SUMMARY
Urea cycle disorders often present as devastating metabolic conditions, resulting in high mortality and significant neuropsychological damage, despite treatment. The Urea Cycle Disorders Longitudinal Study is a natural history study that collects data from regular clinical follow-up and neuropsychological testing. This report examines links between biochemical markers (ammonia, glutamine, arginine, citrulline) and primary neuropsychological endpoints in 3 distal disorders, argininosuccinic acid synthetase deficiency (ASD or citrullinemia type I), argininosuccinic acid lyase deficiency (ASA or ALD) and arginase deficiency (ARGD). Laboratory results and test scores from neuropsychological evaluations were assessed in 145 study participants, ages 3 years and older, with ASD (n=64), ASA (n=65) and ARGD (n=16). Mean full scale IQ was below the population mean of 100 ± 15 for all groups: (ASD = 79 ± 24; ASA = 71 ± 21; ARGD = 65 ± 19). The greatest deficits were noted in visual performance and motor skills for all groups. While ammonia levels remain prominent as prognostic biomarkers, other biomarkers may be equally valuable as correlates of neuropsychological functioning. Cumulative exposure to the biomarkers included in the study proved to be highly sensitive indicators of neuropsychological outcomes, even when below the cut-off levels generally considered toxic. Blood levels of biomarkers obtained on the day of neuropsychological evaluations were not correlated with measures of functioning for any disorder in any domain. The importance of cumulative exposure supports early identification and confirms the need for well-controlled management of all biochemical abnormalities (and not just ammonia) that occur in urea cycle disorders.
Keywords: urea cycle disorders, cognitive outcomes, neuropsychological, argininosuccinic acid synthetase deficiency, argininosuccinic acid lyase deficiency, arginase deficiency
INTRODUCTION
The urea cycle is responsible for the detoxification of ammonia (NH4+) to urea. When enzymes responsible for the sequential steps in the urea cycle are absent or deficient, ammonia builds up in the brain and other tissues (Batshaw et al 1980; Kölker et al 2015). Ammonia accumulation or increases in glutamine (a consequence of hyperammonemia) lead to astrocyte swelling and subsequent damage. Depending on the age of onset and duration and severity of the hyperammonemic episode, brain effects are temporary or permanent and can be mild or devastating (Enns et al, 2008). Other mechanisms of injury may also operate. Treatment varies somewhat depending on the specific location of the enzyme block in the cycle. The basic elements of treatment are dietary protein restriction and ammonia scavenger drugs. Supplemental treatment with citrulline or arginine is required for most of the disorders. Genetic defects in the urea cycle are generally categorized into proximal (mitochondrial) or distal (cytosolic) disorders, plus several transport disorders, which are known by the specific enzyme deficiency or biochemical abnormality.
The Urea Cycle Disorders Consortium (UCDC) is part of the Rare Diseases Clinical Research Network (RDCRN) and is supported jointly by the National Center for Advancing Translational Science, Office of Rare Diseases Research and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD061221). The goals of the RDCRN are to perform natural history studies of rare diseases and to hasten the bringing to market of orphan products to treat rare disorders (Batshaw, et al 2014; Seminara, et al 2010). Philanthropic support supplements these research activities, as well. The UCDC Longitudinal Study, now in its 11th year, collects medical and neuropsychological information on the eight different urea cycle disorders. This paper focuses on three distal disorders, argininosuccinate synthetase deficiency (ASD or citrullinemia type I) (OMIM 215700), argininosuccinic acid lyase deficiency (ASA or ALD) (OMIM 207900) and arginase deficiency (ARGD) (OMM 207800), with the aim of characterizing the associations between biochemical markers (ammonia, glutamine, citrulline and arginine) and neuropsychological outcomes. A better understanding of these biochemical markers and their relationships to specific functional domains has the potential to guide treatment and contribute to the design of clinical drug trials.
METHODS
The Urea Cycle Disorders Longitudinal Study collects data from regular clinical follow-up as well as neuropsychological testing that in many centers would not have been part of routine care. The testing includes age appropriate measures of intelligence and global functioning, verbal abilities, visual performance, motor skills and memory (Table 1). For this paper, we included results from children 3 years of age and older since we focused on neuropsychological outcomes that may not be measurable in younger children. Although some participants (35.2%) were evaluated more than once, we conducted analyses including only the most recent testing results in order to capture long-term risks. Evaluations were performed at a mean of 5.17 ± 5.10 years (median=3.4, range = 0.1-23.6 years) after the last hyperammonemic episode. No one was evaluated while experiencing a hyperammonemic episode.
Table 1.
Neuropsychological domains, sources of data and variables
| DOMAIN | Source | Variables comprising the Domain |
|---|---|---|
| GLOBAL FUNCTIONING | Wechsler Preschool and Primary Scales of Intelligence, Third Edition (WPPSI-III) (Wechsler, 2002) or 4-Subtest Wechsler Abbreviated Intelligence Scale (WASI) (Wechsler, 1999 or 2011) | Full Scale IQ |
| Adaptive Behavior Assessment System, Second Edition (ABAS-II) (Harrison & Oakland, 2003) | General Adaptive Composite (GAC) | |
| Behavior Rating Inventory of Executive Function (BRIEF) | Global Executive Composite (GEC) | |
| Achenbach Child Behavior Checklist (CBCL) (Achenbach & Rescorla, 2000) | Total Problems Score | |
| VERBAL | WPPSI III or WASI | Verbal IQ |
| Developmental NEuroPSYchological Assessment, Second Edition (NEPSY-II) (Korkman, Kirk & Kemp, 2007) or Delas-Kaplan Executive Function System (D-KEFS) (Delis, Kaplan & Kramer, 2001) | Verbal Fluency | |
| ABAS-II Communication Scale | Communication | |
| VISUAL PERFORMANCE | WPPSI III or WASI | Block Design* |
| Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI) (Beery & Beery, 2010) | Visual Motor Integration | |
| MOTOR | Wide Range Assessment of Visual Motor Abilities (WRAVMA) (Adams & Sheslow, 1995) or Lafayette Grooved Pegboard | Pegboard Dominant Hand |
| WRAVMA or Lafayette Grooved Pegboard | Pegboard Non-Dominant Hand | |
| Dynamometer | Grip Strength Dominant Hand | |
| Dynamometer | Grip Strength Non-Dominant Hand | |
| MEMORY | California Verbal Learning Test (Child or Adult) (CVLT-C, CVLT) (Delis et al, 1994; 2001) | List A Trial 1 free recall List A Trial 5 Free Recall |
| Rey Osterreith Complex Figure (ROCF) (Meyers & Meyers, 1995) | ROCF Immediate Recall | |
| Behavior Rating Inventory of Executive Function (BRIEF) (Roth, Isquith and Gioia, 2005) | Working Memory Score |
This subtest also measures aspects of executive functioning.
Biomarkers related to metabolic status included measurements of ammonia, glutamine, arginine, and citrulline. All laboratories for this study were associated with the large medical centers affiliated with the Urea Cycle Consortium and were clinically certified. Certification included rigorous quality control procedures and proficiency testing. In all laboratories, the normal value of blood ammonia was consistently established at less than 50 μmol/L. The arbitrary cut-off of 100 μmol/L to define a hyperammonemic episode was established based on past experience within the Urea Cycle Consortium. Data on laboratory values were obtained from a review of medical records. Parameters related to the biochemical values included the Concurrent Level on day of neuropsychological testing and Mean Lifetime Exposure. If the participant had values that spanned 3 or more years, the mean within each year was first calculated. Then the mean and standard deviations were calculated from these values to obtain Mean Lifetime Exposure. If the participant had values that spanned less than 3 years, means and standard deviations were only calculated if the participant had 1 or more measurements. The Number of Hyperammonemic Episodes (defined as ammonia levels above 100 μmol/L) and the number of Ammonia “Spikes” (defined as an ammonia level greater than 10 μmol/L above the mean for that individual) were also calculated. The degree of dispersion in laboratory values around the mean value for the individual was labeled the Standard Deviation score. Cumulative Exposure was defined as the Lifetime Mean Exposure X Age up to and including the time of the neuropsychological evaluation. Older individuals thus have a higher Cumulative Exposure score. The Standard Deviation and Cumulative Exposure variables as defined above proved to be powerful predictors of outcome in a study of individuals with phenylketonuria (PKU) (Hood et al, 2015). These parameters were included in our analyses because of the possibility that variability and persistence of abnormally elevated levels of biochemical toxins have a similar effect in urea cycle disorders as they do in PKU. In our study, for the individuals who had received a liver transplant to prevent hyperammonemia, cumulative exposure to ammonia was calculated up to the time of the liver transplant (rather than age at testing).
Associations between biomarkers and performance on each of the neuropsychological tests were determined through Pearson correlation analyses for each disorder separately. Scores from the separate tests measuring the same or similar neuropsychological function were converted to z-scores with a mean of 0 and a standard deviation of 1 to create a combined mean “Domain” score. In order to simplify the tables, we report correlation coefficients (r) above r=0.30. Analyses were computed using SAS. Data from male and female participants were combined since no gender differences were noted in the biomarkers or neuropsychological test results.
RESULTS
The total sample included 145 individuals 3 years of age and older, with a mean age of 13.78 years. As shown in Table 2, there were 63 males and 82 females, with the majority diagnosed with ASD (n=64) or ASA (65) and 16 with ARG. In all three distal disorders, onset of symptoms occurred for the majority of babies after the newborn period, with the mean age of urea cycle diagnosis occurring at just under 2 years of age. In this sample 13 (20%) with ASD and 11 (17%) individuals with ASA received liver transplant. Newborn screening, which was instituted during the period of expanded newborn screening (2004 in most states) led to early identification of 25% of infants with ASD and 29% of infants with ASA but none with ARGD. In general, demographic characteristics and health history as well as age at participation in the study were similar for the ASD and ASA groups, while the ARGD group included fewer cases, was older at time of symptom onset, diagnosis, and neuropsychological testing, did not come to attention through newborn screening and did not undergo liver transplantation.
Table 2.
Description of the Sample (Demographics and Health History)
| ASD-Citrullinemia | ASA | ARGD | |
|---|---|---|---|
| Age at most recent evaluation (years) (n, mean ± SD, median, (range)) | n=64 11.43± 7.25 8.86 (3.20–32.56) |
n=65 14.87 ± 10.44 14.06 (3.04–45.60) |
n=16 18.74 ± 11.20 17.97 (3.46–45.53) |
| Sex | Male: n=29(45%) Female: n=35(55%) |
Male: n=29(45%) Female: n=36(55%) |
Male: n=5 (31%) Female: n=11 (69%) |
| Age at diagnosis (years) (n, mean ± SD, median, (range)) | n=64 1.13 ± 3.58 0 (0–21.00) |
n=65 1.82 ± 3.29 0 (0–14.00) |
n=16 4.5± 4.4 3.5 (0–13.00) |
| Neonatal/late onset | Neonatal: n=17 (27%) Late: n=47 (73%) |
Neonatal: n=15 (23%) Late: n=50 (77%) |
Neonatal: n=0 Late: n=16 (100%) |
| Liver transplant | No: n=51 (80%) Yes: n=13 (20%) |
No: n=54 (83%) Yes: n=11 (17%) |
No: n=16 (100%) Yes: n=0 |
| Method of Identification* | Clinical: 43 (67%) Family: 5 (8%) NBS: 16 (25%) |
Clinical: n=38 (59%) Family: n=8 (12%) NBS: n=19 (29%) |
Clinical: n=14 (88%) Family: n=2 (12%) NBS: n=0 |
| Age at time of evaluation for this study (years) | 3–5: 16 (25%) 6–16: 33 (52%) 17+: 15 (23%) |
3–5: 14 (22%) 6–16: 24 (37%) 17+: 27 (42 %) |
3–5: 2 (13%) 6–16: 3 (19%) 17+: 11 (69%) |
n=number of participants; SD = standard deviation;
Method of Identification: Clinical = identified because of clinical symptoms; Family = identified because of an affected family member; NBS = identified through newborn screening
Results from the most recent neuropsychological evaluation are presented in Table 3. Mean full scale IQ was in the range of intellectual disabilities (IQ < 71) for the ASA and ARGD groups and in the borderline range (IQ < 86) for the ASD group. The greatest deficits were noted in the visual performance and motor skills domains for all groups. Figure 1 illustrates the distribution of full scale IQ for each disorder.
Table 3.
Composite z-scores for neuropsychological domains and associated test scores (n, mean± standard deviation, median, (range)).
| ASD Citrullinemia | ASA | ARGD | |
|---|---|---|---|
| GLOBAL FUNCTIONING DOMAIN | 61 −0.75± 1.31 −0.33 (−3.67–2.60) |
64 −1.29± 1.24 −1.03 (−3.67–1.03) |
16 −1.37± 1.43 −0.97 (−3.33–0.69) |
| Full Scale IQ Standard score Normative mean = 100 ± 15 |
52 78.92± 24.06 77.5 (43.0–133.0) |
60 71.47± 21.33 69 (50–141) |
12 65.42± 18.79 60.5 (50.0–111.0) |
| ABAS GAC Standard score Normative mean= 100 ± 15 |
52 79.37± 23.55 83.5 (40.0–117.0) |
52 75.58± 21.75 73.5 (40.0–127.0) |
10 67.10± 26.11 57.5 (40.0–116.0) |
| BRIEF GEC Scaled score Normative mean = 50 ± 10 |
30 58.13± 10.52 58 (39–77) |
28 59.79± 10.92 61.5 (34.0–86.0) |
6 61.00± 16.53 56 (42–82) |
| CBCL Total Problems Scaled score Normative mean = 50± 15 |
42 54.9± 10.1 53.5 (32.0–76.0) |
31 53.29± 10.31 54.0 (36.0–72.0) |
10 60.60± 9.98 60 (45–73) |
| VERBAL DOMAIN | 59 −1.03± 1.37 −0.73 (−3.10–1.60) |
64 −1.55± 1.181.18 −1.57 (−3.00–1.97) |
16 −1.88± 1.19 −2.3 (−3.0–0.3) |
| Verbal IQ Standard score Normative mean = 100 ± 15 |
50 80.46± 23.24 80.5 (52.0–143.0) |
60 74.9± 19.77 71.5 (55.0–139.0) |
12 70.08± 18.74 61.5 (55.0–107.0) |
| Verbal Fluency Scaled score Normative mean= 10 ± 15 |
6 6.17± 3.31 5.5 (2.0–12.0) |
11 4.46± 2.91 4 (1–9) |
3 7.33± 1.53 7 (6–9) |
| ABAS Communication Normative scaled score mean= 10 ± 15 |
52 7.56± 4.46 9 (1–14) |
52 6.3± 4.08 6 (1–16) |
10 5.7± 4.62 4.5 (1.0–12.0) |
|
VISUAL MOTOR PERFORMANCE DOMAIN z-score |
48 −1.39± 1.35 −1.33 (−3.00–2.00) |
59 −1.91± 1.07 −2.33 (−3.00–2.67) |
14 −2.19± 1.10 −2.5 (−3.0–1.0) |
| Block Design Normative scaled score mean= 10 ± 15 |
48 5.83± 4.05 6 (1–16) |
59 4.25± 3.21 3 (1–18) |
14 3.43± 3.30 2.5 (1.0–13.0) |
| Beery VMI Normative standard score mean = 100 ± 15 |
49 80.02± 20.86 80 (45–123) |
36 72.72± 18.97 74.5 (45.0–124.0) |
6 61.83± 19.96 55 (45–88) |
|
MOTOR DOMAIN z-score |
23 −1.83± 2.13 −1.62 (−6.67–1.05) |
31 −2.60± 1.38 −2.42 (−5.70–0.27) |
9 −3.90± 1.37 −4.4 (−5.5– −1.4) |
| Grooved Pegboard (Dominant Hand) Normative standard score mean = 100 ± 15 |
22 69.72± 38.90 75.5 (0–125.0) |
26 51.81± 24.10 46.5 (20.0–109) |
8 32.25± 37.10 24.5 (0.0–117.0) |
| Grooved Pegboard (Non-dominant hand) Normative standard score mean = 100 ± 15 | 22 67.86± 36.73 71 (0–115) |
26 55.54± 28.18 62 (9–104) |
8 28.63± 37.04 18 (0–106) |
| Grip Strength (Dominant Hand) Normative standard score mean = 100 ± 15 | 21 80.38± 31.90 88 (0–121) |
28 67.43± 27.55 71.5 (11–111) |
9 49.44± 16.35 51 (24–72) |
| Grip Strength (Non-dominant hand) Normative standard score mean = 100 ± 15 | 22 78.96± 31.37 86.5 (0–119.0) |
28 69.71± 27.01 74 (3–102) |
9 49.56± 19.02 57 (10–71) |
|
MEMORY DOMAIN z-score |
32 −0.63± 1.29 −0.8 (−2.9–3.6) |
30 −1.61± 0.99 −1.34 (−4.00–0.07) |
6 −1.07± 1.11 −1.2 (−2.4–0.2) |
| CVLT List A Trial 1 z-score |
20 0±1.17 0 (−2.5–2.0) |
22 −1.02± 1.26 −0.75 (−3.50–1.00) |
1 1.50 |
| CVLT List A Trial 5 z-score |
20 −0.40± 1.49 −0.5 (−4.0–2.0) |
22 −2.09± 1.37 −2 (−5–0) |
1 −0.5 |
| BRIEF Working Memory Normative standard score mean=50 ± 10 |
31 61.13± 12.20 59 (36–84) |
28 64.38± 13.08 65 (38–89) |
6 60.17± 9.56 57 (48–74) |
| REY Osterreith Immediate recall Normative standard score mean=50 ± 10 | 14 43.50± 22.88 35.5 (19.0–86.0) |
10 34.8± 16.08 28.5 (20.0–68.0) |
1 20 |
n= number of cases; IQ=intelligence quotient; ABAS GAC=Adaptive Behavior Assessment System, General Adaptive Composite; BRIEF GEC = Behavior Rating Inventory of Executive Function, Global Executive Composite; CBCL=Child Behavior Checklist; VMI=Visual Motor Integration; CVLT=California Verbal Learning test
Figure 1.
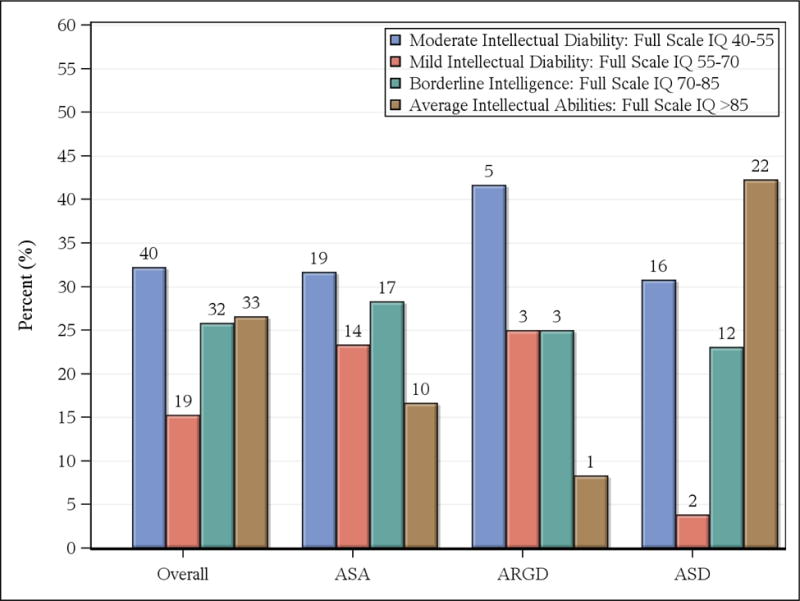
Distribution of Full Scale IQ for overall sample and for argininosuccinic acidemia (ASA), arginine deficiency (ARGD), and argininosuccinic lyase deficiency (ASD). (Numbers above bar lines indicate sample size)
Laboratory values are presented in Table 4. All groups included individuals with hyperammonemic episodes. Mean ammonia levels were lower on the day of neuropsychological testing (for the few who received blood tests on that day) than lifetime mean levels, which were above 100 μmol/L for the ASD and ASA groups and were also relatively elevated for the ARG group. Standard deviation levels, as a measure of variability, were greater than 100 μmol/L for ASD and ASA groups. And all groups included individuals who had “spikes” of ammonia greater than 10 μmol/L as compared to their mean scores.
Table 4.
Laboratory results for Biomarkers (Ammonia, Glutamine, Citrulline and Arginine (n, mean+ standard deviation, median, (range)),
| Biomarker | ASD Citrullinemia | ASA | ARGD |
|---|---|---|---|
|
Ammonia (Reference range: 28–80 umol/L) |
|||
| Number of hyperammonemic episodes | 63 5.6± 7.08 3 (1–32) |
63 4.14± 6.09 1 (1–27) |
15 3.93± 5.82 1 (1–22) |
| Concurrent level | 23 44.71± 43.49 30 (9–206) |
20 25.08± 17.28 22.5 (9.0–82.0) |
2 5.28, 67.0 36.14 (5.28–67.0) |
| Mean lifetime level | 62 127.22± 110.51 108.86 (6.30–539.25) |
59 139.59± 164.89 80 (1–882) |
16 78.87± 51.84 77.5 (5.28–169.33) |
| Standard deviation score* | 53 151.06± 168.03 101.22 (4.03–780.32) |
48 169.02± 251.57 83.76 (3.27–1164.06) |
12 46.46± 34.87 35.31 (5.80–108.93) |
| Number of “spikes”** | 63 4.56± 6.16 2 (0–30) |
60 3.47± 5.67 1 (0–25) |
16 2.38± 4.77 0.5 (0–19) |
|
Glutamine Reference range: 332-1084 umol/L |
|||
| Concurrent | 23 603.55± 1.95.49 606 (121–932) |
24 638.31± 198.80 626 (36–1020) |
3 515.00± 74.28 488 (458–599) |
| Mean | 63 687.16± 205.71 657.65 (347.00–1442.00) |
63 706.94± 222.85 682.36 (334.0–1997.0) |
16 632.71± 167.81 594.6 (374.5–1026.2) |
| Standard deviation score | 61 197.78± 174.11 163.54 (1.41–1223.86) |
55 231.45± 347.49 139.63 (24.04–2444.84) |
16 161.62± 77.86 146.54 (61.94–326.75) |
|
Citrulline Reference range: 2-50 umol/L |
|||
| Concurrent | 23 1804.48± 1284.18 2161.0 (86.4–4766.0) |
24 159.68± 51.15 156 (77–284) |
3 28.33± 5.03 29.0 (23–33) |
| Mean | 62 1857.97± 1141.31 1836.56 (71.31–4752.00) |
63 194.37± 98.06 168.68 (42.00–554.33) |
16 24.16± 5.43 23.44 (17.76–34.67) |
| Standard deviation score | 61 700.62± 810.50 508.00 (26.66–5106.23) |
54 92.36± 105.43 51.01 (6.01–566.31) |
16 6.16± 4.07 5.81 (0–15.82) |
|
Arginine Reference range: 16-149 umol/L |
|||
| Concurrent | 23 69.15± 50.90 54.7 (5.0–194.0) |
24 76.39± 45.89 64 (20–220) |
3 388.67± 85.19 362 (320–484) |
| Mean | 62 128.17± 185.30 101.94 (45.30–1507.33) |
63 107.60± 66.51 95.8 (26.6–444.0) |
16 371.16± 121.26 353.98 (181—535.33) |
| Standard deviation score | 61 96.29± 319.46 44.04 (2.92–2511.93) |
54 71.169± 69.61 55.46 (4.24–445.27) |
16 105.86± 64.13 113.38 (0–283.89) |
n=number of participants
Standard deviation score = the mean degree of dispersion in laboratory values around the mean value for the individual participant was labeled the standard deviation score.
Ammonia “spikes” = an ammonia level greater than 10 umol/L above the mean for the individual participant
Lifetime mean laboratory values were inter-correlated, with citrulline most closely associated with ammonia. In ASD, lifetime mean citrulline levels correlated with lifetime mean ammonia (r=0.42, p=0.0008). In ASA, lifetime mean citrulline correlated with mean ammonia (r=0.33, p=0.11) and mean arginine (r=0.34, p=0.007). In ARGD, lifetime mean citrulline correlated with mean ammonia (r=0.42, p=0.02).
Table 5 presents the associations between biomarkers and neuropsychological domains with correlation coefficients above 0.30. Cumulative exposure emerged as one of the most sensitive indicators of neuropsychological outcomes. Concurrent blood levels of the biomarkers examined were not correlated with measures of functioning for any disorder in any domain.
Table 5.
Associations between biomarkers and composite neuropsychological domain scores with correlation coefficients equal to or greater than +/− 0.30 for argininosuccinic acid synthetase deficiency (ASD) (n=60), argininosuccinic acid lyase deficiency (ASA) (n=62) and arginase deficiency (ARGD) (n=16)
| ASD (Citrullinemia) | ||
|---|---|---|
| Global Functioning (n=60) | Correlation | p-value |
| # hyperammonemic episodes | −0.32 | 0.011 |
| Ammonia mean | −0.40 | 0.002 |
| Ammonia cumulative exposure* | −0.65 | <0.0001 |
| # ammonia “spikes” | −0.33 | 0.011 |
| Glutamine cumulative exposure | −0.34 | 0.008 |
| Citrulline cumulative exposure | −038 | 0.003 |
| Verbal Composite (n=58) | ||
| Ammonia mean | −0.59 | <0.0001 |
| Ammonia cumulative exposure* | −0.55 | <0.0001 |
| Ammonia standard deviation | −0.46 | 0.0011 |
| Citrulline cumulative exposure | −0.35 | 0.008 |
| Memory Composite (n=31) | ||
| None of the biomarkers met criteria | ||
| Visual Performance (n=47) | ||
| # hyperammonemic episodes | −0.43 | 0.002 |
| Ammonia mean | −0.61 | <0.0001 |
| Ammonia cumulative exposure* | −0.62 | <0.0001 |
| Ammonia standard deviation | −0.44 | 0.006 |
| # ammonia “spikes” | −0.50 | 0.0003 |
| Citrulline mean exposure | −0.40 | 0.005 |
| Citrulline cumulative exposure | −0.43 | 0.002 |
| Motor Composite (n=23) | ||
| # hyperammonemic episodes | −0.59 | 0.004 |
| Ammonia cumulative exposure* | −0.68 | 0.0004 |
| # ammonia “spikes” | −0.59 | 0.003 |
| Citrulline mean | −0.62 | 0.002 |
| Citrulline cumulative exposure | −0.61 | 0.002 |
| ASA | ||
|---|---|---|
| Global Functioning (n=62) | Correlation (r) | p-value |
| # hyperammonemic episodes | −0.35 | 0.005 |
| Ammonia mean | −0.35 | 0.007 |
| Ammonia cumulative exposure* | −0.42 | 0.003 |
| Glutamine cumulative exposure | −0.40 | 0.001 |
| Citrulline Mean | −0.44 | 0.0003 |
| Citrulline cumulative exposure | −0.48 | <0.0001 |
| Verbal Composite (n=62) | ||
| # hyperammonemic episodes (n=62) | −0.40 | 0.001 |
| Ammonia mean | −0.44 | 0.0006 |
| Ammonia cumulative exposure* | −0.38 | 0.006 |
| Number of ammonia “spikes” | −0.39 | 0.002 |
| Glutamine cumulative exposure | −0.35 | 0.005 |
| Citrulline mean | −0.53 | <0.0001 |
| Citrulline cumulative exposure | −0.41 | 0.001 |
| Memory Composite (n=28) | ||
| None of the biomarkers met criteria | ||
| Visual Performance (n=57) | ||
| Ammonia mean | −0.37 | 0.007 |
| Ammonia cumulative exposure* | −0.35 | 0.0120 |
| Citrulline mean exposure | −0.42 | 0.001 |
| Citrulline cumulative exposure | −0.36 | 0.007 |
| Motor Composite (n=30) | ||
| Arginine cumulative exposure | −0.47 | 0.009 |
| Arginine standard deviation | −0.49 | 0.011 |
| ARGD | ||
|---|---|---|
| Global Functioning (n=16) | Correlation (r)) | p-value |
| Ammonia cumulative exposure* | −0.39 | 0.13 |
| Citrulline cumulative exposure | −0.32 | 0.23 |
| Arginine cumulative exposure | −0.36 | 0.17 |
| Verbal Composite (n=16) | ||
| # hyperammonemic episodes | −0.38 | 0.17 |
| Memory Composite (n=6) | ||
| Glutamine mean | −0.37 | 0.47 |
| Citrulline mean | −0.58 | 0.23 |
| Arginine mean | −0.41 | 0.43 |
| Arginine standard deviation | −0.63 | 0.26 |
| Visual Performance (n=14) | ||
| # hyperammonemic episodes | −0.38 | 0.20 |
| Motor Composite (n=9) | ||
| Ammonia mean | 0.38 | 0.35 |
| Ammonia standard deviation | 0.30 | 0.56 |
| # ammonia “spikes” | 0.61 | 0.11 |
| Citrulline mean | −0.30 | 0.43 |
| Citrulline standard deviation | −0.47 | 0.20 |
Cumulative exposure was defined as the Mean Exposure X Age up until and including the age at testing. In liver transplant cases, ammonia cumulative exposure was defined as the Mean Exposure X Age up until the time of liver transplant.
For ASD, cumulative exposure to ammonia and citrulline were the most reliable indicators of poorer functioning in every domain, suggesting that long-term exposure poses the greatest risks. Glutamine cumulative exposure was related to global functioning but not to specific functional domains. The total number of hyperammonemic episodes correlated with visual performance and motor abilities, but not with the verbal composite score. Arginine, which tends to be close to normal in ASD, was not associated with performance in any of the neuropsychological domains.
For ASA, the most sensitive biochemical markers for global functioning and verbal abilities were ammonia, glutamine and citrulline. All were in the expected direction, with lower laboratory values correlating with better scores on the neuropsychological tests. Scores in the visual performance and motor domains correlated with arginine levels, indicating that high cumulative arginine exposure and high variability were associated with poorer motor functioning scores. None of the biomarkers correlated with the composite memory score.
For ARGD, cumulative exposure to arginine, ammonia and citrulline correlated at least at a r= 0.30 level with global functioning. In other domains, exposure to elevated arginine and citrulline correlated with poorer functioning. Hyperammonemic episodes were associated with poorer scores on tests of verbal skills and visual performance, but paradoxically, they were associated with better performance on motor tasks.
DISCUSSION
This study provides natural history data related to demographics, metabolic biomarkers and neuropsychological outcomes in three distal urea cycle disorders. As expected, each disorder was characterized by unique biochemical profiles. With ASD, citrulline was significantly elevated while in ASA, ammonia-related biomarkers were most notable and arginine was conspicuous in ARGD. The biomarkers investigated here are not independent variables. Although clinical guidelines were developed over 15 years ago (Summar and Tuchman, 2001), treatment practices appear to vary considerably. Data from this study indicate that arginine and citrulline as well as ammonia and glutamine reflect a patient’s risk for adverse neuropsychological effects and may indicate a need for treatment modifications. Similarly, neuropsychological functioning domains are not independent variables. However, when considered alone and in relation to biochemical parameters, they provide information that may be useful in the selection of clinical outcome variables.
For ASD, poor performance in every domain was associated with cumulative exposure to citrulline as well as to cumulative exposure to ammonia. This is in contrast to a recent publication reporting that neuropsychological function in ASD did not correlate with episodes of hyperammonemia (Baruteau et al, 2017). This may be due to the fact that isolated episodes of hyperammonemia are not as deleterious as cumulative exposure to moderately high levels of ammonia or citrulline.
For ASA, ammonia clearly related to aspects of cognitive outcome, but so did glutamine. Others have suggested that elevated glutamine serves as a harbinger for sudden hyperammonemia (Lee et al, 2016). Our study suggests that glutamine can also be used as a marker for neuropsychological functioning. This is in accordance with Gunz et al (2013) who reviewed studies using magnetic resonance imaging in children with neonatal onset UCD’s. They identified abnormalities in the cerebral cortex, internal capsule, basal ganglia, thalami and brain stem in association with higher glutamine levels and poorer neurological outcomes. In addition, and not reported previously, our study showed that elevated mean lifetime and cumulative citrulline levels were highly correlated with intellectual functioning, verbal skills and visual performance. Furthermore, poor motor skills appeared to be affected by arginine, which was implicated when levels were low.
While each group experienced significant neuropsychological deficits, ARGD appeared to confer the greatest risk for low IQ and poor performance in every domain. There was preliminary evidence that variability in each of the biomarkers led to greater risks for neuropsychological deficits. The impact of elevated ammonia, even when below the cut-off for hyperammonemia (less than 100 μmol/L), should not be underestimated in this disorder. Moreover, elevations in arginine and the standard deviation of arginine levels tended to be associated with poorer functioning in the global and memory domains. On the other hand, the positive correlations between the standard deviation scores for citrulline, glutamine and ammonia on the verbal and memory domains and elevated ammonia on motor functioning are difficult to explain except by recognizing the possibility of spurious correlations derived from the small sample sizes.
This study is limited by small sample sizes as well as heterogeneity in severity of disease (as illustrated by variability in number of hyperammonemic episodes and age at diagnosis). Another shortcoming is the lack of consideration of treatment variables and other potential covariates, such as branched chain amino acids. Small sample sizes also led to a decision to report only correlations of 0.30 or greater. Methods that would have reduced the number of analyses by examining ratios for biochemical parameters or measures of global functioning were considered but would not have allowed for identification of individual biomarkers or specific neuropsychological domains as targets for evaluating clinical outcomes. Since the elapsed time following a hyperammonemic episode was not controlled for, a lack of correlation between concurrent ammonia levels or other biomarkers and scores on cognitive tests needs further investigation. Similarly, an alternative cut-off definition for the number of ammonia “spikes” might have been more informative and requires further study.
Despite the limitations inherent in natural history studies such as this one, (Shapiro et al, 2013), the following general findings from our study may be usefully taken into account when treating these urea cycle disorders and when selecting endpoints in clinical trials.
Metabolic biomarkers obtained at the time of neuropsychological testing do not correlate with performance.
Cumulative exposure to potentially toxic metabolites is closely associated with performance on cognitive and other neuropsychological tests. This may explain why early development in ASA and ARGD appears to be closer to that of typically developing children (Waisbren et al, 2016).
While ammonia levels remain prominent as prognostic biomarkers, other biomarkers may be equally valuable as correlates of neuropsychological functioning. Thus, the study confirms the need for well-controlled management of all biochemical abnormalities associated with the disorder and suggests that the broad biochemical profile should be assessed in clinical trials.
Verbal and visual performance composite scores may be important functional outcome measures. Individuals with ASA, ASD and ARG experienced greatest deficits in the motor skills domain. However, the biomarkers in this study were not as strongly associated with composite motor scores as with the verbal and visual performance composite scores. Memory, although also a particular deficit, was not strongly associated with biomarkers. The likely explanation for this is that early onset hyperammonemia causes irreversible damage to the brain in association with motor and memory functions, as was suggested by the extensive analysis of cases followed by the European IMD Consortium (Posset et al, 2016).
The relevance of the standard deviation score and ammonia spikes in ASD and ASL underscores the need for stability in metabolic control.
The findings of our study empirically substantiate the common sense conclusions that patients with any metabolic disorder do better when diagnosed sooner and remain under good metabolic control. Novel treatments for these urea cycle disorders are now being investigated at an impressive rate. The need for bringing these treatments to market could not be greater. This study provides insights into potential targets for therapies and suggests potential neuropsychological endpoints to determine efficacy in clinical trials for distal UCD’s.
SYNOPSIS.
This natural history study examines links between biochemical markers (ammonia, glutamine, citrulline and arginine) and neuropsychological test scores in three distal urea cycle disorders (argininosuccinate synthetase deficiency (ASD or citrullinemia type I), argininosuccinic acid lyase deficiency (ASA or ALD) and arginase deficiency (ARGD). Cumulative exposure is a sensitive indicator while concurrent blood levels of the biomarkers examined are not correlated with measures of functional outcomes in any disorder in any domain.
Acknowledgments
The authors gratefully acknowledge the support of the Urea Cycle Disorders Consortium (UCDC; U54HD061221), which is part of the Rare Diseases Clinical Research Network (RDCRN) supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Intellectual Developmental Disability Research Centers (IDDRC). The following foundations provided support, without which the neuropsychological follow-up of these patients would not have been possible: the O’Malley Foundation, the Rotenberg Family Fund, the Dietmar-Hopp Foundation, and the Kettering Fund. We thank all the members of the Urea Cycle Consortium, including physicians, coordinators, psychologists, other support staff and especially Jennifer Seminara. We are extremely grateful to the participants in this study who generously volunteered their time and provided us with invaluable information.
Financial Relationships:
The Urea Cycle Disorders Consortium (UCDC) is part of the Rare Diseases Clinical Research Network (RDCRN), and is supported jointly by the National Center for Advancing Translational Science’s Office of Rare Diseases Research and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD061221). The Urea Cycle Disorders Consortium is also supported by the O’Malley Foundation, the Rotenberg Family Fund, the Dietmar-Hopp Foundation, and the Kettering Fund. In addition, support for neuropsychological testing is provided by an NIH grant for Intellectual and Developmental Disability Research Centers (U54HD090257).
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsor.
Relevant financial activities outside the submitted work:
Susan Waisbren consults to Dimension Therapeutics.
Dr. Cederbaum consults to Aeglea Biotherapeutics.
Footnotes
COMPLIANCE WITH ETHICS GUIDELINES
CONFLICTS OF INTEREST
Susan Waisbren consults to Dimension Therapeutics.
Dr. Cederbaum consults to Aeglea Biotherapeutics.
David Cuthbertson, Peter Burgard, Robert McCarter and Amy Holbert have nothing to declare.
The Urea Cycle Disorders Consortium (UCDC) is part of the Rare Diseases Clinical Research Network (RDCRN), and is supported jointly by the National Center for Advancing Translational Science’s Office of Rare Diseases Research and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD061221). The Urea Cycle Disorders Consortium is also supported by the O’Malley Foundation, the Rotenberg Family Fund, the Dietmar-Hopp Foundation, and the Kettering Fund. In addition, support for neuropsychological testing is provided by an NIH grant for Intellectual and Developmental Disability Research Centers (U54HD090257).
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsor.
INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. Proof that informed consent was obtained is available upon request.
DETAILS OF THE CONTRIBUTIONS OF INDIVIDUAL AUTHORS
Susan Waisbren was responsible for overall conduct of the study, including design, data collection, statistical analyses, interpretation of results and manuscript preparation.
David Cuthbertson led the statistical design and analysis team for this study and contributed to interpretation of results and manuscript presentation.
Peter Burgard contributed to designing the study, collecting data, interpreting results and preparing the manuscript.
Amy Holbert contributed to designing the study, statistical analyses and manuscript preparation.
Robert McCarter contributed to the design of the study, statistical analyses, interpretation of results and manuscript preparation.
Members of the Urea Cycle Disorders Consortium contributed to designing the study, data collection, and critical review of the manuscript.
Stephen Cederbaum contributed to designing the study, data collection, data interpretation, and manuscript preparation.
Manuscript approval: All authors
Author who serves as guarantor: Susan Waisbren
INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). This study was approved at each of the study sites through local Institutional Review Boards or Reliance Agreements from Children’s National Health Center, Washington, DC. Written Informed consent was obtained from all participants in this study or from parents/guardians when appropriate.
References
- Achenbach T, Rescorla L. Child Behavior Checklist. Burlington, VT: ASEBA; 2000. [Google Scholar]
- Adams W, Sheslow D. Wide Range Assessment of Visual Motor Abilities. Bloomington, MD: NCS Pearson, Inc; 1995. [Google Scholar]
- Barends M, Pitt J, Morrissy S, Tzanakos N, Boneh A, Newborn Screening Laboratory Staff Biochemical and molecular characteristics of patients with organic acidaemias and urea cycle disorders identified through newborn screening. Mol Genet Metab. 2014 Sep-Oct;113(1–2):46–52. doi: 10.1016/j.ymgme.2014.07.003. Epub 2014 Jul 11. [DOI] [PubMed] [Google Scholar]
- Baruteau J, Jameson E, Morris AA, et al. Expanding the phenotype in argininosuccinic aciduria: need for new therapies. J Inherit Metab Dis. 2017 Mar 1; doi: 10.1007/s10545-017-0022-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batshaw ML, Brusilow SW. Treatment of hyperammonemic coma caused by inborn errors of urea synthesis. J Pediatr. 1980 Dec;97(6):893–900. doi: 10.1016/s0022-3476(80)80416-1. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Tuchman M, Summar M, Seminara J, Members of the Urea Cycle Disorders Consortium A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014 Sep-Oct;113(1–2):127–30. doi: 10.1016/j.ymgme.2014.08.001. Epub 2014 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration. Bloomington, MN: NCS Pearson, Inc; 2010. [Google Scholar]
- Brusilow SW, Horwich AL. Urea cycle enzymes. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic & Molecular Bases of Inherited Diseases. 2001. Chap 85. 8 ed. [Google Scholar]
- Burgard P, Kölker S, Haege G, Lindner M, Hoffmann GF. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders–review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis. 2016 Mar;39(2):219–29. doi: 10.1007/s10545-015-9901-1. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1994, 2000. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Enns GM. Neurologic damage and neurocognitive dysfunction in urea cycle disorders. Semin Pediatr Neurol. 2008 Sep;15(3):132–9. doi: 10.1016/j.spen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Gunz AC, Choong K, Potter M, Miller E. Magnetic resonance imaging findings and neurodevelopmental outcomes in neonates with urea-cycle defects. Int Med Case Rep J. 2013 Aug 19;6:41–8. doi: 10.2147/IMCRJ.S43513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PL, Oakland T. Adaptive Behavior Assessment System, Second Edition. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Hood A, Antenor-Dorsey JA, Rutlin J, et al. Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol Genet Metab. 2015 Jan;114(1):19–24. doi: 10.1016/j.ymgme.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY-II. San Antonio, TX: Psychological Corporation; 2007. [Google Scholar]
- Lafayette Grooved Pegboard, Model 32025, Lafayette Instrument Company, Lafayette, IN.
- Lafayette Hand Dynamometer, Model 78010, Lafayette Instrument Company, Lafayette, IN.
- Lee B, Diaz GA, Rhead W, et al. Gultamine and hyperammonemic crises in patients with urea cycle disorders. Mol Genet Metab. 2016;117(1):27–32. doi: 10.1016/j.ymgme.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Lutz, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- Posset R, Garcia-Cazorla A, Valayannopoulos V, et al. Age at disease onset and peak ammonium level rather than interventional variables predict the neurological outcome in urea cycle disorders. J Inherit Metab Dis. 2016 Sep;39(5):661–72. doi: 10.1007/s10545-016-9938-9. [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- Seminara J, Tuchman M, Krivitsky L, et al. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100(Suppl 1):S97–105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E, Bernstein J, Adams HR, et al. Neurocognitive clinical outcome assessments for inborn errors of metabolism and other rare conditions. Mol Genet Metab. 2016;118:659. doi: 10.1016/j.ymgme.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summar M, Tuchman M. Proceedings of a consensus conference for the management of patients with urea cycle disorders. J Pediatr. 2001 Jan;138(1 Suppl):S6–10. doi: 10.1067/mpd.2001.111831. [DOI] [PubMed] [Google Scholar]
- Waisbren SE, Gropman AL, Members of the Urea Cycle Disorders Consortium (UCDC) Batshaw ML. Improving long term outcomes in urea cycle disorders-report from the Urea Cycle Disorders Consortium. J Inherit Metab Dis. 2016;39:573–84. doi: 10.1007/s10545-016-9942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999, 2011. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Third. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]


