Abstract
Increased adult stature has been associated with risk of testicular germ call tumors (TGCT) in a number of studies. Whether childhood stature is also associated with TGCT is unclear as no studies of measured childhood height and TGCT have been reported. Thus, associations between TGCT in adulthood and childhood height and growth between ages 7 and 13 years were examined in a cohort from the Copenhagen School Health Records Register. Analyses included 162,607 boys born during the years 1930–1989. Development of TGCT was determined via linkage to the Danish Cancer Registry. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression. Between 1968 and 2014, 782 TGCT were diagnosed. Childhood height, per one unit increase in z-score, was associated with risk of TGCT, with HRs ranging from 1.11 (95%CI 1.03–1.20) at age 7 to 1.09 (95%CI=1.01–1.18) at age 13. In a categorical analysis, the shortest boys were at the lowest risk of developing TGCT. Results varied little by TGCT histology (seminoma, nonseminoma). Growth between ages 7 and 13 years was not associated with risk. These findings suggest that risk of TGCT in adulthood was already determined by age 7 years. Although the mechanism requires further investigation, these results provide additional evidence that risk of TGCT is determined at a young age, thus suggesting that additional investigation of early life factors is warranted.
Keywords: height, growth, testicular germ cell tumors
Introduction
Testicular germ cell tumors (TGCT) are rare tumors in the general population, but are the most commonly occurring cancers among males aged 15–44 years in developed countries. Incidence rates of TGCT, including both seminomas and nonseminomas, have been rising in many countries for over 50 years. Incidence rates are particularly high among men of Northern European ancestry, with Denmark having one of the highest TGCT rates in the world 1.
Reasons for the increase in incidence are not clear as few risk factors have been identified. One factor that has been linked to TGCT, however, is adult height, which has been associated with a 13% increased risk in TGCT per 5 cm height increase 2. Whether height in childhood is also associated with TGCT risk is unclear 3, although it is known that height is determined early in life via a combination of genetics, nutrition, and health 4. Rising secular trends in height have been reported 5. While it is unlikely that height has a causal association with TGCT, height may share a common mechanism with an unknown risk factor, which increases the risk 2. Thus, elucidation of the etiologically relevant timeframe for childhood height in association with TGCT could provide insight into possible mechanisms.
The current study, based in records of the Copenhagen School Health Records Register (CSHRR), examined the relationship between childhood height and subsequent risk of TGCT, overall and by histologic subtype (seminoma, nonseminoma).
Methods
As previously described 6, the CSHRR includes 188,360 male children who attended public or private school in Copenhagen, Demark and were born between 1930 and 1989. Annual school health assessments included anthropometric measurement, physical exam, assessment of illness, history of infectious disease, and vaccination status. With the child wearing no shoes, medical personnel measured and recorded height to the nearest half centimeter.
Childhood height z-scores were calculated by age (per month). To generate these z-scores, internal birth-cohort-specific references were utilized. These references were generated in 5-year intervals from the entire cohort (i.e., among boys with and without CPR numbers) using the LMS method 7. For height z-scores at the exact ages (i.e., 7, 8, 9, 10, 11, 12 or 13 years), the z-score that was computed from measured height if the measurement was taken at the child’s exact age (i.e., within the month of the child’s birthday) was used. The z-score was interpolated if two measurements were available on either side of the exact age (±12 months). If only one height measurement was available on either side of the exact age (±12 months), the z-score was extrapolated. If there was not at least one height measurement available within 12 months of the exact age, a z-score was not generated for that age. Height z-scores were also categorized, with cut-points corresponding to approximately the 10th, 25th, 75th, and 90th percentiles of height.
Linkage of the CSHRR to the Danish Cancer Registry and the Danish Civil Registration System (vital statistics) was conducted using a unique national personal identification (ID) number. Beginning in April 1968, Danish citizens of all ages were assigned ID numbers 8. Children attending school in or after 1968 had the ID number recorded on their health card. For indiviudals with no ID number recorded on their health card, probable ID numbers were obtained from the Danish Civil Registration System based on the person’s forename(s), surname, sex, and date of birth. Utilizing this approach, ID numbers were identified for 167,409 males (89%) in the study population. For indiviudals that died or emigrated prior to 1968, an ID number was never issued 6.
Incident testicular cancers, defined by the International Classification of Diseases (ICD), 7th edition diagnostic codes 178, 278, 378, 478, 578, 678 and 878 as well as 10th edition diagnostic code C62, were ascertained by linkage with the Danish Cancer Registry. TGCT was classified using the Danish modified version of the ICD 7th edition codes for seminoma (378) and nonseminoma (278, 478, 578), and the ICD for Oncology, 3rd edition morphology codes for seminoma (9060–9062, 9064) and nonseminoma (9065–9102). Due to small numbers (32 cases), TGCT diagnoses occuring after the age of 60 were excluded. The current analysis included 782 TGCTs, of which 468 were seminomas and 314 were nonseminomas.
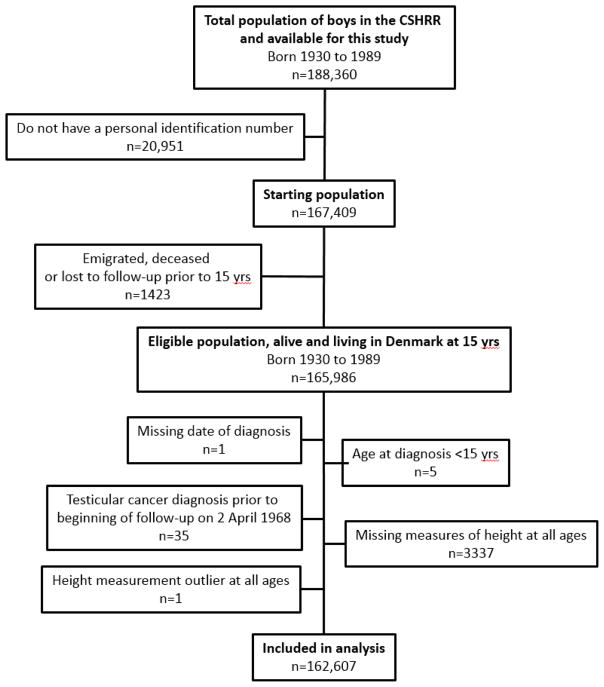
Follow-up began on April 2, 1968, when the Danish Civil Registration System was established, or at age 15 years, and individuals were followed until December 31, 2014. Among 167,409 males with an ID number available, individuals were excluded if they emigrated, died, or were lost to follow-up prior to age 15 years (n=1423); were missing date of diagnosis (n=1) or were diagnosed prior to age 15 (n=5) or April 2, 1968 (n=35); were missing measures of height at all ages (n=3337); or had an outlying height z-score (<–4.5 or >4.5) at all ages (n=1). Thus, 162,607 males were eligible for the study (Figure 1).
Figure 1.
Flow chart of eligible and included participants in the study.
Statistical Analysis
Cox proportional hazard regression analysis was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between height z-score and risk of TGCT, with age as the underlying time metric and baseline hazard stratified by birth cohort (5-year intervals). The proportional hazards assumption was tested by examination of the cumulative hazard plots and using an interaction term between height z-score and the age at diagnosis. The proportional hazards assumption was not observed to be violated (p≥0.05).
To assess the shape of the association between height z-score and testicular cancer risk, restricted cubic splines (five knots) were tested against the linear alternative using the likelihood ratio test. There were no indications of deviations from linearity as none of the tests were statistically significant (all p≥0.05).
Linear growth between 7 and 13 years was examined in the sub-population with height measurement taken at ages 7 and 13. Effects of growth on TGCT risk were examined in categorical models as well as linear models expressed per 0.5 increase in z-score (few boys change their height by more than this amount).
As the CSHRR spans nearly 60 years of births and TGCT incidence is known to be affected by birth cohort, effect measure modification of height z-score and TGCT risk by birth cohort (categorized as 1930–39, 1940–49, 1950–59, 1960–69, 1970–89) was examined. While height increased with each subsequent birth cohort (Supplemental Table 1), there was limited evidence of effect measure modification by birth cohort (all p≥0.05).
Results
Height z-scores were calculated for specific birth cohorts. One z-score unit is equivalent to approximately 5.1 cm at age 7 and approximately 7.6 cm at age 13. As shown in Table 1, taller boys had an increased risk of TGCT at all ages between ages 7 years and 13 years. At age 7, a one unit z-score increase in height was associated with an 11% subsequent increased risk of TGCT (HR=1.11, 95% CI: 1.03–1.20). Risks were similar at all ages. For example, at age 13, a one unit z-score increase in height was associated with a 9% subsequent increased TGCT risk (HR=1.09, 95% CI: 1.01–1.18). Results for seminoma were very similar to the overall results. While the hazard ratios for nonseminoma were also similar to the results for all TGCT and seminoma, the sample size was limited and most did not attain statistical significance.
Table 1.
Hazard ratios for associations between height z-scores and risk of testicular germ cell tumors overall and by histology*
| Age (y) | N | TGCT | Seminoma | Nonseminoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| cases | HR | 95% CI | p-value | cases | HR | 95% CI | p-value | cases | HR | 95%CI | p-value | ||
|
|
|
|
|||||||||||
| 7 | 151835 | 742 | 1.11 | 1.03 – 1.20 | 0.004 | 442 | 1.12 | 1.02 – 1.23 | 0.02 | 300 | 1.10 | 0.98 – 1.23 | 0.10 |
| 8 | 153682 | 757 | 1.13 | 1.05 – 1.21 | 0.001 | 452 | 1.14 | 1.04 – 1.25 | 0.01 | 305 | 1.12 | 1.00 – 1.25 | 0.05 |
| 9 | 147808 | 738 | 1.11 | 1.04 – 1.20 | 0.004 | 442 | 1.12 | 1.02 – 1.24 | 0.02 | 296 | 1.10 | 0.98 – 1.23 | 0.12 |
| 10 | 143766 | 721 | 1.12 | 1.04 – 1.20 | 0.004 | 440 | 1.12 | 1.02 – 1.23 | 0.02 | 281 | 1.10 | 0.98 – 1.24 | 0.10 |
| 11 | 142614 | 708 | 1.10 | 1.02 – 1.19 | 0.01 | 437 | 1.11 | 1.01 – 1.22 | 0.03 | 271 | 1.08 | 0.96 – 1.22 | 0.19 |
| 12 | 141146 | 698 | 1.09 | 1.01 – 1.18 | 0.02 | 431 | 1.10 | 1.00 – 1.21 | 0.06 | 267 | 1.09 | 0.96 – 1.23 | 0.18 |
| 13 | 138542 | 686 | 1.09 | 1.01 – 1.18 | 0.02 | 421 | 1.09 | 0.99 – 1.21 | 0.07 | 265 | 1.09 | 0.97 – 1.23 | 0.17 |
all analyses stratified by birth cohort
Table 2 shows z-scores at ages 7, 10 and 13 years, categorized corresponding to approximately the 10th, 25th, 75th, and 90th percentiles of height. While there was a suggestion that the tallest boys (greater than the 90th percentile of height) at age 7 years had an increased risk of TGCT compared to boys in percentiles 25–50 of height, the results were not statistically significant (HR=1.21, 95% CI: 0.95–1.53). The shortest boys, however, had a significantly decreased risk of TGCT at all three ages (HRage7=0.71, 95% CI: 0.53–0.94; HRage10=0.70, 95% CI: 0.52–0.93; HRage13=0.74, 95% CI: 0.56–0.99).
Table 2.
Hazard ratios of testicular germ cell tumors by categories of height z-scores at 7, 10 and 13 years of age*
| N | cases | HR | 95% CI | |
|---|---|---|---|---|
|
|
||||
| Age 7 years | ||||
| −4.50 to −1.28 | 15824 | 55 | 0.71 | 0.53 – 0.94 |
| −1.28 to −0.68 | 23650 | 119 | 1.02 | 0.83 – 1.26 |
| −0.68 to 0.68 | 76919 | 381 | 1.00 | referent |
| 0.68 to 1.28 | 20880 | 104 | 1.01 | 0.81 – 1.25 |
| 1.28 to 4.50 | 13820 | 83 | 1.21 | 0.95 – 1.53 |
| Age 10 years | ||||
| −4.50 to −1.28 | 14119 | 52 | 0.70 | 0.52 – 0.93 |
| −1.28 to −0.68 | 21482 | 93 | 0.82 | 0.65 – 1.03 |
| −0.68 to 0.68 | 72871 | 388 | 1.00 | referent |
| 0.68 to 1.28 | 20786 | 111 | 1.00 | 0.81 – 1.24 |
| 1.28 to 4.50 | 13787 | 77 | 1.04 | 0.82 – 1.33 |
| Age 13 years | - | |||
| −4.50 to −1.28 | 13813 | 52 | 0.74 | 0.56 – 0.99 |
| −1.28 to −0.68 | 21197 | 98 | 0.91 | 0.73 – 1.14 |
| −0.68 to 0.68 | 69753 | 358 | 1.00 | referent |
| 0.68 to 1.28 | 19671 | 107 | 1.06 | 0.85 – 1.31 |
| 1.28 to 4.50 | 13422 | 71 | 1.03 | 0.80 – 1.33 |
All analyses stratified by birth cohort.
In the growth sub-analyses restricted to the 129,877 boys who had height recorded at both ages 7 and 13 years, results for all TGCT were the same as in the overall analysis, with the hazard ratios ranging from 1.10 (95%CI 1.01–1.18) at age 7 to 1.09 (95%CI=1.00–1.17) at age 13 years (Supplemental Table 2).
Table 3 shows the longitudinal growth analyses of risk of TGCT by cross-classification of height at both ages 7 and 13 years. Compared to boys in the middle height category at both ages, the boys who were in the shortest category at both ages had a suggested, albeit nonsignificant, decreased risk of TGCT (HR=0.70; 95% CI: 0.49–1.01). In contrast, boys in the highest category of height at age 7, but only between the 75th and 90th percentiles at age 13 had a borderline significant 44% increased risk of testicular cancer (HR=1.44, 95% CI: 0.98–2.12). However, numbers were limited to examine this cross-classification of height at ages 7 and 13. As shown in Table 3, very few boys either went from being in the shortest category at age 7 years to the tallest category at age 13 years (n=2), or from the tallest category at age 7 to the shortest category at age 13 (n=1). Finally, when a 0.5 z-score change in height between ages 7 and 13 was examined, growth was not associated with an increased risk of TGCT overall, or separately with either seminoma or nonseminoma (Table 4).
Table 3.
Hazard ratios of testicular germ cell tumors by height z-score categories at 7 and 13 years of age
| Age 7 z-score | Age 13 z-score | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| −4.50 to −1.28 | −1.28 to −0.68 | −0.68 to 0.68 | 0.68 to 1.28 | 1.28 to 4.50 | ||
|
|
||||||
| −4.50 to −1.28 | HR (95% CI) | 0.70 (0.49–1.01) | 0.35 (0.09–1.39) | 0.35 (0.09–1.39) | - | - |
| cases/n | 33/9,081 | 12/3,538 | 2/1,123 | 0/14 | 0/2 | |
| −1.28 to −0.68 | HR (95% CI) | 1.03 (0.63–1.69) | 0.96 (0.70–1.30) | 1.03 (0.73–1.44) | 1.01 (0.14–7.18) | 12.17 (1.70- |
| cases/n | 17/3,215 | 48/9,683 | 39/7,390 | 1/182 | 1/17 | |
| −0.68 to 0.68 | HR (95% CI) | - | 0.97 (0.67–1.37) | Referent | 1.04 (0.75–1.46) | 0.81 (0.40–1.63) |
| cases/n | 0/335 | 33/6,571 | 259/49,727 | 40/7,390 | 8/1,908 | |
| 0.68 to 1.28 | HR (95% CI) | - | - | 0.93 (0.65–1.33) | 0.89 (0.62–1.28) | 1.11 (0.70–1.75) |
| cases/n | 0/0 | 0/19 | 34/6,990 | 34/7,335 | 20/3,467 | |
| 1.28 to 4.50 | HR (95% CI) | - | - | 0.54 (0.13–2.16) | 1.44 (0.98–2.12) | 1.00 (0.71–1.40) |
| cases/n | 0/1 | 0/1 | 2/723 | 29/3,823 | 38/7,285 | |
Table 4.
Association per 0.5 change in height z-score from 7 to 13 years and risk of testicular germ cell tumors
| HR | 95% CI | ||
|---|---|---|---|
|
|
|||
| TGCT | 0.96 | 0.82 – 1.11 | |
| Seminoma | 0.92 | 0.76 – 1.12 | |
| Nonseminoma | 1.01 | 0.80 – 1.28 | |
All analyses stratified by birth cohort.
Discussion
In this large propective analysis, measured childhood height was positively associated with risk of TGCT. In a categorical analysis, the shortest boys were at the lowest risk of subsequently developing TGCT, whereas there was no significant association with risk among the tallest boys. Growth between ages 7 and 13 years, was not associated with risk. Results were similar for seminomas and nonseminomas.
As TGCT is hypothesized to be due to a combination of genetic and early life environmental factors, determination of the association between childhood height and risk could clarify the etiologically relevant timeframe for TGCT development. Only one previous study has examined childhood height in relation to TGCT. The study reported that being taller than one’s peers at ages 9 years (HR=1.55, 95% CI: 0.91–2.64) and 13 years (HR=1.26, 95% CI: 0.78–2.01), compared to being shorter, was associated with nonsignificant increased risks of TGCT. The study, however, was based on self-reported recall of childhood height in comparison to peers, rather than height that was measured and recorded 3. The current study, in contrast, examined height as measured by trained medical professionals by a standard protocol in which height was recorded it to the nearest half centimeter 6. Nevertheless, the studies are in general agreement that increased childhood stature is associated with increased TGCT risk. The results are also in agreement with studies that have examined the relationship between adult height and risk. A 2010 meta-analysis of 14 studies reported a positive association whereby a 5 centimeter increase in adult height was associated with a 13% increased risk of TGCT (HR=1.13, 95% CI: 1.07–1.19).
While risk factors for TGCT are not well characterized, the prevailing hypothesis is that risk is largely determined in utero 9. The association of congenital anomalies, including cryptorchidism, hypospadias and inguinal hernia, lends support to this hypothesis 10. In addition, TGCT has one of the strongest heritabilities of any cancer, with risk particularly increased among men having an affected brother 11. This increased familial risk is consistent with a strong genetic contribution, and a number of genetic markers have been identified 12. A recent study examined whether single nucleotide polymorphisms (SNPs) previously associated with adult height could explain the association seen between height and TGCT. Two of the SNPs evaluated were found to be associated with risk of TGCT (rs6060373 and rs143384). When the association between height and testicular cancer was adjusted for all 15 SNPs analyzed, the association was attenuated, albeit non-significantly. Thus, the authors concluded that there was no strong evidence that this attenuation was greater than what would have been expected with stochastic variation 13.
The reason that height and TGCT are associated is not clear. Adult height is a complex phenotype determined by a combination of genetics, childhood nutrition, and infectious diseases in childhood 4. Hormones essential for normal growth, such as insulin-like growth factor-I (IGF-I) and growth hormone are also key contributors to final adult height 14–16, thus height could be a marker of higher IGF-I levels in childhood. IGF-I is mitogenic and is capable of inhibiting apoptosis in non-malignant and malignant cells 17 and is known to be involved with the regulation of spermatogenesis 18, suggesting that higher IGF-I levels could act to promote TGCT.
IGF-I levels are also known to be affected by childhood diet, in particular by milk intake 19, 20. Due to reported associations between milk and dairy food consumption in childhood and height, several studies have investigated the association been milk and dairy food consumption and TGCT. While two of the studies 21, 22 reported an increased risk associated with higher levels of milk consumption in adolescence, one of the two did not find an increased risk with other dairy food consumption 21, 22. A third study assessed milk and dairy food consumption at several time points between birth and age 18 years and found that consumption of milk with 2% milk fat, in comparison to milk with a higher content of fat, was associated with increased risk 23. Overall dairy consumption, however, was not associated. These results suggest that more research in the area of early life milk consumption, height and TGCT may be warranted.
Social conditions and lifestyle factors have varied dramatically among the birth cohorts included in this study (i.e., 1930–1989). In particular, there has been considerable interest in the cohort born during World War II in Denmark as they had a lower risk of TGCT than prior or subsequent cohorts 24. As shown in Supplementary Table 1, however, childhood height continued to increase, rather than decrease or plateau, among the World War II cohort. As reported previously, weight of the World War II cohort also continued to increase, which is not unexpected as the authors found evidence of only minimal nutritional reduction during the war in Copenhagen 25. These results suggest that the lower TGCT risk of the World War II cohort in Denmark is not related to nutritional deprivation or its effect on height in childhood.
Major strengths of the current analysis include that it was based on a large, prospective cohort study with serially measured heights in childhood and early adolescence. Thus, this is the largest study to investigate an association between childhood height and TGCT, and the only study with prospectively measured height. Introduction of the personal ID number in 1968 permitted linkage with the Danish Cancer Registry, allowing for highly accurate cancer outcome ascertainment 26. In addition, as there was minimal loss to follow-up and a long follow-up period, a large enough number of TGCT cases were diagnosed to investigate birth cohort effects, which were not found. In contrast to these strengths, a limitation of the current study was the inability to adjust for social or lifestyle factors which could have been important confounders 6.
In conclusion, childhood height was associated with an increased risk of TGCT, but growth during childhood was not. Further investigations into environmental influences on height during early childhood may further elucidate the etiology of TGCT.
Supplementary Material
Novelty and Impact.
Although the risk of TGCT has been suggested to be determined in early life, few early like risk factors have been identified. In this first study to examine measured childhood height and risk of TGCT, height by age 7 years was already associated with risk. These results may help to focus new investigations of TGCT epidemiology on the early life period.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, U.S.A.
Footnotes
The authors have no conflicts of interest
References
- 1.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3:4–12. doi: 10.1111/andr.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerro CC, McGlynn KA, Cook MB. A systematic review and meta-analysis of the relationship between body size and testicular cancer. Br J Cancer. 2010;103:1467–74. doi: 10.1038/sj.bjc.6605934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richiardi L, Vizzini L, Pastore G, Segnan N, Gillio-Tos A, Fiano V, Grasso C, Ciuffreda L, Lista P, Pearce N, Merletti F. Lifetime growth and risk of testicular cancer. Int J Cancer. 2014;135:695–701. doi: 10.1002/ijc.28688. [DOI] [PubMed] [Google Scholar]
- 4.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35:263–85. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14:301–8. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 6.Baker JL, Olsen LW, Andersen I, Pearson S, Hansen B, Sorensen T. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38:656–62. doi: 10.1093/ije/dyn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 8.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 9.McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol. 2012;9:339–49. doi: 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 11.Hemminki K, Chen B. Familial risks in testicular cancer as aetiological clues. Int J Androl. 2006;29:205–10. doi: 10.1111/j.1365-2605.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 12.Litchfield K, Shipley J, Turnbull C. Common variants identified in genome-wide association studies of testicular germ cell tumour: an update, biological insights and clinical application. Andrology. 2015;3:34–46. doi: 10.1111/andr.304. [DOI] [PubMed] [Google Scholar]
- 13.Cook MB, Chia VM, Berndt SI, Graubard BI, Chanock SJ, Rubertone MV, Erickson RL, Hayes RB, McGlynn KA. Genetic contributions to the association between adult height and testicular germ cell tumors. Int J Epidemiol. 2011;40:731–9. doi: 10.1093/ije/dyq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee I, Clayton PE. Clinical utility of insulin-like growth factor-I (IGF-I) and IGF binding protein-3 measurements in paediatric practice. Pediatr Endocrinol Rev. 2006;3:393–402. [PubMed] [Google Scholar]
- 15.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 16.Rogers I, Metcalfe C, Gunnell D, Emmett P, Dunger D, Holly J Avon Longitudinal Study of Parents Children Study T. Insulin-like growth factor-I and growth in height, leg length, and trunk length between ages 5 and 10 years. J Clin Endocrinol Metab. 2006;91:2514–9. doi: 10.1210/jc.2006-0388. [DOI] [PubMed] [Google Scholar]
- 17.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 18.Colombo JB, Naz RK. Modulation of insulin-like growth factor-1 in the seminal plasma of infertile men. J Androl. 1999;20:118–25. [PubMed] [Google Scholar]
- 19.Hoppe C, Udam TR, Lauritzen L, Molgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2. 5-y-old Danish children. Am J Clin Nutr. 2004;80:447–52. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 20.Rogers I, Emmett P, Gunnell D, Dunger D, Holly J, Tteam AS. Milk as a food for growth? The insulin-like growth factors link. Public Health Nutr. 2006;9:359–68. doi: 10.1079/phn2006853. [DOI] [PubMed] [Google Scholar]
- 21.Stang A, Ahrens W, Baumgardt-Elms C, Stegmaier C, Merzenich H, de Vrese M, Schrezenmeir J, Jockel KH. Adolescent milk fat and galactose consumption and testicular germ cell cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2189–95. doi: 10.1158/1055-9965.EPI-06-0372. [DOI] [PubMed] [Google Scholar]
- 22.Davies TW, Palmer CR, Ruja E, Lipscombe JM. Adolescent milk, dairy product and fruit consumption and testicular cancer. Br J Cancer. 1996;74:657–60. doi: 10.1038/bjc.1996.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlynn KA, Sakoda LC, Rubertone MV, Sesterhenn IA, Lyu C, Graubard BI, Erickson RL. Body size, dairy consumption, puberty, and risk of testicular germ cell tumors. Am J Epidemiol. 2007;165:355–63. doi: 10.1093/aje/kwk019. [DOI] [PubMed] [Google Scholar]
- 24.Moller H. Clues to the aetiology of testicular germ cell tumours from descriptive epidemiology. Eur Urol. 1993;23:8–13. doi: 10.1159/000474564. discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 25.Angell-Andersen E, Tretli S, Bjerknes R, Forsen T, Sorensen TI, Eriksson JG, Rasanen L, Grotmol T. The association between nutritional conditions during World War II and childhood anthropometric variables in the Nordic countries. Ann Hum Biol. 2004;31:342–55. doi: 10.1080/03014460410001685304. [DOI] [PubMed] [Google Scholar]
- 26.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–5. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.