Abstract
Autism spectrum disorder (ASD) is characterized by a variety of social and non-social behavioral deficits. One potential mechanism that could unify this diverse profile of behaviors is slower processing speed. Seventy-six high-functioning adults with ASD were compared to 64 matched controls on standardized measures of processing speed. Participants with ASD were significantly slower on all measures, and on the composite score from the three tests (d’s > .65). ASD participants with slower processing speeds scored higher on the ADOS Communication and Reciprocal Social Interaction scale (r = .34). These findings provide evidence of slower processing speeds in adults with ASD, and that this may be contributing to impairments in social communication skills. Interventions that improve processing speed might improve social communication abilities in ASD.
Keywords: Autism spectrum disorder, Information processing, Speed of processing, MATRICS
Introduction
Autism spectrum disorder (ASD) is clinically defined by abnormalities in complex behavior, which are currently encapsulated under two criteria: persistent deficits in social communication and interaction, and restricted and repetitive patterns of behavior, interests or activities (DSM 5). Extensive research has defined impairments in higher-order abilities across all neuropsychological domains associated with ASD (Huebner 1992), including in those with greater intellectual ability (Eack et al. 2013a; Fried et al. 2016; Narzisi et al. 2013; Williams et al. 2015). Deficits in emotion perception and regulation (Mazefsky et al. 2013), perspective-taking (Baron-Cohen et al. 1997), pragmatic language (Wang and Tsao 2015), language comprehension (Ricketts et al. 2013), concept formation (Williams et al. 2015), cognitive flexibility (Yeung et al. 2016), face perception (Critchley et al. 2000; Whitaker et al. 2016), self-regulation and motor praxis (Torres et al. 2013; Whyatt and Craig 2013) have been widely replicated (for a review see Gallagher and Varga 2015). The documentation of impairments in complex cognitive and motor abilities has led to research that has attempted to define common cognitive mechanisms that might underlie one or more of these deficits (Happé and Frith 2006; Minshew and Goldstein 1998; Howard et al. 2017).
One potential mechanism that could underlie the diverse profile of neuropsychological deficits observed in ASD is a fundamental impairment in the speed with which affected individuals can process information. ASD is often confounded with intellectual disability (ID), and so there is some ambiguity as to the contribution of ID on processing speed. The examination of processing speed among individuals with comorbid ID has certainly revealed significant impairment (Cornoldi et al. 2014). Studies of processing speed among individuals without ID (i.e., IQ > 70) have yielded inconsistent findings. A growing number of investigations have provided evidence of significant reductions in processing speed in both children (Oliveras-Rentas et al. 2012; Nader et al. 2015; Hedvall et al. 2013; Mayes and Calhoun 2007; Bavin et al. 2016a, b) and in adults with ASD without comorbid ID (Holdnack et al. 2011; Faja et al. 2009; Eack et al. 2013a), and several studies showed relationships between slower processing speed and impaired measures of social cognition (Oliveras-Rentas et al. 2012; Lerner et al. 2013). However, there are several studies that do not show reduced processing speed in children with ASD (Scheuffgen et al. 2000; Wallace et al. 2009; Kenworthy et al. 2013), and even fewer that examine the presence of this impairment in adulthood.
There may be several causes for the discrepancy in the literature. Studies have used a variety of methods to measure processing speed: some used standardized tests from measures of intelligence, for example, the Wechsler Intelligence Scales for Children (Oliveras-Rentas et al. 2012; Nader et al. 2015; Hedvall et al. 2013; Mayes and Calhoun 2007) and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery, which is comprised of tests of neuropsychological functioning (Eack et al. 2013a), whereas others have used tests of inspection time (i.e. reaction time to identify which of two lines appears to be longer; Scheuffgen et al. 2000; Wallace et al. 2009; Kenworthy et al. 2013), language tests (Bavin et al. 2016a, b) or measures confounded with social-cognitive impairment (Faja et al. 2009). When standardized tests have been utilized, the majority of studies have showed impaired processing speed (Oliveras-Rentas et al. 2012; Nader et al. 2015; Hedvall et al. 2013; Mayes and Calhoun 2007; Bavin et al. 2016a, bb; Holdnack et al. 2011; Faja et al. 2009; Eack et al. 2013a). Further, studies have varied in the degree to which case-control differences in overall intellectual ability are accounted for, and those that have accounted for IQ have found that processing speed impairments may not persist beyond impairments in general intellectual functioning (Wallace et al. 2009; Barbeau et al. 2013; Scheuffgen et al. 2000). These findings suggest the possibility that the slower processing speed in ASD is either task dependent, or due to more general intellectual impairments that are not necessarily characteristic of ASD.
Beyond the potential confounds of case-control differences in IQ and the range of experimental methods used, sample sizes have generally tended to be small, often with less than 25 participants in each group (e.g. Holdnack et al. 2011; Wallace et al. 2009; Scheuffgen et al. 2000). Therefore, several of these studies may have been underpowered to determine if there were true differences in processing speed between individuals with ASD and those without the condition. The age of the participants across the studies also varies, making it difficult to ascertain whether any processing speed deficits continue into adulthood, as most research has focused on children. In one of the only studies to examine processing speed in IQ-matched adults with ASD, Faja et al. (2009) found slower processing speeds in 39 adults with ASD compared to 33 neurotypical adults, but they used a facial recognition task, which limits the generalizability of the deficits to facial processing.
The current study was designed to help clarify the nature of processing speed abilities in verbal adults with ASD and without ID compared to age and full-scale IQ-matched neurotypical individuals using the largest matched sample to explore this topic to date. We examined the degree to which verbal adults with ASD evidenced impaired processing speed relative to healthy volunteers, and the degree to which any observed impairments in speed of processing were related to social disability. We hypothesized that there would be significant processing speed deficits in adults with ASD, and that such impairments would be related to greater disability in this population.
Method
Participants
Participants consisted of 76 verbal individuals with autism spectrum disorder (ASD) recruited for studies of Cognitive Enhancement Therapy and 64 healthy control participants. Participants with ASD were recruited from community outreach and support groups, local ASD diagnostic clinics, as well as an established ASD research participant pool. Healthy control participants were recruited for brain imaging studies at the University of Pittsburgh and generally included if they were age 18 years or older, and had an IQ ≥ 80.
Participants completed an initial phone screening interview to assess their eligibility for the study. Eligibility criteria for the ASD group included confirmation of autism (n = 37) or autism spectrum disorder (n = 35) using the Autism Diagnostic Observational Schedule (ADOS)—Module 4 (Lord et al. 2000) or the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), age 16–45, no untreated Attention-Deficit/Hyperactivity Disorder (ADHD), full-scale IQ ≥ 80 assessed by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1997), no significant cognitive disability on the Cognitive Styles and Social Cognition Eligibility Interview (Hogarty et al. 2004), the ability to read English at a Grade 6 level assessed by the Wide Range Achievement Test (WRAT) 4 Word Reading subtest (Wilkinson and Robertson 2006), and were fluent English as assessed through conversation throughout the eligibility assessment. Eligibility testing was conducted by research assistants who were trained to meet research reliability thresholds, and were supervised by a clinical neuropsychologist also trained to be research reliable on the measure. Participants completed the WASI, WRAT-4, and ADOS-Module 4. For participants who did not meet criteria for autism or ASD on the ADOS (n = 3, 3.9%), an ADI-R was completed with their parent, which confirmed an ASD diagnosis in all three cases. A study clinician completed the Cognitive Style and Social Cognition Eligibility Interview. Eligibility for the control group included reporting being free from any current psychiatric diagnosis, including ASD, or met criteria for a current psychiatric disorder on the Structured Clinical Interview for DSM-IV. This study was approved by an Institutional Review Board and performed in accordance with the 1964 Helsinki Declaration. Those who met the phone screen criteria completed eligibility testing after providing written and informed consent. For those who were under 18 years old, the parent or guardian provided written informed consent prior to their child’s participation, and all children provided assent prior to participation.
Table 1 displays demographic information for both groups. In the ASD group, 50% of the participants met the criteria for autism and 46.1% met the criteria for autism spectrum disorder as assessed by the ADOS Module 4. Three participants (3.9%) did not met the criteria for ASD as assessed by the ADOS Module 4 scores for communication, reciprocal communication, and the combined scores, but did using the ADI-R. Both groups consisted of primarily young adult Caucasian males and the groups did not differ significantly in terms of full-scale IQ: t(130.9) = 1.17, p = .24. The majority of participants in both groups had attended some college, but only 27 (35.5%) of the ASD participants were employed at the time of testing and 10 (13.2%) were living independently. Healthy volunteers were significantly older than participants with ASD. No other significant demographic differences were observed between groups.
Table 1.
Demographic, cognitive, and clinical characteristics of ASD and control participants
| ASD group (N = 76) |
Control group (N = 64) |
Between-group difference | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| M/N | SD/% | M/N | SD/% | t/χ2 | df | p | |
| Demographic | |||||||
| Agea | 24.22 | 6.40 | 25.72 | 4.95 | 2.11 | 135.92 | .04 |
| Male | 65 | 85.5% | 49 | 76.6% | 1.85 | 1 | .17 |
| White | 68 | 89.5% | 50 | 78.1% | 3.38 | 1 | .07 |
| Attend some collegeb | 52 | 68.4% | 55 | 85.9% | 7.44 | 2 | .02 |
| Employedb | 27 | 35.5% | 39 | 60.9% | 9.67 | 2 | .01 |
| Living independentlyc | 10 | 13.2% | |||||
| Clinical | |||||||
| Full scale IQ | 108.63 | 14.40 | 106.23 | 9.50 | 1.18 | 130.88 | .24 |
| SAS-IId | 1.67 | 0.59 | |||||
| GAFd | 49.84 | 8.19 | |||||
| Diagnosis | |||||||
| Autism (ADOS) | 38 | 50.0% | |||||
| Autism spectrum (ADOS) | 35 | 46.1% | |||||
| Autism spectrum (ADI) | 3 | 3.9% | |||||
| ADOS subscales | |||||||
| Communication | 3.41 | 1.28 | |||||
| Reciprocal social interaction | 6.96 | 2.45 | |||||
| Imagination/creativity | 1.20 | .69 | |||||
| Stereotyped and repetitive behaviors | 2.50 | 1.74 | |||||
Age variable was log transformed to account for positive skew in distribution
Data available for 73 participants with ASD and 61 control participants
Data available for 73 participants with ASD
Data available for 58 participants with ASD
Measures
Processing Speed
Three standardized measures of processing speed were used in the current study. Each of these tests are included as subtests on the MATRICS Consensus Cognitive Battery (Nuechterlein et al. 2008), which was administered in its entirety to participants as part of the experimental procedures for the ongoing studies of Cognitive Enhancement Therapy (Eack et al. 2013a). Only pre-treatment data were used for analysis. These tests have been shown to have high test–retest reliability (Nuechterlein et al. 2008), and have been validated in large participant groups (Green et al. 2014), including in adolescent individuals (Keheller et al. 2013). While the battery was designed to test neurological functioning in schizophrenia, significant impairments in MATRICS measures have also been reported in adults with ASD and were statistically indistinguishable from adults with schizophrenia (Eack et al. 2013a). Each test is described below.
The Trail Making Test: Part A is a paper test taken from the Army Individual Test Battery (Battery 1994) that requires participants to draw a continuous line to connect numbers 1–25 in ascending order as quickly as possible. Participant completion time in seconds was recorded. This test is included as part of several standardized neuropsychological test batteries and is considered a valid measure of visuomotor tracking and processing speed (Sanchez-Cubillo et al. 2009). Category Fluency: Animal Naming is a widely used test of verbal fluency that requires participants to list the names of as many animals they can in 60 s. Finally, the Brief Assessment of Cognition in Schizophrenia (BACS): Symbol Coding (Keefe et al. 2004) presents participants with a key that displays 9 unique symbols each paired with a number (1–9). Participants must write the correct number that matches each symbol in the space provided. Participants were instructed to complete the coding in order from left to right without skipping any, as quickly as they can for 90 s. The number of items correctly completed in 90 s was recorded. A composite index of processing speed was created by converting raw test scores to a common (z) metric, reverse coding relevant test (Trail A), and averaging across test scores.
Psychosocial Functioning
The Social Adjustment Scale-II (SAS-II; Schooler et al. 1979) and the Global Assessment of Functioning (GAF; Endicott et al. 1976) were used to assess participants’ level of psychosocial functioning. The SAS-II is a standardized semi-structured clinical interview that is designed to assess an individual’s level of functioning and role performance in several different social areas including work, household, external family, social leisure, sexual and romantic relationships, and overall personal well-being and self-care. This measure consists of 44 items that the interviewer rates ranging from 0 (good adjustment) to 4 or 6 (poor adjustment). The GAF is widely used clinical rating of an individual’s level of psychosocial impairment and psychiatric symptom severity. The GAF is a single score that ranges from 1 to 100, with higher scores indicating improved functioning and lower severity of symptoms.
Eighteen individuals with ASD were recruited under a different protocol which did not include the SAS-II or the GAF. Therefore, their data SAS-II and GAF data were not collected, but their processing speed and ADOS scores were still included in the data analysis. Similarly, 41 healthy control participants did not have SAS-II data collected and 21 did not have their GAF data collected.
Procedure
Participants who met all eligibility criteria completed baseline clinical interviews and neuropsychological testing prior to beginning treatment. Baseline clinical interviews were conducted by a research assistant who was intensively trained in the administration and rating of the clinical assessments and was supervised by a senior clinician. Clinical interviews consisted of a comprehensive battery of standardized clinical assessments of psychosocial functioning and symptom severity. Neuropsychological testing was conducted by a research assistant who was trained and supervised by a clinical neuropsychologist. The neuropsychological testing included a battery of standardized neuropsychological tests of memory, attention, processing speed, and social cognition. Only baseline (pre-treatment) clinical assessment and processing speed data were analyzed and reported in the current study.
Data Analysis
To estimate overall processing speed ability for each participant, we calculated a processing speed composite score by averaging each participant’s z-scores on the three processing speed tasks (Trail Making Test A, Symbol Coding, and Category Fluency), after reverse coding participants’ Trail Making Test z-scores. Z-scores allowed for direct comparison between three different metrics of processing speed. To examine between-group differences in processing speed, separate one-way ANCOVAs were performed on participants’ processing speed composite scores, and for participants’ raw scores for each of the three processing speed tasks to illustrate how consistent the findings were in each task. In each analysis, age, education level, and race were controlled for as covariates, as these demographic characteristics differed between the ASD and control groups. For each task, we performed a winsorizing procedure (see Dixon and Tukey 1968) to handle the modest number of outlier scores (Fluency task: 5% of ASD, 3% of controls; Trail task: 9% of ASD; Symbol Coding task: 8% of ASD, 3% of controls) that were ± 2 SD from the total sample mean.
To assess whether processing speed ability was associated with participants’ level of psychosocial functioning in ASD, we first performed a bivariate correlational analysis with participants’ processing speed composite score and measures of social cognition: specifically, their SAS-II composite score, GAF scores, and ADOS Communication and Reciprocal Social Interaction scores. Next a partial correlation analysis was performed with these same variables controlling for IQ, age, and sex, which we hypothesized to be related to psychosocial functioning. The association between processing speed and level of psychosocial functioning was also measured in healthy controls as a comparison; however, SAS-II data were only available for 23 healthy control participants, and GAF data was only available for 43 healthy control participants, and so these correlations should be regarded with caution. Only significant correlations are discussed in the text, and all correlations are show in Table 3.
Table 3.
Correlation coefficients between overall processing speed performance, functional outcomes, and reciprocal social communication skills in individuals with ASD
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. Processing speed composite score | – | − .18 | .19 | − .23* |
| 2. Social adjustment scale IIa | − .09 | – | − .45** | .10 |
| 3. Global assessment of functioning (GAF)a | .24 | − .43** | - | − .04 |
| 4. Communication and social interaction (ADOS)b | − .34** | .06 | − .08 | – |
| 5. IQ | .46** | − .95 | .24 | − .23 |
Zero-order correlations are listed below the diagonal in white, partial correlations are listed above the diagonal in gray
p ≤ .05
p ≤ .01
Data available for 58 participants with ASD.
Data available for 74 participants with ASD.
Results
Do Young Adults with ASD Show a Deficit in Processing Speed?
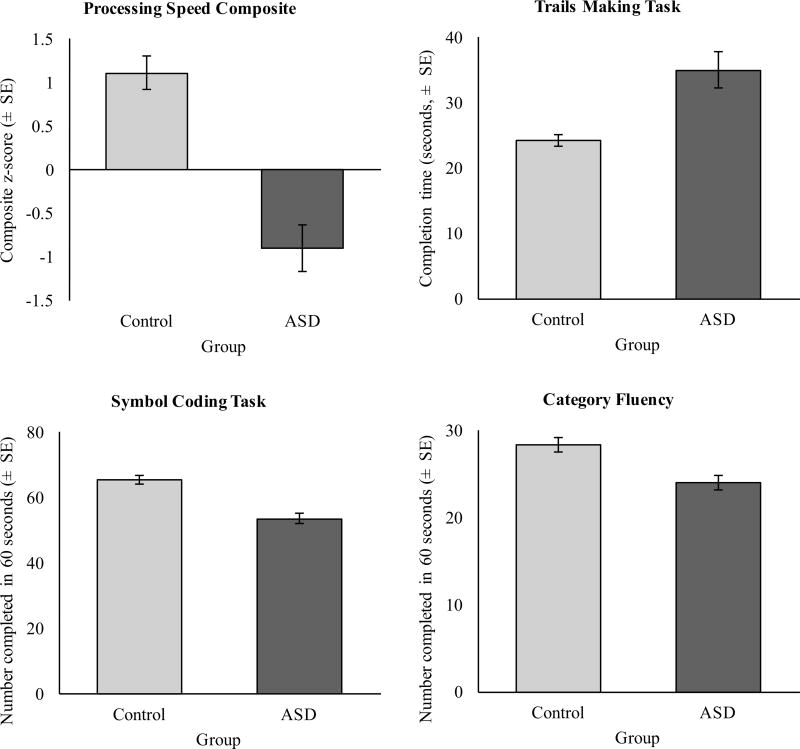
We began our analysis by comparing the ASD and control groups on their composite processing speed scores. The ASD group showed significantly lower processing speed composite scores compared to the control group after controlling for age, race, and education level, F(1, 134) = 32.36, p < .001, d = 1.02 (Fig. 1a; see Table 2 for full means and standard errors). To test whether the group difference in processing speed was due to impaired performance in all three of the subtests, the difference between groups was analyzed separately for the Trail Making Test, Symbol Coding, and Category Fluency tasks.
Fig. 1.
Processing speed scores for ASD and control groups shown as a the composite score, b the completion time in the Trail Making Test, c the number of symbols correctly coded in the Symbols Coding task, and d the number of items reported in the Category Fluency task. All group comparisons were significant (p < .001)
Table 2.
Scores on processing speed tasks for ASD and control participants
| Task | ASD (N = 76) | Control (N = 64) | Between-group difference | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| M | SE | M | SE | F | df | p | d | |
| Processing speed composite | − .92 | .35 | .92 | .35 | 32.36 | 1, 134 | < .001 | − 1.02 |
| Trail making test | 32.82 | 2.08 | 23.76 | 2.07 | 22.35 | 1, 134 | < .001 | .84 |
| Symbol coding | 54.40 | 2.36 | 65.38 | 2.36 | 25.38 | 1, 134 | < .001 | − .90 |
| Category fluency | 22.78 | 1.28 | 27.07 | 1.28 | 13.19 | 1, 134 | < .001 | − .65 |
The ASD group showed significantly slower completion time on the Trail Making Test (M = 32.82 s, SE = 2.08) compared to the control group (M = 23.76 s, SE = 2.07); F(1, 134) = 22.35, p = < .001, d = .84, completed significantly fewer items in 90 s on the Symbol Coding task (M = 54.40, SE = 2.36) compared to the control group (M = 65.38, SE = 2.36) after controlling for age, race, and education level, F(1, 134) = 25.38, p < .001, d = − .90, and ASD participants completed significantly fewer items in 60 s on the Category Fluency task (M = 22.78, SE = 1.28) compared to the control group (M = 27.07, SD = 1.28) after controlling for age, race, and education level, F(1, 134) = 13.19, p < .001, d = − .65.
Are Processing Speed Impairments Related to Psychosocial Functioning?
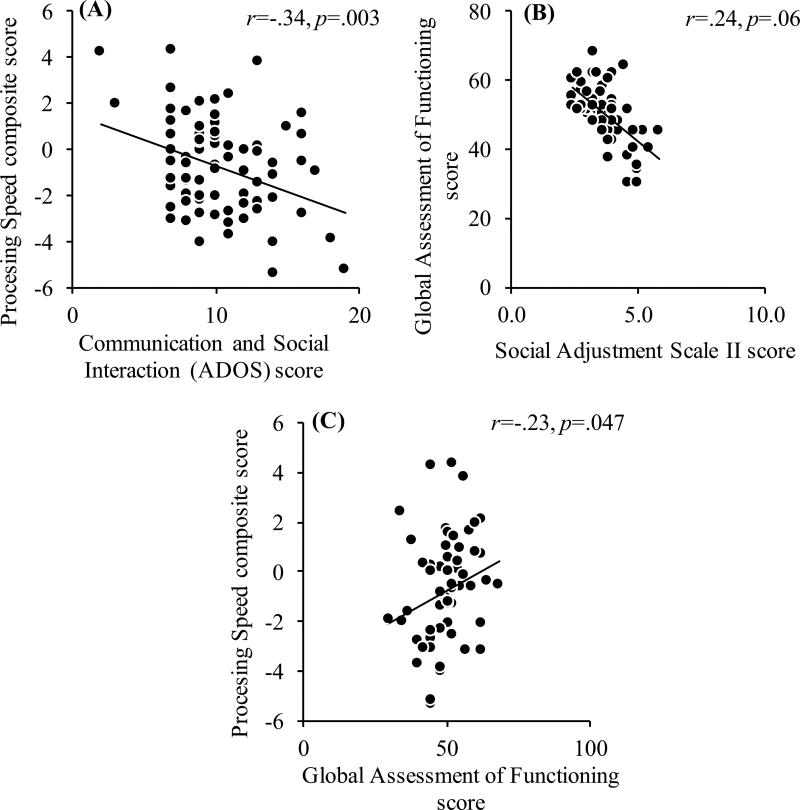
Having found significant deficits in processing speed in ASD, we proceeded to test the relationship between processing speed and measures of psychosocial functioning in ASD. Table 3 displays the correlation coefficients for all analyses conducted. Processing speed composite scores were negatively correlated with ADOS—Communication and Reciprocal Social Interaction scores, r (74) = − .34, p = .003. There was a positive association that was nearing significance with participants’ processing speed composite scores and their GAF scores, r (57) = .24, p = .06, indicating better processing speed was associated with higher levels of functioning. After controlling for IQ, age, and sex, only the association with processing speed remained significant with ADOS Communication and Reciprocal Social Interaction scores, r (71) = − .23, p = .047 (Fig. 2). It is also worth noting that SAS-II and GAF scores were also significantly correlated, r (56) = − .43, p = .001. For the healthy control participants, processing speed correlated with SAS-II scores, r (21) = − .43, p = .039, and with IQ, r (62) = .45, p < .001. However, once IQ was accounted for, the relationship between processing speed and SAS-II scores was no longer significant, r (21) = − .41, p = .061 (Table 4). Taken together, these results suggest that individuals with ASD have a significant and large processing speed deficit, and that such deficits are associated with aspects of communication and social interaction impairments.
Fig. 2.
a The relationship between processing speed composite score and communication and reciprocal social interaction score from the ADOS from 74 ASD individuals, b between processing speed composite score and global assessment of functioning from 58 ASD individuals, and c between global assessment of functioning score and social adjustment scale II score from 58 ASD individuals
Table 4.
Correlation coefficients between overall processing speed performance, functional outcomes, and reciprocal social communication skills in healthy control participants
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Processing speed composite score | – | − .41 | 0.08 |
| 2. Social adjustment scale IIa | − .44* | – | − 0.34 |
| 3. Global assessment of functioning (GAF)b | − .09 | − 0.28 | - |
| 4. IQ | − .44** | − 0.17 | − 0.14 |
Zero-order correlations are listed below the diagonal in white, partial correlations are listed above the diagonal in gray
p ≤ .05
p ≤ .01
Data available for 23 healthy control participants
Data available for 43 healthy control participants
Discussion
The current study was designed to examine processing speed abilities using standardized measures of processing speed in a large sample of verbal adults with ASD without intellectual disability compared to healthy controls who were matched on full-scale IQ. Participants completed three standardized and widely used measures of processing speed: Trail Making Test Part A, Symbol Coding, and Category Fluency. A composite score of these scores was also calculated. The ASD group performed worse on all four measures, indicating a large and significant processing speed deficit. This supports previous findings that even in adults with ASD without intellectual disability, there is a significant deficit in processing speed (Faja et al. 2009; Eack et al. 2013a), suggesting that processing speed is a key deficit in ASD.
The results of the current study contrast the findings from the inspection time studies (Scheuffgen et al. 2000; Wallace et al. 2009; Barbeau et al. 2013) who suggested that processing speed is intact in ASD. The discrepancy between the current findings and the inspection time findings could be due to the inspection tasks producing ceiling effects, and thus it may not have been sensitive enough to detect differences in processing speed in ASD. As mentioned previously, there is some contribution of motor output that might confound any group differences in processing speed: even if processing speed is normal between groups, if motor output is slower in one group over another, then this would result in slower processing speed scores. The three tests used in the MATRICS battery also include a motor-related output, and so the processing speed deficit may be in the perceptual and/or motor systems in ASD. Regardless of the origin of the slower processing speed, the results from the current replicate those reported by Eack et al. (2013a), strongly suggesting that it is the difference in methodology that caused the discrepancy between the inspection time studies (Scheuffgen et al. 2000; Wallace et al. 2009; Barbeau et al. 2013) and the current study.
In addition, the inspection time studies included children with ASD (Scheuffgen et al. 2000; Wallace et al. 2009; Kenworthy et al. 2013), or a broad range of ages from adolescence and adulthood (Barbeau et al. 2013), whereas the current study focused on adults with ASD. There may be a developmental change or delay (Williams et al. 2013) in processing speed in ASD, which could also explain the discrepancy in results. Longitudinal studies tracking the change in processing speed in individuals with ASD across development into adulthood would be able to address this possibility. This discrepancy in the literature also highlights the need to match ASD and neurotypical groups on IQ to ensure that any measurable differences are not due to subtle differences in intelligence. However, it would be interesting to test whether individuals with ASD and lower IQ show exacerbated processing speed deficits, or whether the majority of the deficit can be accounted for by diagnosis.
We also found that participants’ processing speed abilities were associated with some aspects of psychosocial functioning: lower processing speed scores were associated with poorer communication and reciprocal interaction skills as measured by the ADOS-2, and higher processing speed scores showed a weak association with better overall psychosocial functioning as measured by the GAF. These findings support previous studies showing associations between information processing deficits and impairments in social cognition in ASD (Embregts and van Nieuwenhuijzen 2009; Russo-Ponsaran et al. 2015; Worsham et al. 2014; Lerner et al. 2013). However, no significant relationships were observed between processing speed and social adjustment impairments on the SAS-II. Together this suggests that processing speed is slower in ASD and is associated with greater impairment in some aspects of social functioning. Further studies are needed to investigate if processing speed is related specifically to social cognition, or is just more impaired in those who are more symptomatic.
If processing speed is related to social cognition, then processing speed could be a viable target for assessing the effects of treatment. There is some evidence that certain treatments improve processing speed in ASD, for example, after Cognitive Enhancement Therapy (CET). Measures of processing speed in ASD using the MATRICS tasks showed that processing speed fell below the 25th percentile for nearly half of the ASD group (Eack et al. 2013b), and CET had a large effect on improving processing speed in an initial feasibility study (Eack et al. 2013a). CET also improved measures of social cognition, emotion processing, and measures of social adjustment. It is possible that the effects of CET on daily functioning were caused by improvements in processing speed. Regardless of the cause of the improvements, cognitive remediation interventions, such as CET, may help address the deficits in processing speed in ASD, which may then impact daily functioning.
The current study had several limitations that should be considered when interpreting the results. First, the group of adults with ASD was not perfectly matched with the group of healthy control participants. There were significant differences in age, race, and education level between these groups; however, we controlled for these factors in our analyses, and the large group differences persisted. Second, autism diagnostic assessments were not completed with the healthy control participants, and so it is possible that some of the control participants had subclinical autism symptoms. Third, the results did not show a significant relationship between processing speed abilities and the main measure of functional outcomes, the SAS-II. This is a widely used measure of social adjustment, and the absence of associations between processing speed impairment and the SAS-II, suggests that while impairments in processing speed are large in ASD, they may contribute more to communication and reciprocal social interaction deficits rather than social disability, generally. Future studies could compare the magnitude of processing speed deficits with a variety of functional outcomes including social cognition, to examine whether specific functional domains are more closely related to information processing than others. Fourth, this study does not address whether children with ASD are also slower in their processing speed. However, this study does advocate for the use of standardized measures of processing speed to ensure that the test is sensitive enough to detect clinically-related deficits.
In conclusion, when using standardized measures of processing speed, adults with ASD show large deficits compared to IQ-matched healthy controls, and the processing speed deficit is related to their social and communication challenges. These findings have important implications for diagnosis and treatment, and indicate that improving information processing speed may also help improve other more complex symptoms, such as deficits in social communication.
Acknowledgments
This work was supported by NIH grants MH-106450 to Shaun M Eack, MH-85851 to Nancy J Minshew and Shaun M Eack, MH-95783 to Shaun M Eack, RR-24154 to Shaun M Eack, and HD-55748 to Nancy J Minshew, as well as grants from Autism Speaks 05381 to Nancy J Minshew and Shaun M Eack, the Department of Defense AR100344 to Nancy J Minshew and Shaun M Eack, and the Pennsylvania Department of Health SAP #4100047816 to Nancy J Minshew.
Footnotes
Author Contributions SMH wrote the manuscript and helped interpret the data. JAW conducted the study and analyzed the data. CAM and NJM helped with data interpretation and manuscript preparation. SME oversaw the study, helped analyze and interpret the data, and helped write the manuscript. All authors reviewed the manuscript before submission.
Compliance with Ethical Standards
Ethical Approval This study was approved by an Institutional Review Board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest The authors have no conflicts of interest to declare.
References
- Barbeau EB, Soulières I, Dawson M, Zeffiro TA, Mottron L. The level and nature of autistic intelligence III: Inspection time. Journal of Abnormal Psychology. 2013;122(1):295–301. doi: 10.1037/a0029984. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: Evidence from Very high functioning adults with autism or asperger syndrome. Journal of Child Psychology and Psychiatry. 1997;38(7):813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Battery AT. Army individual test battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjunct General’s Office; 1994. [Google Scholar]
- Bavin EL, Kidd E, Prendergast LA, Baker EK. Young children with ASD use lexical and referential information during on-line sentence processing. Frontiers in Psychology. 2016a;7:171. doi: 10.3389/fpsyg.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavin EL, Prendergast LA, Kidd E, Baker E, Dissanayake C. Online processing of sentences containing noun modification in young children with high-functioning autism. International Journal of Language & Communication Disorders. 2016b;51(2):137–147. doi: 10.1111/1460-6984.12191. [DOI] [PubMed] [Google Scholar]
- Cornoldi C, Giofrè D, Orsini A, Pezzuti L. Differences in the intellectual profile of children with intellectual vs. learning disability. Research in Developmental Disabilities. 2014;35(9):2224–2230. doi: 10.1016/j.ridd.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Critchley HD, et al. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Tukey JW. Approximate behavior of the distribution of winsorized t (Trimming/Winsorization 2) Technometrics. 1968;10(1):83–98. doi: 10.1080/00401706.1968.10490537. [DOI] [Google Scholar]
- Eack SM, Bahorik AL, Hogarty SS, Greenwald DP, Litschge MY, Mazefsky CA, Minshew NJ. Brief report: Is cognitive rehabilitation needed in verbal adults with autism? insights from initial enrollment in a trial of cognitive enhancement therapy. Journal of Autism and Developmental Disorders. 2013b;43(9):2233–2237. doi: 10.1007/s10803-013-1774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Bahorik AL, McKnight SAF, Hogarty SS, Greenwald DP, Newhill CE, et al. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophrenia Research. 2013a;148(1–3):24–28. doi: 10.1016/j.schres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embregts P, Van Nieuwenhuijzen M. Social information processing in boys with autistic spectrum disorder and mild to borderline intellectual disabilities. Journal of Intellectual Disability Research. 2009;53(11):922–931. doi: 10.1111/j.1365-2788.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Faja S, Webb SJ, Merkle K, Aylward E, Dawson G. Brief report: Face configuration accuracy and processing speed among adults with high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(3):532–538. doi: 10.1007/s10803-008-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R, et al. A study of the neuropsychological correlates in adults with high functioning autism spectrum disorders. Acta Neuropsychiatrica. 2016;28(5):286–295. doi: 10.1017/neu.2016.12. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Varga S. Conceptual issues in autism spectrum disorders. Current Opinion in Psychiatry. 2015;28(2):127–132. doi: 10.1097/yco.0000000000000142. [DOI] [PubMed] [Google Scholar]
- Green MF, Harris JG, Nuechterlein KH. The MATRICS consensus cognitive battery: What we know 6 years later. American Journal of Psychiatry. 2014;171(11):1151–1154. doi: 10.1176/appi.ajp.2014.14070936. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. http://europepmc.org/abstract/MED/16450045. [DOI] [PubMed] [Google Scholar]
- Hedvall Å, Fernell E, Holm A, Åsberg Johnels J, Gillberg C, Billstedt E. Autism, processing speed, and adaptive functioning in preschool children. The Scientific World Journal. 2013;2013:158263. doi: 10.1155/2013/158263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61(9):866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Holdnack J, Goldstein G, Drozdick L. Social perception and WAIS-IV performance in adolescents and adults diagnosed with asperger’s syndrome and autism. Assessment. 2011;18(2):192–200. doi: 10.1177/1073191110394771. [DOI] [PubMed] [Google Scholar]
- Howard PL, Liversedge SP, Benson V. Investigating the use of world knowledge during on-line comprehension in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2017;47(7):2039–2053. doi: 10.1007/s10803-017-3129-x. [DOI] [PubMed] [Google Scholar]
- Huebner RA. Autistic disorder: A neuropsychological enigma. American Journal of Occupational Therapy. 1992;46(6):487–501. doi: 10.5014/ajot.46.6.487. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief assessment of cognition in schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Clarke MC, Rawdon C, Murphy J, Cannon M. Neurocognition in the extended psychosis phenotype: Performance of a community sample of adolescents with psychotic symptoms on the MATRICS neurocognitive battery. Schizophrenia Bulletin. 2013;39(5):1018–1026. doi: 10.1093/schbul/sbs086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Weinblatt R, Abrams DN, Wallace GL. Motor demands impact speed of information processing in Autism Spectrum Disorders. Neuropsychology. 2013;27(5):529–536. doi: 10.1037/a0033599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MD, McPartland JC, Morris JP. Multimodal emotion processing in autism spectrum disorders: An event-related potential study. Developmental Cognitive Neuroscience. 2013;3:11–21. doi: 10.1016/j.dcn.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Jr, Leventhal B, DiLavore P, et al. The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional-defiant disorder. Child Neuropsychology. 2007;13(6):469–493. doi: 10.1080/09297040601112773. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, White SW. The role of emotion regulation in autism spectrum disorder RH: Emotion regulation in ASD. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(7):679–688. doi: 10.1016/j.jaac.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4(2):129–136. [Google Scholar]
- Nader A-M, Jelenic P, Soulières I. Discrepancy between WISC-III and WISC-IV cognitive profile in autism spectrum: what does it reveal about autistic cognition? PLoS ONE. 2015;10(12):e0144645. doi: 10.1371/journal.pone.0144645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narzisi A, Muratori F, Calderoni S, Fabbro F, Urgesi C. Neuropsychological profile in high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(8):1895–1909. doi: 10.1007/s10803-012-1736-0. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Oliveras-Rentas RE, Kenworthy L, Roberson RB, Martin A, Wallace GL. WISC-IV Profile in high-functioning autism spectrum disorders: Impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of Autism and Developmental Disorders. 2012;42(5):655–664. doi: 10.1007/s10803-011-1289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts J, Jones CRG, Happé F, Charman T. Reading comprehension in autism spectrum disorders: The role of oral language and social functioning. Journal of Autism and Developmental Disorders. 2013;43(4):807–816. doi: 10.1007/s10803-012-1619-4. [DOI] [PubMed] [Google Scholar]
- Russo-Ponsaran NM, McKown C, Johnson JK, Allen AW, Evans-Smith B, Fogg L. Social-emotional correlates of early stage social information processing skills in children with and without autism spectrum disorder. Autism Research. 2015;8(5):486–496. doi: 10.1002/aur.1463. [DOI] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Lago M, Tirapu J, Barceló F. Construct validity of the trail making test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society: JINS. 2009;15(3):438–450. doi: 10.1017/s1355617709090626. [DOI] [PubMed] [Google Scholar]
- Scheuffgen K, Happeé F, Anderson M, Frith U. High “intelligence,” low “IQ”? Speed of processing and measured IQ in children with autism. Development and Psychopathology. 2000;12(1):83–90. doi: 10.1017/s095457940000105x. https://www.cambridge.org/core/article/div-class-title-high-intelligence-low-iq-speed-of-processing-and-measured-iq-in-children-with-autism-div/FDB003BD657D40545D518723B1655CFC. [DOI] [PubMed] [Google Scholar]
- Schooler N, Hogarty G, Weissman M. Social Adjustment Scale II (sas-ii) In: Hargreaves WA, Atkisson CC, Sorenson JE, editors. Resource materials for community mental health program evaluations. Rockville, MD: National Institute of Mental Health; 1979. pp. 290–303. [Google Scholar]
- Torres EB, Brincker M, Isenhower RW, Yanovich P, Stigler KA, Nurnberger JI, Jose JV. Autism: The micro-movement perspective. Frontiers in Integrative Neuroscience. 2013 doi: 10.3389/fnint.2013.00032. [DOI] [PMC free article] [PubMed]
- Wallace GL, Anderson M, Happé F. Brief report: Information processing speed is intact in autism but not correlated with measured intelligence. Journal of Autism and Developmental Disorders. 2009;39(5):809–814. doi: 10.1007/s10803-008-0684-1. [DOI] [PubMed] [Google Scholar]
- Wang J-E, Tsao F-M. Emotional prosody perception and its association with pragmatic language in school-aged children with high-function autism. Research in Developmental Disabilities. 2015;37(Supplement C):162–170. doi: 10.1016/j.ridd.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler adult intelligence scale. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Whitaker L, Jones CRG, Wilkins AJ, Roberson D. Judging the intensity of emotional expression in faces: The effects of colored tints on individuals with autism spectrum disorder. Autism Research. 2016;9(4):450–459. doi: 10.1002/aur.1506. [DOI] [PubMed] [Google Scholar]
- Whyatt C, Craig C. Sensory-motor problems in Autism. Frontiers in Integrative Neuroscience. 2013;7:51. doi: 10.3389/fnint.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT 4: Wide range achievement test; professional manual. Lutz: Psychological Assessment Resources; 2006. [Google Scholar]
- Williams D, Goldstein G, Minshew N. The modality shift experiment in adults and children with high functioning autism. Journal of Autism and Developmental Disorders. 2013;43(4):794–806. doi: 10.1007/s10803-012-1618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ, Goldstein G. Further understanding of complex information processing in verbal adolescents and adults with autism spectrum disorders. Autism. 2015;19(7):859–867. doi: 10.1177/1362361315586171. [DOI] [PubMed] [Google Scholar]
- Worsham W, Gray WE, Larson MJ, South M. Conflict adaptation and congruency sequence effects to social–emotional stimuli in individuals with autism spectrum disorders. Autism. 2014;19(8):897–905. doi: 10.1177/1362361314553280. [DOI] [PubMed] [Google Scholar]
- Yeung MK, Han YMY, Sze SL, Chan AS. Abnormal frontal theta oscillations underlie the cognitive flexibility deficits in children with high-functioning autism spectrum disorders. Neuropsychology. 2016;30(3):281–295. doi: 10.1037/neu0000231. [DOI] [PubMed] [Google Scholar]