Abstract
Little is known about the release of metal engineered nanomaterials (ENMs) from consumer goods including lumber treated with micronized copper. Micronized copper is a recent form of anti-fouling wood preservative containing nano-sized copper particles for use in pressure treated lumber. This study investigated the concentrations released and release rate of total copper over 133 days under freshwater, estuarine, and marine salinity conditions (0, 1, 10, and 30‰) for several commercially available pressure treated lumbers: micronized copper azole (MCA) at 0.96 and 2.4 kg/m3, alkaline copper quaternary (ACQ) at 0.30 and 9.6 kg/m3, and chromated copper arsenate (CCA) at 40 kg/m3. Lumber was tested as blocks and sawdust. Overall, copper was released from all treated lumber samples. Under leaching conditions, total release ranged from 2 to 55% of the measured copper originally in the lumber with release rate constants from the blocks of 0.03 to 2.71 in units of /day. Generally, measured release and modelled equilibrium concentrations were significantly higher in the estuarine conditions compared to freshwater or marine salinities while rate constants showed very limited differences between salinities. Further, organic carbon was released during the leaching and demonstrated a significant relationship with released copper concentrations as a function of salinity. This work indicates copper is released into estuarine/marine waters from multiple wood treatments including lumber amended with nanoparticle size copper.
Keywords: Nanomaterials, copper, ionic copper, pressure treated lumber, leaching, environmental fate
INTRODUCTION
Engineered nanomaterials (ENMs) have unique physical-chemical properties that differ from conventional bulk materials, allowing for a range of evolving applications (Vollath 2013) and potential environmental concerns (Valsami-Jones and Lynch 2015). There are many types of ENMs including the carbon-based fullerenes, graphenes (Ren and Cheng 2014, Zurutuza and Marinelli 2014, Chee et al. 2015) and nanotubes (De Volder et al. 2013), metallic ENMs like metal oxides (e.g., TiO2) (Al-Kattan et al. 2014) and zero-valent metals (e.g., Ag0) (Zhang 2003), nanopolymers (Chee et al. 2015), and quantum dots (Rocha et al. 2017, Xiao et al. 2017). With the different types of ENMs as well as the variety within each type, there are seemingly endless applications for ENMs in medicine, technology, and consumer products. Many of these consumer products utilize metallic ENMs such as silver, titanium dioxide, and copper. Silver ENMs are commonly used as an anti-microbial agent in textiles (Benn and Westerhoff 2008, Mitrano et al. 2014) as well as in components of washing machines (Farkas et al. 2011), personal care products (Quadros et al. 2013, Fröhlich and Roblegg 2012), food (Fröhlich and Roblegg 2012), and toys (Quadros et al. 2013). Titanium dioxide ENMs are found in personal care products such as sunscreen and makeup (Weir et al. 2012), as well as food (Weir et al. 2012), paint (Al-Kattan et al. 2014), and other coatings (Shandilya et al. 2015). Although research on many metallic ENMs is abundant, less work has been focused on copper ENMs, which are currently used as additives in lubricants, polymers, and inks (Wang et al. 2005, Tang et al. 2010), in addition to being used as an anti-fouling wood preservative in paints and pressure treated lumber and agricultural herbicide (Chapman et al. 2013, Anyaogu et al. 2008, Matsunaga et al. 2011, Evans et al. 2008, Platten et al. 2015, Adeleye et al. 2016, Keller et al. 2017).
Although the addition of ENMs to pressure treated lumber is a relatively new application, the product itself is not. The American Wood Protection Association (AWPA), founded in 1904, provides standards for pressure treated lumber based on intended use, environment, and region to ensure effectiveness (www.awpa.com (viewed on 6 July 2017)). These standards include chemical type and amount for each use (e.g., above ground, ground contact, marine submersion). Previously, creosote and chromated copper arsenate (CCA) were used in pressure treated lumber for several decades in the United States. As of 2004, due to concerns with worker health and environmental impacts, the lumber and pesticide industry worked with the U.S. Environmental Protection Agency (EPA) to voluntarily restrict the use of CCA and creosote such that they were no longer available for residential use (https://www.epa.gov/ingredients-used-pesticide-products/chromated-copper-arsenate-cca (viewed on 6 July 2017)). In recent years, other governments have also restricted the use of CCA including Australia (https://apvma.gov.au/node/11751 (viewed on 19 December 2017)), the European Union (http://www.herinst.org/CCAtimber/regulations/Europe.html) (viewed on 19 December 2017)) and the United Kingdom (http://www.legislation.gov.uk/uksi/2007/1596/pdfs/uksi_20071596_en.pdf) (viewed on 19 December 2017)). The newer anti-fouling wood preservative treatments include alkaline copper quaternary (ACQ) and micronized copper azole (MCA) in which the copper is introduced into the lumber in copper oxide (CuO) or copper carbonate (CuCO3) form, respectively. Unlike most lumber treatments including CCA and ACQ, in which the particulate copper (e.g., CuO) is introduced and dissolves into an ionic form in aqueous solution intended to penetrate the wood cells, MCA is introduced to the lumber as an aqueous suspension of extremely fine CuCO3 particles in the nano-size range (McIntyre 2010, Platten et al. 2015).
While some research has been performed on the fate and effects of ENMs in the environment, and more specifically, the marine environment (e.g., Parks et al. 2013, Baker et al. 2014, Parks et al. 2014a,b, Wang et al. 2014a, Wang et al. 2014b, Rocha et al. 2015, Canesi and Corsi 2016, Minetto et al. 2016, Xiao et al. 2017), very little has been conducted on the fate and effects of copper ENMs in the marine benthic community (e.g., Ho et al. 2018).
Due to the applications of pressure treated lumber, the chemicals used for treatment are injected into the outer portion of the lumber and are therefore in direct contact with external environmental media (i.e., air, soil, sediment, water, organisms). Unlike CCA (Stook et al. 2005), MCA and ACQ treated lumber have not been extensively studied to determine the release (i.e., leachability) of copper over time into the marine environment. Particularly important with the MCA treated lumber is the form of copper released into the environment. Although the toxicity of ionic copper has long been well understood (Flemming and Trevors 1989, U.S. EPA 2007, 2016), information on the bioavailability, bioaccumulation and toxicity of nano- and microcopper sized-particles is limited. In addition to size, the rate of release could also be impacted by the lumber treatment type. For example, the MCA treatment chemical is mostly comprised of particles (68%) in the 10 to 700 nm range (McIntyre 2010, Platten et al. 2016), providing a mixture of micro- and nano-sized particles. Other metallic nanoparticles (e.g., silver) have been shown to leach metal ions from their surfaces over time rather than metal nanoparticles (Levard et al. 2013). Compared to traditional copper lumber treatments (i.e., CCA), the MCA treatment may demonstrate reduced rates of copper leaching and lower concentrations released. Hypothetically, ionic copper would need to not only leach out of the wood cells and into the environment, but first dissociate from the copper carbonate and diffuse to the particle surface. This type of treatment could potentially improve the life-span of the treated wood resulting in lower copper concentrations released over longer time periods. In principle, this would lead to a decrease in exposure to surrounding estuarine and marine ecosystems when compared to older conventional treatments.
At this time, CCA, ACQ and MCA treated lumber are all utilized in construction depending on the application. CCA continues to be used for marine submersion applications as it is still the only treatment effective in that environment (www.awpa.com (viewed on 3 August 2017)). MCA and ACQ are both available to the general public for private consumer use and are most often designated as above ground or ground contact lumber. The objective of the present study was to (i) compare the copper concentrations in the aqueous leachate and (ii) evaluate the rate of copper release from untreated (control), MCA-, ACQ-, and CCA-treated lumber in submerged aqueous environments (freshwater, estuarine and marine). Two treatment levels (low and high) corresponding to above ground and ground contact designations were used with MCA and ACQ for a total of six treatment groups. Each group was tested at multiple salinities to identify any differences between freshwater, estuarine and marine environments. Another goal of the present study was to develop what Hendren et al. (2015) designated as ‘functional assays’ to be used to measure fundamental information about the behavior of nanomaterials in the environment. Although most of these lumber treatments are rated for terrestrial use, the data collected in the present study will provide information about a potential worst-case scenario such as prolonged flooding, unintended home or commercial use, or improper disposal, as well as determining if there are any ENM-specific effects on copper leaching from treated lumber.
MATERIALS AND METHODS
Pressure treated lumber materials and copper distributions
The lumber materials used in this study were available commercially from local franchises of national United States distributors (e.g., Ace Hardware, Home Depot, Lowe’s). Descriptions of each type and the dimensions of lumber can be found in Table 1. Of the treatment types, ACQ and CCA are aqueous treatments (i.e., copper was introduced to the lumber in an ionic form) that are not reported to contain nano- or micro-particle copper (Platten et al., 2015). MCA is the only treatment reported to contain nano- and micro-particle copper (McIntyre 2010, Platten et al. 2015). When possible, low and high concentrations of a given treatment type were evaluated. Because the treatment amounts reported by the manufacturers were not copper-specific, subsurface samples (i.e., wood plugs) of each lumber were digested and analyzed by an inductively coupled plasma atomic emission spectroscopy (ICP-AES) for total copper concentrations (Table 1). This was performed on segmented cores and sawdust for each material.
Table 1.
Description of control and pressure treated lumber materials used in this investigation. The micronized copper azole (MCA) treatments are reported to contain copper particles in the nano-size range (10 to 700 nm) (McIntyre 2010, Platten et al. 2016).
| Treatment | Abbreviation | Lumber typea | Lumber Dimensions (cm × cm × cm) |
Sawdust Surface Area (m2/kg)b | Intended use | Manufacturer listing of copper form (based on MSDS) | Reported Amount (kg/m3)c (mg/kg)d |
Measured wood core copper concentration (mg Cu/kg lumber)e | Measured sawdust copper concentration (mg Cu/kg lumber)f |
|---|---|---|---|---|---|---|---|---|---|
| Untreated | Control | SYP #2 | 3.8 × 8.9 × 240 | - | NAg | NA | 0 - |
-- | 0.21±0.20 |
| Micronized copper azole | MCA low | SYP #1 | 3.8 × 8.9 × 240 | 57 ± 14 | Above ground | Copper carbonate expressed as elemental copper | 0.96 (2290)b |
763±284 | 1050±57 |
| Micronized copper azole | MCA high | SYP #2 | 8.9 × 8.9 × 240 | 224 | Ground contact | Copper carbonate expressed as elemental copper | 2.4 (5710)b |
2130±787 | 2560±46 |
| Chromated copper arsenate | CCA | SYP #2 | 6.4 × 19 × 490 | 215 ± 232 | Submergible | Copper oxide | 40 (95200)b |
1930±1660 | 2210±30 |
| Alkaline copper quaternary | ACQ low | SYP #2 | 3.8 × 14 × 240 | 132 | Above ground | Copper complex expressed as copper oxides | 0.3 (714)b |
333±73 | 286±9 |
| Alkaline copper quaternary | ACQ high | SYP #2 | 6.4 × 19 × 310 | 267 ± 177 | Ground contact | Copper complex expressed as copper oxides | 9.6 (22900)b |
1670±1140 | 4720±175 |
a SYP = Southern Yellow Pine; #1 and #2 indicate the quality of the lumber (i.e., #1 is free of knots and other imperfections compared to #2).
b Surface areas for the lumber blocks was approximately 0.6 m2/kg.
c kg/m3 where kg is the total treatment chemical per cubic meter of lumber. Data provided by manufacturer.
d Amount expressed as mg total treatment chemical per kilogram of lumber based on data provided by manufacturer.
e Wood core concentration based on outer 2 cm sections used during the leaching study; the mean ± standard deviation is reported.
f Sawdust was made using all sections of the lumber; the mean ± standard deviation is reported.
g Not applicable.
Segmented wood cores were collected at 1 cm intervals in three dimensions along a plane through the lumber to assess the total copper distribution. Sawdust was created by repeatedly thinly slicing the outer surface of the lumber with a rotary power saw. The sawdust was collected by attaching a clean, polyethylene bag to the sawdust exhaust vent. The wood digestion method was adapted from Watmough and Hutchinson (1996). Sawdust samples and sawdust standard reference material (ERM® CD-100, BAM) digests contained 0.75 to 0.85 grams of sawdust. Samples were added to pre-weighed glass test tubes and dried in the oven for 48 hours at 70 ºC. Three empty glass test tubes were used as method blanks. After obtaining the dry weight, samples were ashed in a muffle furnace at 400 ºC for eight hours. Once cooled, the mass of the ash was recorded. Concentrated nitric acid (2.5 mL) was added to each sample and allowed to digest at room temperature for 24 hours. The digests were then put on a heating block at 80 ºC for four hours. Once cooled, samples were either gravity filtered through a number 42 Whatman filter paper or filtered through a 0.45 μm centrifugal filter (30 minutes at 4000 × g, Pall Macrosep® with Supor® membrane). Ultrapure Milli-Q water was used to rinse the digestion tubes three times into the filter. The filtrate was diluted gravimetrically with Ultrapure Milli-Q water up to 50 g, and then analyzed using ICP-AES for total copper.
Leaching study design
Following the optimization studies (see Supplemental Information), in order to compare the magnitude and rates of copper release between each type of pressure treated lumber, a 133-day static leaching study was performed on the lumber blocks with volume replacement of samples collected for chemical analysis. Each lumber treatment (i.e., untreated, MCA low, MCA high, ACQ low, ACQ high, and CCA) was evaluated in aqueous media consisting of 0 (Milli-Q water), 1, 10, and 30‰ filtered seawater (5 μm) (FSW, Narragansett Bay, RI, USA). Selected lumber blocks (2 cm × 2 cm × 2 cm, approximately 4 grams) were cut from the outer face of the lumber in order to provide an environmentally realistic exposure surface. A 5 mm deep hole was drilled into each block using a 3.2 mm drill bit. Each block was fitted with a 6–32 × 12.7 mm nylon binding machine screw and a 20.3 cm nylon cable tie cut down to 8.5 cm, and placed into a 250 mL polyethylene bottle with 250 mL of Milli-Q water or FSW (Supplemental Information Figure S1). Four lumber blocks were cut for each treatment and matrix pairing, with three sampled for aqueous copper concentration determination over time and one sampled for total organic carbon (TOC) concentration measurements over time. Bottles were mixed on a bench-top platform orbital shaker (New Brunswick™ Innova® 2000) at 110 rpm for the entirety of the experiment. Sampling, 16 or 24 mL per replicate, with equal volume replacement of Milli-Q water or FSW occurred at 8 hours, 1 day, 2 days, 7 days, 14 days, 28 days, and 133 days. Subsamples taken at each time point included water for aqueous TOC analysis, as well as unfiltered water for ICP-AES copper analyses. Ambient pHs of the aqueous samples were measured at the conclusion of the investigation using an Orion electrode and meter (ThermoFisher, Beverly, MA, USA). All ICP-AES samples were acidified with concentrated nitric acid to attain a final concentration of 2.5% nitric acid v/v on the day of sampling and stored in 15 mL metal-free, polypropylene tubes in the dark at 4°C until analysis.
A second leaching study was performed under the same conditions described above using sawdust produced from the MCA low, MCA high, ACQ low, ACQ high, and CCA lumber to compare the copper concentrations and release rates to that of the lumber blocks. Approximately the same mass of lumber sawdust was used (4 grams) and added to 250 mL of aqueous media (i.e., 0, 1, 10, and 30‰ FSW). Sampling with volume replacement occurred from exposure bottles placed on their sides at 8 hours, 1 day, 2 days, 3 days, 7 days, 17 days, and 28 days. During sampling, care was taken to avoid collecting any sawdust inadvertently (e.g., water was withdrawn extremely slowly). To insure inadvertent sampling of sawdust would not affect copper measurements, all samples were filtered using 0.45 μm syringe filters with a polyethersulfone membrane to remove any suspended sawdust prior to acidification. Aqueous TOC was measured at the end of this study for the 0, 1, and 10‰ filtered FSW treatments. The entire volume of the 30‰ treatment was needed for a separate study.
Total organic carbon and surface area analyses
In general, 9 mL of water was sampled at every time point, and analyzed with a Shimadzu TOC-VCPH/CPN Total Organic Carbon Analyzer (Kyoto, Japan) for an operationally-defined aqueous total organic carbon (TOC) measurement. This measurement includes both the dissolved form of organic carbon as well as any carbon associated with very small wood particles inadvertently collected in the samples. However, based on the data described below, we suspect the aqueous phase was dominated by the dissolved form of TOC with particulates making little contribution. A potassium hydrogen phthalate solution was used to calibrate the instrument. Sawdust samples were analyzed for BET surface area using a Quantachrome Instruments Nova 2000e (Boynton Beach, FL, USA).
ICP-AES analysis
To determine the total copper concentrations, each acidified subsample was analyzed for copper at the 324.754 nm emission line against an external calibration using a Horiba Jobin Yvon Ultima 2 ICP-AES equipped with an AS500 autosampler. A single element copper ICP standard (Ultra Scientific, North Kingstown, RI, USA), certified value of 1001 ± 2 mg/L) was used to make calibration standards at 0.12, 1.26, 5, 20, and 50 mg/L in 0, 1, 10, and 30‰ filtered FSW and 2.5% nitric acid. Quality control was maintained by analyzing one of the calibration standards every 10 to 15 samples. These calibration checks did not deviate more than 20% from the reported value with a majority deviating less than 10%. Recoveries for the sawdust SRM were measured on three occasions and averaged 82.7% (± 14.5%) falling within our acceptability range of 80% to 120% based on recommendations from U.S. EPA method 6010C. The copper concentration in each sample was based on an average of three measurements by the instrument. The instrument detection limit (dl) for copper was 2 μg/L with the limits of quantification three to five times the dl.
Statistical Analysis
Statistical analyses were performed using JMP Pro 12.1.0 (SAS Institute Inc). A one-way analysis of variance (ANOVA, p<0.05) was conducted to determine if the measured copper concentration released for each treatment and salinity significantly differed over time. Copper release rate constants (/day) and equilibrium concentrations (mg/L) were estimated based on fitting a non-linear first-order model to the copper concentration data versus time by salinity based on Lin and Wang (2009). A one-way ANOVA (p<0.05) was used to determine significant differences in modelled release rate constants and equilibrium concentrations by salinity for each lumber treatment and by lumber treatment at each salinity. If significant, a Tukey-Kramer HSD post-hoc test was used to determine the identity of the specific differences. Unless otherwise noted, data are reported as mean ± standard deviation for a triplicate sample.
RESULTS AND DISCUSSION
Pressure treated lumber materials and copper distributions
Each treated lumber contained measured total copper concentrations in the high parts-per-million range for the wood core (333 – 2130 mg/kg) and sawdust digests (286 – 4720 mg/kg) (Table 1). With the exception of the ACQ high lumber, the sawdust and core copper concentrations were comparable (i.e., values within 30% of each other). Sawdust digests provided more precise mean values. This is likely due to the sawdust being collected in one batch, homogenized, and sub-sampled for analysis. For MCA lumber, based on the manufacturer provided data, the high treatment contained approximately 2.5 times the amount of copper as the low treatment, which was supported by similar ratios for the copper content in our measured digests. This relationship did not hold true for ACQ treated lumber, which had a copper ratio of 1:32 low:high based on the manufacturer’s MSDS, but an average ratio of 1:16 low:high based on the copper content measured in the sawdust. Although CCA lumber had the highest reported concentration (40 kg/m3) based on the manufacturer data, the amount of measured copper in the lumber was similar to the MCA high treatment; for example, 1930 versus 2130 mg/kg for CCA and MCA, respectively, for lumber, and 2210 versus 2560 mg/kg for CCA and MCA, respectively, for sawdust. Direct comparison of the manufacturer amount data expressed in mg/kg (Table 1) to measured values, in mg copper/kg, was problematic, with the amount values being higher than the measured values for every type of treatment. This discrepancy likely occurs because it is uncertain what exact form of copper the manufacturers reported in their MSDSs.
Qualitatively, the cross-sectional photographs of the lumber provided insight into the variation in treatment concentration and distribution (SI Figure S2). Wood cores were sectioned and digested to provide quantitative support to those observations (SI Figure S2). MCA low and high lumber had relatively consistent amounts of copper throughout with 550 to 1000 mg/kg and 1300 to 2800 mg/kg, respectively (SI Figure S2a, S2b). ACQ low had the lowest measured copper in the very center (139 mg/kg) but the outer edges ranged from 300 to 400 mg/kg (SI Figure S2c). ACQ high and CCA provided the most drastic visual confirmation of copper concentrations (SI Figures S2d, S2e) with the treatment most visible on the outer edges of the cross-sections. Measured copper concentrations reflected those visual trends with relatively lower concentrations in the centers of these two treatments compared to the outer edges: 720 (center) to 3000 mg/kg for ACQ high and 700 (center) to 4100 mg/kg for CCA. This combination of analytical copper measurements and visual information demonstrate that, in general, for aqueous environmental exposures, the water-outer lumber surface interface is where the greatest exposure concentrations will likely occur, as opposed to exposure via a deep crack in the lumber exposing the interior surfaces.
Magnitude of copper release
For the lumber blocks, over the first 28-day time period, copper was detected above background in the leachate of each treatment group, excluding the control (Figure 1). No copper was detected in the untreated control lumber aqueous media. Copper concentrations showed a gradual increase in the aqueous media with increasing time until a concentration plateau was established. Copper concentrations ranged from approximately 5 mg/L up to 15 mg/L after 28 days with little evidence of those concentrations changing with time up to 133 days except for two treatments (SI Figure S3). For ACQ high and CCA (SI Figure S3 c,e), the concentrations of copper increased with time in the higher salinity aqueous media (e.g., 30‰), reaching levels of up to 20 mg/L after 133 days. The magnitude of total copper released from the lumber was directly related to the amount of copper (mg/kg) measured in the lumber (Table 1). MCA high lumber had approximately 2.5 times more treatment chemical than MCA low and the leaching results showed a difference of approximately 1.4 times more copper in the high compared to the low. CCA and MCA high had similar, although not identical, copper concentrations in the digested wood cores, which were reflected by similar total copper concentrations in the leachate. However, when comparing the copper release in different aqueous media there was a reproducible trend. Frequently, the intermediate (i.e., estuarine) salinities (1 and 10‰) demonstrated slightly higher copper concentrations than the deionized water (0‰) and seawater salinity (30‰) (Figure 2). However, as noted above, for ACQ high and CCA, the higher salinity treatments released more copper after 133 days of leaching (Figure S3). For a relative comparison, Stook et al. (2005), using a leaching system based on the U.S. EPA toxicity characteristic leaching procedure (TCLP), and arguably a harsher design than the one described here (and therefore not directly comparable), reported copper concentrations of 5 to 10 mg/L released from CCA in an artificial seawater (~3.2 ‰) and DI solution. They also found copper concentrations of 30 to 40 mg/L for ACQ in the same matrices.
Figure 1.
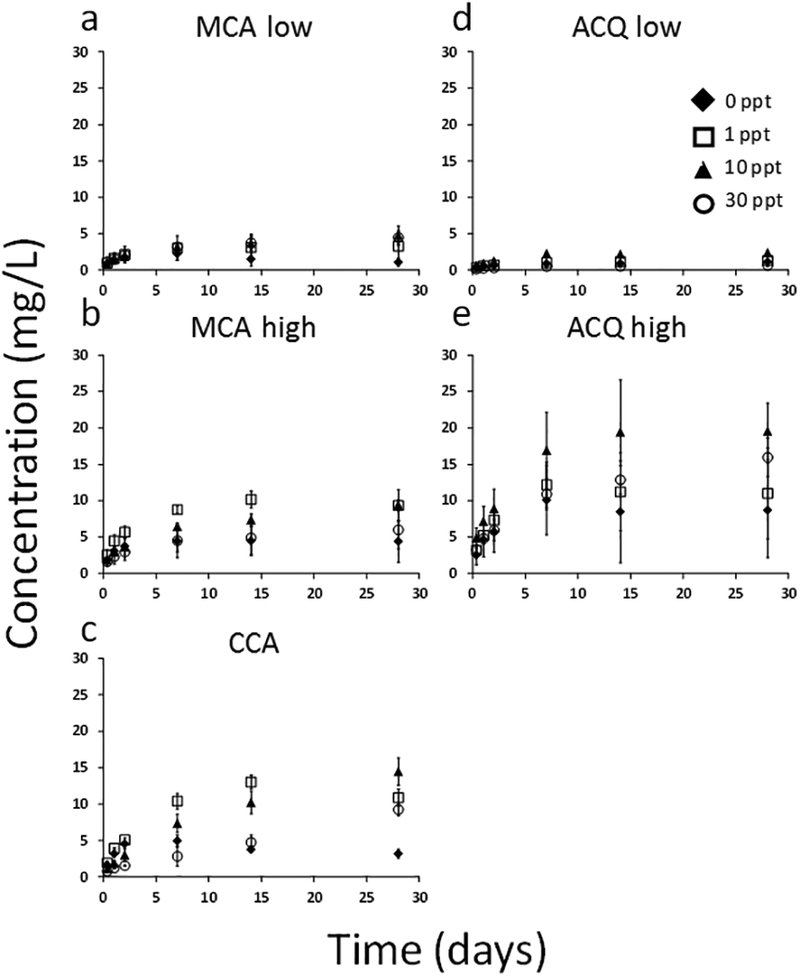
Concentration of copper released from pressure treated lumber blocks over 28 days under four different salinities. Total copper released from MCA low (a), MCA high (b), CCA (c), ACQ low (d), and ACQ high (e). Salinity is expressed as parts per thousand (ppt).
Figure 2.
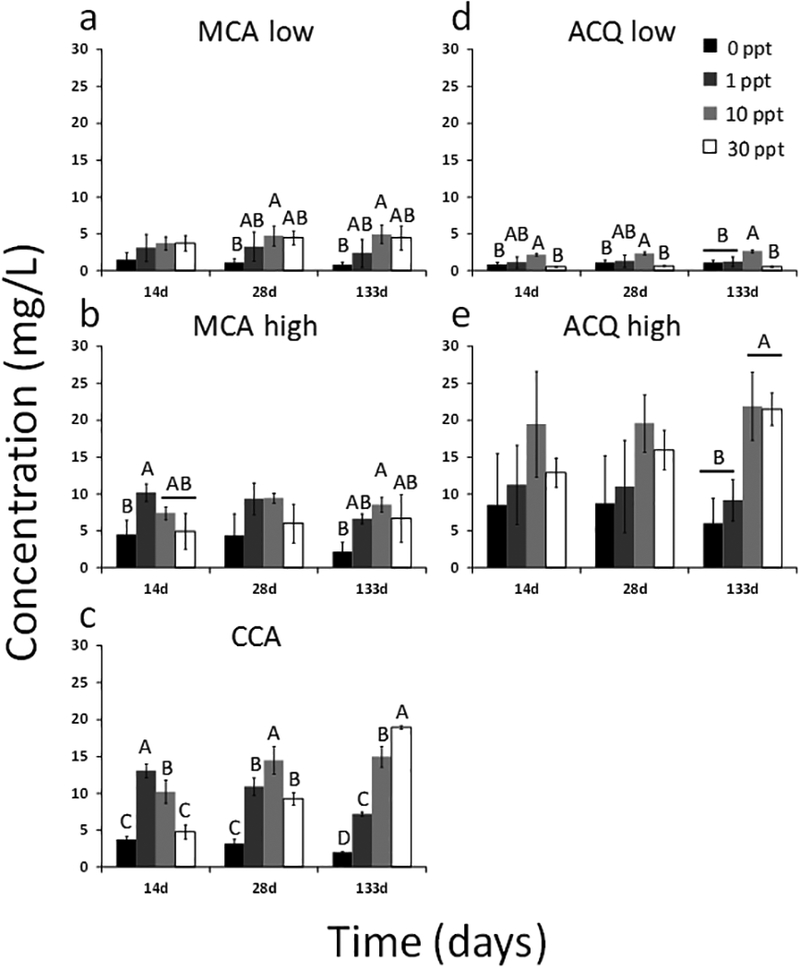
Statistically significant differences in copper concentrations in the lumber block leachate due to salinity for MCA low (a), MCA high (b), CCA (c), ACQ low (d), and ACQ high (e) on days 14, 28, and 133. Different letters denote significant differences between salinities within a given sampling time. Salinity is expressed as parts per thousand (ppt).
During the study, the pH of the water columns decreased compared to the pHs of the initial aqueous media. For example, the 30‰ aqueous media pH was 7.72 and it decreased in the leaching system by 1.70±0.30 by the end of the study. Similarly, deionized water, 1‰ and 10‰ showed decreases in pH of 0.33±0.86, 1.92±0.76 and 2.04±0.46, respectively, compared to initial aqueous media pHs of 5.50, 6.88 and 7.49, respectively. The lower salinity treatments demonstrated the most substantial and variable changes in pH, reflecting their limited buffering capacity to the acidic groups released from the lumber (e.g., carboxylic and benzoic acids) and CO2 from the atmosphere compared to the 10‰ and 30‰ treatments. For example, the deionized water treatment showed a range in mean pH change of −1.02 to 1.38 relative to the media pH.
When statistical differences did exist, in 52% of comparisons of the treatment without any salinity (0 ppt) to treatments with salinity (1, 10, 30 ppt), the presence of salinity resulted in greater copper release than in the deionized water (Figure 2). For example, in the MCA low, after 28 and 133 days, the 10‰ had significantly higher copper concentrations than the deionized water but not different from the 1 or 30‰ treatments. In contrast, as noted, the deionized water, which was an imperfect surrogate for natural freshwater, never released more copper than the saline treatments. The actual differences in leaching copper concentrations, when significant, were up to approximately a factor of 10× for CCA (Figure 2c). For MCA low and high, the differences were about a factor of 2 to 4 (Figure 2 a, b). These results suggest that over a month, the intermediate estuarine salinities (i.e., 1 and 10 ppt) significantly increased the release of copper, as compared to the marine salinity (30 ppt), from the types of pressure treated lumber studied in this investigation. However, after longer durations, the ACQ high and CCA did release more copper into higher marine salinity water.
The trend discussed above (i.e., Figure 2) regarding greater release of copper from the saline treatments was also evident when examining copper loss relative to the original copper concentrations in the lumber blocks (SI Figure S4). Overall, the total amount of copper released from the lumber blocks ranged from 5 to 55% of the total copper measured in the outer 2 cm core sections (Figure S4). Typically, a greater percentage of copper was present in the leachate under saline conditions (1, 10, or 30 ‰), with the exception of MCA high and ACQ low. Of all the treatment types, ACQ high released the highest percent copper (25 to 55%) with most of the other treatments reaching a maximum release of 25 to 40%.
Unlike the lumber blocks with their slow release, sawdust provided a rapid pulse input of copper to the aqueous media early in the study (Figure 3). Like the lumber blocks, no copper was detected in the control sawdust aqueous media. Following the initial pulse, concentrations decreased and then levelled. This may be due to the higher exposed surface area of the sawdust particles compared to the lumber block surfaces. For example, sawdust surface areas ranged from 57 to 267 m2/kg while the surface areas for the lumber blocks were only approximately 0.6 m2/kg (Table 1). The magnitude of total copper released from the sawdust was slightly less than that released by the lumber blocks but followed a similar trend with values ranging from approximately 2 to 4 mg/L, except for ACQ high which released the highest copper concentrations ranging from approximately 8 to 16 mg/L. Some statistically significant differences in copper concentrations as a function of salinity were observed, as noted with the lumber blocks. In many cases, the intermediate salinities (1 and 10‰) demonstrated higher copper concentrations than the deionized water (0‰) and seawater salinities (30‰). This trend was evident for MCA low, MCA high and ACQ low (Figure 4a, b, d). Contradicting this observation was the ACQ high treatment (Figure 4e), which showed that after 17 and 28 days, releases into the higher salinity media (e.g., 30‰) were the largest. This trend was also observed for ACQ high in the lumber block studies (Figure 2).
Figure 3.
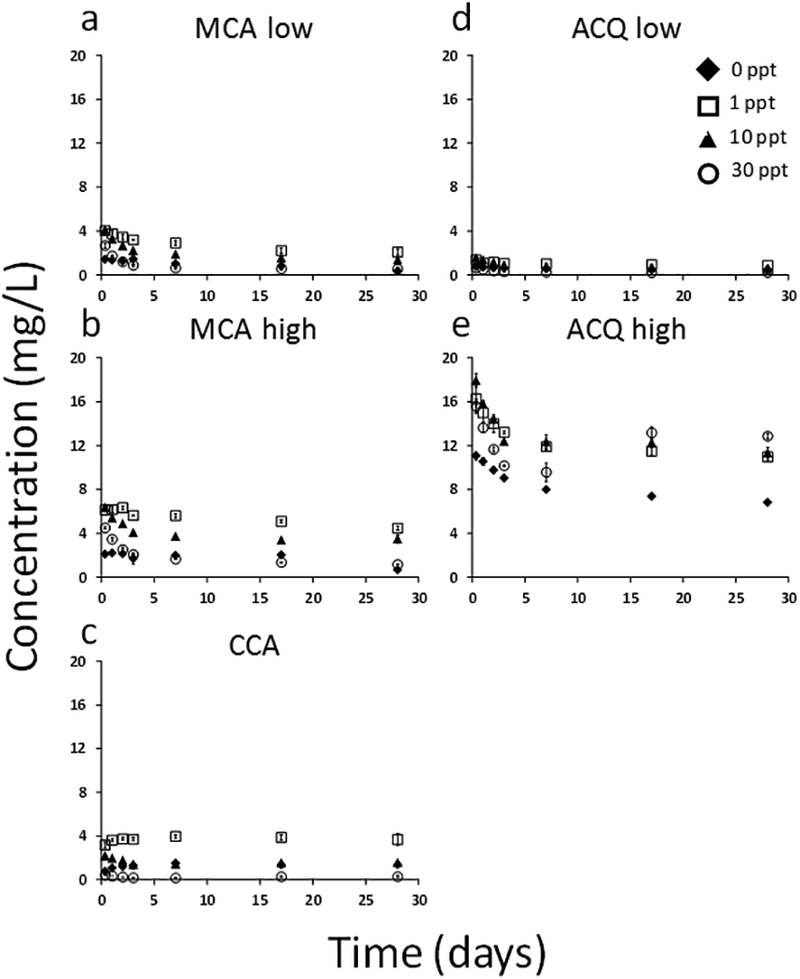
Concentration of copper released from sawdust prepared from pressure treated lumber over 28 days under four different salinities. Total copper released from MCA low (a), MCA high (b), CCA (c), ACQ low (d), and ACQ high (e). Salinity is expressed as parts per thousand (ppt).
Figure 4.
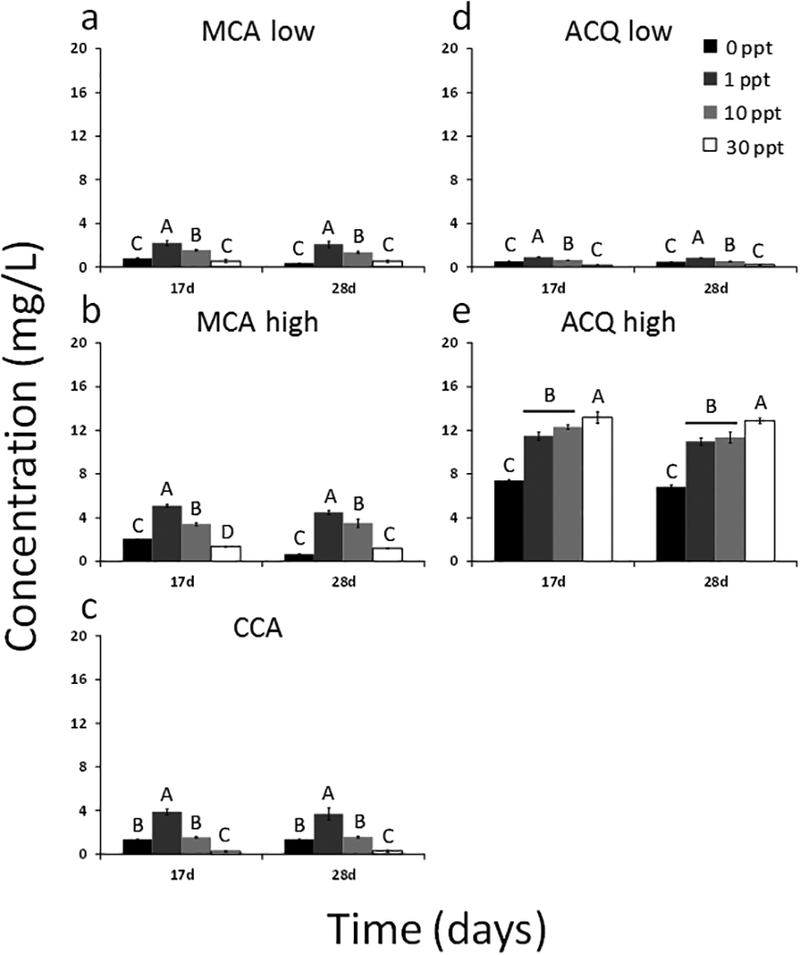
Significant differences in copper concentrations in the sawdust leachate due to salinity for MCA low (a), MCA high (b), CCA (c), ACQ low (d), and ACQ high (e) on days 17 and 28. Different letters denote significant differences between salinities within a given sampling time. Salinity is expressed as parts per thousand (ppt).
Overall, the amount of copper released from the sawdust ranged from 1 to 20% of the total copper measured in the sawdust (SI Figure S5). Like the lumber blocks, more copper was present in the leachate for intermediate salinities (1‰ and 10‰). However, unlike the lumber blocks, the ACQ low treatment released the most copper in the 1‰ salinity media. In contrast, ACQ high released consistently larger percentages of total copper in the 1, 10, and 30‰ salinity media with values ranging from 15 to 17%. After 17 days, the percent of total copper released from MCA low and MCA high sawdust was nearly identical. This observation held true for each salinity.
As noted, in many cases, copper concentrations released from the sawdust decreased slightly after the first day (Figure 3e illustrates this observation). As noted earlier, mechanistically, this could result because of the higher surface area of the sawdust particles compared to the lumber blocks (approximately a factor of 450 difference) (Table 1), providing sites for copper to re-associate via chelation to the exposed woody organic material. In addition, we observed, in several of the lumber samples, significantly elevated copper concentrations in the aqueous phase with estuarine salinities (1 and 10‰) compared to the deionized water treatment (Figures 2 and 4). This may reflect copper associated with the copper carbonate and copper oxide originally used in the pressure treated lumber (Table 1) being displaced by the presence of the much higher concentrations of naturally-occurring cations in seawater (e.g., sodium, calcium). Salinity has been shown to be a key variable in explaining the behavior of nanocopper in aquatic systems (Adeleye et al. 2014, 2016, Conway et al. 2015). Hypothetically, if this mechanism does explain the elevated copper concentrations under estuarine salinities, we might also expect elevated copper concentrations in the marine salinity treatments (30‰). However, this was not always observed and, in some cases, the copper concentrations in the 30‰ treatments were significantly less than the estuarine salinities (1‰ and 10‰). In principal, however, the duration of these studies may have been too brief for us to observe the thermodynamically stable final distributions of copper in the different salinities. Some of the treatments, like ACQ high and CCA, did demonstrate higher copper concentrations in the 30‰ treatments after 133 days (Figures 2, S4), suggesting that under completely equilibrium conditions, the seawater salinity treatments may ultimately host the highest copper concentrations.
Finally, as noted above, the pHs of the aqueous phases in the leaching systems were observed to decrease in the study including in the untreated control lumber treatment. Although pH was not controlled in this investigation, the decrease was expected because of the anticipated release of organic acidic groups from the lumber blocks. The lowest pHs were in the deionized water and 1‰ treatments with final values around 5, which may be expected to result in enhanced copper release from the blocks. However, the lowest concentrations of released copper were observed in the deionized water treatment (Figure 2), suggesting pH was not affecting copper release substantially. Conversely, the 1‰ treatment demonstrated some instances of elevated copper release.
Rates of copper release
For the lumber blocks, copper concentrations in all of the leachates increased over time until levelling between 7 and 28 days (Figure 1). A non-linear first-order kinetic model taking into consideration initial copper concentrations was fit to the concentration data versus time to determine the rate constants and estimated equilibrium concentrations for copper release from the lumber blocks under each experimental condition (lumber and aqueous salinity). Overall, the summary statistics demonstrated good model fits (SI Table S1) with elevated r2 values, significant regressions, and very few failures to meet model assumptions. Total copper release rate constants ranged from 0.026 1/d (CCA 30‰) to 2.71 1/d (MCA low 0‰). As a function of salinity by wood treatment, in only two cases were there statistically significant differences between rate constants. Specifically, the MCA low and CCA 0‰ treatments were significantly larger than the other salinities (Table 2). For the other salinities within a given treatment, no differences were detected between rate constants (Table 2). As a function of salinity by wood treatment, modelled equilibrium copper concentrations based on the non-linear first-order model were often larger for the estuarine salinities than the freshwater or marine salinities (Table 2). This finding agreed with the higher measured copper concentrations in the estuarine salinities discussed earlier. For two of the wood treatments (MCA high and ACQ low), at least one of the estuarine salinities equilibrium concentrations was statistically significantly greater than the fresh and marine salinities equilibrium concentrations. The wood treatment, CCA, experienced a poor model fitting in one of the 30‰ salinity replicates which compromised the statistical analysis (Table 2). When the compromising 30‰ replicate data was removed from the analysis, the estuarine and marine salinities had statistically larger equilibrium concentrations than the freshwater.
Table 2.
Predicted release rate constants and equilibrium concentrations of copper from five types of pressure treated lumber blocks as a function of salinity based on a non-linear first-order model. Salinity is expressed as parts per thousand (‰).
| Treatment | Salinity (‰) | Copper release rate constant (1/d) | Significance groupinga | Equilibrium concentration (mg/L) | Significance groupinga |
|---|---|---|---|---|---|
| MCA low | 0 | 2.71 ± 1.13 | A | 1.68 ± 0.76 | NS |
| 1 | 0.79 ± 0.15 | B | 3.15 ± 1.86 | ||
| 10 | 0.29 ± 0.09 | B | 4.25 ±1.10 | ||
| 30 | 0.44 ± 0.12 | B | 3.94 ± 0.83 | ||
| MCA high | 0 | 1.82 ± 1.49 | NS | 4.47 ± 1.96 | B |
| 1 | 0.57 ± 0.07 | 9.48 ± 1.25 | A | ||
| 10 | 0.32 ± 0.11 | 8.31 ± 0.97 | AB | ||
| 30 | 0.58 ± 0.17 | 5.22 ± 2.49 | AB | ||
| CCA | 0 | 1.66 ± 0.27 | A | 4.12 ± 0.50 | A |
| 1 | 0.33 ± 0.05 | B | 12.0 ± 0.15 | A | |
| 10 | 0.09 ± 0.04 | B | 16.0 ± 2.62 | A | |
| 30 | 0.03 ± 0.03 | B | 1400 ± 2400b | A | |
| ACQ low | 0 | 0.57 ± 0.24 | NS | 1.00 ± 0.25 | B |
| 1 | 0.49 ± 0.43 | 1.24 ± 0.72 | B | ||
| 10 | 0.49 ± 0.12 | 2.31 ± 0.13 | A | ||
| 30 | 0.53 ± 0.06 | 0.59 ± 0.04 | B | ||
| ACQ high | 0 | 0.78 ± 0.29 | NS | 9.09 ± 6.17 | A |
| 1 | 0.73 ± 0.31 | 11.6 ± 5.22 | A | ||
| 10 | 0.37 ± 0.02 | 19.2 ± 5.44 | A | ||
| 30 | 0.28 ± 0.10 | 14.4 ± 2.24 | A |
NS = not a statistically significant difference between salinities within a treatment.
a Different letters denote significant differences between salinities with a treatment. Treatments sharing two letters (e.g., ‘AB’) are not different from either salinities ‘A’ or ‘B’.
b Poor model fitting.
Comparisons between wood treatments by salinity found no differences between rate constants in the 0‰ and 1‰ salinities (SI Table S2). However, in the 10‰ salinity, the CCA rate constant was statistically lower than the ACQ low and high and MCA high treatments while MCA low was not statistically different from any other treatment (SI Table S2). At the highest salinity evaluated (30‰), the CCA rate constant was not significantly different from ACQ high but was statistically lower than the MCA low and high and ACQ low rate constants. The ACQ high rate constant was significantly lower than MCA high but not different from ACQ low or MCA low (SI Table S2). Despite the presence of statistical differences between rate constants by salinity (with very few differences within a wood treatment type), no clear trends were observed. This conclusion also holds for examining the estimated equilibrium concentrations between wood treatments one salinity at a time (SI Table S2). Several statistical differences were identified but few trends were clear.
Release rates for the copper in the sawdust leachate were not calculated due to the nearly instantaneous release observed in the measured leachate over time (Figure 3). For many treatments, the 8 hour or one-day sampling time resulted in the highest copper concentrations. Based on the lumber block data, the hypothesis that micronized copper in the MCA treated lumber would release total copper at a slower rate than conventional pressure treatments (i.e., in which copper is added in an ionic form, not as nanoparticles (e.g., CCA)) was not supported.
Aqueous copper concentrations and total organic carbon
In aquatic systems, total organic carbon (TOC) is an important environmental phase affecting the behavior of copper (Impellitteri et al., 2002). Specifically, ionic copper has a higher affinity for binding with TOC than do other divalent transition metals of toxicological concern like cadmium and nickel. Consequently, in this study, we examined the interaction of copper and aqueous TOC in the leaching systems. Total organic carbon was measured over the course of the leaching study performed on the lumber blocks (SI Figure S6) as well as at the end of the sawdust leaching study (SI Figure S7). In this study, the aqueous TOC was likely to consist of water soluble and acidic degraded forms of common wood organic molecules including cellulose, hemicellulose and lignins (Boerjan et al. 2003). The concentration of aqueous TOC in the lumber block leachates increased over time and levelled between 14 and 28 days, similar to the copper concentrations in the leachate. Since aqueous TOC was measured in one subsample per treatment per time (replicate samples were used for copper analyses), no statistical analysis could be performed to determine any possible differences in aqueous TOC released by salinity. In general, aqueous TOC concentrations, once stabilized, in each of the lumber block treatments were fairly similar, with control, MCA low, MCA high, ACQ low and CCA aqueous TOC concentrations having values of approximately 25 mg/L, 50 mg/L, 50 mg/L, 100 mg/L and 50 mg/L, respectively, regardless of salinity. The one exception, ACQ high showed at least two trends: the 0, 1 and 30‰ salinities had aqueous TOC values of about 200 mg/L while the 10‰ treatment was about 100 mg/L. Aqueous TOC released in the sawdust study ranged in concentration from 100 mg/L to approximately 300 mg/L with little apparent relationship to lumber treatment or salinity (Figure S7). The significance of TOC is its ability to chelate and accumulate ionic copper and potentially serve as a transport vector for copper and, possibly, nanocopper to the surrounding aqueous environment (Gustafsson and Gschwend 1997). For this research, copper was measured in the total form, making it difficult to distinguish which species of copper was being detected (i.e., ionic copper, nanocopper or copper-TOC complexes, or all of them).
Regression analysis of the total copper and aqueous TOC concentration relationships for the lumber blocks suggests that as the salinity increased, the significance of the interactions between these variables also increased. That is, as salinity increased so did the coefficient of determinations (r2) while the p-values decreased. Specifically, as salinity increased the correlation could explain more of the variability associated with the relationship and that relationship became increasingly more statistically significant. For example, for MCA low, the r2 values increased from 0.00, 0.22, 0.74 to 0.65 while the p-value decreased from 0.94, 0.03, <0.0001 and <0.0001 when going from 0‰ to 1‰ to 10‰ to 30‰ (SI Table S3, SI Figure S8). Other treatments demonstrated similar trends. If copper is interacting with aqueous TOC as it is released from the treated lumber, one potential environmental benefit is that copper associated with TOC has been shown to be far less bioavailable to aquatic organisms than the ionic form of copper, thus limiting the toxic effects (Sunda and Lewis, 1978), at least temporarily. The effect of dissolved organic carbon on nanocopper dissolution and bioavailability in aqueous systems is only now beginning to be understood (Jiang et al. 2017).
CONCLUSIONS
This investigation found copper was released from all of the copper-treated lumber, including the nanocopper treated lumber (i.e., MCA). Relative to marine systems, salinity did appear to be a significant factor in explaining the amount of copper released especially at estuarine salinities (1‰ and 10‰). The rate of copper release based on the modelled rate constants was not as clearly affected by salinity while the estimated equilibrium concentrations supported the measured concentration data. The role of pH on copper release was less clear and requires further inquiry. Given this finding, a critical area of future research is to better understand the form of copper released. As noted earlier, the fate and effects of the nanocopper is not well understood while our understanding of the fate and effects of ionic copper is fairly sophisticated (Flemming and Trevors 1989, U.S.EPA 2007, 2016). This includes data from freshwater leaching and dermal exposure studies with nanocopper treated lumber that found ionic copper and copper associated with macro-particles of wood was released (i.e., not nanocopper particles) (Platten et al. 2015, 2016). This suggests that if nanocopper is an environmental hazard, in some circumstances, it may be released at higher concentrations in estuarine environments which are recognized as containing particularly sensitive and often commercially important habitats (Kennish 2002). This research demonstrates that along with copper, TOC is also released from the pressure treated lumbers investigated. An understanding of the relationship between ionic copper, nanocopper and TOC in the marine systems investigated remains incomplete. However, regression analysis suggests that as salinity increases the interaction between released copper and TOC becomes stronger. The importance of this observation is not entirely clear but suggests that at higher salinities copper may associate with TOC at an accelerated rate representing another transport mechanism for copper in aquatic environments. Finally, as discussed, this research was performed in a ‘functional assay’ designed to determine under simplified conditions if copper was released from nanocopper treated lumber. The functional assay was not designed to and cannot address precisely the release of copper under actual environmental conditions which would result in water exchanges caused by currents, tides and additional sources of water including rainfall and snowmelt. To address those conditions a more elaborate and expensive investigation would need to be mounted. A key implication of the findings of the current investigation is that such a study is worth consideration with a focus on the form of copper released.
Supplementary Material
ACKNOWLEDGEMENTS
The authors appreciate the insightful comments on the draft manuscript by the internal reviewers Adeyemi Adeleye, Denise Champlin, Wayne Munns and Julia Sullivan, and two anonymous reviewers. In addition, Adam Kopacsi (Urban Contractors, Narragansett, RI, USA) is thanked for the preparation of the lumber blocks and sawdust and Sandra Fogg’s support of this research in the laboratory is greatly appreciated. Portions of this work was performed while ANP was a National Research Council post-doctoral research associate at the U.S. EPA’s ORD/NHEERL Atlantic Ecology Division (Narragansett, RI, USA).
Footnotes
This is NHEERL Contribution ORD-022606.
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the U.S. Environmental Protection Agency. The EPA does not endorse any commercial products, services, or enterprises.
REFERENCES
- Adeleye AS, Conway JR, Perez T, Rutten P, Keller AA. 2014. Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles. Environ Sci Technol 48:12561–12568. [DOI] [PubMed] [Google Scholar]
- Adeleye AS, Oranu EA, Tao M, Keller AA. 2016. Release and detection of nanosized copper from a commercial antifouling paint. Wat Res 102:374–382. [DOI] [PubMed] [Google Scholar]
- Al-Kattan A, Wichser A, Zuin S, Arroyo Y, Golanski L, Ulrich A, Nowack B. 2014. Behavior of TiO2 released from nano-TiO2-containing paint and comparison to pristine nano-TiO2. Environ Sci Technol 48:6710–6718. [DOI] [PubMed] [Google Scholar]
- Anyaogu KC, Fedorov AV, Neckers DC. 2008. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 24:4340–4346. [DOI] [PubMed] [Google Scholar]
- Baker TJ, Tyler CR, Galloway TS. 2014. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut 186:257–271. [DOI] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. 2008. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Ann Rev Plant Biol 54:519–549. [DOI] [PubMed] [Google Scholar]
- Canesi L, Corsi I. 2016. Effects of nanomaterials on marine invertebrates. Sci Tot Environ 565:933–940. [DOI] [PubMed] [Google Scholar]
- Chapman J, Le Nor L, Brown R, Kitteringham E, Russell S, Sullivan T, Regan F. 2013. Antifouling performances of macro- to micro- to nano-copper materials for the inhibition of biofouling in its early stages. J Mater Chem B 1:6194–6200. [DOI] [PubMed] [Google Scholar]
- Chee WK, Lim HN, Huang NM, Harrison I. 2015. Nanocomposites of graphene/polymers: a review. RSC Advances 5:68014–68051. [Google Scholar]
- Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA. 2015. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ Sci Technol 49:2749–2756. [DOI] [PubMed] [Google Scholar]
- De Volder MFL, Tawfick SH, Baughman RH, Hart AJ. 2013. Carbon nanotubes: present and future commercial applications. Science 339:535–539. [DOI] [PubMed] [Google Scholar]
- Evans P, Matsunaga H, Kiguchi M. 2008. Large-scale application of nanotechnology for wood protection. Nature Nanotechnol 3:577–577. [DOI] [PubMed] [Google Scholar]
- Farkas J, Peter H, Christian P, Gallego Urrea JA, Hassellöv M, Tuoriniemi J, Gustafsson S, Olsson E, Hylland K, Thomas KV. 2011. Characterization of the effluent from a nanosilver producing washing machine. Environ Internat 37:1057–1062. [DOI] [PubMed] [Google Scholar]
- Flemming CA, Trevors JT. 1989. Copper toxicity and chemistry in the environment: a review. Water Air Soil Pollut 44:143–158. [Google Scholar]
- Fröhlich E, Roblegg E. 2012. Models for oral uptake of nanoparticles in consumer products. Toxicology 291:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson O, Gschwend PM. 1997. Aquatic colloids: concepts, definitions, and current challenges. Limnol Oceanogr 42:519–528. [Google Scholar]
- Hendren CO, Lowry GV, Unrine JM, Wiesner MR. 2015. A functional assay-based strategy for nanomaterial risk forecasting. Sci Total Environ 536:1029–1037. [DOI] [PubMed] [Google Scholar]
- Ho KT, Portis L, Chariton AA, Pelletier M, Cantwell MG, Katz D, Cashman M, Parks AN, Baguley JG, Conrad-Forrest N, Boothman W, Luxton T, Simpson SL, Fogg S, Burgess RM. 2018. Effects of micronized and nano-copper azole on marine benthic communities. Environ Toxicol Chem 37:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellitteri CA, Lu Y, Saxe JK, Allen HE, Peijnenburg WJGM. 2002. Correlation of the partitioning of dissolved organic matter fractions with the desorption of Cd, Cu, Ni, Pb and Zn from 18 Dutch soils. Environ Internat 28:401–410. [DOI] [PubMed] [Google Scholar]
- Jiang C, Castellon BT, Matson CW, Aiken GR, Hsu-Kim H. 2017. Relative contributions of copper oxide nanoparticles and dissolved copper to Cu uptake kinetics of Gulf killifish (Fundulus grandis) embryos. Environ Sci Technol 51:1395–1404. [DOI] [PubMed] [Google Scholar]
- Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, Cherr GN, Hong J, Gardea-Torresdey JL, Godwin HA, Hanna S, Ji Z, Kaweeteerawat C, Lin S, Lenihan HS, Miller RJ, Nel AE, Peralta-Videa JR, Walker SL, Taylor AA, Torres-Duarte C, Zink JI, Zuverza-Mena N. 2017. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7:28–40. [Google Scholar]
- Kennish MJ. 2002. Environmental threats and environmental future of estuaries. Environ Conserv 29:78–107. [Google Scholar]
- Levard C, Mitra S, Yang T, Jew AD, Badireddy AR, Lowry GV, Brown GE. 2013. Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ Sci Technol 47:5738–5745. [DOI] [PubMed] [Google Scholar]
- Lin J, Wang L. 2009. Comparison between linear and non-linear forms of psuedo-first-order and psuedo-second order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Engin China 3:320–324. [Google Scholar]
- Matsunaga H, Kataoka Y, Kiguchi M, Evans P. 2011. Copper nanoparticles in southern pine wood treated with a micronised preservative: nanodistirbution of copper in the pit membrane and border of an earlywood bordered pit. The International Research Group on Wood Protection; Queenstown, New Zealand. [Google Scholar]
- McIntyre CR. 2010. Comparison of micronized copper particles sizes. The International Research Group on Wood Protection; Biarritz, France. [Google Scholar]
- Minetto D, Volpi Ghirardini A, Libralato G. 2016. Saltwater ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C60 engineered nanoparticles: An overview. Environ Internat 92–93:189–201. [DOI] [PubMed] [Google Scholar]
- Mitrano DM, Rimmele E, Wichser A, Erni R, Height M, Nowack B. 2014. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 8:7208–7219. [DOI] [PubMed] [Google Scholar]
- Parks AN, Burgess RM, Ho KT, Ferguson PL. 2014a. On the likelihood of single-walled carbon nanotubes causing adverse marine ecological effects. Integr Environ Assess Manage 10:472–474. [Google Scholar]
- Parks AN, Chandler GT, Portis LM, Sullivan JC, Perron MM, Cantwell MG, Burgess RM, Ho KT, Ferguson PL. 2014b. Effects of single-walled carbon nanotubes on the bioavailability of PCBs in field-contaminated sediments. Nanotoxicology 8:111–117. [DOI] [PubMed] [Google Scholar]
- Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, Ho KT, Chandler GT, Ferguson PL. 2013. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ Toxicol Chem 32:1270–1277. [DOI] [PubMed] [Google Scholar]
- Platten W, Luxton TP, Harmon S, Sylvest N, Bradham K, Rogers K. 2015. Release of Micronized Copper Particles from Pressure Treated Wood Products. 600/R-14/365. U.S. Environmental Protection Agency, Office of Research and Development; Washington, DC, USA. [Google Scholar]
- Platten WE, Sylvest N, Warren C, Arambewela M, Harmon S, Bradham KI, Rogers K, Thomas T, Luxton TP. 2016. Estimating dermal transfer of copper particles from the surface of pressure-treated lumber and implications for exposure. Sci Total Environ 548–549:441–449. [DOI] [PubMed] [Google Scholar]
- Quadros ME, Pierson R, Tulve NS, Willis R, Rogers K, Thomas TA, Marr LC. 2013. Release of silver from nanotechnology-based consumer products for children. Environ Sci Technol 47: 8894–8901. [DOI] [PubMed] [Google Scholar]
- Ren W, Cheng H-M. 2014. The global growth of graphene. Nat Nanotechnol 9:726–730. [DOI] [PubMed] [Google Scholar]
- Rocha TL, Gomes T, Sousa VS, Mestre NC, Beianno MJ. 2015. Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: An overview. Mar Environ Res 111:74–88. [DOI] [PubMed] [Google Scholar]
- Rocha TL, Mestre NC, Sabóia-Morais SMT, Bebianno MJ. 2017. Environmental behaviour and ecotoxicity of quantum dots at various trophic levels: A review. Environ Internat 98:1–17. [DOI] [PubMed] [Google Scholar]
- Shandilya N, Le Bihan O, Bressot C, Morgeneyer M. 2015. Emission of titanium dioxide nanoparticles from building materials to the environment by wear and weather. Environ Sci Technol 49:2163–2170. [DOI] [PubMed] [Google Scholar]
- Stook K, Tolaymat T, Ward M, Dubey B, Townsend T, Solo-Gabriele H, Bitton G. 2005. Relative leaching and aquatic toxicity of pressure-treated wood products using batch leaching tests. Environ Sci Technol 39:155–163. [DOI] [PubMed] [Google Scholar]
- Sunda WG, Lewis JAM. 1978. Effect of complexation by natural organic ligands on the toxicity of copper to a unicellular alga, Monochrysis lutheri. Limnol Oceanogr 23:870–876. [Google Scholar]
- Tang X-F, Yang Z-G, Wang W-J. 2010. A simple way of preparing high-concentration and high-purity nano copper colloid for conductive ink in inkjet printing technology. Colloid Surf A: Physico Engineer Aspects 360:99–104. [Google Scholar]
- U.S. EPA. 2007. Aquatic Life Ambient Freshwater Quality Criteria—Copper 2007 Revision. 822-R-07–001. Office of Science and Technology, Washington, DC, USA. [Google Scholar]
- U.S. EPA. 2016. Draft Aquatic Life Ambient Estuarine/Marine Water Quality Criteria For Copper - 2016. 822-R-03–026. Office of Science and Technology, Washington DC, USA. [Google Scholar]
- Valsami-Jones E, Lynch I. 2015. How safe are nanomaterials? Science 350:388–389. [DOI] [PubMed] [Google Scholar]
- Vollath D. 2013. Nanomaterials: An Introduction to Synthesis, Properties and Applications. John Wiley & Sons, New York, NY, USA. [Google Scholar]
- Wang X-L, Xu B-S, Xu Y, Yu HL, Shi PJ, Liu Q. 2005. Preparation of nano-copper as lubrication oil additive. J Centr S Univ Technol, 12:203–206. [Google Scholar]
- Wang H, Burgess RM, Cantwell MG, Portis LM, Perron MM, Wu F, Ho KT. 2014a. Stability and aggregation of silver and titanium dioxide nanoparticles in seawater: Role of salinity and dissolved organic carbon. Environ Toxicol Chem 33:1023–1029. [DOI] [PubMed] [Google Scholar]
- Wang H, Ho KT, Scheckel KG, Wu F, Cantwell MG, Katz DR, Horowitz DB, Boothman WS, Burgess RM. 2014b. Toxicity, bioaccumulation, and biotransformation of silver nanoparticles in marine organisms. Environ Sci Technol 48:13711–13717. [DOI] [PubMed] [Google Scholar]
- Watmough SA, Hutchinson TC. 1996. Analysis of tree rings using inductively coupled plasma mass spectrometry to record fluctuations in a metal pollution episode. Environ Pollut 93:93–102. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, Von Goetz N. 2012. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Ho KT, Burgess RM, Cashman M. 2017. Aggregation, sedimentation, dissolution, and bioavailability of quantum dots in estuarine systems. Environ Sci Technol 51:1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W-X. 2003. Nanoscale iron particles for environmental remediation: an overview. J Nano Res 5:323–332. [Google Scholar]
- Zurutuza A, Marinelli C. 2014. Challenges and opportunties in graphene commercialization. Nat Nanotechnol 9:730–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


