Abstract
Dehydrogenation of (partially) saturated heterocycles provides an important route to heteroaromatic compounds. A heterogeneous cobalt oxide catalyst, previously employed for aerobic oxidation of alcohols and amines, is shown to be effective for aerobic dehydrogenation of various 1,2,3,4-tetrahydroquinolines to the corresponding quinolines. The reactions proceed in good yields under mild conditions. Other N-heterocycles are also successfully oxidized to their aromatic counterparts.
Graphical abstract
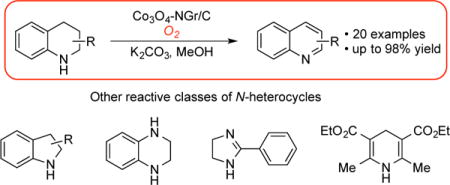
(Hetero)aromatic molecules are ubiquitous in pharmaceutics and other biologically active molecules.1 The introduction of substituents on the aromatic framework is typically accomplished by sequential introduction of groups onto a preformed aromatic ring. Recently, our group has been exploring aerobic dehydrogenation of (partially) saturated cyclic compounds as a complementary route to prepare substituted arenes and heteroarenes.2 In many cases, these methods enable synthesis of aromatic compounds with substitution patterns and/or functional groups that are difficult to access via traditional aromatic functionalization reactions. This strategy has been employed by us and others in Pd-catalyzed methods for the conversion of cyclohexanone and cyclohexenone derivatives into substituted phenols,3 aryl ethers4 or anilines5 and for the conversion of cyclohexene derivatives into substituted arenes.6 More recently, we began exploring methods for aerobic dehydrogenation of partially saturated N-heterocycles to heteroarenes. Our initial efforts to employ Pd-based catalysts led to unsatisfactory results, and we turned our attention to bioinspired ortho-quinone-based catalysts that are effective for the aerobic dehydrogenative aromatization of (partially) saturated nitrogen heterocycles.7-9 Collectively, the aerobic dehydrogenation methods represent compelling alternatives to established methods that use stoichiometric reagents, such as DDQ or sulfur.10 In light of the challenges we encountered in the use of Pd-based catalysts for N-heterocycle dehydrogenation, specifically to access quinoline products (Scheme 1A), we elected to test heterogeneous Co-oxide-based catalysts for this application. Here, we present the results of this effort.
Scheme 1.
Dehydrogenation 1,2,3,4-Tetrahydroquinolines to Quinolines
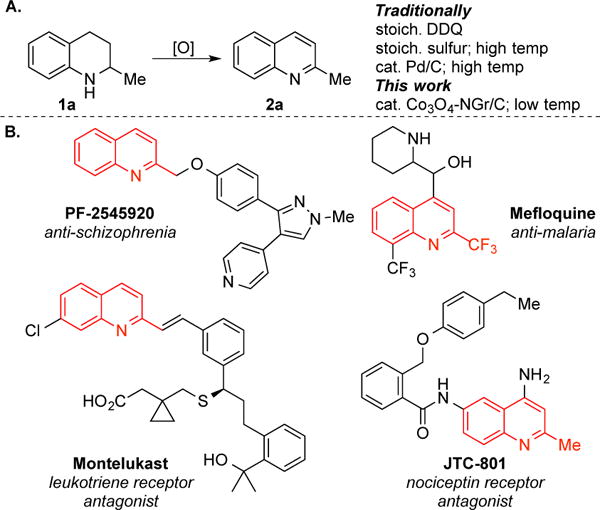
For our initial studies we chose 1,2,3,4-tetrahydroquinaldine (1a) as a test substrate (Table 1). The product of this dehydrogenation, 2-methylquinoline or quinaldine (2a), resembles the core of compounds that exhibit important pharmaceutical activity, including those for the treatment of schizophrenia (PF-2545920),11 HIV infection (styrylquino lines),12 malaria (Mefloquine),13 asthma (Montelukast)14 and pain (JTC-801)15. Therefore, it represents a simple proof-of-concept for use in catalyst screening (Scheme 1B). Our previously reported homogeneous Pd-based dehydrogenation catalysts3a,16 were tested, but they led to only modest yields (49–55%) with rather poor mass balance (Table 1, entries 1-3). In an effort to improve the results, we considered other catalysts, including heterogeneous variants. Heterogeneous Pd catalysts have been successfully used for the dehydrogenation of cyclohexanones and cyclohexenones.3d,e,4b,5e Other heterogeneous catalysts composed of noble metals (Ru, Au, Pd3Pb) have also been shown to be active for aerobic dehydrogenation of the parent 1,2,3,4-tetrahydroquinoline;17 however, the reactions proceed at high temperatures (>100 °C), they are accompanied by catalyst deactivation, and the results were not extended to substituted tetrahydroquinoline derivatives. During the course of these initial studies, Beller and coworkers reported a unique Co-oxide-based catalyst for aerobic alcohol oxidation (oxidative esterification of primary alcohols).18,19 More recently, the same researchers have applied these and related catalysts to the conversion of alcohols and amines to nitriles.20 Overall, these catalysts represent appealing base-metal alternatives to heterogeneous Pd/C and other supported noble metals, and we reasoned that similar catalysts could promote aerobic dehydrogenation of heterocycles.21
Table 1.
Low Temperature Dehydrogenation of 1,2,3,4-Tetrahydroquinaldine
| ||||
|---|---|---|---|---|
|
| ||||
| entry | catalyst (mol %) | solvent (concn [M]) | temp | yield (conv)b |
| 1 | Pd(TFA)2 (5)/2-NMe2-py (10)/pTsOH (20) | DMSO (1) | 80 | 52 (88) |
| 2 | Pd(TFA)2 (5)/DMSO (10) | AcOH (0.2) | 80 | 55 (100) |
| 3 | Pd(TFA)2 (5)/4,5-diazafluoren-9-one (5) | DMSO (0.2) | 80 | 49 (61) |
| 4a,c | CoOx-phen/AB | MeOH (0.125) | 60 | 92 (94) |
| 5a | CoOx-phen/AB | MeOH (0.125) | 60 | 43 (43) |
| 6a,c | CoOx-bpy/AB | MeOH (0.125) | 60 | 0 (1) |
| 7a,c | CoOx-terpy/AB | MeOH (0.125) | 60 | 82 (100) |
| 8a,c | CoOx-phd/AB | MeOH (0.125) | 60 | 67 (74) |
| 9a,c | MnOx-phen/AB | MeOH (0.125) | 60 | 16 (32) |
| 10a,c | FeOx-phen/AB | MeOH (0.125) | 60 | 0 (52) |
| 11a,c | NiOx-phen/AB | MeOH (0.125) | 60 | 0 (13) |
| 12a,c | CoOx-phen/Vulcan XC72R | MeOH (0.125) | 60 | 92 (100) |
| 13a | Pd/C | Xylene (0.2) | 60 | 0 (0) |
| 14a | Pd/C | MeOH (0.125) | 60 | 13 (30) |
| 15a,d,e | Activated C | Xylene (0.2) | 60 | 29 (66) |
| 16a,d,f | Co(salen) | EtOH (0.066) | 60 | 28 (63) |
|
| ||||
1a (0.10 mmol), solvent, 2.5 mol % [cat.], 1 atm O2, orbital mixing, 60 °C, 4 h.
% Yields and conv determined by 1H NMR.
1 equiv K2CO3 added.
Magnetic stirring was employed.
100 wt % catalyst, 24 h.
1 mol % catalyst was used, 24 h. C = carbon; AB = Acetylene Black.
The Co-oxide catalysts were prepared according to the protocol reported by Beller and coworkers.18 Briefly, cobalt(II) acetate was stirred with different bi- and tridentate nitrogen containing ligands in ethanol for 30 minutes, then acetylene black (AB) or Vulcan XC72R was added as a conductive carbon black support, and the mixture refluxed. After drying, the black powder was pyrolyzed under an argon flow at 800 °C for 2 hours. This procedure enabled the synthesis of cobalt based nanoparticles of varying sizes, encapsulated by nitrogen-rich graphene-like layers. This specific architecture has been suggested to account for the notable reactivity of these catalysts.18
Using the procedure just noted, a number of related catalysts were prepared, in which the identities of the nitrogen-containing ligand, the metal source and/or the carbon support was varied. Our initial studies were performed with acetylene black (AB) as the carbon support because it was already available in our lab. A catalyst prepared with this material, using Co(OAc)2 as the Co source and 1,10-phenanthroline (phen) as the nitrogen source, provided a 92% yield of quinaldine in the presence of one equivalent of K2CO3 as a base. The reaction was complete within 4 h in MeOH at only 60 °C (Table 1, Entry 4). Without any base, the catalyst was still active, but led to a lower final yield (43%, Entry 5).22 Catalysts prepared analogously using other nitrogen-based ligands instead of phen were also investigated (Entries 6-8).22 With 2,2′-bipyridine (bpy) as the nitrogen source, no product was obtained, while with 2,6-di(2-pyridyl)pyridine (terpy) and 1,10-phenanthroline-5,6-dione (phd) 82% and 67% yields were obtained, albeit lower than when using phen. Further investigations revealed that the ratio of cobalt to phen in the catalyst preparation procedure had little impact on this dehydrogenation.22 Next, catalysts with metals other than cobalt were prepared using phen as the nitrogen source and acetylene black as the carbon source. These heterogeneous Mn, Fe and Ni oxide materials were tested under the reaction conditions. The MnOx-based catalyst provided low yields of product, while the rest were inactive (Entries 9-11). Upon obtaining a sample of Vulcan XC72R, a Co(OAc)2/phen-derived catalyst was prepared with this carbon source (Entry 12). This catalyst led to a product yield similar to that obtained with the catalyst supported on AB, but it showed increased initial activity.22
Control reactions were also conducted to verify the necessity of the pyrolysis, heterogeneity and “nitrogen-doping” of the catalyst.22 Co(OAc)2 alone was not active for the reaction, either in the presence of a nitrogenous ligand or carbon black. The carbon black support (AB) was also completely inactive for the dehydrogenation, with or without pyrolysis and/or nitrogen doping. Finally, a mixture of Co(OAc)2/1,10-phenanthroline impregnated on acetylene black, but not pyrolyzed, was also inactive. Collectively, the results show that pyrolysis of Co(OAc)2:phen in a 1:2 mixture on AB and Vulcan XC72R at 800 °C under Ar generates effective catalysts. These compositions align with the catalyst previously optimized and characterized by Beller and coworkers, and their nomenclature “Co3O4-NGr/C” is adopted below.
The Co3O4-NGr/C catalyst outperforms other commonly used dehydrogenation catalysts, such as Pd/C10a and activated carbon. Moreover, it exhibits good activity at 60 °C, which is lower than temperatures typically required for dehydrogenation. When Pd/C was tested under comparable conditions, none of the dehydrogenation product was obtained in xylene and only low yield was observed in methanol (Table 1, Entries 13, 14). Activated carbon has been shown to promote dehydrogenation of 1,2,3,4-tetrahydroquinolines at 120 °C under O2 with 100 wt% loading of carbon.23 At the lower temperatures used here, however, use of 100 wt% activated carbon affords only 29% yield of product (Entry 15). Co(salen) has been used as a catalyst previously for dehydrogenation of the parent 1,2,3,4-tetrahydroquinoline at 60 °C,24 but the presence of the 2-methyl group in tetrahydroquinaldine significantly attenuates the yield (28%, Entry 16).
The Co3O4-NGr/C catalyst was then tested in the dehydrogenation of a variety of 1,2,3,4-tetrahydroquinolines under analogous reaction conditions (Scheme 2).25 The parent quinoline 2b was obtained in 90% yield and 2-phenylquinoline 2c in 92% yield after 6 h. The presence of functional groups on a 2-aryl substituent influenced the dehydrogenation reactivity. Substrates containing -SMe and -CF3 groups reacted well (2d, 2e), while those containing −NO2 and −CN groups needed more catalyst and longer reaction times to reach completion, and afforded lower yields of the desired products (2f, g). Substrates with 2-thienyl and 2-furyl groups led to high yields of the corresponding quinolines (2h, 2i), showing no inhibition by heterocyclic substituent. The results with 2c–2i are noteworthy in demonstrating the compatibility of the catalyst with 2-arylquinolines, a motif present in pharmaceutically active compounds. The reaction forming 3-methylquinoline (2j) exhibited lower mass balance and product yield; however, 4-methyl derivatives were obtained in high yields (1k, 1l).
Scheme 2. Substrate Scope for the Dehydrogenation of 1,2,3,4-Tetrahydroquinolines to Quinolinesa.
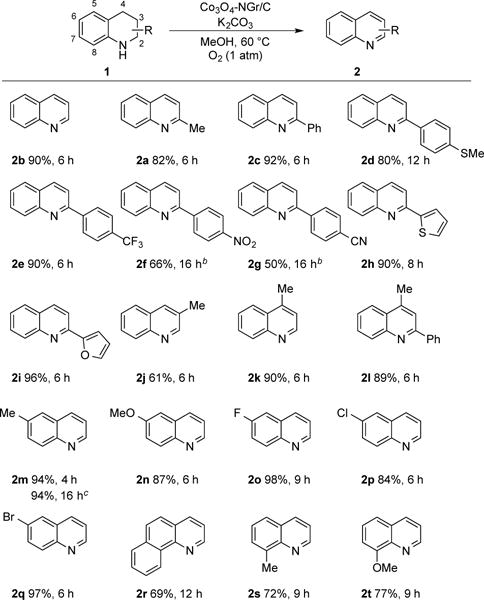
aConditions: 1 (0.5 mmol), 4 mL MeOH, 25 mg catalyst (2.5 mol %), 1 equiv K2CO3, 1 atm O2, orbital stirring, 60 °C. b50 mg of catalyst was used. cReaction on 10 mmol scale of 1m, magnetic stirring.
A number of tetrahydroquinolines with substitution at positions 6-8 were investigated (Scheme 2). Derivatization at these positions is usually accessible via selective electrophilic aromatic substitution reactions of tetrahydroquinolines. In contract, the reactivity of quinolines in similar reactions is usually unselective, yielding multiple products. First, substitution at the 6-position was explored. Substrates with electron-donating substituents, such as methoxy and methyl, underwent dehydrogenation in high yields (2m, 2n). The dehydrogenation of 1m was also carried out on 10 mmol scale (1.47 g) and the product was obtained in a 94% yield. The presence of halogens F-, Cl- and Br- at the 6-position led to good yields of dehydrogenation product 2o–2q. These observations highlight the advantages of this Co-based catalyst over Pd/C catalysts for dehydrogenations reactions, as the latter can promote undesired reactivity with aryl halides, and the −Cl or −Br substituents are appealing functional groups for subsequent derivatization. The 6,7-benzo-fused derivative 1r underwent dehydrogenation in good yield to the benzoquinoline 2r. Dehydrogenation of substrates with substituents in the 8 position also led to products in good yield (77% for the −OMe and 72% for the –Me derivatives 2s and 2t. The reactions involving substrates 1r-1t were somewhat slower, presumably due to steric effects.
With these successful results in hand, we tested a number of other partially saturated N-heterocycles containing secondary amines for their reactivity toward dehydrogenation. The reaction of 1,2,3,4-tetrahydroquinoxaline 3 under the standard reaction condition led to quinoxaline 4 in 97% yield. The readily accessible substrates, indoline 5 and 2-methyl indoline 7 exhibited excellent reactivity under the reaction conditions and afforded high yields of products at room temperature in a short time. This reactivity compares favorably with the previously reported aerobic dehydrogenation of indolines with 10 mol% of a molecular Co-salen catalyst.26 Hayashi and coworkers also reported dehydrogenation of the parent indoline 5 using 100 wt% of activated carbon, although a longer reaction time and higher temperature were required.27 A tertiary amine substrate, N- methyl indoline 9 was less reactive, affording only 33% yield of the desired indole. The oxidation of Hantzsch ester 11 was also successful, exhibiting a yield of 95% after only 2 hours at room temperature, which again compares favorably with results obtained with activated carbon.27b Finally, the more challenging dehydrogenation of 2-phenylimidazoline 13, led to a 43% product yield, with increased catalyst loading. Empirical optimization efforts with this substrate showed that the best catalyst was prepared with a 1:1 ratio of Co:phen. In this case, activated carbon is a more effective reagent for dehydrogenation, affording the desired imidazole in 84% yield at 120 °C.27c
In conclusion, we have shown that the dehydrogenation of 1,2,3,4-tetrahydroquinolines and other important N-heterocycles can be achieved using a cobalt-oxide-based catalyst supported on nitrogen-doped carbon under mild conditions. This heterogeneous first-row transition-metal catalyst represents a promising alternative to stoichiometric DDQ or other catalysts based on Pd for the synthesis of biologically active heteroaromatic compounds.
Supplementary Material
Scheme 3. Dehydrogenation of Other Classes of N-Heterocyclesa.
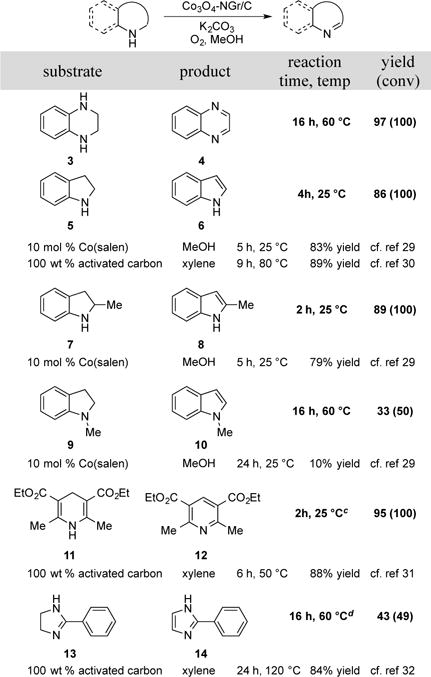
Conditions: Substrate (0.1 mmol), 0.8 mL MeOH, 5 mg catalyst, 1 equiv K2CO3, 1 atm O2, orbital stirring. bYields and conv determined by 1H NMR. cEtOH was used as solvent. dCatalyst prepared with Co:phen ratio of 1:1. 10 mg of catalyst used.
Acknowledgments
We thank the following individuals for assistance: Dr. R. Pokhrel (synthesis of catalysts), Dr. E. J. Popczun, M. S. Ahmed and Y. Preger (pXRD) and J. C. King (XPS) (all from UW-Madison). This work was funded by NIH (R01-GM100143). NMR facilities were partially supported by the NSF (CHE-0342998, CHE-1048642) and NIH (S10 RR08389). MS instrumentation was partially supported by NIH (S10 RR024601).
Footnotes
Supporting Information. Optimization data, experimental procedures and spectroscopic characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 2.For leading references, see:; (a) Izawa Y, Pun D, Stahl SS. Science. 2011;333:209. doi: 10.1126/science.1204183. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Izawa Y, Zheng C, Stahl SS. Angew Chem Int Ed. 2013;52:3672. doi: 10.1002/anie.201209457. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pun D, Diao T, Stahl SS. J Am Chem Soc. 2013;135:8213. doi: 10.1021/ja403165u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Iosub AV, Stahl SS. J Am Chem Soc. 2015;137:3454. doi: 10.1021/ja512770u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Imahori T, Tokuda T, Taguchi T, Takahata H. Org Lett. 2012;14:1172. doi: 10.1021/ol300145g. [DOI] [PubMed] [Google Scholar]; (b) Kikushima K, Nishina Y. RSC Adv. 2013;3:20150. [Google Scholar]; (c) Zhang J, Jiang Q, Yang D, Zhao X, Dong Y, Liu R. Chem Sci. 2015;6:4674. doi: 10.1039/c5sc01044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Simon MO, Girard SA, Li CJ. Angew Chem Int Ed. 2012;51:7537. doi: 10.1002/anie.201200698. [DOI] [PubMed] [Google Scholar]; (b) Sutter M, Sotto N, Raoul Y, Metay E, Lemaire M. Green Chem. 2013;15:347. [Google Scholar]
- 5.(a) Girard SA, Hu X, Knauber T, Zhou F, Simon MO, Deng GJ, Li CJ. Org Lett. 2012;14:5606. doi: 10.1021/ol3027279. [DOI] [PubMed] [Google Scholar]; (b) Hajra A, Wei Y, Yoshikai N. Org Lett. 2012;14:5488. doi: 10.1021/ol302568b. [DOI] [PubMed] [Google Scholar]; (c) Xie Y, Liu S, Liu Y, Wen Y, Deng GJ. Org Lett. 2012;14:1692. doi: 10.1021/ol3002442. [DOI] [PubMed] [Google Scholar]; (d) Hong WP, Iosub AV, Stahl SS. J Am Chem Soc. 2013;135:13664. doi: 10.1021/ja4073172. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sutter M, Duclos MC, Guicheret B, Raoul Y, Métay E, Lemaire M. ACS Sustainable Chem Eng. 2013;1:1463. [Google Scholar]
- 6.See ref 2d and the following:; Kandukuri SR, Oestreich M. J Org Chem. 2012;77:8750. doi: 10.1021/jo301088f. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wendlandt AE, Stahl SS. J Am Chem Soc. 2014;136:506. doi: 10.1021/ja411692v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wendlandt AE, Stahl SS. J Am Chem Soc. 2014;136:11910. doi: 10.1021/ja506546w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For other catalytic aerobic dehydrogenation routes to substituted heterocycles, see:; (a) Flanagan JCA, Dornan LM, McLaughlin MG, McCreanor NG, Cook MJ, Muldoon MJ. Green Chem. 2012;14:1281. [Google Scholar]; (b) Han B, Yang XL, Wang C, Bai YW, Pan TC, Chen X, Yu W. J Org Chem. 2012;77:1136. doi: 10.1021/jo2020399. [DOI] [PubMed] [Google Scholar]; (c) Yuan H, Yoo WJ, Miyamura H, Kobayashi S. Adv Synth Catal. 2012;354:2899. [Google Scholar]; (d) Dawsey AC, Li V, Hamilton KC, Wang J, Williams TJ. Dalton Trans. 2012;41:7994. doi: 10.1039/c2dt00025c. [DOI] [PubMed] [Google Scholar]; (e) Chen S, Hossain MS, Foss FW. ACS Sustainable Chem Eng. 2013;1:1045. [Google Scholar]; (f) Chen Z, Chen J, Liu M, Ding J, Gao W, Huang X, Wu H. J Org Chem. 2013;78:11342. doi: 10.1021/jo401908g. [DOI] [PubMed] [Google Scholar]; (g) Yu J, Xu J, Lu M. Appl Organometal Chem. 2013;27:606. [Google Scholar]
- 9.For other homogeneous catalytic methods for dehydrogenation of 1,2,3,4-tetrahydroquinolines, see:; (a) Yamaguchi R, Ikeda C, Takahashi Y, Fujita K-I. J Am Chem Soc. 2009;131:8410. doi: 10.1021/ja9022623. [DOI] [PubMed] [Google Scholar]; (b) Wu J, Talwar D, Johnston S, Yan M, Xiao J. Angew Chem Int Ed. 2013;52:6983. doi: 10.1002/anie.201300292. [DOI] [PubMed] [Google Scholar]; (c) Chakraborty S, Brennessel WW, Jones WD. J Am Chem Soc. 2014;136:8564. doi: 10.1021/ja504523b. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fu PP, Harvey RG. Chem Rev. 1978;78:317. [Google Scholar]; (b) Buckle DR. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Inc; New York: 2010. [Google Scholar]
- 11.Verhoest PR, Chapin DS, Corman M, Fonseca K, Harms JF, Hou X, Marr ES, Menniti FS, Nelson F, O’Connor R, Pandit J, Proulx-LaFrance C, Schmidt AW, Schmidt CJ, Suiciak JA, Liras S. J Med Chem. 2009;52:5188. doi: 10.1021/jm900521k. [DOI] [PubMed] [Google Scholar]
- 12.Mouscadet JF, Desmaële D. Molecules. 2010;15:3048. doi: 10.3390/molecules15053048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft A, Garner P. BMJ. 1997;315:1412. doi: 10.1136/bmj.315.7120.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belley ML, Leger S, Labelle M, Roy P, Xiang YB, Guay D. 5,565,473. US Patent. 1996 Oct 15;
- 15.Sestilli I, Borioni A, Mustazza C, Rodomonte A, Turchetto L, Sbraccia M, Riitano D, Del Giudice MR. Eur J Med Chem. 2004;39:1047. doi: 10.1016/j.ejmech.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.(a) Diao T, Stahl SS. J Am Chem Soc. 2011;133:14566. doi: 10.1021/ja206575j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Diao T, Wadzinski TJ, Stahl SS. Chem Sci. 2012;3:887. doi: 10.1039/C1SC00724F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For heterogeneous catalysts based on Pd:; (a) Furukawa S, Suga A, Komatsu T. Chem Commun. 2014;50:3277. doi: 10.1039/c4cc00024b. Ru. [DOI] [PubMed] [Google Scholar]; (b) Yamaguchi K, Mizuno N. Angew Chem Int Ed. 2003;42:1480. doi: 10.1002/anie.200250779. [DOI] [PubMed] [Google Scholar]; (c) Li F, Chen J, Zhang Q, Wang Y. Green Chem. 2008;10:553. [Google Scholar]; (d) Yamaguchi K, Kim JW, He J, Mizuno N. J Catal. 2009;268:343. and Au. [Google Scholar]; (e) So MH, Liu Y, Ho CH, Che CH. Chem Asian J. 2009;4:1551. doi: 10.1002/asia.200900261. [DOI] [PubMed] [Google Scholar]
- 18.Jagadeesh RV, Junge H, Pohl M-M, Radnik J, Brückner Beller M. J Am Chem Soc. 2013;135:10776. doi: 10.1021/ja403615c. [DOI] [PubMed] [Google Scholar]
- 19.See also:; Zhu J, Kailasam K, Fischer A, Thomas A. ACS Catal. 2011;1:342. [Google Scholar]
- 20.(a) Jagadeesh RV, Junge H, Beller M. Nat Commun. 2014;5:4123. doi: 10.1038/ncomms5123. [DOI] [PubMed] [Google Scholar]; (b) Jagadeesh RV, Junge H, Beller M. ChemSusChem. 2015;8:92. doi: 10.1002/cssc.201402613. [DOI] [PubMed] [Google Scholar]
- 21.For a related recent application, see:; Cui X, Li Y, Bachmann S, Scalone M, Surkus AE, Junge K, Topf C, Beller M. J Am Chem Soc. 2015;137:10652. doi: 10.1021/jacs.5b05674. [DOI] [PubMed] [Google Scholar]
- 22.See Supporting Information for details.
- 23.Tanaka T, Okunaga K-i, Hayashi M. Tetrahedron Lett. 2010;51:4633. [Google Scholar]
- 24.Nishinaga A, Yamazaki S, Matsuura T. Tetrahedron Lett. 1988;29:4115. [Google Scholar]
- 25.CAUTION: Because of the flammability of methanol and O2, large scale reactions should be conducted below the LOC of MeOH (approx. 7-8%):; Osterberg PM, Niemeier JK, Welch CJ, Hawkins JM, Martinelli JR, Johnson TE, Root TW, Stahl SS. Org Process Res Dev. 2015 doi: 10.1021/op500328f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inada A, Nakamura Y, Morita Y. Chem Lett. 1980:1287. [Google Scholar]
- 27.Nomura Y, Kawashita Y, Hayashi M. Heterocycles. 2007;74:629. [Google Scholar]; (b) Nakamichi N, Kawashita Y, Hayashi M. Synthesis. 2004;7:1015. [Google Scholar]; (c) Haneda S, Okui A, Ueba C, Hayashi M. Tetrahedron. 2007;63:2414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


