Colorectal cancer (CRC) is the third leading cause of cancer death among women (American Cancer Society, 2015). If individuals received screening per recommended guidelines, it is estimated that 60% of lives would be saved (American Cancer Society, 2014). Healthy People 2020, acknowledged CRC screening rates as a high-priority issue and created a target screening rate of 70.5% among the U.S. population (U. S. Department of Health and Human Services, 2013). Colorectal cancer screening rates among women are 3.4% lower that among men and remain lower than breast and cervical cancer screening rates (American Cancer Society, 2015). Currently, only 55% of women are up to date with colorectal cancer screening (American Cancer Society, 2014). CRC screening rates have not made significant gains despite public service announcements, and numerous types of interventions to increase CRC screening (Powe, Faulkenberry, & Harmond, 2010; U. S. Department of Health and Human Services, 2013). Research further indicates that CRC interventions should be delivered multiple times to increase the likelihood that CRC screening would be completed. Hence, suggesting that CRC interventions should be started prior age 50, when CRC screening is to begin, and that interventions should be done well in advance of a primary care or health care related appointment where a decision needs to be made (Powe, et al., 2010). Previous research indicates that factors influencing informed decisions about CRC screening intention and adherence are different for women when compared to men (Brittain, Loveland-Cherry, Northouse, Caldwell, & Taylor, 2012; Brittain & Murphy, 2015). Yet, many CRC screening interventions are not specifically designed for women, and they are not accessible to patients whenever they would like to use them in advance of a health care provider visit (Christy et al., 2013; Rawl et al., 2008).
Today, many more options for increasing informed decisions about CRC screening exist. One such option is providing the CRC screening intervention on the smartphone or tablet via a mobile application (mobile app). According to Pew Research, fifty-eight percent of adults have a smartphone (Perrin & Duggan, June 2015). Of the smartphone owners, 53% are Caucasian and 59% are African American and among women ages 50–64, 49% of women ages 50–64 have smartphones (Perrin & Duggan, June 2015). The use of smartphones has grown and of smartphone owners, 52% of have used their phone to obtain health-related information (Smith, 2011). Smartphones have increased access to the internet for many people. Among individuals ages 50–64, 46% of Caucasians and 41% of African Americans own smartphones and 42% of Americans have a tablet computer. About 19% of smartphone owners have downloaded an app to track or manage health and 60% of that group tracks weight, diet or exercise routine (Duggan, Ellison, Lampe, Lenhart, & Madden, 2015; Perrin & Duggan, June 2015). However, there is a lack of theory based and cancer specific interactive mobile apps designed to increase informed decisions about CRC screening. Mobile technology, like smartphones and tablets, hold the possibility of increasing informed decisions about CRC screening, increasing shared decision making between the patient and provider about CRC screening and increasing CRC screening rates.
Using the results of past research and the promise of mobile technology, mobile apps may offer a new strategy in increasing CRC screening rates among women. The purpose of this study is to explore the usability, acceptability, and satisfaction with and establish the effects of a theory based mobile app designed to increase CRC screening intention among 44–70-year-old women.
The specific aims for this feasibility study were to: (1) develop a targeted mobile app to increase CRC health beliefs to support CRC screening, informed decision-making about CRC screening and CRC screening intention among women age 44–70 years old, (2) to evaluate the participants’ satisfaction with the mobile app, and (3) determine the feasibility of using an app designed for increasing colorectal cancer screening participation.
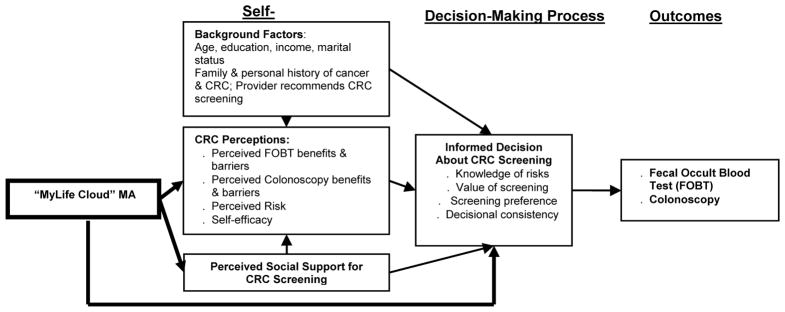
Using conceptual framework that synthesizes the Preventive Health Model (PHM) and The Decision Support Framework (Myers, 2005; O’Connor et al., 1998), we developed our mobile app to (1) assess the woman’s personal health history to determine her CRC risk; (2) assess and affect her CRC perceptions; (3) assess the woman’s level of social support for CRC screening completion; (4) measure the woman’s level of an informed decision about CRC screening by assessing her knowledge of CRC screening risks and benefits, CRC screening values and preferences, and (5) provide the woman with the CRC screening option (Fecal Occult Blood Test (FOBT) or colonoscopy) that is best suited to her based on her responses to the questions in the mobile app. (Figure 1). Our conceptual model posits that the self-system comprised of background factors (age, CRC screening history, etc.) affects CRC perceptions, and that perceived social support for CRC screening affects CRC beliefs. Furthermore, background factors and CRC perceptions affect an informed decision about CRC screening.
Figure 1.
Conceptual Model: Synthesis of the Preventive Health Model (PHM) and The Decision Support Framework (DSF) (Myers, 2005, O’Connor et al, 1998)
METHODS
Study Design, Setting, and Sample
Participant eligibility for this feasibility study included self-identifying as either an African American or Caucasian woman; age 44 years to 70 years old; able to speak English; no personal history of CRC; have insurance for CRC screening, and report having used a mobile application at least once. We chose the eligibility criteria to closely mirror the sample from the previous research on gender differences and the factors that influence an informed decision about CRC screening (Brittain, Loveland-Cherry, et al., 2012; Brittain, Taylor, Loveland-Cherry, Northouse, & Caldwell, 2012). Potential participants who lived in the Midwest were recruited via social networks, community organizations and beauty salons.
Procedures
After receiving Institutional Review Board approval for the study, potential participants contacted the Principal Investigator who assessed whether the participant met the eligibility criteria. Once the potential participant was determined to be eligible, those willing to participate indicated their consent by either signing a hard copy consent form for those participants choosing to complete hard copy questionnaire or an electronic version of the consent form for those who chose to complete the web-based questionnaire. After the participants completed the questionnaire, they received instructions on how to download the appropriate “MyLife Cloud” mobile app based on the participant either having an iPhone/iPad or Android phone/tablet. After using the mobile app, participants were asked to complete an evaluation of the mobile app. For this feasibility study, all participants received the intervention and there was no control group. All participants received a $25 gift card for participating in the study.
Mobile Application Intervention
The “MyLife Cloud” mobile app was designed to increase colorectal cancer screening (fecal occult blood test (FOBT) or colonoscopy) adherence among women who have never been screened or who are not adherent to the recommended CRC screening guidelines for FOBT and/or colonoscopy. For the feasibility study, the results from previous studies (Brittain, Loveland-Cherry, et al., 2012; Brittain, Taylor, et al., 2012) were used to guide the development of the interactive mobile app that would provide information on FOBT and colonoscopy (preparation, risks, benefits, values, and preferences) as well as solicit her CRC screening values and preferences based on the FOBT and colonoscopy information presented. Additionally, the mobile app assess key factors related to an informed decision about CRC screening as identified by previous research so that the women receives a CRC screening recommendation from the mobile app that she is more likely to complete. In order to make sure that the women would receive appropriate CRC screening guidance and information, it was necessary to include a CRC risk assessment as the first part of the mobile app. The researchers used the “CRC Risk Assessment Tool”, a risk calculator that helps estimate a person’s risk of developing CRC retrieved from the National Cancer Institute’s website. Before the woman begins the interacting with the CRC screening and social support content of the app, the woman completes the CRC Risk Assessment tool on her mobile device. Her responses are calculated and she is given a CRC risk estimation. The eligibility criteria of the study were such that all participants would be categorized as average risk and subsequent CRC screening information would be appropriate for a women of average risk for CRC.
Selecting Mobile App Content
Next, participants used the mobile app to respond to questions related to the factors found to be significant predictors of CRC screening intention which are CRC perceptions and social support (Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015). CRC perception questions used in the app were from the 35 item CRC Perceptions Scale (Brittain, Loveland-Cherry, et al., 2012; Green & Kelly, 2004). The CRC Perceptions Scale measures the individual’s beliefs about CRC susceptibility, severity, benefits and barriers. Based on previous research, 15 of the original 35 CRC perceptions items were used in the mobile app (Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012). The 15 CRC perceptions scale questions used assessed CRC susceptibility (n = 5), CRC worries/expected outcomes (n = 5), and CRC barriers/self-efficacy (n =5). Previous research indicates that social support is associated with an informed decision about CRC screening and CRC screening adherence (Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012). Thus, 7 questions about the participant’s perception of social support related to CRC screening were used in the mobile app. Questions about social support addressed tangible support such as transportation to colonoscopy, informational support and emotional support related to CRC screening decision making. Components that comprise an informed decision about CRC screening were included as content on the risks and benefits of FOBT and colonoscopy, FOBT and colonoscopy preparation were adapted from the CDC and/or a link was provided in the app to the CDC website. An assessment of family influence used in a previous study was included because an important component of an informed decision is to acknowledge that CRC screening information can come from any source including family(Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012).
Study Measures
Prior to using the mobile app, each participant completed a demographic questionnaire that included age, race, educational attainment, and CRC screening history. Additionally, participants completed the CRC Perceptions Scale, Medical Outcomes Social Support Survey, and Informed Decision about CRC Screening Scale prior to and after using the mobile app.
Colorectal Cancer Perceptions Scale has 35 items that assess CRC susceptibility, severity, benefits and barriers to screening using a 5-point Likert scale, with 1 corresponding with strongly disagree and 5 representing strongly agree. High scores on the scale indicate that the respondent has positive perceptions about colorectal cancer and colorectal cancer screening (α = 0.92)(Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012).
An informed decision about colorectal cancer screening was assessed using a 28-item scale was used to assess colorectal cancer screening preferences between fecal occult blood testing(FOBT) and colonoscopy, understanding of colorectal cancer screening, knowledge of risks related to colorectal cancer screening, value of colorectal cancer screening and decisional consistency. Low scores indicate a low informed decision (α = .68)(Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012).
Social Support was assessed using the Medical Outcomes Study Social Support Survey (MOS-SSS). The 19 item MOS-SSS measures perceived availability of social support that includes: emotional support, informational support, tangible support, affectionate support and positive social interactions. A high total score indicates high perceived social support (α = .93)(Brittain, Loveland-Cherry, et al., 2012; Brittain & Murphy, 2015; Brittain, Taylor, et al., 2012).
Process Evaluation
Using the Technology Acceptance Model, participants’ satisfaction with the app was evaluated and 3 characteristics of the app were assessed: (1) perceived usefulness, (2) perceived ease of use and usability, and (3) satisfaction with the overall experience of using the app. Subscale scores ranged from 1 (Strongly Disagree) to 4 (Strongly Agree). Participants were asked to give the mobile app an overall grade ranging from A (1) to D (4). Additionally, participants were asked to provide comments on the app and suggest areas to add to or improve the app.
Data Analyses
Data collected to address aim 2 of the study which was to evaluate the participants’ satisfaction with the mobile app was analyzed using descriptive statistics. Data collected to address aim 3 of the study which was to determine the feasibility of using a mobile app designed to increase CRC screening participation was analyzed using descriptive statistics examining enrollment and retention rates.
Results
Sample
Potential participants were recruited from social media, social networks, community organizations, local businesses and non-profit organizations. Of the 48 women recruited, 45 were eligible. The two women who were ineligible were because of age. Of the remaining 45 women who were eligible, 41 completed the intervention (retention = 91%). Loss of participants were due to inability to contact for study completion (n = 4). Table 1 provides the demographic characteristics of the final sample (N = 45). Table 1 indicates that more than 61% of the sample had a previous CRC screening test. Most often it was a colonoscopy. Table 1 also indicates that most of the participants had no family history of colorectal cancer.
Table 1.
Sample Characteristics
| Characteristics | n | % |
|---|---|---|
| Gender (N = 45) | ||
| Female | 45 | 100 |
| Age (N = 45) | ||
| 44–49 | 16 | 36 |
| 50–59 | 22 | 49 |
| 60–65+ | 7 | 15 |
| Race/ethnicity (N = 44) | ||
| African American/Black | 16 | 36 |
| Caucasian/White | 28 | 64 |
| Marital Status (N = 45) | ||
| Married | 31 | 69 |
| Single/never married/divorced/widow | 14 | 31 |
| Education (N = 45) | ||
| High school graduate | 5 | 11 |
| Some college | 15 | 33 |
| College/Graduate school graduate | 25 | 56 |
| Income (N = 44) | ||
| Less than $9,000 | 2 | 5 |
| $10,000–$29,000 | 4 | 9 |
| $30,000–$49,000 | 8 | 18 |
| $50,000–$69,000 | 10 | 23 |
| $70,000–$90,000+ | 20 | 45 |
Study Measures
No differences were found between demographic variables (ie, age, education) and participants’ scores on CRC perceptions, MOS-SSS, and informed decision about CRC screening. Due to the small sample size there may have been insufficient power to detect existing differences.
Satisfaction with the Mobile App
Table 2 displays the results of the process evaluation and indicates that 80.6% of the participants strongly agree/agreed the app made them think about CRC screening. Among the participants, 83.8% strongly agree/agreed that the mobile app provided enough information to make a decision about CRC screening. Most of the participants, 86.1%, strongly agree/agreed that a mobile app like the one in the current study could help them talk with their provider about CRC screening. Family & spouse were identified most often (63.2%) as social support the participants would discuss their CRC screening decision with. Most participants gave the app an “A” (Mean = 1.5; S.D. = 0.7) and would recommend the app to another woman.
Table 2.
Process Evaluation Results
| Question | Na | Mb (S.D) |
|---|---|---|
| Understand the information in the mobile app | 36 | 3.6 (0.8) |
| The app took too much time | 37 | 1.8 (0.7) |
| Using the app made me nervous | 37 | 1.8 (0.6) |
| Enjoyed using the app | 37 | 3.0 (0.6) |
| Information received was important to me | 37 | 3.2 (0.8) |
| Very interested in the information from the app | 36 | 3.2 (0.7) |
| The app made me think about having a colon test | 36 | 3.0 (0.7) |
| The messages made sense to me | 36 | 3.2 (0.7) |
| …information does not relate to me | 37 | 1.9 (0.6) |
| …information was interesting | 37 | 3.0 (0.7) |
| Since using the mobile app, I have enough info to make a decision about colon testing | 37 | 3.0 (0.8) |
| Time passed quickly when I used the mobile app | 36 | 3.0 (0.7) |
| I carefully read the info in the mobile app | 36 | 3.0 (0.7) |
| …information in the mobile app was easy to understand | 36 | 3.1 (0.7) |
| I can use the information from the mobile app in my daily life | 36 | 3.0 (0.7) |
| The information was relevant to me | 35 | 2.9 (0.6) |
| I would like to learn more about colon testing | 36 | 2.6 (0.5) |
| I don’t need the information in the mobile app | 36 | 2.1 (0.6) |
| I had trouble paying attention to the mobile app | 35 | 1.8 (0.7) |
| A mobile app like this could help me talk with my doctor about colon testing | 36 | 3.0 (0.6) |
| I would recommend this mobile app to other people | 36 | 3.0 (0.8) |
Numbers may not be equal due to missing data;
4 = Strongly Agree; 3 = Agree; 2 = Disagree; 1 = Strongly Disagree
Feasibility of Mobile App Delivery
Enrollment and retention rates were examined as indicators of the mobile apps feasibility. The enrollment and retention rates were 91%.
Discussion
Our study used previous research to develop, examine the satisfaction with and feasibility of a mobile app specifically for CRC screening among women age 45–70 years old. In terms of satisfaction with the app, most of the women stated that they would recommend the app to another woman and overall gave the app an “A”. The participants reported finding the app useful in terms of the information provided, level of detail, thinking about colorectal cancer screening, and assisting them in making an informed decision about colorectal cancer screening. The women found the mobile app easy to use, understand, and enjoyed using the app. Additionally, the women thought that the app could help them talk with their doctor about colorectal cancer screening. This finding is important as this study is one of the few CRC intervention studies to address the recommendations of previous research that suggests that CRC screening information be presented at multiple time points (Powe, et al., 2010). For this study, we decided to try presenting CRC screening information to women prior to age 50 as a way to address the previous research recommendation. The results of our study indicates that women younger than 50 are very willing to learn about CRC screening and make an informed decision about CRC screening prior to age 50. It is possible that if CRC screening information was presented regularly and in wider audiences similarly to breast cancer screening information that women would come prepared to make a shared decision with their health care provider that is consistent with their CRC screening values and preferences which could increase routine CRC screening rates. There is not much previous research among women over 40 and mobile apps for increasing colorectal cancer screening informed decisions thus it is hard for us to make comparisons to our results.
An essential component of the development of the mobile app was the collaboration between the nurse scientist who conducted the previous research on the conceptual model, and instruments/subscales on social support, colorectal cancer screening perceptions, and an informed decision about colorectal cancer screening and the engineers with the experience in mobile app development and testing. The nurse and engineers worked together to develop the interactive mobile app based on the conceptual model that was shown in a previous study to be appropriate for women. Using the variables that were significantly related to an informed decision about CRC screening among women, the mobile app was developed. Also important to the development of the mobile app was the participation of an advisory group of women who provided early feedback on the mobile app.
Limitations
There were a few notable limitations to our study. In addition to the small sample size, it is not possible to determine what could have influenced the results of our study as this study did not have a control group. Additionally, the use of self-reports and participation incentives could have led to respondent bias. Finally, only African American and Caucasian women were recruited for this study, thus our results are not generalizable to women in of other racial/ethnic backgrounds.
Conclusion
Despite the aforementioned limitations, this study is one of the first to use a conceptual model and validated instruments to develop an interactive mobile app to increase informed decisions about CRC screening. The participants found the mobile app easy to use and useful in making CRC screening decisions. Our results indicate that future health related mobile apps for women 40 and older, should be interactive and assess social support as previous research indicates that positive social support is important in making an informed decision about colorectal cancer screening. It is important for individuals involved with public health prevention to find new strategies that patients find engaging, and have the potential to increase patient-provider communication. Furthermore, using an interactive mobile app could be a useful tool in primary care practices to increase the quality of shared decision making, increase patient satisfaction with the services provided by the health care provider, and increase colorectal cancer screening adherence.
Acknowledgments
Funding for the pilot study was awarded to Kelly Brittain through a grant from the Center for Future Technologies in Cancer Care at Boston University (NIH/National Institute of Biomedical Imaging and Bioengineering; U54EB01540; PI: Kapperich). Kelly Brittain is an assistant professor in the College of Nursing at Michigan State University in East Lansing, Michigan. Kendra Kamp is a doctoral student in the College of Nursing at Michigan State University in East Lansing, Michigan. Christos Cassandras is a professor in Systems Engineering at Boston University in Boston, Massachusetts. Zachary Salaysay is a Registered Nurse at Sparrow Health System in East Lansing, Michigan. José Gómez-Márquez is the Manager of the Little Devices Lab at Massachusetts Institute of Technology.
References
- American Cancer Society. Colorectal Cancer Facts & Figures 2014–2016. Atlanta, Ga: American Cancer Society; 2014. [Google Scholar]
- American Cancer Society. Cancer facts & figures 2015. 2015. [Google Scholar]
- Brittain K, Loveland-Cherry C, Northouse L, Caldwell CH, Taylor JY. Sociocultural Differences and Colorectal Cancer Screening Among African American Men and Women. Oncology Nursing Forum. 2012;39(1):100–107. doi: 10.1188/12.onf.100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain K, Murphy VP. Sociocultural and Health Correlates Related to Colorectal Cancer Screening Adherence Among Urban African Americans. Cancer Nursing. 2015;38(2) doi: 10.1097/NCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain K, Taylor J, Loveland-Cherry C, Northouse L, Caldwell CH. Family Support and Colorectal Cancer Screening Among Urban African Americans. The Journal for Nurse Practitioners. 2012;8(7):522–533. doi: 10.1016/j.nurpra.2011.12.003. doi: http://dx.doi.org/10.1016/j.nurpra.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy SM, Perkins SM, Tong Y, Krier C, Champion VL, Skinner CS, … Rawl SM. Promoting colorectal cancer screening discussion: A randomized controlled trial. American Journal of Preventive Medicine. 2013;44:325–329. doi: 10.1016/j.amepre.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan M, Ellison NB, Lampe C, Lenhart A, Madden M. Social Media Update 2014. Pew Research Center; 2015. [Google Scholar]
- Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nursing. 2004;27(3):206–215. doi: 10.1097/00002820-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Myers RE. Decision Counseling in Cancer Prevention and Control. Health Psychology. 2005;24(4):S71–S77. doi: 10.1037/0278-6133.24.4.S71. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, … Drake E. A Decision Aid for Women Considering Hormone Therapy After Menopause: Decision Support Framework and Evaluation. Patient Education and Counseling. 1998;33:267–279. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- Perrin A, Duggan M. Americans’ Internet Access: 2000–2015. Washington, D.C: Pew Research Center; Jun, 2015. [Google Scholar]
- Powe BD, Faulkenberry R, Harmond L. A Review of Intervention Studies that Seek to Increase Colorectal Cancer Screening Among African Americans. American Journal of Health Promotion. 2010;25(2):92–99. doi: 10.4278/ajhp.080826-LIT-162. [DOI] [PubMed] [Google Scholar]
- Rawl SM, Champion VL, Scott LL, Zhou H, Monahan P, Ding Y, … Skinner CS. A Randomized Trial of Two Print Interventions to Increase Colon Cancer Screening Among First-Degree Relatives. Patient Education and Counseling. 2008;71:215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Smartphone. Pew Internet & American Life Project; 2011. [Google Scholar]
- U. S. Department of Health and Human Services. Healthy People 2020. 2013 Retrieved from http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf.