Abstract
Human infants are often exposed to opiates chronically but the mechanisms by which opiates induce dependence in the infant are not well studied. In the adult the brain regions involved in the physical signs of opiate withdrawal include the periaqueductal gray area, the locus coeruleus, amygdala, ventral tegmental area, nucleus accumbens, hypothalamus, and spinal cord. Microinjection studies show that many of these brain regions are involved in opiate withdrawal in the infant rat. Our goal here was to determine if these regions become metabolically active during physical withdrawal from morphine in the infant rat as they do in the adult. Following chronic morphine or saline treatment, withdrawal was precipitated in 7-day-old pups with the opiate antagonist naltrexone. Cells positive for Fos-like immunoreactivity were quantified within select brain regions. Increased Fos-like labeled cells were found in the periaqueductal gray, nucleus accumbens, locus coeruleus, and spinal cord. These are consistent with other studies showing that the neural circuits underlying the physical signs of opiate withdrawal are similar in the infant and adult.
Keywords: opiate, morphine, dependence, withdrawal, Fos, periaqueductal gray, nucleus accumbens, spinal cord, locus coeruleus
INTRODUCTION
Large numbers of seriously ill infant patients are treated with opiates to alleviate pain; other human infants are exposed in utero to illicit opiate drugs such as heroin and to prescribed opiates such as methadone. It is therefore important to understand the effects of acute and chronic exposure to drugs such as these on the immature organism. In the adult prolonged exposure to opiates results in distinct biochemical and physiological changes in individual neurons and in entire interacting circuits of neurons, which in turn can result in long-term alterations in cellular communication (Carlezon et al., 1997; Ingram, Vaughan, Bagley, Connor, & Christie, 1998; Koob & Bloom, 1988; Redmond & Krystal, 1984). These neural changes form the basis of the psychological and physical dependence that is seen after chronic exposure to the opiate. Yet it is not known if the same mechanisms mediate withdrawal in the infant.
Animal models of opiate dependence have defined the neuroanatomical regions that are involved in the physical signs of opiate withdrawal in the adult. In an early study injection of 3H-naloxone into the lateral ventricle and basal cisterns of morphine dependent rats, produced strong withdrawal behaviors as a result of its spread to the periaqueductal gray (PAG) of the midbrain, the locus coeruleus (LC), and the periventricular gray substance (Laschka, Teschemacher, Mehraein, & Herz, 1976). More recent studies have shown that physical withdrawal can be induced by the microinjection of opiate antagonists into the amygdala, nucleus accumbens (NAcc), medial thalamus, preoptic hypothalamus, PAG, LC, and nucleus raphe magnus (Druhan, Walters, & Aston-Jones, 2000; Maldonado, Fournié-Zaluski, & Roques, 1992). Affective signs of withdrawal can be induced by the microinjection of antagonists into the NAcc and amygdala (Frenois, Stinus, Di Blasi, Cador, & Le Moine, 2005; Stinus, Le Moal, & Koob, 1990).
Opiate withdrawal increases regional cerebral glucose utilization or activation of c-fos in the amygdala, hypothalamus, LC, NAcc, medial and lateral preoptic areas, thalamus, and spinal cord (Adams & Wooten, 1990; Chieng, Keay, & Christie, 1995; Geary & Wooten, 1985; Hayward, Duman, & Nestler, 1990; Kimes & London, 1989; Rohde, Detweiler, & Basbaum, 1996a; Stornetta, Norton, & Guyenet, 1993; Veinante & Freund-Mercier, 2003). When these studies are taken together, a circuit evolves for the regulation of physical dependence in the adult rodent whose major components include (but are not limited to) the locus coeruleus, periaqueductal gray, amygdala, spinal cord, hypothalamus, thalamus, and nucleus accumbens.
As with adult human and rats, human infants and rat pups display physical withdrawal behaviors when long-term opiate exposure is terminated. These behaviors in human infants include altered sleep patterns, high-pitched crying, respiratory and gastrointestinal dysfunction, irritability, and tremors (Anand & Arnold, 1994; Finnegan, 1985; Franck, Vilardi, Durand, & Powers, 1998). Treatment options in the human infant in withdrawal are limited and include tapering with opiates such as methadone, opium or more recently buprenorphine or adjuvant treatment with barbiturates or benzodiazepines (Kraft et al., 2008; Osborn, Cole, & Jeffery, 2002; Osborn, Jeffery, & Cole, 2002).
In the rodent model there are qualitative changes in physical withdrawal behaviors that are seen throughout development. For instance, common behaviors seen in adult rats include wet dog shakes, teeth chattering, ptosis, piloerection, and jumping (Blasig, Herz, Reinhold, & Zieglgansberger, 1973; Buckett, 1964; Maldonado, Stinus, Gold, & Koob, 1992); behaviors that are unique to the 7-day-old rat include head movements, paw movements, twisting, stretching, rolling, and increased ultrasonic vocalizations (Barr & Wang, 1992; Jones & Barr, 1995; Thornton, Wang, & Smith, 1997; Windh, Little, & Kuhn, 1995). One explanation for the different behavioral and physiological changes that are seen during ontogeny may be the relative immaturity of the neurons that comprise the individual brain loci and/or the pathways that interconnect these loci.
Little is known of the regions involved in opiate withdrawal in the infant rodent. There are dramatic increases in levels of c-fos mRNA in the olfactory bulb and small increases in the hypothalamus and medulla of 7-day-old morphine dependent rats after naloxone-induced withdrawal (Maeda et al., 2002), but more regional specificity is not known. In addition, whole brain and spinal cord show changes in c-fos mRNA (Akbarian et al., 2002; Jones et al., 2002) in an acute opiate withdrawal model. These and other loci are also associated with withdrawal when microinjected with an opiate antagonist (Jones & Barr, 2001) and with conditioned place preference when microinjected with morphine (Barr & Rossi, 1992).
Thus although withdrawal can be precipitated by antagonist injection into similar brain regions in the infant and adult, there are no data on specific brain loci that are activated by withdrawal in the infant. It is our general hypothesis that the neural circuitry of withdrawal in adults and infants is concordant despite differences in withdrawal behavior. Here we hypothesized that despite the relative immaturity of these same regions, areas that show activity during opiate withdrawal in the adult rat would show activity in the young rat. Thus Fos, a marker of cellular activity, the expression of which is rapidly activated in response to neuronal stimulation, was quantified. The presence of the protein Fos and its mRNA has been used successfully as a measure of neuronal activation throughout the CNS of adult (Le Guen, Catheline, & Besson, 1999; Morgan, Cohen, Hempstead, & Curran, 1987) and developing rats (Andersen, LeBlanc, & Lyss, 2001; Boucher, Jennings, & Fitzgerald, 1998; Joyce & Barr, 1995; Wiedenmayer & Barr, 2001a; Williams, Evan, & Hunt, 1990; Yi & Barr, 1995). Therefore, for the current experiment we precipitated physical withdrawal, using the opiate antagonist naltrex-one, in morphine dependent 7-day-old rat pups and quantified the number of Fos-like immunoreactive (Fos-LIR) cells in brain and spinal cord.
GENERAL METHODS
Subjects
Subjects were offspring of Long-Evans Hooded rats (Harlan Sprague Dawley) mated in our colony room. The parents (and offspring) were housed in plastic tubs under standard conditions. Tubs were checked twice daily (≈09:00 and 17:00 hr) and any new pups found at either time were recorded as 0 days old. The experiment began on postnatal Day 1 (PD-1), and at that time the litter was culled to eight pups, without regard to sex.
Induction of Morphine Dependence
All procedures for this study included adequate measures to minimize pain or discomfort and were conducted in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory animals and approved by the internal IACUC of Hunter College. At PD-1 pups were removed from the dam and transferred to a smaller plastic container, containing beta chips from the home cage. Using India ink, each pup was permanently tattooed on the ventral surface of the paws (Geller & Geller, 1966). All pups from a litter were weighed and given an intraperitoneal (i.p.) injection with either the vehicle (saline) or the opiate agonist morphine sulfate (Henry Schein, Indianapolis, IN; 10 mg/kg, 0.01 ml/g). After injection of the final animal, the entire litter was placed in an incubator (maintained at 32 C) for 30 min before being returned to the dam. The weighing was repeated once a day (each morning) for 7 days. The saline or morphine injections were given twice daily (≈8:00 and 17:00) for 6 days and one more time on the morning of the 7th day. This regimen and dose has been shown to consistently produce morphine dependence in the 7-day-old rat pup (Jones & Barr, 1995; McPhie & Barr, 2000).
Precipitation of Withdrawal
Four hours after the final morning injection on the 7th day, all pups were removed from the dam and placed in a plastic container in the testing room. One pup was randomly removed from its littermates. It was weighed, sexed and injected (i.p.) with either saline or the long-acting opiate antagonist naltrexone hydrochloride (Sigma, St. Louis, MO; 1 mg/kg, 0.01 ml/g). The pup was then returned to the huddle. A second pup was treated similarly with the other drug/vehicle. The drugs were assigned randomly and the experimenter was blind to the drug each pup received. The presence or absence of withdrawal behaviors was noted and all pups in the morphine-naltrexone group exhibited classic neonatal withdrawal signs (Jones & Barr, 1995). None of the control groups expressed withdrawal. No more than one pup per litter was included in any experimental group; data for males and females were combined and not analyzed separately.
Fixation of Tissue/Perfusion
After 2 hr, pups were sacrificed with an i.p. injection of a lethal dose of sodium pentobarbital and perfused with 4% paraformaldehyde (Morgan et al., 1987; Wiedenmayer & Barr, 2001b; Yi & Barr, 1997). The brains and spinal cords were frozen and coronally sectioned (30 μm for spinal cord and locus coeruleus, 50 μm for periaqueductal gray and nucleus accumbens) on a cryostat. Sections were floated in PBS in isolated wells of a plastic container. One of the floated sections was stained for cresyl violet to locate anatomical regions; the other floated section was stained for Fos. The third cut section was stored at −80°C.
Immunohistochemistry
Sections were processed using a modified protocol of the avidin-biotin-peroxidase system of Hsu, Raine, and Fanger (1981). The sections were then incubated for 48 hr at 4°C on a shaker in the primary antibody, rabbit anti-Fos (Ab-5, Oncogene Science, Union, NY) diluted 1:2,000 in PBS with Triton-X and 1% goat serum. Sections were stained using diaminobenzidine peroxidase substrate tablets (Sigma). Assay controls included tissue without the primary antibody; no staining was seen. In prior experiments preabsorption of the Fos antibody with excess of the Fos protein abolished staining (Yi & Barr, 1995). Tissue from control pups and treated pups were processed together and the experimenter was blind to the treatment of each pup.
Fos Cell Quantitation
Fos-like immunoreactive (LIR) positive cells were visualized using a microscope (Nikon-Optiphot) equipped with a drawing tube. A person unaware of the chronic and acute treatment of the animal counted all Fos-LIR cells, regardless of the density of the staining (as long as it was distinct from the background; Fig. 1). For each animal, the mean number of Fos-LIR cells per brain area was calculated by averaging counts from all sections.
FIGURE 1.
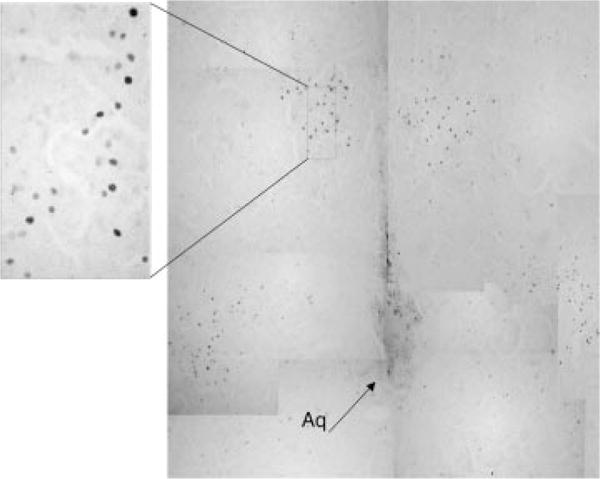
Photomicrograph of Fos-like immunoreactive cells in the periaqueductal gray area of 7-day-old rats that were treated chronically with morphine and given an acute injection of naltrexone (M-N) to precipitate withdrawal.
RESULTS
All sections containing the regions of interest were counted and averaged. There were 2–5 sections for the nucleus accumbens; 4–8 sections for the periaqueductal gray and locus coeruleus; and 2–5 sections in the lumbar enlargement of the spinal cord. Preliminary analyses showed that there were no significant differences between the left and right sides in any of the regions so the values were pooled together for subsequent analysis.
A factorial analysis of variance (ANOVA) was performed for each of the brain regions and spinal cord. The experimental group was (1) chronic morphine + acute naltrexone (M–N). The three controls were: (1) chronic morphine + acute saline (M-S); (2) chronic saline acute naltrexone (S-N); and (3) chronic saline acute + saline (S-S). The controls did not differ from each +other and were combined to form a single control group for analysis. However, for clarity the data from the controls are displayed as separate groups in the figures.
Nucleus Accumbens
There were significantly higher levels of Fos-LIR in the entire nucleus accumbens of the M-N group compared to all the controls, F (1,9) = 9.66, P < 0.01 (Fig. 2). In addition, the shell showed higher levels of Fos-LIR than the core in the treated group, F (1,8) = 8.24, P < 0.05.
FIGURE 2.
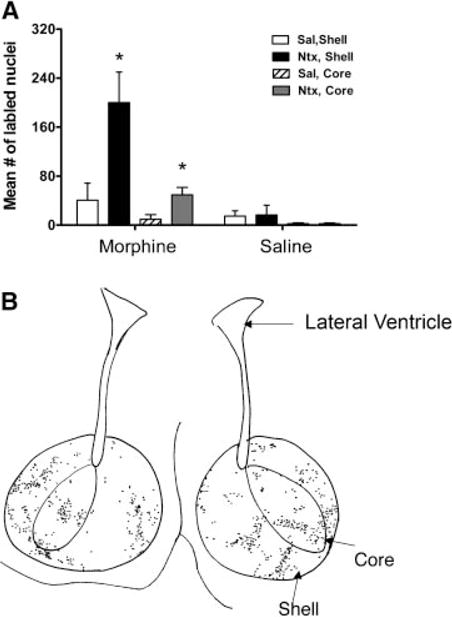
(A) Mean number of Fos-like immunoreactive cells in the nucleus accumbens of 7-day-old rats that were chronically exposed to morphine (M) or saline (S) (labeled on the X-axis) and then given an acute injection of naltrexone (N) or saline (S) (N = 5/group; mean ± SE). Asterisk (*) denotes significance between the M-N group and the combined controls (M-S, S-S, and S-N). Note: The scale of this figure differs from that of Figures 3–5. (B) Camera lucida drawing of the shell and core of the nucleus accumbens. Note: The increased number of Fos labeled cells in the shell than the core.
Periaqueductal Gray Area
Significantly higher levels of Fos-LIR were found in the entire periaqueductal gray of the M-N animals compared to the controls, F (1,8) = 9.94, P < 0.05 (Fig. 3). There were no significant differences in Fos-LIR levels between the dorsal and ventrolateral regions of the periaqueductal gray (data not shown).
FIGURE 3.
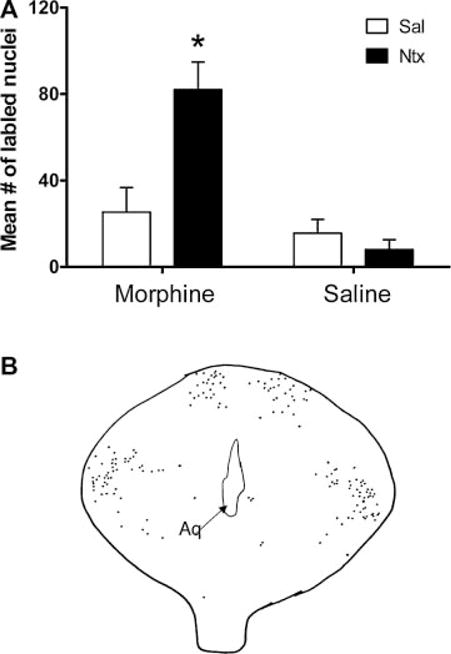
(A) Mean number of Fos-like immunoreactive cells in the periaqueductal gray of 7-day-old rats that were chronically exposed to morphine (M) or saline (S) (labeled on the X-axis) and then given an acute injection of naltrexone (N) or saline (S) (N = 5/group; mean significance ± SE). Asterisk (*) denotes between the M-N group and the combined controls (M-S, S-S, and S-N). (B) Camera lucida drawing of the periaqueductal gray area. There were no differences between dorsal and ventrolateral regions.
Spinal Cord
There were significant increases in the levels of Fos-LIR in the M-N group compared to the control, F (1,7) = 42.3, P < 0.01. Note: these data include only a M-S control because prior work had shown no or minimal Fos expression in pups treated with chronic saline (Fig. 4). The changes occurred largely in Lamina I and II (or dorsal surface) of the dorsal horn.
FIGURE 4.

(A) Mean number of Fos-like immunoreactive cells in the dorsal horn of the spinal cord of 7-day-old rats that were chronically exposed to morphine (M) or saline (S) (labeled on the X-axis) and then given an acute injection of naltrexone (N) or saline (S) (N = 8/group; mean ± SE). Asterisk (*) denotes significance between the M-N group and the control group (M-S). (B) Camera lucida drawing of the spinal cord. More label cells can be seen in the dorsal horn (top of drawing).
Locus Coeruleus
There were significantly higher levels of Fos-LIR in the M-N treated group compared to the controls, F (1,12) = 6.73, P < 0.05 (Fig. 5).
FIGURE 5.
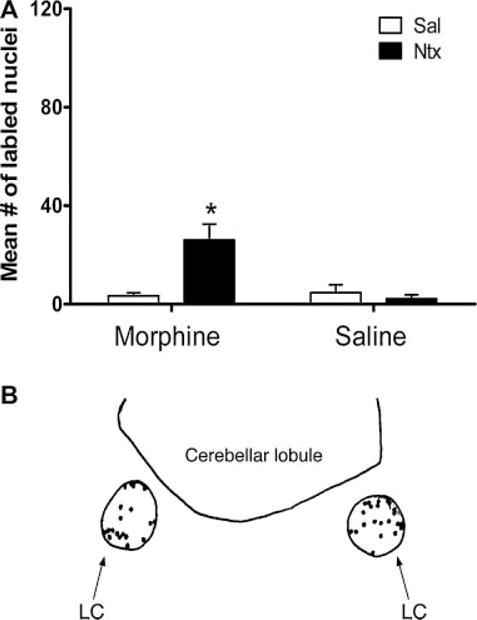
(A) Mean number of Fos-like immunoreactive cells in the locus coeruleus of 7-day-old rats that were chronically exposed to morphine (M) or saline (S) (labeled on the X-axis) and then given an acute injection of naltrexone (N) or saline (S) (N = 8 for M-N and M-S, N = 6 for S-S and S-N; mean ± SE). Asterisk (*) denotes significance between the M-N group and the combined controls (M-S, S-S, and S-N). (B) Camera lucida drawing of the locus coeruleus.
Other Regions
Although we did not quantify other brain regions, we noted substantially increased staining in chronic morphine treated animals in withdrawal in a variety of areas, all of which have been described previously in adults. These include the hypothalamus, including the paraventricular, dorsomedial, medial preoptic, and anterior nuclei. In addition there was substantial Fos staining in the midline thalamic nuclei, in particular the paraventricular nucleus, and the arcuate of the hypothalamus that was dense in morphine treated animals regardless of whether or not they received naltrexone. In addition, there was inconsistent and sporadic staining in the anterior dorsal thalamus, dorsal interpeduncular nucleus, and medial and central nuclei of the amygdala.
DISCUSSION
Withdrawal in the 7-day-old morphine dependent rat pup, precipitated by the long-acting opiate antagonist naltrexone, increased Fos-like immunoreactivity in several brain regions. These included the periaqueductal gray area, the nucleus accumbens, the dorsal horn of the spinal cord, and the locus coeruleus. The assumption of this and most studies with Fos is that the increased transcription of Fos indicates increased cellular activity.
The increased levels of Fos activity in the nucleus accumbens following opiate withdrawal was comparable to adult studies where behavioral withdrawal precipitated by the injection of opiate antagonists into the NAcc caused an increase in levels of the c-fos protein (Hayward et al., 1990), c-fos mRNA (Hayward et al., 1990), and glucose utilization (Geary & Wooten, 1985, 1986). In addition, the systemic administration of naltrexone caused increased Fos-related antigen immunoreactivity in chronically morphine treated rats (Walters, Aston-Jones, & Druhan, 2000) and increased CREB-mediated transcription in chronically morphine treated mice (Shaw-Lutchman et al., 2002).
The nucleus accumbens is part of the mesocorticolim-bic-dopamine pathway and a critical site for the reinforcing properties of opiates (Dworkin, Guerin, Goeders, & Smith, 1988; Koob, Wall, & Bloom, 1989; Shaw-Lutchman et al., 2002; Wise, 1987). Lever pressing in morphine dependent adult rats can be significantly reduced when the opiate antagonist methylnaloxonium is microinjected into the nucleus accumbens (Koob et al., 1989; Stinus et al., 1990). Place aversions cannot be conditioned in rats at PD-7 but can at PD-14 (Barr & Goodwin, 1997) and separation-induced ultrasonic vocalizations (USVs) (Barr & Wang, 1992) can be elicited in withdrawing rat pups younger than PD-3. Therefore the increased levels of Fos-LIR elicited in this current study may represent the cellular parallel of the increased ultrasonic vocalizations.
In addition to the nucleus accumbens, there were increased levels of Fos-LIR in the periaqueductal gray area of the 7-day-old rat, consistent with findings in the adult animal (Chieng et al., 1995). There were no differences between its dorsal and ventrolateral subregions. The periaqueductal gray contains a large number of opiate receptors in the adult rodent brain (Vaughan & Christie, 1997) and is therefore sensitive to the administration of opiate antagonists and agonists. The direct injection of methylnaloxonium into the ventral periaque-ductal gray elicits withdrawal behaviors similar to those seen after the systemic injection of naltrexone in the infant and adult animal (Aghajanian, 1978; Jones & Barr, 2001; Laschka et al., 1976; Maldonado, Negus, & Koob, 1992). When combined with the current data, there is strong evidence that the periaqueductal gray becomes increasingly active during opiate withdrawal in the 7-day-old rat, as in the adult rat.
The dorsal horn of the spinal cord also showed significantly increased levels of Fos-LIR upon the precipitation of morphine withdrawal in the 7-day-old rat. As the initial CNS structure to receive afferent information, the spinal cord plays an important regulatory role and modulates noxious input. Moreover, it has a significant role in opiate dependence and physical withdrawal in the adult rodent. For instance, pretreatment with an intrathecal administration of an opiate antagonist blocks the development of dependence (Delander & Takemori, 1983) and the precipitation of withdrawal increases levels of Fos-LIR within the dorsal horn of the spinal cord (Rohde, Detweiler, & Basbaum, 1996b). In the infant rat, there is evidence for the role of the spinal cord in opiate tolerance and withdrawal. In an isolated spinal cord preparation from 7-day-old rats that had been chronically treated with morphine, the addition of naltrexone to the bath increased the magnitude of the electrically evoked slow ventral root potentials (Zhu & Barr, 2003, 2004).
The final brain structure quantified here where levels of Fos-LIR that were significantly increased during precipitated withdrawal was the locus coeruleus. This finding was expected considering the vast amount of published research that has focused on the locus coeruleus as a model for the long-term changes resulting from opiate exposure. Microinjections of opiate antagonists into the locus coeruleus of morphine dependent adult (Maldonado, Negus et al., 1992; Maldonado, Stinus et al., 1992) and infant rats (Jones & Barr, 2001) cause withdrawal signs similar to that seen by systemic injections.
Human infants are exposed to opiates either when the fetus is exposed passively to opiates used by the mother, or when premature infants (as early as 22–23 weeks of gestation) are treated for pain. We chose 7 days of age because we wanted to model exposure within the early human infant age range. It is not possible to draw direct equivalents between developmental stages across species because different systems have different developmental trajectories, although that effort has been attempted since the rat was first used as a developmental model (e.g., Donaldson, 1918). More recent attempts have examined specific nervous system regions and suggest that humans at birth are approximately equivalent to 10- and 14- day old rat pups (Porterfield & Hendrich, 1993; Romijn, Hofman, & Gramsbergen, 1991). In the most ambitious effort to date, Clancy developed a complex model to provide equivalents across species (Clancy, Darlington, & Finlay, 2001). In her model, there are clear regional differences but birth in the rat is roughly equal to a 4- to 6-month-old human fetus. On non-neural measures, the rat is far more altricial; thermoregulation, eye opening, and visual processing, pinna detachment, all present in the human newborn, do not happen until late in the second week of a rat’s life. Therefore, to the extent comparisons are possible, we would expect similar neural mechanisms mediating withdrawal in the human fetus and human adult. And although it is likely true that human mothers do not use opiates only in the second half of pregnancy, it is equally true that human opiate abusers almost never use only a single drug; nor do they have the adequate nutrition that laboratory rats receive. Thus although this is clearly not a model of what happens in the human condition, it is a reasonable model to understand mechanisms of withdrawal during early development.
Although decades of research have gone into understanding underlying mechanisms of opiate withdrawal, little is still known about infants and opiate dependence. Are the events that unfold in the infant rodent (and human) during chronic exposure to opiates qualitatively similar, somewhere in between, or completely different from those seen in adult rodents? We show here that, as in the adult, there is increased cellular activity (as measured by levels of Fos-LIR) following precipitated withdrawal in the locus coeruleus, nucleus accumbens, periaqueductal gray, and spinal cord. Mechanisms mediating that increase might include activation of nitric oxide synthesis (Adams, Kalicki, Meyer, & Cicero, 1993; Bhargava & Thorat, 1996; Buccafusco, Terry, & Shuster, 1995; Kimes, Vaupel, & London, 1993; Zhu & Barr, 2000), and activation of PKC, PKA, and ERK kinases. Given that withdrawal can be precipitated by injections of antagonist in the same regions of the adult and infant brain, it appears that some of the neural circuitry, and perhaps some intracellular signaling paths, that mediate withdrawal in adult and infant are similar. Nonetheless there are differences that lie in the ability of chronic opiates, but not acute opiates, to engage mechanisms that include, but may not be limited to NMDA glutamate receptors (Jones et al., 2002; Zhu & Barr, 2000, 2001, 2003, 2004). Furthermore, although the use of an opiate antagonist is standard in the animal studies to understand mechanisms of withdrawal, in humans withdrawal is more gradual. We know of no developmental work on the effects of spontaneous withdrawal in animal models. Understanding the similarities and differences in mechanisms between infants and adults is necessary for the development of more rational treatment of opiate exposed infants.
Acknowledgments
NOTES
The authors would like to thank Dr. Christoph Wiedenmayer, Shaoning Wang, and Jianxin Cheng for their assistance with the ICC and tissue samples. Supported by a minority supplement from the National Institute on Drug Abuse, NIH grants DA-06600, DA-00325, and RR03037, the National Science Foundation and a CUNYAGEP fellowship.
Contract grant sponsor: NIH
Contract grant numbers: DA-06600, DA-00325, RR03037
Contract grant sponsor: National Science Foundation
Contract grant sponsor: CUNYAGEP fellowship
References
- Adams ML, Kalicki JM, Meyer ER, Cicero TJ. Inhibition of the morphine withdrawal syndrome by a nitric oxide synthase inhibitor, N(G)-nitro-L-arginine methyl ester. Life Sciences. 1993;52:PL245–PL249. doi: 10.1016/0024-3205(93)90472-f. [DOI] [PubMed] [Google Scholar]
- Adams RE, Wooten GF. Dependence and withdrawal following intracerebroventricular and systemic morphine administration: Functional anatomy and behavior. Brain Research. 1990;518:6–10. doi: 10.1016/0006-8993(90)90946-9. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Rios M, Liu RJ, Gold SJ, Fong HF, Zeiler S, et al. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. Journal of Neuroscience. 2002;22(10):4153–4162. doi: 10.1523/JNEUROSCI.22-10-04153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ, Arnold JH. Opioid tolerance and dependence in infants and children. Critical Care Medicine. 1994;22:334–342. doi: 10.1097/00003246-199402000-00027. [DOI] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41(4):345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Barr GA, Goodwin GA. Precipitated morphine withdrawal induces a conditioned aversion in the preweaning rat. Pharmacology Biochemistry and Behavior. 1997;57(4):779–783. doi: 10.1016/s0091-3057(96)00382-6. [DOI] [PubMed] [Google Scholar]
- Barr GA, Rossi G. Conditioned place preference from ventral tegmental injection of morphine in neonatal rats. Brain Research. Developmental Brain Research. 1992;66:133–136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. European Journal of Pharmacology. 1992;215:35–42. doi: 10.1016/0014-2999(92)90605-4. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Thorat SN. Evidence for a role of nitric oxide of the central nervous system in morphine abstinence syndrome. Pharmacology. 1996;52(2):86–91. doi: 10.1159/000139371. [DOI] [PubMed] [Google Scholar]
- Blasig J, Herz A, Reinhold K, Zieglgansberger WS. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacology. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Boucher T, Jennings E, Fitzgerald M. The onset of diffuse noxious inhibitory controls in postnatal rat pups: A C-Fos study. Neuroscience Letters. 1998;257(1):9–12. doi: 10.1016/s0304-3940(98)00779-4. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV, Jr, Shuster L. Spinal NMDA receptor—Nitric oxide mediation of the expression of morphine withdrawal symptoms in the rat. Brain Research. 1995;679:189–199. doi: 10.1016/0006-8993(95)00203-3. [DOI] [PubMed] [Google Scholar]
- Buckett WR. A new test for morphine-like physical dependence (addiction liability) in rats. Psychopharmacology. 1964;4:410–416. doi: 10.1007/BF00429568. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, et al. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Chieng B, Keay KA, Christie MJ. Increased fos-like immunoreactivity in the periaqueductal gray of anaesthetised rats during opiate withdrawal. Neuroscience Letters. 1995;183:79–82. doi: 10.1016/0304-3940(94)11119-4. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Delander GE, Takemori AE. Spinal antagonism of tolerance and dependence induced by systemically administered morphine. European Journal of Pharmacology. 1983;94(1–2):35–42. doi: 10.1016/0014-2999(83)90439-9. [DOI] [PubMed] [Google Scholar]
- Donaldson HH. A comparison of growth changes in the nervous system of the rat with corresponding changes in the nervous system of man. Proceedings of the National Academy of Sciences. 1918;4(3):280–283. doi: 10.1073/pnas.4.9.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druhan JP, Walters CL, Aston-Jones G. Behavioral activation induced by D(2)-like receptor stimulation during opiate withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2000;294(2):531–538. [PubMed] [Google Scholar]
- Dworkin SI, Guerin GF, Goeders NE, Smith JE. Kainic acid lesions of the nucleus accumbens selectively attenuate morphine self-administration. Pharmacology Biochemistry & Behavior. 1988;29:175–181. doi: 10.1016/0091-3057(88)90292-4. [DOI] [PubMed] [Google Scholar]
- Finnegan LP. Effects of materanl opiate abuse on the newborn. Federation Proceedings. 1985;44:2314–2317. [PubMed] [Google Scholar]
- Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. American Journal of Critical Care. 1998;7(5):364–369. [PubMed] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. Journal of Neuroscience. 2005;25(6):1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary WAI, Wooten GF. Dose effects of naloxone on fixed morphine dependence: Simultaneous behavioral and 2-deoxyglucose study in the rat. Brain Research. 1985;332:69–78. doi: 10.1016/0006-8993(85)90390-7. [DOI] [PubMed] [Google Scholar]
- Geary WAI, Wooten GF. Time action profiles of regional cerebral glucose utilization during naloxone-precipitated morphine withdrawal. Brain Research. 1986;399:181–184. doi: 10.1016/0006-8993(86)90616-5. [DOI] [PubMed] [Google Scholar]
- Geller LM, Geller ES. A simple technique for permanent marking of newborn albino rats. Psychological Reports. 1966;18:221–222. doi: 10.2466/pr0.1966.18.1.221. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Duman RS, Nestler EJ. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Research. 1990;525:256–266. doi: 10.1016/0006-8993(90)90872-9. [DOI] [PubMed] [Google Scholar]
- Hsu S, Raine L, Fanger H. Use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry & Cytochemistry. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. Journal of Neuroscience. 1998;18(24):10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behavioral Neuroscience. 1995;109:1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Injections of an opioid antagonist into the locus coeruleus and periaqueductal gray but not the amygdala precipitates morphine withdrawal in the 7-day-old rat. Synapse. 2001;39(2):139–151. doi: 10.1002/1098-2396(200102)39:2<139::AID-SYN5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Jones KL, Zhu H, Jenab S, Du T, Inturrisi CE, Barr GA. Attenuation of acute morphine withdrawal in the neonatal rat by the competitive NMDA receptor antagonist LY235959. Neuropsychopharmacology. 2002;26(3):301–310. doi: 10.1016/S0893-133X(01)00347-5. [DOI] [PubMed] [Google Scholar]
- Joyce MP, Barr GA. Ontogeny of Fos protein-like immunoreactivity in the suprachiasmatic nucleus. Synapse. 1995;21(1):54–59. doi: 10.1002/syn.890210108. [DOI] [PubMed] [Google Scholar]
- Kimes AS, London ED. Glucose utilization in the rat brain during chronic morphine treatment and naloxone-precipitated morphine withdrawal. Journal of Pharmacology & Experimental Therapeutics. 1989;248:538–545. [PubMed] [Google Scholar]
- Kimes AS, Vaupel DB, London ED. Attenuation of some signs of opioid withdrawal by inhibitors of nitric oxide synthase. Psychopharmacology. 1993;112:521–524. doi: 10.1007/BF02244904. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology. 1989;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: A randomized trial. Pediatrics. 2008;122(3):e601–e607. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschka E, Teschemacher HH, Mehraein P, Herz A. Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacology. 1976;46:141–147. doi: 10.1007/BF00421383. [DOI] [PubMed] [Google Scholar]
- Le Guen S, Catheline G, Besson JM. Effects of NMDA receptor antagonists on morphine tolerance: A c-Fos study in the lumbar spinal cord of the rat. European Journal of Pharmacology. 1999;373(1):1–11. doi: 10.1016/s0014-2999(99)00272-1. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kishioka S, Inoue N, Shimizu N, Fukazawa Y, Ozaki M, et al. Naloxone-precipitated morphine withdrawal elicits increases in c-fos mRNA expression in restricted regions of the infant rat brain. Japanese Journal of Pharmacology. 2002;90(3):270–275. doi: 10.1254/jjp.90.270. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Fournié-Zaluski MC, Roques BP. Attenuation of the morphine withdrawal syndrome by inhibition of catabolism of endogenous enkephalins in the periaqueductal gray matter. Naunyn-Schmiedebergs Archives of Pharmacology. 1992;345:466–472. doi: 10.1007/BF00176626. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31(12):1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. Journal of Pharmacology and Experimental Therapeutics. 1992;261:669–677. [PubMed] [Google Scholar]
- McPhie AA, Barr GA. The role of opioid receptors in morphine withdrawal in the infant rat [In Process Citation]. Brain Research. Developmental Brain Research. 2000;124(1–2):73–80. doi: 10.1016/s0165-3806(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Cole MJ, Jeffery HE. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database of Systematic Reviews. 2002;(3):CD002059. doi: 10.1002/14651858.CD002059. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database of Systematic Reviews. 2002;(3):CD002053. doi: 10.1002/14651858.CD002053.pub2. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development–current perspectives. Endocrinology Reviews. 1993;14(1):94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Krystal JH. Multiple mechanisms of withdrawal from opioid drugs. Annual Review of Neuroscience. 1984;7:443–478. doi: 10.1146/annurev.ne.07.030184.002303. [DOI] [PubMed] [Google Scholar]
- Rohde DS, Detweiler DJ, Basbaum AI. Spinal cord mechanisms of opioid tolerance and dependence: Fos-like immunoreactivity expression increases in subpopu-lations of spinal cord neurons during withdrawal. Neuroscience. 1996a;72:233–242. doi: 10.1016/0306-4522(95)00529-3. [DOI] [PubMed] [Google Scholar]
- Rohde DS, Detweiler DJ, Basbaum AI. Spinal cord mechanisms of opioid tolerance and dependence: Fos-like immunoreactivity increases in subpopulations of spinal cord neurons during withdrawal [corrected] [published erratum appears in Neuroscience 1996 September; 74(1) 296] Neuroscience. 1996b;72(1):233–242. doi: 10.1016/0306-4522(95)00529-3. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Human Development. 1991;26(1):61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, et al. Regional and cellular mapping of cAMP response-element mediated transcription during naltrexone-precipitated morphine withdrawal. Journal of Neuroscience. 2002;22(9):3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Norton FE, Guyenet PG. Autonomic areas of rat brain exhibit increased fos-like immunoreactivity during opiate withdrawal in rats. Brain Research. 1993;624:19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Thornton SR, Wang AF, Smith FL. Characterization of neonatal rat morphine tolerance and dependence. European Journal of Pharmacology. 1997;340:161–167. doi: 10.1016/s0014-2999(97)01434-9. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. Journal of Physiology—London. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier M. Branching patterns of central amygdaloid nucleus efferents in the rat: Single-axon reconstructions. Annals of the New York Academy of Sciences. 2003;985:552–553. [Google Scholar]
- Walters CL, Aston-Jones G, Druhan JP. Expression of fos-related antigens in the nucleus accumbens during opiate withdrawal and their attenuation by a D2 dopamine receptor agonist. Neuropsychopharmacology. 2000;23(3):307–315. doi: 10.1016/S0893-133X(00)00113-5. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behavioural Brain Research. 2001a;126(1–2):147–157. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in responsivity to threat are stimulus-specific in rats. Developmental Psychobiology. 2001b;39(1):1–7. doi: 10.1002/dev.1022. [DOI] [PubMed] [Google Scholar]
- Williams S, Evan G, Hunt SP. Spinal c-fos induction by sensory stimulation in neonatal rats. Neuroscience Letters. 1990;109:309–314. doi: 10.1016/0304-3940(90)90013-y. [DOI] [PubMed] [Google Scholar]
- Windh RT, Little PJ, Kuhn CM. The ontogeny of mu opiate tolerance and dependence in the rat: Anti-nociceptive and biochemical studies. Journal of Pharmacology and Experimental Therapeutics. 1995;273(3):1361–1374. [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacology & Therapeutics. 1987;35(1–2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Yi DK, Barr GA. The induction of Fos-like immunoreactivity by noxious thermal, mechanical and chemical stimuli in the lumbar spinal cord of infant rats. Pain. 1995;60:257–265. doi: 10.1016/0304-3959(94)00119-y. [DOI] [PubMed] [Google Scholar]
- Yi DK, Barr GA. Formalin-induced c-fos expression in the spinal cord of fetal rats. Pain. 1997;73(3):347–354. doi: 10.1016/S0304-3959(97)00119-X. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Naltrexone-precipitated morphine withdrawal in infant rat is attenuated by acute administration of NOS inhibitors but not NMDA receptor antagonists. Psychopharmacology (Berlin) 2000;150(3):325–336. doi: 10.1007/s002130000442. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Opiate withdrawal during development: Are NMDA receptors indispensable? Trends in Pharmacological Sciences. 2001;22(8):404–408. doi: 10.1016/s0165-6147(00)01792-2. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Ontogeny of NMDA receptor-mediated morphine tolerance in the postnatal rat. Pain. 2003;104(3):437–447. doi: 10.1016/S0304-3959(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. The role of AMPA and metabotropic glutamate receptors on morphine withdrawal in infant rats. International Journal of Developmental Neuroscience. 2004;22(5–6):379–395. doi: 10.1016/j.ijdevneu.2004.06.005. [DOI] [PubMed] [Google Scholar]


