paris E, Tron F, Thuillez C, et al. Involvement of antibody-dependent apoptosis in graft rejection. Transplantation. 2000;69(12):2601–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]


