Highlights
-
•
We report an elder case of cerebral amyloid angiopathy-related inflammation (CAA-ri) in the right occipital lobe.
-
•
It appears like an intraparenchymal tumor at first, and can be finally diagnosed only through biopsy.
-
•
CAA-ri should be considered among the differential diagnoses for unprovoked seizure onset, when associated with petechial hemorrhages on MRI.
-
•
Recently DFS staining has been considered more desirable to ascertain the deposition of amyloid protein.
Abbreviations: CAA, cerebral amyloid angiopathy; Aβ, β-amyloid; CAA-ri, cerebral amyloid angiopathy-related inflammation; NCSE, nonconvulsive status epilepticus; EEG, electroencephalogram; CT, computed tomography; MRI, magnetic resonance imaging; WI, weighted image; FLAIR, fluid-attenuated inversion recovery; Gd, gadolinium; T2*-GRE, T2*-weighted gradient-recalled echo; SWI, susceptibility-weighted imaging; PET, positron emission tomography; H&E, hematoxylin and eosin; DFS, direct fast scarlet; GFAP, glial fibrillary acidic protein
Keywords: Cerebral amyloid angiopathy-related inflammation, Epilepsy, Susceptibility-weighted imaging, β-Amyloid protein, Mimicking brain tumor
Abstract
Introduction
Cerebral amyloid angiopathy-related inflammation (CAA-ri), a rare and treatable variant of cerebral amyloid angiopathy, lacks specific imaging and clinical features, and requires invasive brain biopsy to confirm the diagnosis. We report the case of a patient with nonconvulsive status epilepticus (NCSE) caused by CAA-ri in the right occipital lobe.
Presentation of case
A 78-year-old man with a history of hypertension and rheumatoid arthritis was admitted to our hospital following an episode of seizures. CT scan showed a low-attenuating subcortical lesion in the right occipital lobe. MRI revealed the lesion as hypointense on T1-weighted imaging (WI) and hyperintense on T2-WI, showing no enhancement on T1-WI contrast-enhanced with gadolinium. In addition, T2*-weighted gradient-recalled echo (T2*-GRE) and susceptibility-weighted imaging (SWI) revealed extensive cortical microbleeds. Biopsy to determine the exact diagnosis revealed histological findings of reactive changes and perivascular inflammatory infiltration associated with amyloid deposition in vessel walls. These findings were consistent with CAA-ri. Corticosteroid therapy with dexamethasone was initiated for a short period as a diagnostic and therapeutic maneuver, resulting in marked reductions in the lesion.
Discussion
CAA is generally associated with intracerebral hemorrhage, dementia, and small cerebral infarctions in the elderly population, but in a small proportion of cases is related to inflammatory responses to vascular deposits of Aβ, as so-called CAA-ri.
Conclusion
CAA-ri should be considered among the differential diagnoses for causes of unprovoked seizure onset in elderly individuals, when associated with petechial hemorrhages on T2*-GRE and SWI sequences on MRI.
1. Introduction
Cerebral amyloid angiopathy (CAA), a common small vessel disease of the brain, is characterized by progressive deposition of β-amyloid (Aβ) protein in the walls of small to medium-sized arteries and capillaries in the cerebral cortex and overlying leptomeninges [[1], [2], [3], [4]]. The typical presentation of CAA is spontaneous lobar intracerebral hemorrhage in an elderly patient [2,4]. However, CAA can also manifest as subacute cognitive impairment, dementia, transient neurological symptoms, or epilepsy [1]. This could be a manifestation of an uncommon subtype of CAA, distinguished as CAA-related inflammation (CAA-ri, also known as Aβ-related angiitis) [3,[5], [6], [7]]. CAA-ri represents the coexistence of CAA and vascular inflammation, and is thought to result from an inflammatory response to Aβ protein in the blood vessel walls. Cognitive and behavioral changes are the most common symptoms of CAA-ri, followed by focal neurological signs, headache, and seizures [3,5]. In addition to this, CAA-ri can also mimic brain tumor from clinical and radiological point of view [3,4,8,9]. In this paper, we report an elder case of nonconvulsive status epilepticus (NCSE) due to CAA-ri in the right occipital lobe. It appears like an intraparenchymal tumor at first, and can be finally diagnosed only through biopsy. Therefore, we also demonstrat the pathological features of this unique and cryptic entity.
Thiswork has been reported in line with the SCARE criteria [10].
2. Case description
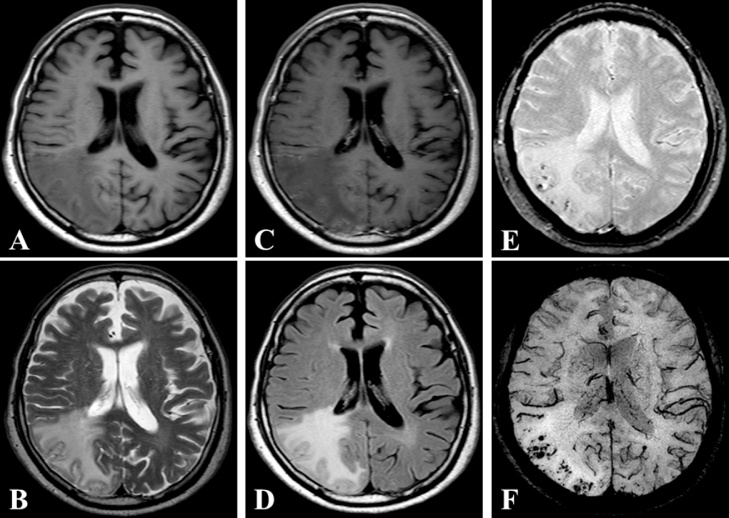
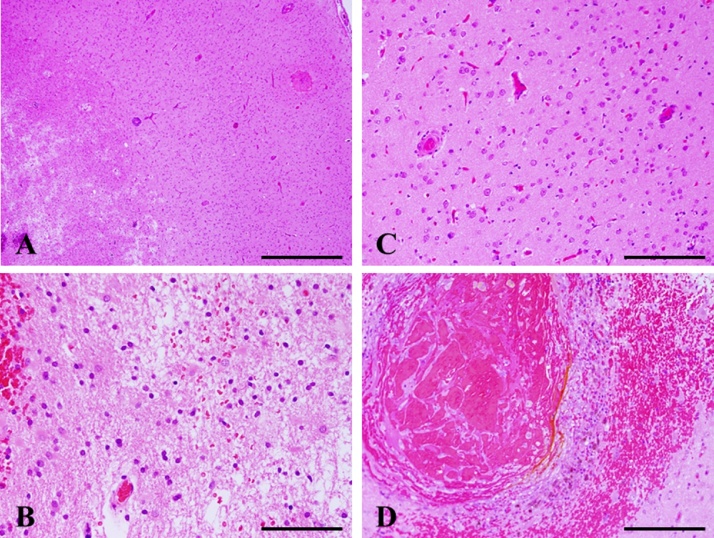
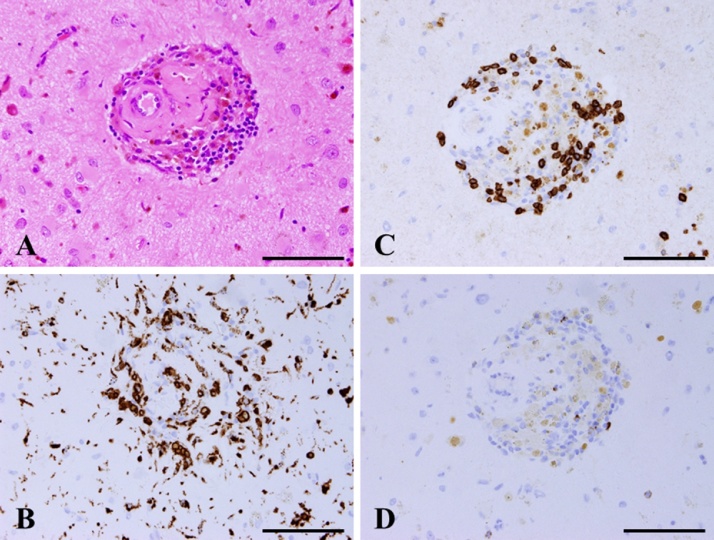
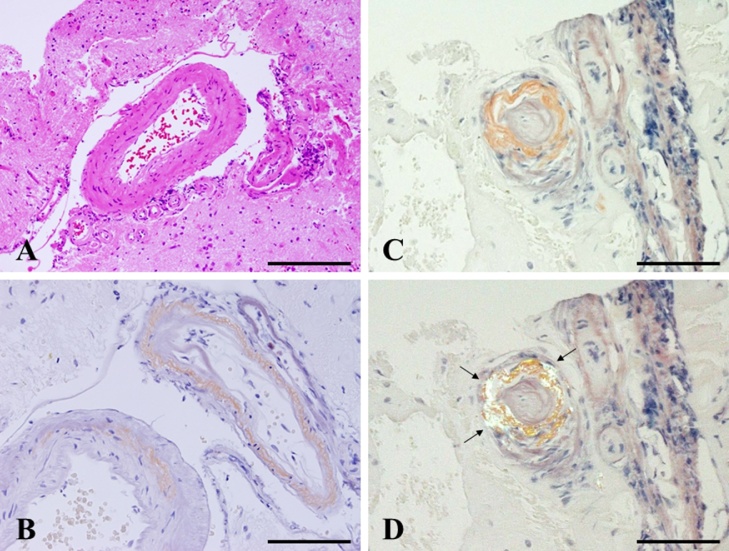
A 78-year-old man with a history of hypertension and rheumatoid arthritis was admitted to our hospital with suspected unprovoked seizure. His family had first noted new onset of irritability and confusion as uncharacteristic behaviors. Neurological examination on admission showed mild left-sided hemiparesis and a visual field defect. Laboratory examinations, including cerebrospinal fluid studies, revealed no abnormal findings, and the concentrations of tumor markers remained with normal limits. Routine scalp electroencephalogram (EEG) showed frequent epileptogenic discharges in the right occipital region. Computed tomography (CT) scan of the head showed a low-attenuating subcortical lesion in the right occipital lobe. Magnetic resonance imaging (MRI) demonstrated the lesion as hypointense on T1-weighted imaging (WI) and hyperintense on T2-WI and fluid-attenuated inversion recovery (FLAIR) imaging, without enhancement on T1-WI using gadolinium (Gd) contrast. In addition, T2*-weighted gradient-recalled echo (T2*-GRE) and susceptibility-weighted imaging (SWI) demonstrated extensive cortical microbleeds (Fig. 1). Cerebral angiography did not show any vascular abnormalities. Positron emission tomography (PET) scan of the brain revealed slightly increased uptake of methionine in the right occipital region, consistent with the lesion shown on MRI (maximum standardized uptake value for methionine, 2.4). On initial consideration, the history and results of laboratory examinations and radiological studies seemed most consistent with multifocal glioma, rather than any infectious, an inflammatory disease, or acute stroke. Therefore, the patient was first loaded with levetiracetam for epilepsy control. The dose of levetiracetam was increased after confirming a recurrent episode of intermittent confusion and aphasia as a manifestation of NCSE. In order to obtain the exact histological diagnosis and plan effective treatment for the primary disease, we performed surgical biopsy of the right occipital lesion with the assistance of image-guided navigation. Postoperative histopathology obtained from hematoxylin and eosin (H&E) staining demonstrated reactive changes including vacuolization, which suggests edema and gliosis, and thickened blood vessels (Fig. 2A–C). Most subcortical glial cells were small and pyknotic, indicating ischemic changes. Some subcortical vessels showed evidence of thrombosis and hypertrophied vessel walls. Fresh perivascular hemorrhage and several areas of hemosiderin deposition, and infiltration of small inflammatory cells were also present (Fig. 2D). Lymphocytes and epithelioid macrophages had infiltrated not only around the blood vessels, but also into the vessel walls (Fig. 3A). Immunohistochemical studies were performed using antibodies for CD3, CD20, CD68, Congo red and direct fast scarlet (DFS). Perivascular inflammatory cells mostly comprised monocyte/microglial cells (positive staining for CD68) (Fig. 3B). T lymphocytes (positive staining for CD3) were also present (Fig. 3C), whereas a neoplastic increase in B lymphocytes was absent (negative staining for CD20) (Fig. 3D). In addition, Congo red and DFS staining after permanganic acid treatment showed Aβ deposition in the vascular walls (Fig. 4). The H&E staining and immunohistochemical studies revealed morphological characteristics consistent with CAA-ri. Corticosteroid therapy with dexamethasone was started as a diagnostic and therapeutic maneuver. Two weeks after starting this treatment, MRI showed marked size reduction of hyperintense lesions on T2-WI (Fig. 5) and we stopped the administration of dexamethasone. The postoperative course was uneventful, with no relapse of epileptic symptoms. With entirely resolving of speech difficulties and confusion, he could communicate with good response, and follow verbal commands normally. Also, left-sided hemiparesis became unnoticeable. At the time of 6 months after starting treatment, MRI showed no aggravation of the lesion and no neurological deficits were apparent without taking dexamethasone.
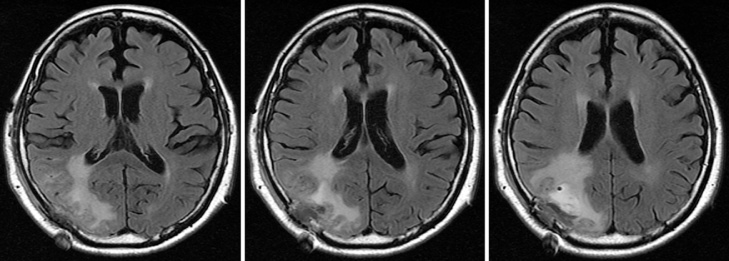
Fig. 1.
Images from preoperative axial T1-weighted imaging (A), T2-weighted imaging (B), gadolinium (Gd)-enhanced T1-weighted imaging (C), fluid-attenuated inversion recovery (FLAIR) (D), T2*-weighted gradient-recalled echo (T2*-GRE) (E), and susceptibility-weighted imaging (SWI) (F) show an area of abnormal intensity in the right occipital lobe. The lesion demonstrates no enhancement with Gd. T2*-GRE and SWI. images reveal multiple spotty cortical and subcortical hypointensities in the same region.
Fig. 2.
Histopathology of the biopsy specimen. H&E staining shows reactive changes, including vacuolization suggestive of edema and astrocytosis (A–C). Most subcortical glial cells are small and pyknotic, and perivascular hemorrhage and infiltration of small inflammatory cells are detected (D). Magnification, A: ×100. Scale bar, 1000 μm. B, C: ×400. Scale bar, 100 μm. D: ×200. Scale bar, 500 μm.
Fig. 3.
H&E staining shows lymphocytes and macrophages infiltrating not only around the blood vessels, but also in their walls (A). These immune cells show positive staining for CD68 (B) and CD3 (C), but negative staining for CD20 (D). Magnification, ×400. Scale bar, 100 μm.
Fig. 4.
Pathological findings show thickened blood vessels with perivascular hemosiderin deposition on H&E staining (A). Direct fast scarlet (DFS) staining (B) and Congo red staining (C: bright field; D: polarizing microscope) after permanganic acid treatment shows amyloid deposition in the vascular walls, displayed as apple green in (D) (black arrow). Magnification, A: ×200. Scale bar, 500 μm. B–D: ×400. Scale bar, 100 μm.
Fig. 5.
Serial FLAIR images two weeks after treatment with dexamethasone. The right occipital subcortical lesion has clearly decreased in size.
3. Discussion
CAA is generally associated with intracerebral hemorrhage, dementia, and small cerebral infarctions in the elderly population, but in a small proportion of cases is related to inflammatory responses to vascular deposits of Aβ, as so-called CAA-ri [[1], [2], [3], [4],11]. CAA-ri generally presents with subacute cognitive decline, mild headaches and new-onset seizures, rather than the chronic types of dementia or hemorrhagic strokes associated with CAA. The pathophysiology is considered to result from autoimmune responses to Aβ deposition in cerebral small or medium arteries and leptomeninges [12,13]. CAA-ri has histologically been described as primary angiitis of the central nervous system associated with CAA, including cerebral amyloid inflammatory vasculopathy [8,13].
CAA-ri is found in 23–57% of the asymptomatic elderly population, with increased rates among individuals with dementia, epilepsy or intracerebral hemorrhage [13,14]. Men and women are equally affected, and its onset is generally in the seventh decade, significantly younger than hemorrhagic CAA [13,15,16]. These clinical manifestations are associated with asymmetrical white matter lesions and multiple cortical-subcortical micro hemorrhages [16]. MRI findings in CAA-ri include fluctuating multifocal white matter T2 hyperintensity with petechial hemorrhages on T2*-GRE and SWI [13,15]. In previous reports, the detection of asymmetrical white-matter hyperintense signal on T2-WI and of multiple, scattered, cortical or subcortical microhemorrhages is highly predictive of CAA-ri [3,13,15,16]. Therefore, in the presence of delirium- and stroke-like symptoms as not quite matching the findings on routine CT and MRI, performance of MRI for T2*-GRE and/or SWI should be considered for the detection of cerebral micro-bleeds. Since CAA-ri lacks specific clinical and radiological features, brain biopsy is essential to make the exact diagnosis of CAA-ri [5,8].
Microscopically, the histopathological characteristics of CAA-ri demonstrate amyloid deposition within vessel walls and perivascular, transmural, or intramural inflammatory changes with or without granuloma formation [3,4,8,13]. These findings are basically consistent with CAA and the details are as follows. H&E staining showed reactive changes including vacuolization suggestive of edema and astrocytosis or gliosis [2,13]. Glial fibrillary acidic protein (GFAP) staining highlighted reactive astrocytes. H&E staining also revealed thickened blood vessels with intraluminal thrombosis and perivascular hemosiderin deposition [2,13]. In addition, confirming the presence of Aβ deposition in blood vessel walls by Congo red staining, DFS staining or Aβ immunohistochemistry is very important [2,13]. While Congo red staining is widely known as a conventional dye for amyloid protein, recently DFS staining has been considered more desirable to ascertain the deposition of amyloid protein, and many facilities perform this staining routinely [2]. In the present case, we used DFS staining to detect amyloid protein in addition to Congo red staining. Moreover, the diagnosis of CAA-ri requires clear histological findings of angiitis of the central nervous system in addition to the findings mentioned above [[2], [3], [4],16]. Characteristically, perivascular inflammatory cells mostly comprise monocyte/microglial cells (positive staining for CD68). T lymphocytes (positive staining for CD3) are also present, whereas neoplastic increases in B lymphocytes are absent (negative staining for CD20) [4]. In the present case, the biopsy specimen from the right occipital lobe revealed amyloid deposition in subarachnoidal and cortical vessel walls and transmural infiltration of numerous T lymphocytes and macrophages. The patient was therefore diagnosed with central nervous system vasculitis associated with cerebral amyloid angiopathy, representing so-called CAA-ri.
The optimal treatment for CAA-ri remains to be determined [8]. Several published cases and case series have described immunosuppressive treatment, including administration of corticosteroids with or without additional immunosuppressive therapies such as methotrexate, mycophenolate mofetil, or, most commonly, cyclophosphamide [8,13]. Rapid clinical and radiologic responses have been reported after introduction of steroids alone or in conjunction with other immunosuppressive agents such as cyclophosphamide. However, the optimal duration of treatment remains unclear, and relapse upon withdrawal of treatment has been reported [8,13]. While spontaneous improvement has also been reported, the rate of favorable outcomes has tended to be higher among patients who received steroids (with or without cyclophosphamide) than among those who received no treatment (78% vs 58%) [4,8]. In the present case, only a short course of dexamethasone proved sufficient to elicit rapid and persistent clinical and radiologic improvements, resulting in excellent epilepsy control throughout follow-up period of 6 months.
4. Conclusion
CAA-ri is an unusual form of CAA, which could be treatable at a high rate. Therefore, this entity should be considered among the differential diagnoses of the cause for unprovoked seizures in elderly populations, particularly in association with fluctuating multifocal T2 hyperintensities and petechial hemorrhages on MRI. Further experience with therapy for this pathological entity and longer patient follow-up is required.
Conflicts of interest
None of the authors have any commercial or financial involvement in connection with this study that represents or appears to represent any conflicts of interest.
Sources of funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
The clinical study of the above-mentioned case report was approved by the Ethics Committee for Clinical Research of Ehime University Hospital, and informed consent was obtained from the patient prior to initiating the study.
Consent
Written informed consent was obtained from the patient’s family for publication of this case report and any accompanying images. A copy of the written consent is available for review by Editor-in-Chief of this journal on request.
Author contribution
All authors in this manuscript contributed to the interpretation of data, and drafting and writing of this manuscript. KK is first and AI is corresponding author of this paper. They and TK conceived and designed the study and drafted the manuscript. KK, AI, SM, MK, RK, HW and TK were engaged in patient’s care in his hospital including surgery under the supervision of TK. All the authors read and approved the final manuscript.
Registration of research studies
This manuscript is case report, so we cannot register any type of research.
Guarantor
Takeharu Kunieda, MD, PhD.
Acknowledgments
The authors would like to express their gratitude to Taichi Furumochi and Yasuhiro Shiraishi of the Department of Neurology, Ehime University Hospital, Japan, and Satsuki Myoga of the Department of Pathology, Ehime University Hospital, Japan, for their help in obtaining pathological and radiological image findings.
References
- 1.Charidimou A., Gang Q., Werring D.J. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 2.Okamura A., Kawamoto Y., Yoshioka H., Murakami T., Yonezawa K., Kaneko M. A case of cerebral amyloid angiopathy-related inflammation where MR spectroscopy was helpful for differential diagnosis. Jpn. J. Stroke. 2013;35:97–102. [Google Scholar]
- 3.Castro Caldas A., Silva C., Albuquerque L., Pimentel J., Silva V., Ferro J.M. Cerebral amyloid angiopathy associated with inflammation: report of 3 cases and systematic review. J. Stroke Cerebrovasc. Dis. 2015;24:2039–2048. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Ronsin S., Deiana G., Geraldo A.F., Durand-Dubief F., Thomas-Maisonneuve L., Formaglio M. Pseudotumoral presentation of cerebral amyloid angiopathy-related inflammation. Neurology. 2016;86:912–919. doi: 10.1212/WNL.0000000000002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinnecom C., Lev M.H., Wendell L., Smith E.E., Rosand J., Frosch M.P. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68:1411–1416. doi: 10.1212/01.wnl.0000260066.98681.2e. [DOI] [PubMed] [Google Scholar]
- 6.Salvarani C., Brown R.D., Jr, Calamia K.T., Christianson T.J., Huston J., 3rd, Meschia J.F. Primary central nervous system vasculitis: comparison of patients with and without cerebral amyloid angiopathy. Rheumatology. 2008;47:1671–1677. doi: 10.1093/rheumatology/ken328. [DOI] [PubMed] [Google Scholar]
- 7.Danve A., Grafe M., Deodhar A. Amyloid beta-related angiitis: a case report and comprehensive review of literature of 94 cases. Semin. Arthritis Rheum. 2014;44:86–92. doi: 10.1016/j.semarthrit.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Chung K.K., Anderson N.E., Hutchinson D., Synek B., Barber P.A. Cerebral amyloid angiopathy related inflammation: three case reports and a review. J. Neurol. Neurosurg. Psychiatry. 2011;82:20–26. doi: 10.1136/jnnp.2009.204180. [DOI] [PubMed] [Google Scholar]
- 9.Kotsenas A.L., Morris J.M., Wald J.T., Parisi J.E., Campeau N.G. Tumefactive cerebral amyloid angiopathy mimicking CNS neoplasm. AJR Am. J. Roentgenol. 2013;200:50–56. doi: 10.2214/AJR.12.8500. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Ringheim G.E., Conant K. Neurodegenerative disease and the neuroimmune axis (Alzheimer’s and Parkinson’s disease, and viral infections) J. Neuroimmunol. 2004;147:43–49. doi: 10.1016/j.jneuroim.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Vinters H.V. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 13.Tolchin B., Fantaneanu T., Miller M., Helgager J., Lee J.W. Status epilepticus caused by cerebral amyloid angiopathy-related inflammation. Epilepsy Behav. Case Rep. 2016;6:19–22. doi: 10.1016/j.ebcr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coria F., Rubio I. Cerebral amyloid angiopathies. Neuropathol. Appl. Neurobiol. 1996;22:216–227. [PubMed] [Google Scholar]
- 15.Eng J.A., Frosch M.P., Choi K., Rebeck G.W., Greenberg S.M. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann. Neurol. 2004;55:250–256. doi: 10.1002/ana.10810. [DOI] [PubMed] [Google Scholar]
- 16.Crosta F., Orlandi B., De Santis F., Passalacqua G., DiFrancesco J.C., Piazza F. Cerebral amyloid angiopathy-related inflammation: report of a case with very difficult therapeutic management. Case Rep. Neurol. Med. 2015;54:2137–2146. doi: 10.1155/2015/483020. [DOI] [PMC free article] [PubMed] [Google Scholar]