Abstract
Background/Objectives
Our aim was to validate recent epidemiologic trends and describe the distribution of TIGAR-O risk factors in chronic pancreatitis (CP) patients. Methods The NAPS-2 Continuation and Validation (NAPS2-CV) study prospectively enrolled 521 CP patients from 13 US centers from 2008 to 2012. CP was defined by definitive changes in imaging, endoscopy, or histology. Data were analyzed after stratification by demographic factors, physician-defined etiology, participating center, and TIGAR-O risk factors.
Results
Demographics and physician-defined etiology in the NAPS2-CV study were similar to the original NAPS2 study. Mean age was 53 years (IQR 43, 62) with 55% males and 87% white. Overall, alcohol was the single most common etiology (46%) followed by idiopathic etiology (24%). Alcohol etiology was significantly more common in males, middle-aged (35–65 years), and non-whites. Females and elderly (≥65 years) were more likely to have idiopathic etiology, while younger patients (<35 years) to have genetic etiology. Variability in etiology was noted by participating centers (e.g., alcohol etiology ranged from 27 to 67% among centers enrolling ≥25 patients). Smoking was the most commonly identified (59%) risk factor followed by alcohol (53%), idiopathic (30%), obstructive (19%), and hyperlipidemia (13%). The presence of multiple TIGAR-O risk factors was common, with 1, 2, ≥3 risk factors observed in 27.6, 47.6, and 23.6% of the cohort, respectively.
Conclusion
Our data validate the current epidemiologic trends in CP. Alcohol remains the most common physician defined etiology, while smoking was the most commonly identified TIGAR-O risk factor. Identification of multiple risk factors suggests CP to be a complex disease.
Keywords: Chronic pancreatitis, Epidemiology, Risk factors
Background
Chronic pancreatitis (CP) is a complex inflammatory disorder of the pancreas caused by a myriad of factors, which ultimately results in progressive pancreatic fibrosis leading to end-organ damage manifested as chronic pain, maldigestion, diarrhea, diabetes mellitus, and pancreas cancer [1–4].
Recent studies have improved our understanding of the current epidemiology of CP [5–12]. Increasing recognition of the genetic factors and the presence of disease in only a fraction of individuals with known environmental risks have led to the proposal of the Toxic Idiopathic Genetic Autoimmune Recurrent Obstructive [TIGAR-O] etiologic risk factor classification [13–15].
We organized the North American Pancreatitis Study 2 (NAPS2) with the overarching goal to better understand the complex environmental, metabolic, and genetic mechanisms underlying CP in human subjects [1]. Multiple reports from this cohort have been published [5, 16–21]. Patients in the current study, the NAPS2-CV (CV, continuation and validation), were ascertained to recruit additional subjects to increase sample size of the cohort for genome-wide association studies, candidate gene-based analyses, and additional molecular-based investigations. The aim of this study was to validate the prior NAPS2 study and current epidemiologic trends in chronic pancreatitis.
Methods
NAPS2 Program and NAPS2-CV Study
The NAPS2-CV study prospectively enrolled 521 CP subjects from 13 US centers (12 referrals) with specific interest in pancreatic diseases from 2008 to 2011. Enrollment criteria for the NAPS2-CV study were similar to the initial NAPS2 study. All subjects provided a blood sample or buccal swab using standard procedure for future genetic and serological analyses.
Participating Centers
All 13 participating centers enrolled CP patients. Of these, eight participated in the initial NAPS2 study (Brigham and Women’s Hospital, Dartmouth-Hitchcock, Indiana University, Medical University of South Carolina, Mayo Clinic Jacksonville, University of Michigan, University of Pittsburgh, St. Louis University) and five were new sites (Aurora Healthcare, University of Alabama at Birmingham, University of Florida, Griffin Hospital—Yale Affiliate, Virginia Commonwealth University). The study was approved by the institutional review board of individual participating centers, and all study subjects signed an informed consent before enrollment.
Definition of Chronic Pancreatitis
CP was defined by the presence of characteristic changes in abdominal imaging studies [computerized tomography (CT) scan, magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP)] (Cambridge classification) or endoscopic ultrasound (EUS; presence of 5 or more findings or presence of calcifications) or histology. Of the 521 CP patients in the final cohort, the number of patients fulfilling the entry criteria on individual studies was—CT scan (n = 323), ERCP (n = 176), MRI/MRCP (n = 194), EUS (n = 132), and histology (n = 25).
Patient and Physician Questionnaires
Patient and physician questionnaires were administered by a trained nurse coordinator. Information collected was similar to the initial NAPS2 study. Additional information was collected from all patients on pain symptoms (pain frequency and duration), McGill pain questionnaire [22], PROMIS pain impact score [23], on alcohol exposure (in the years before acquiring pancreatitis using quantity-frequency method), and on alcohol exposure in a subset of patients (lifetime drinking history) [24], and in a subset of patients on tobacco exposure (lifetime smoking history, exposure to second-hand smoke).
Etiology and Risk Factors for CP
In the initial NAPS2 study, the final etiology was assigned using a hierarchical algorithm based on information on the working diagnosis of CP (one or more potential diagnoses) identified by the enrolling physician. In the NAPS2-CV study, physicians were asked to choose one etiologic classification which in their opinion best explained the patients CP from the following list—alcohol, genetic, idiopathic (early-onset, late-onset; age of onset cut point of 35 years), obstructive, autoimmune, autoimmune-associated, post-necrotic, hypertriglyceridemia, and miscellaneous causes (e.g., trauma, post-ERCP, other). All physicians participating in the study are experts in pancreatic diseases and well aware of the workup, definitions, and classifications for etiologies of CP. The etiologic classification for each patient was thus based on the enrolling physician’s assessment of the patient’s history and work up until the time of enrollment.
Statistics
Descriptive analyses are presented as proportions for categorical data and mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous data. Bivariate comparisons were made using Chi-squared or Fischer’s exact test for categorical data, and Student’s t test was used for continuous data. Data were analyzed collectively and also after stratification by etiology and by enrolling centers. Data analysis was performed using GraphPad Quick Calcs; Open Epi; and SAS version 9.2 programs. Two-tailed p values <0.05 were considered statistically significant.
Results
NAPS2-CV Validates the Initial NAPS2 Cohort Demographics and Physician-Defined Etiologies
As listed in Table 1, the distribution of demographic factors and physician-defined etiology was similar between the NAPS2 and NAPS2-CV cohorts. Of the 521 CP patients in the NAPS2-CV cohort, 55% were male, 86% were white, and the median age at the time of enrollment was 53 years (IQR 43, 62). Similar to the NAPS2 cohort, overall, alcohol was the single most common physician defined etiology for CP (46%). Other etiologies in descending order were late-onset idiopathic (17%), genetic (10%), early-onset idiopathic (7%), obstructive (7%), autoimmune (2%) followed by various other causes (12%). When both idiopathic groups (early- and late-onset) were combined, 24% of the NAPS2-CV cohort had no clearly defined etiology for their CP, making it the second largest etiologic group after alcohol.
Table 1.
Comparison of demographic factors and physician-defined etiology NAPS2 and NAPS2-CV cohorts
| NAPS2 | NAPS2-CV | |
| Enrollment time period | 2000–2006 | 2008–2011 |
| Academic centers (n)a | 19 | 13 |
| Chronic pancreatitis subjects (n) | 540 | 521 |
| Median age at enrollment (years) (median, IQR) | 49 (39, 60) | 53 (43, 62) |
| Males (%) | 283 (53) | 287 (55) |
| White (%) | 455 (84) | 453 (87) |
| Physician-defined etiology groups in CP patients [n (%)] | ||
| Alcoholb | 240 (45) | 238 (46) |
| Idiopathicc | 154 (29) | 126 (24) |
| Genetic | 47 (9) | 51 (10) |
| Obstructive | 47 (9) | 36 (7) |
| Autoimmune | 12 (2) | 8 (2) |
| Other | 39 (7) | 62 (12) |
One additional primary care practice enrolled only control subjects
In NAPS2 study, alcohol etiology was assigned to patients in whom physicians indicated alcohol only as a working diagnosis or risk factor; in NAPS2-CV study, alcohol etiology includes patients where physicians specifically chose alcohol as the etiology
Idiopathic represents combined early- and late-onset idiopathic categories
NAPS2-CV Physician-Defined Etiologies Varied by Sex, Age, Race, and Participating Centers
Sex
When stratified by sex (Table 2), the three most common etiologies (alcohol, late-onset idiopathic, and genetic factors) remained the same for both genders, but other differences became more apparent and statistically relevant. Males were more likely than females to have alcohol etiology (58.2 vs. 30.3%, OR 3.2, 95% CI 2.2–4.7). Conversely, an obstructive etiology was observed more frequently in females (12.4 vs. 2.4%, OR 5.6, CI 2.3–14.4), and late-onset idiopathic was observed more frequently in females (22.5 vs. 11.9%, OR 2.2, 95% CI 1.3–3.6). When idiopathic groups (early- and late-onset) were combined, females were more likely to have an unknown cause of CP when compared with males (31.6 vs. 18.1%, OR 2.1, 95% CI 1.3–3.2). No other physician-defined CP groups were found to be statistically significant different based on sex.
Table 2.
Comparison of physician-defined etiology for chronic pancreatitis in the NAPS2-CV cohort by sex
| Etiology | Males (n = 287) | Females (n = 234) |
| Alcohol* | 167 (58.2) | 71 (30.3) |
| Idiopathic* | 52 (18.1) | 74 (31.6) |
| Early-onset | 18 | 21 |
| Late-onset | 34 | 53 |
| Genetic | 22 (7.7) | 29 (12.4) |
| Obstructive* | 7 (2.4) | 29 (12.4) |
| Autoimmune Pancreatitis | 4 (1.4) | 4 (1.7) |
| Miscellaneous | 35 (12.2) | 27 (11.5) |
| Hyperlipidemia | 11 | 4 |
| Post-necrotic | 5 | 6 |
| Autoimmune disease | 1 | 4 |
| Others | 18 | 13 |
Statistically significant; p < 0.05
Age
About half (269/521, 52%) of the patients were diagnosed with CP between ages 35–65 years, while 37% (195/521) were diagnosed before age 35 years, and 11% (57/521) after 65 years of age. Differences were seen in physician defined etiology based on the age at the time of CP diagnosis. Patients in whom CP was diagnosed between ages 35–65 years were most likely to have alcohol etiology (155/269, 58%) when compared with those diagnosed before age 35 years (70/195, 36%, OR 2.4, 95% CI 1.6–3.6) or after 65 years (13/57, 23%, OR 4.6, 95% CI 2.3–9.5). Genetic etiology was most common among patients diagnosed before age 35 years (40/195, 21%) when compared with those diagnosed between ages 35–65 years (9/269, 3%, OR 8.4, 95% CI 3.7–20) or after 65 years (3/57, 5%, OR 4.6, 95% CI 1.3–19.7). Lastly, about half of the patients diagnosed after age 65 years (25/57, 44%) had idiopathic CP when compared to about a fourth of patients diagnosed before age 35 years (48/195, 25%, OR 4.0, 95% CI 1.9–8.4) or between 35 and 65 years (53/269, 20%, OR 2.9 95% CI 1.4–6).
Race
As mentioned above, the NAPS2-CV cohort was 87% white, while the remaining patients were either black (11%) or other race (2%). When stratified by race, nonwhites were more likely to have an alcohol etiology when compared with whites (68 vs. 42%, OR 3.3 95% CI 1.9–6.0) and whites were more likely to have physician defined genetic etiology (11 vs. 1%, OR 8.3 95% CI 1.2–164.7).
Variability Among Participating Centers
As displayed in Fig. 1, among centers enrolling 20 or more patients, high variability was observed in physician-defined etiology of CP. For example, patients enrolled from Virginia Commonwealth University were more likely to have alcohol etiology when compared with St. Louis University (68 vs. 27%, OR 5.6, 95% 2.5–12.9), genetic etiology was more common in patients enrolled from the University of Pittsburgh when compared with Indiana University (18 vs. 6%, OR 3.7, 95% CI 0.98–16.1), and patients enrolled from the University of Michigan were more likely to have idiopathic CP when compared with St. Louis University (42 vs. 18%, OR 3.3, 95% 1.1–10).
Fig. 1.
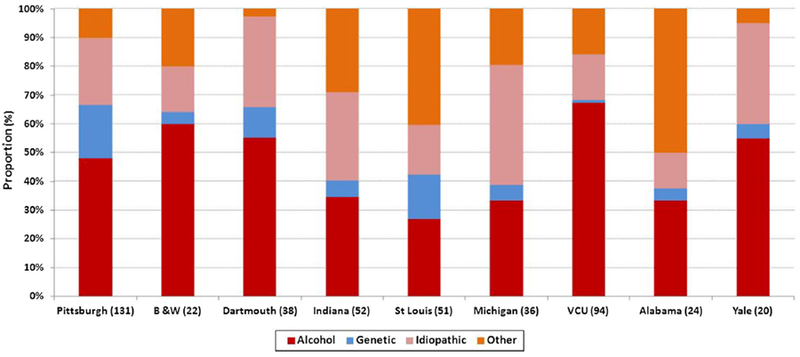
Distribution of physician-defined etiology in the NAPS2-CV study by participating center. Data shown for centers that enrolled 20 or more patients
Multiple Etiologic Risk Factors Were Identified in the NAPS2-CV Cohort
The TIGAR-O classification was applied to each study participant and compared to its physician-defined etiology. As listed in Table 3, the top five TIGAR-O risk factors in descending order were smoking (59%), alcohol (53%), idiopathic (30%), obstruction (19%), and hyperlipidemia (13%). Although the overall number of patients identified to have alcohol as a risk factor or etiology by physicians was higher in the CV study than the original NAPS2 study (53 vs. 45%, p < 0.05) [5], this difference was due to a very high fraction of patients with alcohol etiology (64/94, 68%) and risk factor (82/94, 87%) from one center (Virginia Commonwealth University-VCU). At centers other than VCU, the number of patients with alcohol etiology (174/427, 40.7%) or risk factor (194/427, 45.4%) was similar to the prior report.
Table 3.
Distribution of TIGAR-O risk actors in the NAPS2-CV cohort—overall and within physician-defined etiology groups
| TIGAR-O risk factors | All (521) | Physician-defined etiology |
||||
| Alcohol (238) | Idiopathic (126) | Genetic (51) | Obstructive (36) | Others (70) | ||
| Toxic metabolic (%) | 381 (73) | 238 (100) | 62 (49) | 11 (22) | 18 (50) | 52 (74) |
| Alcohol | 276 (53) | – | 16 (13) | 6 (12) | 3 (9) | 13 (19) |
| Tobacco | 306 (59) | 194 (86) | 48 (38) | 6 (12) | 14 (39) | 44 (63) |
| Hyperlipidemia | 66 (13) | 24 (12) | 11 (9) | 1 (2) | 3 (9) | 27 (39) |
| Chronic renal failure | 10 (2) | 2 (1) | 6 (5) | 0 (0) | 0 (0) | 2 (3) |
| Idiopathic | 158 (30) | 6 (3) | – | 13 (26) | 4 (11) | 9 (13) |
| Genetic | 56 (11) | 4 (2) | 0 (0) | – | 0 (0) | 1 (1) |
| Autoimmune | ||||||
| Autoimmune pancreatitis | 15 (3) | 2 (1) | 2 (2) | 0 (0) | 0 (0) | 11 (16) |
| Autoimmune-associated diseases | 18 (3) | 2 (1) | 6 (5) | 2 (4) | 0 (0) | 8(11) |
| RAP- and SAP-associated | 35 (7) | 10 (4) | 3 (2) | 2 (4) | 2 (6) | 18 (26) |
| Obstructive | 98 (19) | 23 (10) | 22 (18) | 8 (16) | – | 9 (13) |
| Pancreas divisum | 47 (9) | 14 (6) | 4(3) | 5 (10) | 23 (68) | 1 (1) |
| Sphincter of Oddi dysfunction | 20 (4) | 2 (1) | 11 (9) | 1 (2) | 5 (16) | 1 (1) |
| Duct obstruction | 16(3) | 4 (2) | 3 (3) | 1 (2) | 5 (16) | 1 (1) |
| Other obstructive | 27 (5) | 6 (3) | 8 (7) | 3 (6) | 6 (19) | 3 (4) |
| Miscellaneous | 50 (10) | 9 (4) | 17 (14) | 0 (0) | 2 (6) | 22 (31) |
| Gallstones | 42 (8) | 7 (3) | 14 (11) | 0 (0) | 2 (6) | 19 (27) |
| Other factors | 13 (2) | 3 (1) | 5 (4) | 0 (0) | 0 (0) | 5 (7) |
TIGAR-O risk factors not shown in the table are—hypercalcemia (2); medications (7); post-irradiation (1); post-trauma duct stricture (2); intraductal papillary mucinous neoplasm (2) Number of patients with inflammatory bowel disease were—9 (6 Crohn’s disease, 3 ulcerative colitis) Proportions are rounded to zero decimal spaces
It is also apparent that many CP subjects had multiple risk factors for CP. In fact, one risk factor was identified by physicians in only a quarter of patients (150/521, 28.8), while two risk factors were identified in about half (248/521, 47.6%), and three or more risk factors were identified in the remaining quarter of patients (123/521, 23.6%).
Table 3 shows data on the distribution of TIGAR-O risk factors in physician-defined etiology groups. In addition to the presence of multiple risk factors within each category, distribution of the type of risk factors provides an insight into the complex nature of the disease. For example, in patients considered to have alcoholic CP, physicians recognize a potential role of elevated triglycerides (12%), obstructive factors (10%), or genetic factors (2%) in a subset of patients. Moreover, in 3% patients with diagnosed alcoholic CP, physicians also checked idiopathic factors in the TIGAR-O risk factor category, indicating that in these patients the enrolling physician felt that alcohol alone may not explain the patients’ disease completely. Similarly, contribution of risk factors other than the primary etiologic category was identified in other major etiology groups. In patients with idiopathic CP, physicians also identified smoking as a risk factor in 38%, and obstructive factors in 18% and alcohol as a contributing factor in 13% patients. Similarly, in patients with obstructive pancreatitis, smoking was identified as a risk factor in 39% and idiopathic in 11% of patients.
Discussion
NAPS2-CV Validates the Recent Epidemiologic Trends in CP
The NAPS2-CV replicates data on the demographic and physician-defined etiology distributions of the initial NAPS2 study. Combined results of the two NAPS2 studies (initial and CV) with >1000 patients provide robust estimates of the current demographic and etiologic distribution of CP patients treated at US referral centers.
While the overall demographic and etiologic distributions in the NAPS2 cohort are similar to the vast literature on CP, the primary differences include a higher prevalence of females, a lower overall prevalence of alcohol etiology, and a higher prevalence of idiopathic CP. Several potential explanations for these observations have already been discussed—i.e., preferential referral of patients with no identifiable etiology to academic centers, improved recognition of the role of genetic factors, and enhanced resolution of imaging studies enabling earlier detection of disease [5]. Thus, while the results of this large multicenter study are important, it still may not completely reflect the entire US population despite efforts to enroll CP patients from various regions of the country.
No uniform criteria have been established for reporting etiology of chronic pancreatitis. Although empiric data are available on the level of alcohol consumption that independently increases the risk of pancreatitis (4 drinks/day according to a meta-analysis and 5 drinks/day according to the NAPS2 data), it has also been acknowledged that alcohol may play a disease-modifying role at lower levels of drinking. Moreover, assigning the most likely etiology of a patient’s chronic pancreatitis often takes into consideration not only history of alcohol consumption and smoking, but also the results of other testing (e.g., genetic testing and ductal abnormalities). The perceived etiology of chronic pancreatitis may therefore vary between physician based on patient’s history, evaluation and their own clinical experience and opinion.
To evaluate the relationship between physician-assigned etiologies between participating physicians empirically, we did an internal analysis to assess the correlation between patient’s self-reported alcohol consumption and physician diagnosis of alcohol etiology. A very tight correlation was seen in patients self-report and physician assignment overall (r = 0.752; p < 0.001) and between centers, and the correlation was very tight at the extremes with some variability in patients with light-moderate drinking categories.
Expert physicians considered alcohol to be the primary etiology in about half of the patients with CP—it accounted for the majority of cases among males, but only a minority among females. In a quarter of patients, no clear etiology of CP was identified even after a comprehensive evaluation. Our study confirms the high prevalence of alcohol etiology in blacks indicating a higher susceptibility to disease when compared with whites. Differences were also noted based on age—genetic factors were more frequent in younger patients, while the majority of elderly had idiopathic disease.
NAPS2-CV Identifies Multiple Risk Factors in CP Patients
Since initially proposed, no study has evaluated the distribution and utility of TIGAR-O classification in patients with CP [13]. The NAPS2-CV study for the first time provides estimates for the distribution of TIGAR-O risk factors in a well-defined cohort of CP patients.
There are no universal guidelines for assigning etiology of CP, and except for certain genetic mutations which can explain a patient’s disease (e.g., PRSS1 mutation, compound heterozygote status for CFTR mutations proven to be associated with pancreatitis) [25], for the majority of patients, no diagnostic tests are currently available to confidently determine the etiology of CP. Unlike other organs (e.g., liver), biopsies from the pancreas are not performed (and even if available, no pathognomonic findings may be seen) to provide clues to the potential etiology of CP. A lack of knowledge of the early events in human pancreatic disease has led to limited understanding of the pathogenesis of disease, in general or from specific etiologic causes. While stellate cells are known to play an important role in disease development [26, 27], they represent a final common pathway for fibrosis for all etiologies of CP.
The assignment of etiology, therefore, is based on circumstantial evidence. As an example, in a patient with CP who also drinks heavily, alcohol is considered to be the most likely etiology (often irrespective of the presence of other factor(s)). Similarly, the presence of pancreas divisum in a CP patient who does not have any other identifiable etiology can be considered to be the cause by many physicians. Only when the known risk factors are absent (or when alcohol consumption is present but not considered enough to explain an individual’s disease), an idiopathic etiology is assigned.
Interpretation of the data on TIGAR-O risk factors therefore needs a conceptual framework of the rationale used by clinicians in assigning etiology of CP. In about 70% patients, physicians identified at least one TIGAR-O risk factor in addition to the primary etiology of CP. Such a high number in part is explained by recognition of smoking as a risk factor in a large fraction of patients—in fact, smoking was the single most commonly identified risk factor (59%) by physicians in the NAPS2-CV cohort, ahead of alcohol (53%). However, other risk factors were also considered important by physicians in all etiology groups. While the recognition of additional factor(s) may reflect uncertainty of the physician in attributing the cause of CP to the primary etiology based on available evidence, this provides empiric data that CP is a complex disease resulting from interaction of multiple risk factors.
The prevalence of TIGAR-O risk factors in CP patients also provides crude estimates of association between a risk factor and CP. While smoking is now an established risk factor for CP, association of some other factors of importance is discussed here. Hypertriglyceridemia (typically ≥1000 mg/dl) is a well-recognized risk factor for AP and RAP. The prevalence of serum TG >500 and >1000 mg/dl in adult population is about 2 and 0.05% [28], respectively. Although a cutoff was not predefined for the NAPS2-CV study, using a conservative estimate of 500 mg/dl, the expected prevalence of HTG >500 mg/dl in the study population would be 2%. Physicians, however, identified HTG to be the cause in 4% and a risk factor in 13% indicating an increase risk of CP with HTG to be about twofold-sixfold. Similarly, the prevalence of IBD in the NAPS2 cohort was about 3.5-fold higher than US adults (1700 vs. 450/100,000) [29]. The odds would be still higher if the data are limited to nonalcohol etiologies. The prevalence of chronic renal failure was 5%, which is similar to the initial NAPS2 study, and was significantly higher than controls (5 vs. 1.2%) [5]. Finally, the prevalence of pancreas divisum in the overall cohort was not much different that the reported prevalence in the general population (9 vs. 7%) questioning the role of pancreas divisum as the sole cause of CP.
In conclusion, this NAPS2-CV study confirms the evolving trends in CP epidemiology. Several risk factors coexist in CP patients, indicating that development of disease in an individual may be a result of interaction between multiple factors.
Acknowledgments
Funding NIH (DCW—DK061451, U01DK108306), NIH (DY—DK077906, U01DK108306), and UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. NIH (DC—U01DK108327) and NIH (CEF—U01DK108320).
Footnotes
Author’s contribution DLC, DY, and DCW were involved in study design. DLC, DY, PAB, BSS, SS, SA, TBG, MAA, CMW, MDL, TM, CEF, GAC, NMG, REB, AG, and AS were involved in acquisition of data. DLC and DY were involved in analysis and interpretation. DLC and DY were involved in drafting of manuscript. All authors were involved in critical review. YT, SRW, DLC, and DY were involved in statistical analysis.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somogyi L, Martin SP, Venkatesan T, et al. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology. 2001;120:708–717. [DOI] [PubMed] [Google Scholar]
- 3.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–1490. [DOI] [PubMed] [Google Scholar]
- 4.Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013;144:1282–1291. e3. [DOI] [PubMed] [Google Scholar]
- 5.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–273. (quiz e27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav D, Slivka A, Sherman S, et al. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology. 2010;10:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frulloni L, Falconi M, Gabbrielli A, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis. 2010;42:S381–S406. [DOI] [PubMed] [Google Scholar]
- 8.Spanier B, Bruno MJ, Dijkgraaf MG. Incidence and mortality of acute and chronic pancreatitis in the Netherlands: a nationwide record-linked cohort study for the years 1995–2005. World J Gastroenterol. 2013;19:3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez-Munoz JE, Lucendo A, Carballo LF, et al. A Spanish multicenter study to estimate the prevalence and incidence of chronic pancreatitis and its complications. Rev Esp Enferm Dig. 2014;106:239–245. [PubMed] [Google Scholar]
- 10.De-Las-Heras-Castano G The study of chronic pancreatitis epidemiology—the big challenge. Rev Esp Enferm Dig. 2014; 106:237–238. [PubMed] [Google Scholar]
- 11.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14:490–496. [DOI] [PubMed] [Google Scholar]
- 12.Levy P, Dominguez-Munoz E, Imrie C, et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United Eur Gastroenterol J. 2014;2:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. [DOI] [PubMed] [Google Scholar]
- 14.Aoun E, Chang CC, Greer JB, et al. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS One. 2008;3:e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–2270. [DOI] [PubMed] [Google Scholar]
- 16.Greer JB, LaRusch J, Brand RE, et al. ABO blood group and chronic pancreatitis risk in the NAPS2 cohort. Pancreas. 2011;40:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton F, Alkaade S, Collins D, et al. Use and perceived effectiveness of non-analgesic medical therapies for chronic pancreatitis in the United States. Aliment Pharmacol Ther. 2011;33: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality of life in chronic pancreatitis: a case-control study from the North American Pancreatitis Study 2 cohort. Pancreas. 2013;42:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand H, Diergaarde B, O’Connell MR, et al. Variation in the gamma-glutamyltransferase 1 gene and risk of chronic pancreatitis. Pancreas. 2013;42:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melzack R The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. [DOI] [PubMed] [Google Scholar]
- 23.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. [DOI] [PubMed] [Google Scholar]
- 25.LaRusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011;27:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316–320. [DOI] [PubMed] [Google Scholar]
- 27.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41. [DOI] [PubMed] [Google Scholar]
- 28.Christian JB, Bourgeois N, Snipes R, et al. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol. 2011;107:891–897. [DOI] [PubMed] [Google Scholar]
- 29.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. [DOI] [PubMed] [Google Scholar]


