Abstract
Human infants may be exposed to opiates through placental transfer from an opiate-using mother or through the direct administration of such drugs to relieve pain (e.g., due to illness or neonatal surgery). Infants of many species show physical dependence and tolerance to opiates. The magnitude of tolerance and the nature of withdrawal differ from those of the adult. Moreover, the mechanisms that contribute to the chronic effects of opiates are not well understood in the infant but include biological processes that are both common to and distinct from those of the adult. We review the animal research literature on the effects of chronic and acute opiate exposure in infants and identify mechanisms of withdrawal and tolerance that are similar to and different from those understood in adults. These mechanisms include opioid pharmacology, underlying neural substrates, and the involvement of other neurotransmitter systems. It appears that brain circuitry and opioid receptor types are similar but that NMDA receptor function is immature in the infant. Intracellular signaling cascades may differ but data are complicated by differences between the effects of chronic versus acute morphine treatment. Given the limited treatment options for the dependent infant patient, further study of the biological functions that are altered by chronic opiate treatment is necessary to guide evidenced-based treatment modalities.
Keywords: dependence, glutamate, infant, morphine, opiate, protein kinase, rodent model, tolerance, withdrawal
Introduction
Opiate tolerance to and withdrawal from opium and its derivatives have long been recognized, and reports of the deleterious effects of opiates on infants are over 100 years old (Happel 1892; Tate 1899). Today, infants may be exposed to opiates for medical reasons; for example, iatrogenic induction of opiate dependence is not uncommon in preterm infants in the neonatal intensive care unit (Anand et al. 2010). In addition, opiate use by pregnant women can result in dependence in the fetus. The rates of such use are difficult to ascertain and estimates vary widely, but some are as high as 2–4% of pregnant women (Keegan et al. 2010; Lester et al. 2001; Shannon et al. 2010).
Most of the research on mechanisms underlying tolerance and dependence has used animal, and especially rodent, models, so we provide comparisons of the developmental stages of rats and humans. These comparisons are at best estimates and clearly depend on the metric compared.
In terms of overall rates of protein synthesis in the brain, the rat is altricial and at birth is roughly equivalent to an early-third-trimester human fetus (Dobbing 1981); birth in the human would be equal to a 7-day-old pup (Figure 1). A more fine-grained analysis (Clancy et al. 2007) describes a range of developmental equivalencies depending on brain site. In this model, the birth of the rat infant “translates” to the human as the start of the second trimester for noncortical and limbic structures and the early third trimester for cortical structures. This places the rat at about 18 days of postnatal age when the human is born.
Figure 1.
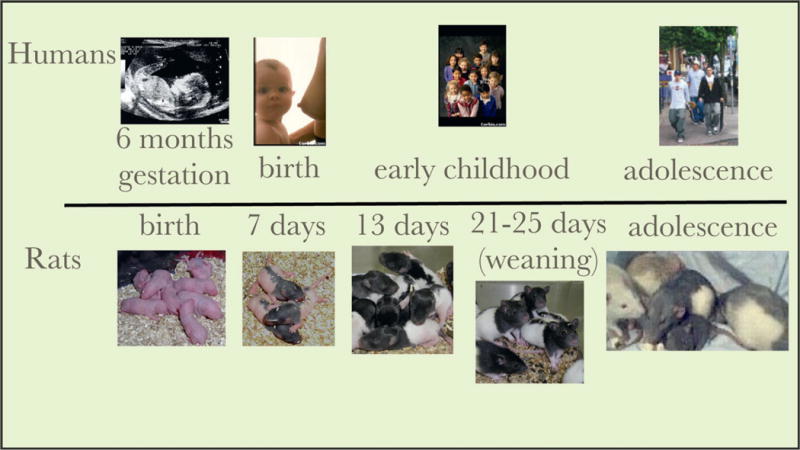
Illustration of rough equivalent ages for a rat and human based on rate of protein synthesis in the brain (Dobbing 1981). Other schemes, based on other criteria, show slightly different age equivalencies (Clancy et al. 2007) and individual brain regions and physiological functions develop at individual rates. Despite slight differences the rat is altricial and is developmentally similar at birth to the human fetus at 6 months.
In this review we focus on mechanisms of tolerance and withdrawal after repeated or acute exposure of infants to opiates (mostly morphine), building on the work of others who have addressed similar topics (Noda and Nabeshima 2004; Richardson et al. 2006). (For reviews of the long-term effects of in utero and neonatal opiate exposure, see Lester and Lagasse 2010; Schempf 2007; Vathy 2002; and Yanai et al. 2003.)
Effects of Chronic Opiate Exposure in the Infant
Tolerance
Tolerance is defined as a decreased response to a drug after administration of or exposure to the drug; empirically it is defined as a shift to the right in the dose-response curve with an increased effective dose (ED50) or effective concentration (EC50) (Figure 2). The degree of tolerance may differ for different drug effects. It usually follows multiple administrations of the drug but can occur after a single injection (acute tolerance or tachyphylaxis). The mechanisms of tolerance to acute (single-dose) and chronic (repeated) exposure to the drug may differ. The maximum effect can also be reduced in some cases.
Figure 2.

Schematic demonstrating tolerance. When a drug that can induce tolerance is given repeatedly, the dose response curve shifts to the right. (Sensitization, not shown, would be a shift to the left.) The dose that affects 50% of the subjects (ED50) is then increased.
Numerous studies have shown that human and nonhuman infants become tolerant to the analgesic effects of opiates (Anand et al. 2010; Barr et al. 1986; Barr and Wang 1992; Ceger and Kuhn 2000; Richardson et al. 2006; Tempel et al. 1988; Thornton and Smith 1997; Thornton et al. 1997; Windh et al. 1995; Zissen et al. 2007). The degree to which tolerance develops is less for infants than that for adults for reasons that are not known but are likely to be pharmacokinetic and pharmacodynamic.
In addition to tolerance to the drug’s analgesic actions, a variety of species (humans, rodents, sheep, and guinea pigs) exhibit tolerance to sedation, arousal changes in sleep state, and EEG and respiratory depression (Choe and Smith 2000; Eaton et al. 1992; Szeto et al. 1988, 1990). In contrast, there is no tolerance to morphine-induced suppression of distress vocalizations (Barr and Wang 1992). We are not aware of any data on the development of tolerance to the gastrointestinal effects (e.g., constipation) of morphine. Tolerance can also occur to drugs that target kappa-opioid receptors (Barr et al. 1986).
There are no data suggesting that the age of the human infant influences the magnitude or rapidity of tolerance to opiates. Indeed, tolerance in the infant rat is typically of a lesser degree than that of the older pup or adult rat (van Praag and Frenk 1991; van Praag et al. 1993; Windh et al. 1995; Zhu and Barr 2003).
Studies of acute tolerance, in which the test dose of the opiate takes place shortly after an initial dose, are rare but show that it does occur in the older preweaning rodent (Huidobro and Huidobro 1973). In one study a single injection of morphine to 2-day-old rat pups resulted in tolerance revealed by testing 3 weeks later (Bardo and Hughes 1981) but not when the initial injection was at 5, 9, or 13 days of age. This approach is not a test of “acute tolerance” in the strict sense, but it demonstrates long-term effects of opiate treatment in the infant and can indicate whether there is a critical period for the development of long-term consequences, although to our knowledge this critical period has not been clearly defined.
Dependence and Withdrawal
Dependence and withdrawal can be physical or psychological or both. Their intensity is a function of the drug, duration of use, dose, and the kinetics of the drug in addition to the age of the subject.
Physical dependence entails the presence of physical signs when the drug is withdrawn or when an antagonist to the drug’s action is given. Such signs can be minor (e.g., a caffeine withdrawal headache) or life threatening (e.g., seizures from barbiturate or alcohol withdrawal). Physical signs of opiate withdrawal in human and nonhuman adults include flu-like symptoms such as muscle aches, runny nose, abdominal pain and diarrhea, dilated pupils, and nausea and vomiting.
Psychological dependence is a need to continue drug use and does not require physical signs. It can last well beyond the resolution of physical withdrawal signs. Humans have also described a state of dysphoria that exceeds in severity the actual physical symptoms.
Unconditioned Behaviors
The mechanisms by which opiates produce withdrawal in adults are well studied if not fully understood (e.g., Frenois et al. 2005a; Mao 1999; McClung 2006; Nestler et al. 1993). We focus here on mechanisms specific to the infant.
As mentioned above, very early studies demonstrated that a pregnant woman’s use of opiates can have deleterious effects on the infant (Happel 1892; Shute and Davis 1933; Tate 1899), who may become passively dependent through placental transfer and experience abrupt withdrawal at birth. Withdrawal can also occur in neonates that have received opiates for pain management.
Upon withdrawal the infant experiences behavioral and state regulation disturbances, called the neonatal abstinence syndrome (NAS), which includes increased irritability, increased movements and activity, sucking and swallowing disturbances, sleep deprivation, and disorganized and fragmented sleep-wake states (Franck and Vilardi 1995; Gewolb et al. 2004; Hutchings 1990; O’Brien and Jeffery 2002). Among infants whose dependence is due to maternal opiate use there is no strong relationship between the type or dose of opiate and the severity and duration of withdrawal (Coghlan et al. 1999; Kuschel et al. 2004). In infants treated medically with opiates, withdrawal is measured by a recently validated psychometric tool that takes into account the presence and intensity of multiple withdrawal symptoms (Franck et al. 2008).
Animal models of opiate withdrawal have been developed only in the past 30 years. Part of the difficulty of defining withdrawal in infants is that its manifestations are fundamentally different from those of the adult. Opiate withdrawal in human or nonhuman adults includes, among other signs, activation of the sympathetic nervous system, but in the infant these processes are immature (Myers et al. 1992; Quigley et al. 1996); thus classic signs in the adult rodent, such as teeth chattering, jumping, diarrhea, “wet-dog shakes,” and ptosis (which cannot occur in infant rodents, whose eyes have not yet opened), do not occur between 14 and 21 days of age in the rat (Jones and Barr 1995).
Early rodent studies reported that infants in withdrawal show altered activity and sleep-wake rhythms (Hutchings et al. 1979, 1980; Kirby 1981). Subsequent work demonstrated that, in addition to increased behavioral activation, there are clearly defined behaviors for the NAS in rodent infants, postweanlings, and indeed throughout development (Figure 3; Barr et al. 1998; Jones and Barr 1995; Thornton and Smith 1997; Thornton et al. 1997; Windh et al. 1995). Infant behaviors include increased ultrasonic vocalizations upon separation from the dam and littermates, head swaying, paw movement, and rolling (Table 1; Barr et al. 1998; Jones and Barr 1995; Thornton and Smith 1997; Thornton et al. 1997; Windh et al. 1995). Administration of naloxone after a single opiate injection induces similar withdrawal signs (Jones et al. 2002; Perez-Saad et al. 1996). Some withdrawal behaviors also occur in the fetal rat after precipitated withdrawal when the dam has been treated with morphine (Ceger and Kuhn 2000; Jones and Barr 2000; Kirby 1981).
Figure 3.
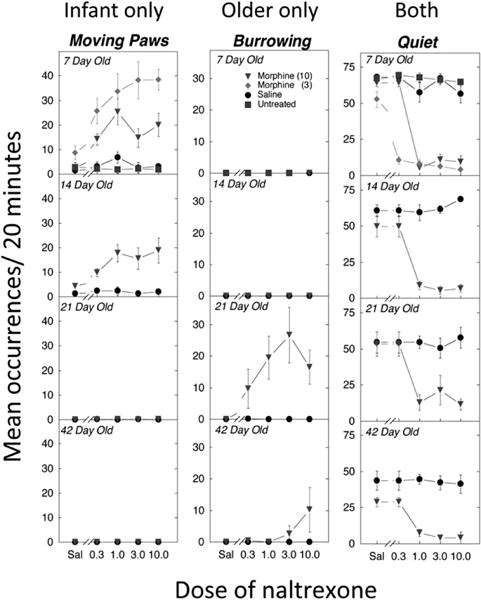
Examples of behaviors that are unique to infant rats (7 days old), occur only in older rats (21+ days old), or occur throughout the lifespan (adapted from Jones and Barr 1995). See Table 1 for definitions of the behaviors. Numbers after morphine are the chronic doses (3 or 10 mg/kg 2×/day) begun 7 days before testing. Sal, saline
Table 1.
Precipitated withdrawal behaviors in the infant rat
| Behavior | Definition |
|---|---|
| Burrowing | Sliding the body under shavings in the observation chamber |
| Head swaying | Lateral and/or rotary motion of the head |
| Paw movement | Continuous movement of the hind paws without walking |
| Quiet | “Sedated” appearance without movement |
| Rolling | Turning the body over at least one full rotation |
| Ultrasonic vocalization | Vocalizations in the ultrasonic range (typically ~40 kHz) that imply distress in infant rodents |
| Together | Bodily contact with one or more littermates |
| Walking | Moving forward at least one step |
| Wall climbing | Putting both forepaws on the wall of the observation chamber, typically with movement |
Adapted from Zhu and Barr (2004).
Sensitization
Hyperalgesia (heightened sensitization to pain) can occur during either chronic administration or withdrawal, but there is no consensus about the frequency or circumstances of its occurrence (for reviews, Bannister and Dickenson 2009; Bekhit 2010). In animal models only one laboratory has examined sensitization after opiate treatment in infants (Sweitzer et al. 2004a,b; Zhang and Sweitzer 2008; Zissen et al. 2007). Withdrawal induced mechanical allodynia and thermal hyperalgesia in rat pups as early as 7 days of age and in some cases lasted weeks (Zhang and Sweitzer 2008). The sensitization occurred after acute, chronic, or intermittent morphine treatment, although there were differences among the treatment regimens (Sweitzer et al. 2004a; Zissen et al. 2006, 2007).
Sensitization may be the result of central or peripheral processes, and there is enhanced nociception during the second phase of the formalin test (a measure of central and peripheral sensitization; Zissen et al. 2006) and enhanced slow ventral root potential (sVRP1), an electrophysiological correlate of nociception in an ex vivo spinal cord preparation (Sweitzer et al. 2004b).
Affective Consequences
In adult humans and animals, opiate withdrawal is associated with a strong negative affect (Fendt and Mucha 2001; Handelsman et al. 1992; Kanof et al. 1993; Koob et al. 1989; Mucha et al. 1986). In the human infant, the interpretation of negative affective consequences of withdrawal is drawn from increased fussiness, increased crying, and a decreased ability to be soothed. With opiates that have a short half-life (e.g., heroin, fentanyl), it is possible that the human fetus undergoes withdrawal after exposure to the opiate. It is not known whether withdrawal induces a negative affective state in the fetus (Handelsman et al. 1992; Kanof et al. 1993). If so, the fetus, which can learn in utero (e.g., DeCasper and Fifer 1980; Smotherman 2002; Stickrod et al. 1982; for review, Moon and Fifer 2000), could associate maternal cues (e.g., odors) with that aversive state. That association might affect later attachment to the mother, although to our knowledge there are no data that bear on this speculation.
The question of whether opiate withdrawal in the infant is aversive has been the subject of animal studies. In the infant rodent, withdrawal from a variety of dysphoric drugs increases ultrasonic vocalizations, a behavior normally expressed under stressful conditions such as cold ambient temperature. At 7 days of age, pups spontaneously withdrawn from chronic morphine cry more than controls 6 hours after the last injection and show altered ultrasonic vocalization patterns 3 days later (Barr and Wang 1992).
It is not clear, however, that the aversive properties of withdrawal can be conditioned in infant rats, in part because, unlike older pups and adults, they are resistant to learning to associate cues with aversive stimuli (Sullivan et al. 2009): in a conditioned odor aversion paradigm, pups in precipitated withdrawal did not learn to avoid an odor associated with withdrawal at 7 days of age, but did so at 14 days (Barr and Goodwin 1997). This is not due to an inherent inability to learn aversions since younger pups can learn these associations under certain circumstances (Barr et al. 1994; reviewed by Sullivan et al. 2009). Thus, although conditioned aversions are not learned early (7 days of age), unconditioned responses associated with an aversive state are present quite early and show the negative affective component of opiate withdrawal.
Mechanisms of Opiate Tolerance and Withdrawal in the Infant
There are multiple possible reasons for different withdrawal syndromes in the neonate and the adult. Given the immaturity of the central nervous system (CNS) in the infant, it is likely that the neural mechanisms that mediate tolerance and withdrawal in the infant differ from those of the adult in at least two ways. First, there may be age-related differences in cellular mechanisms that mediate withdrawal, including changes in receptor populations, their ability to be internalized, intracellular messengers, and/or transcription factors. Second, different neural circuitry may mediate withdrawal in the infant and the adult. In their most simple form, the CNS circuits may be similar across ages, whereas the output mechanisms— the autonomic nervous system, for example—may differ. As discussed in the following sections, the literature suggests that the anatomical circuits are at least similar and that the opioid receptor involved is the same. In contrast, the role of intracellular signals, in particular those related to glutamate N-methyl-d-aspartate (NMDA1) receptors, appears to differ.
Opiate Receptor Development
Because opioid receptors develop at different stages (Leslie and Loughlin 1993)—for example, in rats the delta opioid receptor does not appear until the second week of life, comparable to early childhood in humans (De Vries et al. 1990; Leslie et al. 1982; Spain et al. 1985)—it is possible that different classes of opioid receptors mediate withdrawal in infants and adults. Indeed, studies have shown that the effects of chronic morphine treatment on receptor dynamics are age dependent. When pups are treated with morphine starting at postnatal day (PND1) 1, there is a downregulation of mu opioid receptor numbers at PND 4 that is not seen at PND 8 or older, even with continued treatment (Stoller et al. 2002; Tempel 1991; Tempel et al. 1988). This downregulation may be because of unique properties of opioid receptors shortly after birth, but what those properties may be is not known.
Withdrawal is mediated by similar opioid receptor types: delta (DOR), kappa (KOR), and mu (MOR). In young pups (less than a week old) mu opioid receptors are the major receptor type involved in withdrawal (McPhie and Barr 2000), although the animals may develop tolerance to drugs that prefer the kappa opioid receptor (Barr et al. 1986). Antagonists to the delta or kappa opioid receptor did not precipitate behavioral withdrawal in the 7-day-old pup, whereas an antagonist to the mu opioid receptor did; similarly, in adults, mu opioid receptors regulate morphine-induced tolerance and dependence (Dumas and Pollack 2008; Raehal and Bohn 2005). In pups treated from PND 14 to PND 17 with morphine, the kappa agonist U50,488 is less effective than in controls, suggesting that chronic morphine exposure alters kappa receptor function (Stoller et al. 2007). Although the mu opioid receptor density and affinity are unaltered in the older pups, they still exhibit tolerance and withdrawal (Stoller et al. 2002; Tempel et al. 1988).
Changing Neural Circuitry
One possible reason for differences in the withdrawal behaviors of infants and adults is differential involvement of neural circuits: the periaqueductal gray (PAG1), locus coeruleus (LC), amygdala, ventral tegmental area, nucleus accumbens, hypothalamus, and spinal cord (for adult circuitry see Chieng et al. 1995; Druhan et al. 2000; Frenois et al. 2005b; Maldonado et al. 1992). It appears, however, that similar neural circuits are involved at both stages of life. Direct injection of an opiate antagonist into either the PAG or the LC precipitated withdrawal in a morphine-dependent 7-day-old pup, whereas injections into the amygdala did not (Jones and Barr 2001). This latter finding is not necessarily inconsistent with the adult literature. The amygdala mediates the aversive properties of opiate withdrawal in the adult (Maldonado et al. 1992) but learned aversions to opiate withdrawal are not present in the infant rat at PND 7 (Barr and Goodwin 1997).
Late maturation of the ability to learn a conditioned aversion is not limited to opiate withdrawal as the immaturity of amygdala function limits it ability to regulate other learned aversions (Sullivan et al. 2009). Moreover, withdrawal activates the same brain circuits—in the olfactory bulb, nucleus accumbens, hypothalamus, PAG, LC, medulla oblongata, and spinal cord (assessed by Fos protein or messenger RNA expression)—in both the infant and the adult (Maeda et al. 2002; McPhie and Barr 2009). However, there are developmental differences in activation patterns between the infant and adult medulla (Maeda et al. 2002).
Role of Glutamate Neurotransmission
NMDA Receptors: Chronic Opiate Exposure
In the adult animal, NMDA blockers reduce the development and expression of opiate withdrawal and tolerance (Noda and Nabeshima 2004; Trujillo 2000), whereas in the infant these blockers are less effective in alleviating either tolerance or withdrawal until the animal begins weaning (for review, Noda and Nabeshima 2004; Zhu and Barr 2001).
Tolerance and withdrawal can be defined by behavioral changes or by changes in the sVRP after dorsal root simulation in the isolated spinal cord (Yanagisawa et al. 1984). One advantage of the in vitro spinal cord preparation is that it bypasses changes that might be due to other physiological systems.
In pups younger than 8 days of age, coadministration of the NMDA antagonists MK-801 or dextromethorphan with morphine does not reduce tolerance or withdrawal but rather can exacerbate both (Bell and Beglan 1995a,b; Zhu and Barr 2000, 2003). In older animals NMDA blockers become effective in reversing behavioral tolerance or withdrawal— they are somewhat effective at PND 14 and fully effective at PND 21 in reducing both tolerance and withdrawal (Zhu and Barr 2000, 2003). In pups 12 to 17 days of age, 3 days of twice-daily morphine treatment downregulated glutamate transporter activity and may thus have increased NMDA receptor activation because of the resulting higher levels of extracellular glutamate (Thomson et al. 2006). Further research is necessary to determine whether this mechanism occurs in younger animals, when NMDA antagonists do not have the ability to prevent withdrawal.
Acute treatment with MK-801 affects the expression (but not the development) of withdrawal in older animals but is mostly ineffective in the 6- to 7-day-old pup. Although it decreased head moves, it increased walking, wall climbing, and overall locomotor activity (Zhu and Barr 2000). In contrast, it reduced behavioral tolerance in older pups (14 days of age), reduced the sVRP in the isolated spinal cord preparation (likely by a synergistic action with morphine; Bell and Beglan 1995b), and inhibited excitatory postsynaptic currents (EPSCs) in voltage-clamped cells in spinal slices (Zeng et al. 2006).
Fos expression in the olfactory bulb, hypothalamus, and medulla of the infant rat is stimulated during withdrawal (Maeda et al. 2002). This heightened level of expression was reduced by concurrent treatment with MK-801 and morphine from PND 2 to PND 7 in the olfactory bulb and hypothalamus but not in the medulla (Maeda et al. 2002).
In a different approach, we used two strains of mice to assess the role of the NMDA glutamate receptor in tolerance to morphine during early development. Adult mice of the 129 strain display little or no analgesic tolerance to morphine, whereas other strains such as CD-1 and Swiss-Webster mice become tolerant (Crain and Shen 2000; Kest et al. 2002; Kolesnikov et al. 1998; Liang et al. 2006). It has been hypothesized that the mouse strains differ in functional NMDA receptor function (Kolesnikov et al. 1998) and that deficiencies in GM1 ganglioside regulate excitatory opioid receptor function (Crain and Shen 2000). Thus the 129 strain either lacks or has an impaired receptor.
If the NMDA receptor is not important in tolerance in the infant then the lack of its functionality should have no consequence—the 129 and CD-1 infant mice should respond the same to chronic morphine exposure; in older mice, for which the NMDA receptor is important, the strains should differ. To assess this hypothesis we injected CD-1 and 129S6 pups with morphine starting on either PND 2 or PND 16 for 7 days and tested for analgesic tolerance. At PND 8, both types of mice showed tolerance in a tail flick test, whereas by PND 22 the 129S6 pups no longer did (Figure 4). The data are consistent with adult data showing that there is no role for the NMDA receptor in tolerance in the 8-day-old mouse but a necessary involvement at PND 21 (Perez and Barr, unpublished).
Figure 4.
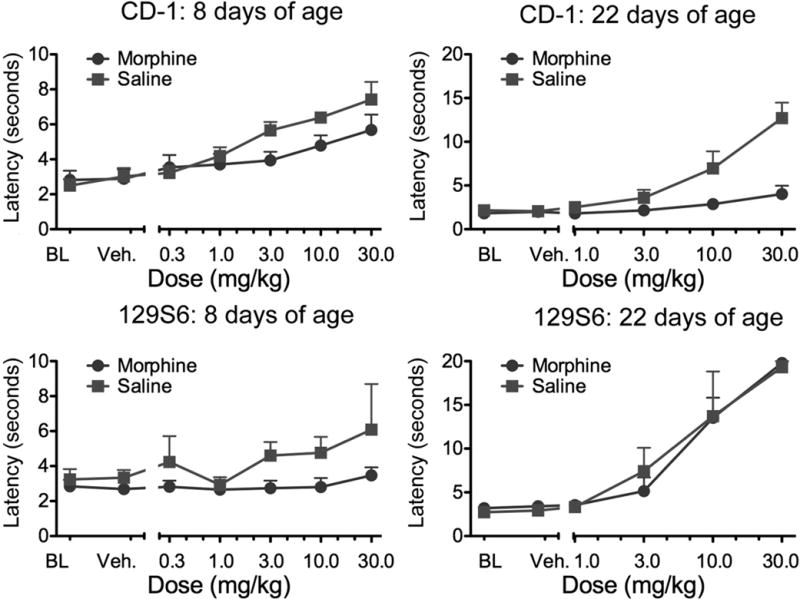
The development of tolerance in CD-1 and 129S6 mice. Pups were injected twice daily for 6½ days starting at either 1 or 15 days of age (N = 3–9 per condition). At 8 or 22 days of age, respectively, they were tested for analgesia in a cumulative dose response paradigm with morphine using the tail immersion test. At 8 days of age, both strains showed tolerance, but at 22 days only the CD-1 mice were tolerant. Thus the deficit in the 129S6 mouse—whether a lack of functional NMDA receptors or deficiencies in GM1 ganglioside–regulated excitatory opioid receptor function—has no influence on tolerance in the infant. In the older pup, the deficit has functional consequences (Perez and Barr, unpublished data). BL, baseline; NMDA, N-methyl-d-aspartate; veh, vehicle
NMDA Receptors: Acute Opiate Exposure
A single injection of morphine can induce tolerance and establish dependence, and a subsequent injection of an NMDA blocker reduces withdrawal, tolerance, and Fos expression (Jones et al. 2002). Acute morphine treatment does not induce many of the neuroplastic changes—for example on receptors or second messenger systems (for review, Zhang et al. 2009)—that chronic treatment would. In the first week of life in the rat, these changes, induced by chronic morphine exposure, are not NMDA dependent. NMDA antagonists are effective, however, when given either after or with chronic treatment with morphine after PND 21.
Non-NMDA Glutamate Receptor Effects
We are aware of only one study that examined non-NMDA glutamate receptor effects after chronic opiate exposure during early development (Zhu and Barr 2004). Use of both a behavioral model and the isolated spinal cord preparation showed that an acutely administered group II metabotropic glutamate agonist and/or an AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) blocker reduced withdrawal in 7-day-old rat pups. Whether they would block neuroplastic changes if given concurrently with morphine is not known.
Nitric Oxide
The production of nitric oxide via activation of the NMDA receptor may facilitate the development and expression of morphine-induced tolerance and dependence (Elliott et al. 1995; Inturrisi 1997; Thorat et al. 1994; Trujillo 1995; Vaupel et al. 1995a,b). Very little is known about nitric oxide and its effects on withdrawal in infants but the one existing study showed that acute inhibition of nitric oxide synthase by either L-NAME (NG-nitro-L-arginine methyl ester) or 7-nitroindazole blocked withdrawal behaviors (Zhu and Barr 2000).
Other Neurosubstrates: Substance P
There is a single study on the role of substance P (SP) in tolerance in infants (Thomson et al. 2008). The data, from 12- to 17-day-old pups, show a downregulation of the neurokinin-1 (NK1) receptor and a loss of SP effects in lamina 1 of the spinal cord, but not in the dorsal root ganglia, after twice-daily morphine treatment (Thomson et al. 2008). This result is the opposite of that found in adults, in which continuously infused morphine increased NK1 receptor internalization (King et al. 2005). Thomson and colleagues attributed the results mostly to differences in the treatment protocol (twice daily injection vs. continuous infusion), but they may also be age dependent because SP has a late-developing role in nociception in the infant (King and Barr 2003; King et al. 2000a,b). More research is necessary to improve understanding of the role of substance P in opiate tolerance.
Protein Kinase Signaling Cascades
Withdrawal from Acute Morphine Exposure
In the adult animal, morphine alters intracellular signaling pathways (Figure 5; reviewed by Chen and Sommer 2009; Zhai et al. 2008; Zhang et al. 2009), but few studies have examined these cascades in early development. We summarize those here.
Figure 5.

Schematic diagram of protein kinase (PK) A and PKC intracellular signaling pathways by which G protein–coupled receptors (GPCRs) activate cAMP and other signaling molecules and thus affect gene expression. Adapted from SABiosciences/Protein Lounge.
A broadly acting protein kinase (PK1) C antagonist blocked spontaneous or precipitated withdrawal after a single injection of morphine both in vivo in rats and in vitro (sVRP in isolated rat spinal cord) at PND 7 (Sweitzer et al. 2004b). Calcium-independent PKC antagonists (but not calcium-dependent PKCγ antagonists) blocked precipitated thermal hyperalgesia and the increased sVRP response; antagonists to both PKCs blocked spontaneous withdrawal (Sweitzer et al. 2004b). Sweitzer and colleagues (2003) also examined the role of PKCγ and PKCε in naloxone-precipitated withdrawal, which induced both allodynia and withdrawal behaviors at 7 and 21 days of age. When withdrawal was precipitated shortly after acute morphine (30 minutes), PKCε but not PKCγ contributed to withdrawal at PND 7. Both isoforms were involved at PND 21. With later withdrawal (120 minutes after morphine administration), both isoforms contributed to withdrawal at both ages.
Because PKCε is located in dorsal root ganglia and PKCγ is concentrated in the spinal cord, both of which are immature at PND 7, these effects point to the need to consider age-related anatomical changes in the circuits that regulate the effects of morphine.
Withdrawal from Chronic Morphine Exposure
In experiments with rodents that examined the role of PKC and PKA, we found little evidence for their involvement in either tolerance or withdrawal at PND 7 after repeated twice-daily morphine treatment (Figure 6; McPhie and Barr, unpublished). Acute injection of a general PK blocker or of specific PKC or PKA antagonists did not reduce tolerance after 13 twice-daily morphine injections (administered over 6½ days; Figure 6). These drugs also do not reduce behavioral withdrawal signs (data not shown). Concurrent blockade of protein kinases during the establishment of tolerance and dependence might be effective but those experiments have not been conducted.
Figure 6.
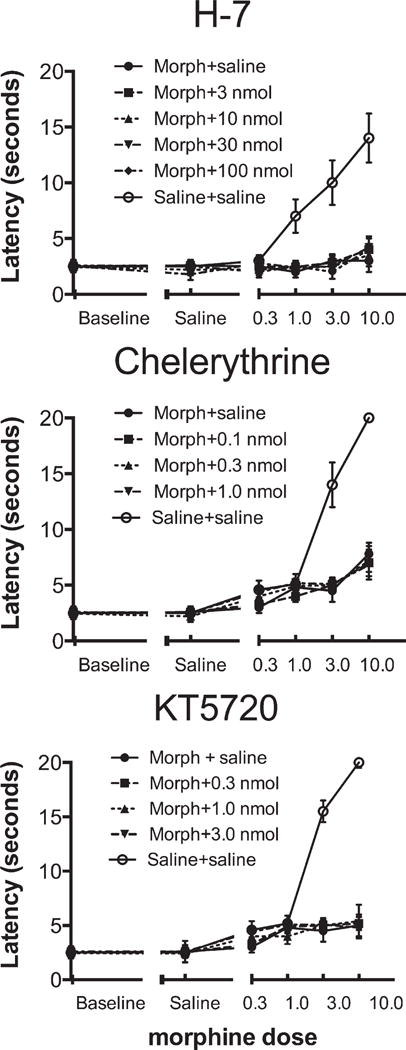
Rat pups were made tolerant to morphine by 13 twice-daily injections (10 mg/kg) from postnatal day (PND) 1 to PND 7, after which we used a cumulative dose-response paradigm to test for tolerance to the drug’s analgesic effect. We injected H-7, chelerythrine, and KT5720—broadly acting protein kinase (PK), PKC, and PKA antagonists, respectively—before the tolerance test (a thermal tail immersion test of nociception). None of these drugs reduced tolerance at any dose (McPhie-Lalmansingh and Barr, unpublished data). Morph, morphine; nmol, nanomole
In a different set of experiments, we examined changes in levels of other signaling molecules. These protein kinases— pAkt, pERK,2 pCREB,3 and pCaMKIIa4—are regulated by opioid signaling and also modulate neuronal plasticity, transcription, and cell survival in the adult. We injected pups twice daily starting at PND 1 or PND 14 and assayed them either 4 hours after the last morphine injection (to assess tolerance) or after precipitated withdrawal. We then performed immunohistochemistry and counted cells stereologically in the PAG and spinal cord; we present the PAG data here (Riley and Barr, unpublished). Fos expression in both groups of animals was increased by chronic morphine and augmented further after the administration of naltrexone to precipitate withdrawal (data not shown). pAkt and pERK were increased by chronic morphine but not further enhanced in withdrawal (Figure 7). pCREB was unaltered in the PAG (Figure 7) but enhanced in the spinal cord (not shown).
Figure 7.

pAkt, pCREB, and pERK in the periaqueductal gray. Rat pups were treated as described in Figure 6. At postnatal day (PND) 7 and 21, pAkt and pERK were enhanced by chronic morphine but not further increased in withdrawal (Riley and Barr, unpublished data). There were no changes in pCREB at either age for any treatment. pCREB, phosphorylated cyclic adenosine monophosphate (cAMP) response element binding [protein]; pERK, phosphorylated extracellular-signal-regulated kinase
These differences in intracellular signaling molecules do not easily map to age-dependent differences in behavior. Perhaps the levels of activated phosphorylated proteins are less important than the dynamics that stabilize their absolute levels even as their functional activity is altered. For example, basal activity of the isoforms PKCα and PKCγ remains unchanged by prenatal heroin exposure, whereas the cholinergic receptor–induced translocation and activation of PKCγ and PKCβII were lost (Shahak et al. 2003; Yaniv et al. 2004). Unfortunately, the only studies on PK isoforms are on acute withdrawal, so their role in the longer-term effects of chronic morphine is not known.
Endothelin
Endothelins are strong vasoconstrictors. Endothelin-1 is released at the site of tissue injury, interacts with its receptors, and enhances pain in both adults and infants (McKelvy et al. 2007; McKelvy and Sweitzer 2008). Endothelin-1 injection in a rat’s rear paw in infancy, on PND 7 or PND 11, decreased morphine-induced analgesia (i.e., tolerance) at PND 21 (McKelvy and Sweitzer 2009) in a sex-dependent manner, and the treatment on PND 7 reduced mu opioid receptor expression in the hindpaw skin (McKelvy and Sweitzer 2009).
Acetylcholine
Cholinergic neurotransmission has been suggested to play a role in morphine withdrawal in the infant rat. Acute withdrawal (head shaking) precipitated in 9-day-old pups by naloxone or nalorphine can be blocked by spiroperidol, clonidine, and scopolamine (Perez-Saad et al. 1996). However, only scopolamine shifted dose-response curves without altering maximum effect, showing that it is specific and the others are not. The authors argue for a specific role of cholinergic neurotransmission in morphine withdrawal in the infant rat. Unfortunately, we are not aware of any follow-up studies to confirm or extend these data.
Treatment
There is no strong evidence of effectiveness for any treatments of human neonatal abstinence syndrome other than the use of opiates for tapering (for recent thorough reviews on treatment of infants for opiate dependence, Osborn et al. 2010a,b). Environmental manipulations such as dimming lights or reducing noise are typically unsuccessful. The careful administration of opiates likely reduces both the time to regain lost birth weight and the duration of required supportive care, although such use may lengthen hospital stays.
Opiates are also likely superior to clonidine, phenobarbitone, and diazepam in reducing infant withdrawal syndrome. Osborn and colleagues (2010a,b) recommend initial opiate treatment for NAS infants but point to methodologic limitations in most studies and suggest that further research is needed to address many questions, including, for example, the effects of adding barbiturates or clonidine to opiates.
Summary
It is clear that human and nonhuman infants can become tolerant to opiates and experience withdrawal. The pattern of withdrawal differs and tolerance is less profound than in adults, likely because of the immaturity of neural systems that mediate both experiences. Although the nature of that immaturity is not known, there is reason to believe that it includes glutamate neurotransmission. The mechanisms underlying acute and chronic tolerance and dependence differ, and the latter probably involve neuroplastic changes that are not associated with acute tolerance or dependence. However, there have been leads not followed; for example, the roles of acetylcholine, substance P, and the endothelins remain to be clarified.
Further research in all these areas will enhance understanding of the mechanisms underlying opiate-induced neural changes.
Acknowledgments
The empirical research described in this review was supported in part by National Institutes of Health (NIH) grants (R01 DA06600 and K02 DA00325; Barr). Trainee support was provided by an NIH minority supplement (F31 DA15274), the National Science Foundation, a fellowship from the City University of New York (CUNY) Alliances for Graduate Education and the Professoriate (AGEP; McPhie-Lalmansingh), and NIH grants (G12 RR003037, Perez, through Jennifer Rabb, PI; and T32 MH18264, Riley, through Michael Myers, PI).
The research described in this article was conducted at and supported by the Department of Psychology at Hunter College in New York City; the Biopsychology and Behavioral Neuroscience Doctoral Program of the Graduate Center of City University of New York (CUNY); and the Department of Developmental Neuroscience at the New York State Psychiatric Institute.
Footnotes
Abbreviations that appear ≥3x throughout this article: NMDA, N-methyl-d-aspartate; PAG, periaqueductal gray of the midbrain; PK, protein kinase; PND, postnatal day; sVRP, slow ventral root potential
pERK, phosphorylated extracellular-signal-regulated kinase
pCREB, phosphorylated cyclic adenosine monophosphate (cAMP) response element binding [protein]
pCaMKII, calcium (Ca2+)/calmodulin-dependent protein kinase II
Contributor Information
Gordon A. Barr, James Battaglia Endowed Chair in Pediatric Pain Medicine and Director of the Division of Basic Science Research in the Department of Anesthesiology and Critical Care Medicine at Children’s Hospital of Philadelphia, and Associate Professor of Psychology in Anesthesiology and Critical Care Medicine at the Perelman School of Medicine at the University of Pennsylvania.
Anika McPhie-Lalmansingh, Participated as a predoctoral student and is now a review analyst at MANILA Consulting Group in McLean, Virginia.
Jessica Perez, Participated as an undergraduate honors student and now works in the private sector.
Michelle Riley, Participated as a postdoctoral fellow and is now Coordinator of the Research Infrastructure in Minority Institutions (RIMI) Program at Mercy College in Dobbs Ferry, New York.
References
- Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125:e1208–e1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care. 2009;4:1–5. doi: 10.1097/SPC.0b013e328335ddfe. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Hughes RA. Single-dose tolerance to morphine-induced analgesic and hypoactive effects in infant rats. Dev Psycho biol. 1981;14:415–423. doi: 10.1002/dev.420140504. [DOI] [PubMed] [Google Scholar]
- Barr GA, Goodwin GA. Precipitated morphine withdrawal induces a conditioned aversion in the preweaning rat. Pharmacol Biochem Behav. 1997;57:779–783. doi: 10.1016/s0091-3057(96)00382-6. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. Eur J Pharmacol. 1992;215:35–42. doi: 10.1016/0014-2999(92)90605-4. [DOI] [PubMed] [Google Scholar]
- Barr GA, Paredes W, Erickson KL, Zukin RS. Kappa opioid receptor-mediated analgesia in the developing rat. Brain Res. 1986;394:145–152. doi: 10.1016/0165-3806(86)90090-8. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S, Carden S. Aversive properties of the k opioid agonist U50,488 in the week-old rat pup. Psychopharmacol. 1994;113:422–428. doi: 10.1007/BF02245218. [DOI] [PubMed] [Google Scholar]
- Barr GA, Zmitrovich A, Hamowy AS, Liu PY, Wang S, Hutchings DE. Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacol Biochem Behav. 1998;60:97–104. doi: 10.1016/s0091-3057(97)00596-0. [DOI] [PubMed] [Google Scholar]
- Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17:498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- Bell JA, Beglan CL. Co-treatment with MK-801 potentiates naloxone-precipitated morphine withdrawal in the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1995a;294:297–301. doi: 10.1016/0014-2999(95)00548-x. [DOI] [PubMed] [Google Scholar]
- Bell JA, Beglan CL. MK-801 blocks the expression but not the development of tolerance to morphine in the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1995b;294:289–296. doi: 10.1016/0014-2999(95)00547-1. [DOI] [PubMed] [Google Scholar]
- Ceger P, Kuhn CM. Opiate withdrawal in the neonatal rat: Relationship to duration of treatment and naloxone dose. Psychopharmacology (Berl) 2000;150:253–259. doi: 10.1007/s002130000413. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- Chieng B, Keay KA, Christie MJ. Increased fos-like immunoreactivity in the periaqueductal gray of anaesthetised rats during opiate withdrawal. Neurosci Lett. 1995;183:79–82. doi: 10.1016/0304-3940(94)11119-4. [DOI] [PubMed] [Google Scholar]
- Choe CH, Smith FL. Sedative tolerance accompanies tolerance to the analgesic effects of fentanyl in infant rats. Pediatr Res. 2000;47:727–735. doi: 10.1203/00006450-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Coghlan D, Milner M, Clarke T, Lambert I, McDermott C, McNally M, Beckett M, Matthews T. Neonatal abstinence syndrome. Ir Med J. 1999;92:232–233. 236. [PubMed] [Google Scholar]
- Crain SM, Shen K. Enhanced analgesic potency and reduced tolerance of morphine in 129/SvEv mice: Evidence for a deficiency in GM1 ganglioside-regulated excitatory opioid receptor functions. Brain Res. 2000;856:227–235. doi: 10.1016/s0006-8993(99)02446-4. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Hogenboom F, Mulder AH, Schoffelmeer ANM. Ontogeny of mu-, delta- and kappa-opioid receptors mediating inhibition of neurotransmitter release and adenylate cyclase activity in rat brain. Dev Brain Res. 1990;54:63–69. doi: 10.1016/0165-3806(90)90065-7. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Dobbing J. Later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Paediatrics. London: William Heinemann; 1981. pp. 744–759. [Google Scholar]
- Druhan JP, Walters CL, Aston-Jones G. Behavioral activation induced by D(2)-like receptor stimulation during opiate withdrawal. J Pharmacol Exp Ther. 2000;294:531–538. [PubMed] [Google Scholar]
- Dumas EO, Pollack GM. Opioid tolerance development: A pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008;10:537–551. doi: 10.1208/s12248-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DG, Wertheim D, Oozeer R, Royston P, Dubowitz L, Dubowitz V. The effect of pethidine on the neonatal EEG. Dev Med Child Neurol. 1992;34:155–163. doi: 10.1111/j.1469-8749.1992.tb14982.x. [DOI] [PubMed] [Google Scholar]
- Elliott K, Kest B, Man A, Kao B, Inturrisi CE. N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995;13:347–356. doi: 10.1016/0893-133X(95)00083-P. [DOI] [PubMed] [Google Scholar]
- Fendt M, Mucha RF. Anxiogenic-like effects of opiate withdrawal seen in the fear-potentiated startle test, an interdisciplinary probe for drug-related motivational states. Psychopharmacology (Berl) 2001;155:242–250. doi: 10.1007/s002130100709. [DOI] [PubMed] [Google Scholar]
- Franck L, Vilardi J. Assessment and management of opioid withdrawal in ill neonates. Neonatal Netw. 1995;14:39–48. [PubMed] [Google Scholar]
- Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The Withdrawal Assessment Tool-1 (WAT-1): An assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9:573–580. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Le Moine C, Cador M. The motivational component of withdrawal in opiate addiction: Role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Rev Neurosci. 2005a;16:255–276. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005b;25:1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewolb IH, Fishman D, Qureshi MA, Vice FL. Coordination of suck-swallow-respiration in infants born to mothers with drug-abuse problems. Dev Med Child Neurol. 2004;46:700–705. [PubMed] [Google Scholar]
- Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Kanof PD. The dysphoria of heroin addiction. Am J Drug Alcohol Abuse. 1992;18:275–287. doi: 10.3109/00952999209026067. [DOI] [PubMed] [Google Scholar]
- Happel J. Morphinism in its relation to the sexual functions and appetite and its effect on the off-spring of the users of the drug. Med Surg Rep. 1892;68:403–407. [Google Scholar]
- Huidobro JP, Huidobro F. Acute morphine tolerance in new born and young rats. Psychopharmacologia. 1973;28:27–34. doi: 10.1007/BF00413954. [DOI] [PubMed] [Google Scholar]
- Hutchings DE. Issues of risk assessment: Lessons from the use and abuse of drugs during pregnancy. Neurotoxicol Teratol. 1990;12:183–189. doi: 10.1016/0892-0362(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Feraur E, Gorinson HS, Golden R. The effects of prenatal exposure to methadone on the rest-activity cycle of the preweanling rat. Neurobehav Toxicol Teratol. 1979;1:33–40. [PubMed] [Google Scholar]
- Hutchings DE, Towey JP, Bodnarenko SR. Effects of prenatal methadone on activity level in the preweanling rat. Neurotoxicol Teratol. 1980;2:231–235. [Google Scholar]
- Inturrisi CE. Preclinical evidence for a role of glutamatergic systems in opioid tolerance and dependence. Sem Neurosci. 1997;9:110–119. [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behav Neurosci. 1995;109:1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Opiate withdrawal in the fetal rat: A behavioral profile. Pharmacol Biochem Behav. 2000;66:419–424. doi: 10.1016/s0091-3057(00)00209-4. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Injections of an opioid antagonist into the locus coeruleus and periaqueductal gray but not the amygdala precipitates morphine withdrawal in the 7 day-old rat. Synapse. 2001;39:139–151. doi: 10.1002/1098-2396(200102)39:2<139::AID-SYN5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Jones KL, Zhu H, Jenab S, Du T, Inturrisi CE, Barr GA. Attenuation of acute morphine withdrawal in the neonatal rat by the competitive NMDA receptor antagonist LY235959. Neuropsychopharmacology. 2002;26:301–310. doi: 10.1016/S0893-133X(01)00347-5. [DOI] [PubMed] [Google Scholar]
- Kanof PD, Aronson MJ, Ness R. Organic mood syndrome associated with detoxification from methadone maintenance. Am J Psychiatry. 1993;150:423–428. doi: 10.1176/ajp.150.3.423. [DOI] [PubMed] [Google Scholar]
- Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. J Addict Dis. 2010;29:175–191. doi: 10.1080/10550881003684723. [DOI] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: A survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- King TE, Barr GA. Functional development of neurokinin peptides substance P and neurokinin A in nociception. Neuroreport. 2003;14:1603–1607. doi: 10.1097/00001756-200308260-00012. [DOI] [PubMed] [Google Scholar]
- King TE, Cheng J, Wang S, Barr GA. Maturation of NK1 receptor involvement in the nociceptive response to formalin. Synapse. 2000a;36:254–266. doi: 10.1002/(SICI)1098-2396(20000615)36:4<254::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- King TE, Heath MJ, Debs P, Davis MB, Hen R, Barr GA. The development of the nociceptive responses in neurokinin-1 receptor knockout mice. Neuroreport. 2000b;11:587–591. doi: 10.1097/00001756-200002280-00031. [DOI] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Effects of morphine and naloxone on spontaneous activity of fetal rats. Exp Neurol. 1981;73:430–439. doi: 10.1016/0014-4886(81)90277-6. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: Evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacol. 1989;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- Kuschel CA, Austerberry L, Cornwell M, Couch R, Rowley RS. Can methadone concentrations predict the severity of withdrawal in infants at risk of neonatal abstinence syndrome? Arch Dis Child Fetal Neonatal Ed. 2004;89:F390–F393. doi: 10.1136/adc.2003.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE. Ontogeny and plasticity of opioid systems. In: Hammer RP, editor. The Neurobiology of Opiates. Boca Raton: CRC Press; 1993. pp. 85–123. [Google Scholar]
- Leslie FM, Tso S, Hurlbut DE. Differential appearance of opiate receptor subtypes in neonatal rat brain. Life Sci. 1982;31:1393–1396. doi: 10.1016/0024-3205(82)90389-7. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29:259–276. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HH, Huestis MA, Finnegan LP, Maza PL. The Maternal Lifestyle Study: Drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kishioka S, Inoue N, Shimizu N, Fukazawa Y, Ozaki M, Yamamoto H. Naloxone-precipitated morphine withdrawal elicits increases in c-Fos mRNA expression in restricted regions of the infant rat brain. Jpn J Pharmacol. 2002;90:270–275. doi: 10.1254/jjp.90.270. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exper Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Mao J. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Brain Res Rev. 1999;30:289–304. doi: 10.1016/s0165-0173(99)00020-x. [DOI] [PubMed] [Google Scholar]
- McClung CA. The molecular mechanisms of morphine addiction. Rev Neurosci. 2006;17:393–402. doi: 10.1515/revneuro.2006.17.4.393. [DOI] [PubMed] [Google Scholar]
- McKelvy AD, Sweitzer SM. Endothelin-1 exposure on postnatal day 7 alters expression of the endothelin B receptor and behavioral sensitivity to endothelin-1 on postnatal day 11. Neurosci Lett. 2008;451:89–93. doi: 10.1016/j.neulet.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvy AD, Sweitzer SM. Decreased opioid analgesia in weanling rats exposed to endothelin-1 during infancy. Neurosci Lett. 2009;466:144–148. doi: 10.1016/j.neulet.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvy AD, Mark TR, Sweitzer SM. Age- and sex-specific nociceptive response to endothelin-1. J Pain. 2007;8:657–666. doi: 10.1016/j.jpain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- McPhie AA, Barr GA. The role of opioid receptors in morphine withdrawal in the infant rat. Dev Brain Res. 2000;124:73–80. doi: 10.1016/s0165-3806(00)00102-4. [DOI] [PubMed] [Google Scholar]
- McPhie AA, Barr GA. Regional Fos expression induced by morphine withdrawal in the 7-day-old rat. Dev Psychobiol. 2009;51:544–552. doi: 10.1002/dev.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CM, Fifer WP. Evidence of transnatal auditory learning. J Perinatol. 2000;20(8 Pt 2):S37–S44. doi: 10.1038/sj.jp.7200448. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Gritti MD, Kim C. Aversive properties of opiate withdrawal studied in rats. NIDA Res Monogr. 1986;75:567–570. [PubMed] [Google Scholar]
- Myers MM, Shair HN, Hofer MA. Feeding in infancy: Short- and long-term effects on cardiovascular function. Experientia. 1992;48:322–333. doi: 10.1007/BF01923426. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: A model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Noda Y, Nabeshima T. Opiate physical dependence and N-methyl-D-aspartate receptors. Eur J Pharmacol. 2004;500:121–128. doi: 10.1016/j.ejphar.2004.07.017. [DOI] [PubMed] [Google Scholar]
- O’Brien CM, Jeffery HE. Sleep deprivation, disorganization and fragmentation during opiate withdrawal in newborns. J Paediatr Child Health. 2002;38:66–71. doi: 10.1046/j.1440-1754.2002.00724.x. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010a:CD002059. doi: 10.1002/14651858.CD002059. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010b:CD002053. doi: 10.1002/14651858.CD002053. [DOI] [PubMed] [Google Scholar]
- Perez-Saad H, Urba-Holmgren R, Holmgren B. Pharmacological analysis of acute morphine dependence in infant rats: Close molecular relationship of head-shaking precipitated by opiate antagonists and cholinergic neurotransmission. Arch Med Res. 1996;27:139–144. [PubMed] [Google Scholar]
- Quigley KS, Shair HN, Myers MM. Parasympathetic control of heart period during early postnatal development in the rat. J Auton Nerv System. 1996;59:75–82. doi: 10.1016/0165-1838(96)00010-0. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Yohay AL, Gauda EB, McLemore GL. Neonatal animal models of opiate withdrawal. ILAR J. 2006;47:39–48. doi: 10.1093/ilar.47.1.39. [DOI] [PubMed] [Google Scholar]
- Schempf AH. Illicit drug use and neonatal outcomes: A critical review. Obstet Gynecol Surv. 2007;62:749–757. doi: 10.1097/01.ogx.0000286562.31774.76. [DOI] [PubMed] [Google Scholar]
- Shahak H, Slotkin TA, Yanai J. Alterations in PKCgamma in the mouse hippocampus after prenatal exposure to heroin: A link from cell signaling to behavioral outcome. Brain Res Dev Brain Res. 2003;140:117–125. doi: 10.1016/s0165-3806(02)00607-7. [DOI] [PubMed] [Google Scholar]
- Shannon LM, Havens JR, Hays L. Examining differences in substance use among rural and urban pregnant women. Am J Addict. 2010;19:467–473. doi: 10.1111/j.1521-0391.2010.00079.x. [DOI] [PubMed] [Google Scholar]
- Shute E, Davis ME. The effect on the infant of morphine administered in labor. Surg Gynecol Obstet. 1933;57:727–736. [Google Scholar]
- Smotherman WP. Classical conditioning in the rat fetus: Involvement of mu and kappa opioid systems in the conditioned response. Dev Psychobiol. 2002;40:104–115. doi: 10.1002/dev.10016. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Pharmacol Biochem Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Stoller DC, Thornton SR, Smith FL. Loss of antinociceptive efficacy in rat pups infused with morphine from osmotic minipumps. Pharmacology. 2002;66:11–18. doi: 10.1159/000063250. [DOI] [PubMed] [Google Scholar]
- Stoller DC, Sim-Selley LJ, Smith FL. Role of kappa and delta opioid receptors in mediating morphine-induced antinociception in morphine-tolerant infant rats. Brain Res. 2007;1142:28–36. doi: 10.1016/j.brainres.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Moriceau S, Raineki C, Roth TL. Ontogeny of infant fear learning and the amygdala. In: Gazzaniga M, editor. Cognitive Neuroscience IV. Cambridge MA: MIT Press; 2009. pp. 889–904. [Google Scholar]
- Sweitzer SM, Shumilla JA, Zisson MH, Kendig JJ. Protein kinase C epsilon and gamma: Roles in age-specific modulation of acute opioid-withdrawal allodynia. Sem Pain Med. 2003;1:206–219. [Google Scholar]
- Sweitzer SM, Allen CP, Zissen MH, Kendig JJ. Mechanical allodynia and thermal hyperalgesia upon acute opioid withdrawal in the neonatal rat. Pain. 2004a;110:269–280. doi: 10.1016/j.pain.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Wong SM, Tjolsen A, Allen CP, Mochly-Rosen D, Kendig JJ. Exaggerated nociceptive responses on morphine withdrawal: Roles of protein kinase C epsilon and gamma. Pain. 2004b;110:281–289. doi: 10.1016/j.pain.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Zhu YS, Amione J, Clare S. Prenatal morphine exposure and sleep-wake disturbances in the fetus. Sleep. 1988;11:121–130. doi: 10.1093/sleep/11.2.121. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Zhu Y-S, Cai LQ. Central opioid modulation of fetal cardiovascular function: Role of mu- and delta-receptors. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1453–R1458. doi: 10.1152/ajpregu.1990.258.6.R1453. [DOI] [PubMed] [Google Scholar]
- Tate MA. The transmissibility of morphine. Cincinnati Lancet–Clinic. 1899;43:598–603. [Google Scholar]
- Tempel A. Visualization of mu opiate receptor downregulation following morphine treatment in neonatal rat brain. Brain Res Dev Brain Res. 1991;64:19–26. doi: 10.1016/0165-3806(91)90204-v. [DOI] [PubMed] [Google Scholar]
- Tempel A, Habas J, Paredes W, Barr GA. Morphine-induced downregulation of mu-opioid receptors in neonatal rat brain. Brain Res. 1988;469:129–133. doi: 10.1016/0165-3806(88)90176-9. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Zeng J, Terman GW. Differential effect of glutamate transporter inhibition on EPSCs in the morphine naive and morphine tolerant neonatal spinal cord slice. Neurosci Lett. 2006;407:64–69. doi: 10.1016/j.neulet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Terman GW, Zeng J, Lowe J, Chavkin C, Hermes SM, Hegarty DM, Aicher SA. Decreased substance P and NK1 receptor immunoreactivity and function in the spinal cord dorsal horn of morphine-treated neonatal rats. J Pain. 2008;9:11–19. doi: 10.1016/j.jpain.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorat SN, Barjavel MJ, Matwyshyn GA, Bhargava HN. Comparative effects of NG-monomethyl-L-arginine and MK-801 on the abstinence syndrome in morphine-dependent mice. Brain Res. 1994;642:153–159. doi: 10.1016/0006-8993(94)90917-2. [DOI] [PubMed] [Google Scholar]
- Thornton SR, Smith FL. Characterization of neonatal rat fentanyl tolerance and dependence. J Pharmacol Exp Ther. 1997;281:514–521. [PubMed] [Google Scholar]
- Thornton SR, Wang AF, Smith FL. Characterization of neonatal rat morphine tolerance and dependence. Eur J Pharmacol. 1997;340:161–167. doi: 10.1016/s0014-2999(97)01434-9. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. Effects of noncompetitive N-methyl-D-aspartate receptor antagonists on opiate tolerance and physical dependence. Neuropsychopharmacology. 1995;13:301–307. doi: 10.1016/0893-133X(95)00088-U. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- van Praag H, Frenk H. Evidence for opiate tolerance in newborn rats. Dev Brain Res. 1991;60:99–102. doi: 10.1016/0165-3806(91)90160-k. [DOI] [PubMed] [Google Scholar]
- van Praag H, Falcon M, Guendelman D, Frenk H. The development of analgesic, pro- and anti-convulsant opiate effects in the rat. Ann Ist Super Sanita. 1993;29:419–429. [PubMed] [Google Scholar]
- Vathy I. Prenatal opiate exposure: Long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology. 2002;27:273–283. doi: 10.1016/s0306-4530(01)00049-x. [DOI] [PubMed] [Google Scholar]
- Vaupel DB, Kimes AS, London ED. Comparison of 7-nitroindazole with other nitric oxide synthase inhibitors as attenuators of opioid withdrawal. Psychopharmacol (Berl) 1995a;118:361–368. doi: 10.1007/BF02245935. [DOI] [PubMed] [Google Scholar]
- Vaupel DB, Kimes AS, London ED. Nitric oxide synthase inhibitors: Preclinical studies of potential use for treatment of opioid withdrawal. Neuropsychopharmacology. 1995b;13:315–322. doi: 10.1016/0893-133X(95)00138-4. [DOI] [PubMed] [Google Scholar]
- Windh RT, Little PJ, Kuhn CM. The ontogeny of mu opiate tolerance and dependence in the rat: Antinociceptive and biochemical studies. J Pharmacol Exper Ther. 1995;273:1361–1374. [PubMed] [Google Scholar]
- Yanagisawa M, Murakoshi T, Tamai S, Otsuka M. Tail-pinch method in vitro and effects of some antinociceptive compounds. Eur J Pharmacol. 1984;106:231–239. doi: 10.1016/0014-2999(84)90710-6. [DOI] [PubMed] [Google Scholar]
- Yanai J, Huleihel R, Izrael M, Metsuyanim S, Shahak H, Vatury O, Yaniv SP. Functional changes after prenatal opiate exposure related to opiate receptors’ regulated alterations in cholinergic innervation. Int J Neuropsychopharmacol. 2003;6:253–265. doi: 10.1017/S1461145703003523. [DOI] [PubMed] [Google Scholar]
- Yaniv SP, Naor Z, Yanai J. Prenatal heroin exposure alters cholinergic receptor stimulated activation of the PKCbetaII and PKCgamma isoforms. Brain Res Bull. 2004;63:339–349. doi: 10.1016/j.brainresbull.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA, Terman GW. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26:12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: Implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Sweitzer SM. Neonatal morphine enhances nociception and decreases analgesia in young rats. Brain Res. 2008;1199:82–90. doi: 10.1016/j.brainres.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong W, Lin X, Ma X, Yu LC. Receptor trafficking induced by mu-opioid-receptor phosphorylation. Neurosci Biobehav Rev. 2009;33:1192–1197. doi: 10.1016/j.neubiorev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Naltrexone-precipitated morphine withdrawal in infant rat is attenuated by acute administration of NOS inhibitors but not NMDA receptor antagonists. Psychopharmacology (Berl) 2000;150:325–336. doi: 10.1007/s002130000442. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Opiate withdrawal during development: Are NMDA receptors indispensable? Trends Pharmacol Sci. 2001;22:404–408. doi: 10.1016/s0165-6147(00)01792-2. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Ontogeny of NMDA receptor-mediated morphine tolerance in the postnatal rat. Pain. 2003;104:437–447. doi: 10.1016/S0304-3959(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. The role of AMPA and metabotropic glutamate receptors on morphine withdrawal in infant rats. Int J Dev Neurosci. 2004;22:379–395. doi: 10.1016/j.ijdevneu.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zissen MH, Zhang G, Kendig JJ, Sweitzer SM. Acute and chronic morphine alters formalin pain in neonatal rats. Neurosci Lett. 2006;400:154–157. doi: 10.1016/j.neulet.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Zissen MH, Zhang G, McKelvy A, Propst JT, Kendig JJ, Sweitzer SM. Tolerance, opioid-induced allodynia and withdrawal associated allodynia in infant and young rats. Neuroscience. 2007;144:247–262. doi: 10.1016/j.neuroscience.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]


