Abstract
Background parenchymal enhancement (BPE) is the degree to which normal breast tissue enhances on contrast‐enhanced magnetic resonance imaging (MRI). MRI‐density is a volumetric measure of breast density that is highly correlated with mammographic density, an established breast cancer risk factor. Endogenous estrogen concentrations are positively associated with postmenopausal breast cancer risk and BPE has been shown to be sensitive to hormonal exposures. The objective of our study was to examine the relationship between BPE and MRI‐density and serum hormone concentrations in postmenopausal women. This was a study of cancer‐free postmenopausal women undergoing contrast‐enhanced breast MRI (N = 118). At the time of MRI all women completed a self‐administered questionnaire and blood samples were collected for hormone analyses. Serum concentrations of estrone (E1), estradiol (E2) and bioavailable E2 were examined by category of BPE and MRI‐density. Compared to women with “minimal” BPE, those who had “marked” BPE had significantly higher serum concentrations of E1, E2 and bioavailable E2 (90% increase, p trend across all categories = 0.001; 150% increase, p trend = 0.001; and 158% increase, p trend = 0.001, respectively). These associations were only affected to a minor extent by adjustment for BMI and other variables. After adjustment for BMI, no significant associations between MRI‐density and serum E1, E2 and bioavailable E2 were observed. Serum estrogen concentrations were significantly positively associated with BPE. Our study provides further evidence of the hormone‐sensitive nature of BPE, indicating a potential role for BPE as an imaging marker of endogenous and exogenous hormonal exposures in the breast.
Keywords: MRI, background parenchymal enhancement, breast cancer, hormones
Short abstract
What's new?
Background parenchymal enhancement (BPE) on breast MRI is an emerging marker of breast cancer risk. Both MRI‐density, an established risk factor, and BPE are knwon to be sensitive to hormonal exposures, but the relationship between MRI‐density, BPE, and serum hormone levels remains unstudied. Here, no significant associations between MRI‐density and serum E1, E2 and bioavailable E2 were observed after adjustment for body mass index in postmenopausal women without cancer. Serum estrogen concentrations were, however, positively associated with BPE, providing further evidence of its hormone‐sensitive nature, and suggesting its potential role as an imaging marker of hormonal exposures in the breast.
Abbreviations
- BI‐RADS
Breast Imaging Reporting and Data System
- BMI
body mass index
- BPE
background parenchymal enhancement
- CI
confidence interval
- DCIS
ductal carcinoma in situ
- E1
estrone
- E2
estradiol
- FGT
fibroglandular tissue
- MHT
menopausal hormone therapy
- MPD
mammographic percent density
- MRI
magnetic resonance imaging
- MSK
Memorial Sloan Kettering Cancer Centre
- SERM
selective estrogen receptor modulator
- SHBG
sex hormone binding globulin
Introduction
Mammographic percent density (MPD) is a measure of the proportion of the normal breast occupied by fibroglandular tissue (FGT), seen as dense (white) areas on a mammogram. Studies have consistently demonstrated a strong relationship between MPD and risk of breast cancer.1 In a large meta‐analysis, an overall relative risk (RR) for incident breast cancer of 4.6 (95% confidence interval (CI) 3.6–5.9) was observed for women with ≥75% vs. <5% MPD.1 Current recommendations in the United States are for women with a lifetime risk of breast cancer of ≥20% to undergo screening with contrast‐enhanced breast magnetic resonance imaging (MRI) as well as mammography.2 As is the case for mammography, FGT and fat are the two primary components observed on breast MRI, which provides a volumetric assessment of FGT in the breast (MRI‐density). This measure has been shown to be highly correlated with MPD,3, 4, 5 and like MPD, has been associated with breast cancer risk.6
Contrast‐enhanced MRI uses a contrast agent, which is injected intravenously, to aid in the visualization of tumors which often generate a distinct pattern of contrast dispersal.7, 8 This pattern, termed enhancement, helps to identify suspicious regions of interest for biopsy or follow‐up. Normal FGT also enhances to varying degrees on contrast‐enhanced MRI and is called background parenchymal enhancement (BPE). Having a high amount of BPE has been associated with breast cancer risk,6, 9 independent of the amount of MRI‐density.6
Estrogen is central to the etiology of breast cancer and circulating estrogen levels are associated with postmenopausal breast cancer risk.10 MPD is known to be influenced by hormonal exposures including reduced MPD at menopause11 and with tamoxifen treatment.12, 13 An increase in MPD is seen with use of menopausal hormone therapy (MHT), and this is more pronounced with the use of combined estrogen and progestin therapy.14, 15, 16, 17 Studies examining the association between MPD and serum hormone concentrations have largely been null.18, 19, 20, 21, 22, 23, 24
Like MPD, both MRI‐density25, 26, 27 and BPE25–31 have been shown to be sensitive to hormonal exposures, but the relationship between MRI‐density, BPE and serum hormone levels has not been examined. The objective of our study was to examine the relationship between BPE and MRI‐density and serum hormone concentrations in a population of postmenopausal women, without cancer, undergoing breast MRI screening at Memorial Sloan Kettering Cancer Center (MSK).
Materials and Methods
Our study was reviewed and approved by the MSK Institutional Review Board, and written informed consent was obtained at the time of recruitment from all study participants.
Study population
Between August 2012 and March 2014, 505 women who had no prior history of any cancer (including DCIS, but excluding non‐melanoma skin cancer) as noted in their medical record were approached in the MRI clinic at MSK as part of a larger study of BPE and breast cancer risk factors. The majority of women screened at the clinic meet the criteria to be classified as high risk (i.e., ≥20% lifetime risk of breast cancer). Of the 451 women who volunteered to participate in the study, 16 women ultimately did not participate or were determined to be ineligible (insufficient proficiency in English: n = 2, or prior personal history of cancer not previously identified during medical record review: n = 14). An additional 14 women were excluded because their study questionnaire was incomplete (n = 2) or they were diagnosed with breast cancer within the 6 months following their breast MRI (n = 12). This left a study population of 421 women, 220 of whom were postmenopausal. Of these postmenopausal women, 159 (72%) consented to a blood draw for hormone analyses. For the purpose of the analysis reported here, 23 of these 159 women were excluded because they reported current use of MHT (n = 4) or of a selective estrogen receptor modulator (SERM) (tamoxifen n = 2, raloxifene n = 16) or aromatase inhibitor (n = 1). This left 136 postmenopausal women with hormone measures available for the current analysis (Fig. 1).
Figure 1.
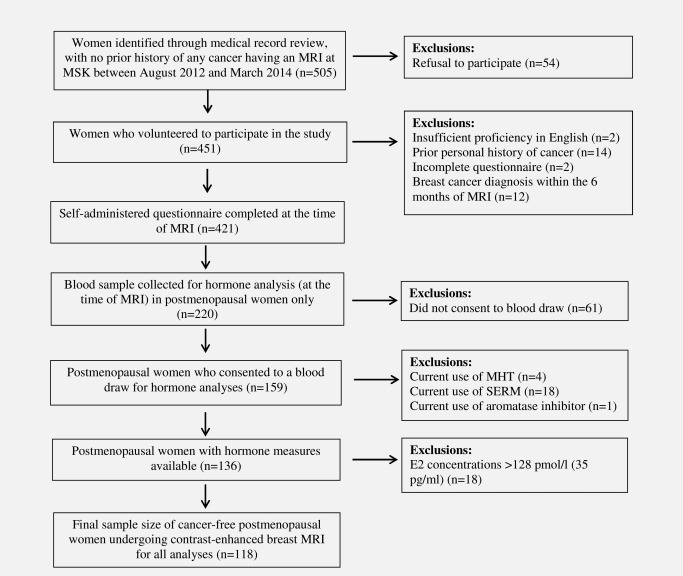
Summary of patient recruitment.
Data collection
At the time of MRI, all women completed a self‐administered questionnaire collecting information on established breast cancer risk factors, including: age at menarche, age at menopause, parity (number of full‐term pregnancies, age at first full‐term pregnancy, time since last pregnancy), use of hormonal medications at the time of MRI (i.e., MHT, tamoxifen, raloxifene, aromatase inhibitors), current weight and height, family history of breast cancer and history of oophorectomy. Medical records were reviewed to confirm questionnaire data as available. Women were considered to be postmenopausal at the time of MRI if they had not had a menstrual period in the preceding six months.
Biospecimen collection
Non‐fasting peripheral blood samples were collected for serum in red top Vacutainer™ collection tubes. Samples were collected at the time of MRI, just prior to contrast administration, and allowed to clot for 30 min at room temperature. Samples were then processed within 2 hr of collection, and aliquoted into barcoded cryotubes. All samples were stored at −20°C until the time of hormone analyses.
Contrast‐enhanced MRI and assessment of BPE and MRI‐density
Breast MRI examinations were performed using a dedicated surface breast coil with the patient in the prone position. Standard imaging protocols were followed and included a localizing sequence, followed by a T2‐weighted fat suppressed sequence, a T1‐weighted non‐fat saturated sequence, and a bilateral T1‐weighted simultaneous sagittal fat‐suppressed sequence performed before and three times after a rapid bolus injection of 0.1 mmol/l per kg of body weight of the contrast agent, gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals, Montville, NJ) delivered through an in‐dwelling IV catheter. The first post‐contrast MRI series were run following a 1 min delay and were run consecutively with each series taking approximately 2 min.
BPE was assessed using the T1‐weighted fat‐saturated sequence from the pre‐contrast and first post‐contrast series (the first post‐contrast series starts 1 min following the pre‐contrast series) and the subtraction image (subtraction of the pre‐contrast from the first post‐contrast images) in accordance with the recommendations of the proposed American College of Radiology Breast Imaging Reporting and Data System (BI‐RADS).32, 33 A single reader (JS) categorized BPE using the BI‐RADS four‐point scale as: minimal (<25% of FGT demonstrating enhancement); mild (25–50%); moderate (>50% to 75%) or marked (>75%).33 MRI‐density was classified using the BI‐RADS criteria of: predominantly fatty (<25% of the breast comprised of FGT); scattered (25–50%); heterogeneously dense (>50% to 75%) or extremely dense (>75%).34
Although BPE and MRI‐density are usually both very similar in both breasts,33 readings were conducted for both breasts and any potential discordance noted. In the single patient were there was discordance, the higher value was taken. Once all reads were complete, a random set of MRIs (n = 19) were re‐read for both BPE and MRI‐density to assess agreement between repeat reads. All these readings were made without knowledge of the serum hormone results.
Serum hormone analysis
Serum estrone (E1) and estradiol (E2) concentrations were quantified by validated radioimmunoassays (RIAs) after organic solvent extraction and Celite column partition chromatography.35, 36, 37 Chromatographic separation of the steroids was achieved by using different concentrations of toluene in isooctane and ethyl acetate in isooctane. A highly specific antibody was used in each RIA in conjunction with an iodinated tracer. Sex hormone binding globulin (SHBG) was quantified by use of a solid‐phase, two‐site, chemiluminescent immunometric assay using the Immulite analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). SHBG concentration was utilized in a validated algorithm with E2 to calculate bioavailable (i.e., non‐SHBG‐bound) E2. (Note: with this validated algorithm, free E2 is a simple proportion of bioavailable E2.)38, 39, 40 Quality control samples containing low, medium and high levels of the hormones and SHBG were included in each assay. The inter‐assay coefficients of variation ranged between 8% and 13%. The assay sensitivities for E1, E2 and SHBG were 15 pmol/l, 7.5 pmol/l and 1 nmol/l, respectively.
Statistical analysis
Women were excluded if they had E2 concentrations >128 pmol/l (35 pg/ml), indicating that they may not have been truly postmenopausal at the time of MRI/blood draw (n = 18).23 The final sample size for all analyses was 118 postmenopausal women (Fig. 1).
Prior to analysis, serum SHBG, E1, E2 and bioavailable E2 concentrations were transformed using a Box‐Cox transformation to achieve approximately normal distributions.41 This procedure finds the power transformation (λ) of the values that best approximates a normal distribution (note: λ = 0 is the log transformation). With λ values of 0.25, −0.25, −0.75 and −0.25 for SHBG, E1, E2 and bioavailable E2 respectively, the data for all serum measures were normally distributed based on the Shapiro–Wilk test for normality42 and visual inspection of histograms and QQ plots.
The mean concentrations and 95% confidence intervals (CI), of SHBG, E1, E2 and bioavailable E2 were calculated for each category of BPE and MRI‐density using the “means” statement in the glm procedure in the statistical analysis package program SAS 9.4 (SAS Institute, Inc., Cary, NC).
MRI‐density is a measure of the proportion of the breast occupied by FGT. Thus, for a fixed amount of FGT, MRI‐density decreases with increasing BMI,43 and for this reason, all analyses involving MRI‐density included an adjustment for BMI. In BPE analyses all values were compared to the “minimal” BPE category using ANOVA and adjusting for multiple comparisons using the Dunnett's t‐test. Similarly, in MRI‐density analyses all values were compared to the “predominantly fatty” category. Statistical significance for a linear trend across categories of BPE and MRI‐density were calculated using the “contrast” statement in the glm procedure. Variables that were considered as potential confounders included age at MRI, BMI (BPE analysis only), race/ethnicity, age at menarche, parity, BRCA mutation status, oophorectomy, first degree family history of breast cancer and alcohol consumption.
Cohen's kappa coefficients were used to determine the concordance between repeat BPE and MRI‐density reads by the study radiologist. All statistical analyses were conducted using SAS 9.4.
Results
Characteristics of the study population are shown in Table 1. The median age at MRI was 57 years and 91% of study participants were White/Caucasian. The majority of women (92%) were undergoing breast MRI for high‐risk breast cancer screening. Other reasons for MRI included an abnormal screening mammogram and/or the presence of a lump in the breast (Table 1). None of these women were diagnosed with breast cancer at the time of MRI or in the following 6 months. Most women (72%) had a first degree family history of breast cancer (mother or daughter), and about a fifth were known BRCA1 or BRCA2 mutation carriers. The majority of women (55%) had “minimal” BPE and 54% had “heterogeneously dense” breasts. There was no significant association between BPE and MRI‐density (Spearman rank correlation coefficient r = −0.08, p = 0.40) (Table 2). Weighted Cohen's kappa coefficients for BPE and MRI‐density repeat reads were 0.93 (95% CI 0.80, 1.00) and 0.94 (95% CI 0.83, 1.00) respectively, i.e., “almost perfect agreement.”
Table 1.
Characteristics of the study population N = 118
| Patient characteristics | |
|---|---|
| Age at MRI (years) | |
| Median (IQR) | 57.0 (54.4, 61.8) |
| Reason for MRI, N (%) a | |
| Abnormal screening mammogram | 19 (16.1) |
| Lump in breast | 5 (4.2) |
| High‐risk breast cancer screening | 109 (92.4) |
| Other | 8 (6.8) |
| Race/ethnicity, N (%) | |
| White/Caucasian | 107 (90.7) |
| Black/African American | 7 (5.9) |
| Asian | 2 (1.7) |
| Other | 2 (1.7) |
| Body mass index (BMI, kg/m b ) | |
| Median (IQR) | 24.3 (22.0, 28.1) |
| <25 | 70 (59.3) |
| 25 to <30 | 23 (19.5) |
| ≥30 | 25 (21.2) |
| Age at menarche (years) | |
| Median (IQR) | 12.3 (12.0, 13.0) |
| Parity, N (%) | |
| Nulliparous | 32 (27.1) |
| Parous | 86 (72.9) |
| First degree family history of breast cancer, N (%) | |
| No | 33 (28.0) |
| Yes | 84 (71.2) |
| Unknown | 1 (0.8) |
| BRCA mutation status, N (%) b | |
| Negative | 22 (18.6) |
| Positive | 25 (21.2) |
| Unknown | 71 (60.2) |
| Bilateral oophorectomy, N (%) | |
| No | 78 (66.1) |
| Yes | 40 (33.9) |
| Number of drinks per week, N (%) | |
| None | 28 (23.7) |
| <7 | 73 (61.9) |
| ≥7 | 17 (14.4) |
| Smoking status at MRI, N (%) | |
| No | 116 (98.3) |
| Yes | 2 (1.7) |
Abbreviations IQR: inter‐quartile range.
Women were asked to indicate all that apply. “Other” category includes women with a personal history of atypical hyperplasia or LCIS.
BRCA positive women includes those who were BRCA1 positive (n = 14), BRCA2 positive (n = 10) and a single carrier with a BRCA1 variant of unknown significance.
Table 2.
Distribution of MRI measurements (N = 118)
| Background parenchymal enhancement (BPE) | ||||||
|---|---|---|---|---|---|---|
| Minimal | Mild | Moderate | Marked | Total, N (%) | ||
| MRI‐density | Fatty | 12 | 5 | 1 | 0 | 18 (15.3) |
| Scattered | 11 | 15 | 3 | 1 | 30 (25.4) | |
| Heterogeneous | 37 | 22 | 3 | 2 | 64 (54.2) | |
| Dense | 5 | 0 | 0 | 1 | 6 (5.1) | |
| Total, N (%) | 65 (55.1) | 42 (35.6) | 7 (5.9) | 4 (3.4) | 118 | |
Spearman rank correlation coefficient: r = −0.08, p = 0.40.
Forty women reported having had a bilateral oophorectomy (Table 1). Median age, BMI and serum E2 and E1 concentrations did not differ by oophorectomy status (results not shown). An additional 11 women reported having had a simple hysterectomy. These women ranged in age from 44 to 64 years. Though for some of these women their menopausal status could be considered ambiguous, all had E2 levels lower than the cut‐off for inclusion (i.e., 128 pmol/l), and all had higher concentrations of E1 than E2. This indicates that these women were postmenopausal at the time of MRI/blood draw, and all were retained in the analysis. Furthermore, when analyses were repeated using a more stringent E2 cut‐point (i.e., 92 pmol/l or 25 pg/ml), only one additional woman was excluded and the study results were unchanged.
Though exclusions for MHT use at the time of MRI were made (n = 4, see Fig. 1), it is possible that women could have recently used MHT. Of the women included in the current analysis, 16 reported ever use of MHT or evidence of MHT use was found in the electronic medical record (EMR). Of these women, two had ambiguous data on when MHT use stopped. Another two women reported that they stopped using MHT in the same year of the MRI. For the remaining women the average number of years between stopping MHT and MRI was 8.6 years (range: 1–13). Given this information no further exclusion for MHT use were made.
Figure 2 shows the unadjusted mean concentrations and 95% CIs of serum hormones by category of BPE. There was no association between BPE and serum SHBG. Significant positive associations were seen between BPE and serum E1, E2 and bioavailable E2. Compared to women with “minimal” BPE, women who had “marked” BPE had significantly higher serum concentrations of E1 (90% increase, p trend across all categories = 0.001), E2 (150% increase, p trend = 0.001) and bioavailable E2 (158% increase, p trend = 0.001). As expected, BMI was positively associated with E1, E2 and bioavailable E2 concentrations. There was no significant association between BPE and BMI in this data. Still, including BMI in the model strengthened the association between serum estrogens and BPE somewhat leading to a 138% increase in E1 from minimal to marked enhancement (p trend = 0.001), a 239% increase for E2 (p trend = 0.001) and a 193% increase for bioavailable E2 (p trend = 0.001). The relationship between BPE and serum hormones did not vary appreciably following adjustment for age at MRI, family history of breast cancer or BRCA mutation status. Furthermore, results did not differ in analyses restricted to Caucasians, the only group with appreciable numbers (results not shown).
Figure 2.
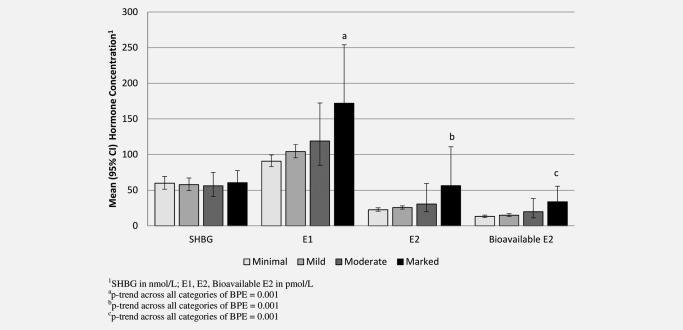
Association between serum hormone concentrations and degree of BPE in postmenopausal women.
Figure 3 shows the corresponding results for MRI‐density (including an adjustment for BMI). There was a positive association between MRI‐density and serum SHBG: compared to women with “predominantly fatty” breasts, those with “dense” breasts had 67% higher serum concentrations of SHBG (p trend = 0.011). As MRI‐density increased, serum concentrations of E1 E2 and bioavailable E2 tended to decrease (Fig. 3). However, no statistically significant relationships were observed (p trend = 0.96, p trend = 0.42 and p trend = 0.07, respectively). After further adjustment for SHBG, the relationship between MRI‐density and bioavailable E2 was further attenuated (p trend = 0.31).
Figure 3.
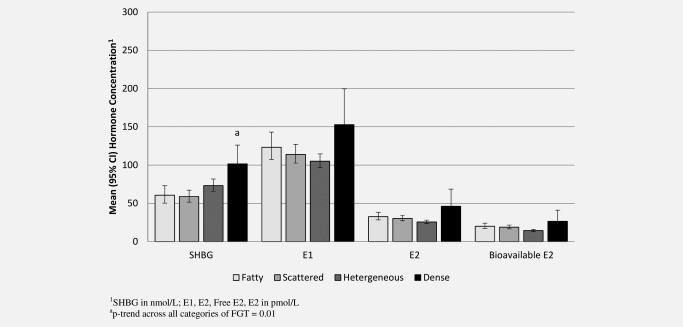
Association between serum hormone concentrations and degree of MRI‐density in postmenopausal women, adjusted for BMI.
Discussion
BPE and MRI‐density are characteristics of normal breast tissue that are routinely assessed by radiologists from standard contrast‐enhanced MRI. Both measures have been shown to be influenced by hormonal exposures. This is the first study to examine the relationship between BPE and MRI‐density and serum hormone concentrations. We found a significant positive association between BPE and serum concentrations of E1, E2 and bioavailable E2. This significant positive association persisted following adjustment for multiple factors including BMI.
We6 and others9 have found BPE to be positively associated with breast cancer risk. BPE has also been shown to decrease with menopause,25, 28 oophorectomy29 and in response to tamoxifen26, 30 or aromatase inhibitor27, 31 treatment. It is hypothesized that BPE may reflect the proliferative activity of the breast, influenced by both endogenous and exogenous hormonal exposures, and that it is not just how much breast tissue you have (i.e., MPD or MRI‐density) but how biologically active that tissue is that determines breast cancer risk.
We found no relationship between MRI‐density and serum concentrations of E1, E2 or bioavailable E2. Results from studies of the association between serum estrogens and MPD have been mixed, but have largely shown no association.18, 19, 20, 21, 22, 23, 24 A number of these studies initially found an inverse association between MPD and serum E2, but in all 18, 20, 23 but one of these44 the association disappeared after adjustment for BMI. The influence of MHT use on MPD has also been shown to be largely restricted to women taking combined estrogen plus progestin therapy, rather than estrogen alone.14, 15, 16, 17 This provides further support for the results of our study and others, which have not found a strong relationship between MPD and circulating estrogen concentrations in postmenopausal women.
Finally, we found a positive association between MRI‐density and SHBG. Though most studies have also found positive associations between MPD and SHBG,18, 19, 21, 23 the biological basis of this finding is unclear. It has been suggested by others that it may be due to residual confounding by adiposity.19, 20
Our study has a number of strengths. First, we were able obtain blood samples immediately prior to MRI, to ensure that serum hormone concentrations reflected the hormonal environment at the time of MRI. Furthermore, information on BMI, use of hormonal medications and other breast cancer risk factors were also collected at the time of MRI allowing these variables to be taken into account in terms of participant eligibility and/or analysis. A further strength of the study was that we were able to exclude women who had a cancer diagnosis within the six months following MRI to ensure that we were working with a cancer‐free study population. This limits the potential effect of the presence of a tumor on MRI measurements and serum hormone concentrations. We also examined the relationship between serum androgens (androstenedione, total and bioavailable testosterone) but no difference in serum concentration was observed across any category of BPE or MRI‐density. For simplicity these results have not been presented.
BPE is a promising new marker of breast cancer risk that appears to be independent of breast density, one of the strongest known risk factors for breast cancer. This is the first study to examine the relationship between serum hormone concentrations and BPE and MRI‐density, and as such requires replication. The hormonally responsive nature of BPE, supported by this, and prior studies, suggests that BPE could be a novel imaging marker of risk used to further stratify high‐risk women undergoing breast cancer screening with MRI.
Along with enhanced screening with MRI and mammogram, high‐risk women are also targeted for preventive measures including prophylactic surgeries (e.g., mastectomy, oophorectomy) and chemoprevention (e.g., tamoxifen), both of which can significantly impact quality of life. Efforts are underway to improve breast cancer risk stratification to inform population‐based programs of breast cancer screening and preventive intervention. It is thought that BPE could be used to further optimize risk stratification, supporting efforts to further individualize recommendations for breast cancer screening and prevention, such that women at the highest risk of disease can be targeted for more aggressive interventions and those at lower risk, who will benefit less from the intervention, will not have to undergo an unnecessary procedure or experience the associated side‐effects.
As a new imaging marker of risk we are still working to understand the role BPE might play in breast cancer risk and the relationship to hormonal exposures. If our results are confirmed, future studies should address the impact of including BPE in risk prediction algorithms but should also address the potential of BPE in assessing the impact of other hormonal exposures on the breast. This could include using BPE as an imaging marker of hormonal treatment response in women with breast cancer, and perhaps for use in the identification of novel chemotherapeutic agents.
Acknowledgements
We would like to extend special thanks to the study participants for volunteering for our study.
Conflict of interest: There are no conflicts of interest to disclose
References
- 1. McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta‐analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159–69. [DOI] [PubMed] [Google Scholar]
- 2. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89. [DOI] [PubMed] [Google Scholar]
- 3. Thompson D, Leach M, Kwan‐Lim G, et al. Assessing the usefulness of a novel MRI‐based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: the UK MARIBS study. Breast Cancer Res 2009;11:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klifa C, Carballido‐Gamio J, Wilmes L, et al. Magnetic resonance imaging for secondary assessment of breast density in a high‐risk cohort. Magn Reson Imaging 2010;28:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee NA, Rusinek H, Weinreb J, et al. Fatty and fibroglandular tissue volumes in the breasts of women 20–83 years old: comparison of X‐ray mammography and computer‐assisted MR imaging. Am J Roentgenol 1997;168:501–6. [DOI] [PubMed] [Google Scholar]
- 6. King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin of N Am 2007;45:863–80. [DOI] [PubMed] [Google Scholar]
- 8. Turnbull LW. Dynamic contrast‐enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed 2009;22:28–39. [DOI] [PubMed] [Google Scholar]
- 9. Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 2015;276:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Endogenous Hormones and Breast Cancer Collaborative Group . Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606–16. [DOI] [PubMed] [Google Scholar]
- 11. Boyd NF, Lockwood GA, Byng JW, et al. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998;10:1133–1144. [PubMed] [Google Scholar]
- 12. Nyante SJ, Sherman ME, Pfeiffer RM, et al. Longitudinal change in mammographic density among ER‐positive breast cancer patients using tamoxifen. Cancer Epidemiol Biomarkers Prev 2016;25:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuzick J, Warwick J, Pinney E, et al. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 2004;96:621–8. [DOI] [PubMed] [Google Scholar]
- 14. Stomper PC, Voorhis BJV, Ravnikar VA, et al. Mammographic changes associated with postmenopausal hormone replacement therapy: a longitudinal study. Radiology 1990;174:487–90. [DOI] [PubMed] [Google Scholar]
- 15. Greendale GA, Reboussin BA, Slone S, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst 2003;95:30–7. [DOI] [PubMed] [Google Scholar]
- 16. Laya MB, Gallagher JC, Schreiman JS, et al. Effect of postmenopausal hormonal replacement therapy on mammographic density and parenchymal pattern. Radiology 1995;196:433–7. [DOI] [PubMed] [Google Scholar]
- 17. Colacurci N, Fornaro F, De Franciscis P, et al. Effects of different types of hormone replacement therapy on mammographic density. Maturitas 2001;40:159–64. [DOI] [PubMed] [Google Scholar]
- 18. Boyd NF, Stone J, Martin LJ, et al. The association of breast mitogens with mammographic densities. Br J Cancer 2002;87:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bremnes Y, Ursin G, Bjurstam N, et al. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer 2007;121:2506–11. [DOI] [PubMed] [Google Scholar]
- 20. Verheus M, Peeters P, van Noord P, et al. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect‐EPIC cohort. Breast Cancer Res 2007;9:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warren R, Skinner J, Sala E, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2006;15:1502–8. [DOI] [PubMed] [Google Scholar]
- 22. Woolcott C, Courneya K, Boyd N, et al. Association between sex hormones, glucose homeostasis, adipokines, and inflammatory markers and mammographic density among postmenopausal women. Breast Cancer Res Treat 2013;139:255–65. [DOI] [PubMed] [Google Scholar]
- 23. Sprague B, Trentham‐Dietz A, Gangnon R, et al. Circulating sex hormones and mammographic breast density among postmenopausal women. Horm Cancer 2011;2:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamimi RM, Hankinson SE, Colditz GA, et al. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2005;14:2641–7. [DOI] [PubMed] [Google Scholar]
- 25. King V, Gu Y, Kaplan J, et al. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 2012;22:2641–7. [DOI] [PubMed] [Google Scholar]
- 26. King V, Kaplan J, Pike MC, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement and cysts on breast magnetic resonance imaging. Breast J 2012;18:527–34. [DOI] [PubMed] [Google Scholar]
- 27. King V, Goldfarb S, Brooks JD, et al. Impact of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue on breast MRI. Radiology 2012;264:670–8. [DOI] [PubMed] [Google Scholar]
- 28. Scaranelo A, Carrillo M, Fleming R, et al. Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology 2013;267:692–700. [DOI] [PubMed] [Google Scholar]
- 29. Price ER, Brooks JD, Watson EJ, et al. The impact of bilateral salpingo‐oophorectomy on breast MRI background parenchymal enhancement and fibroglandular tissue. Eur Radiol 2014;24:162–8. [DOI] [PubMed] [Google Scholar]
- 30. Schrading S, Schild H, Kühr M, et al. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast‐enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology 2014;271:45–55. [DOI] [PubMed] [Google Scholar]
- 31. Mousa N, Eiada R, Crystal P, et al. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause 2012;19:420–5. [DOI] [PubMed] [Google Scholar]
- 32. Morris EA, Comstock CE, Lee CH, et al. ACR BI‐RADS Magnetic Resonance Imaging. In ACR BI‐RADS Atlas, Breast Imaging and Reporting System. Reston VA: American College of Radiology, 2013. [Google Scholar]
- 33. Morris EA. Diagnostic breast MR imaging: current status and future directions. Magn Reson Imaging Clin N Am 2010;18:57–74. [DOI] [PubMed] [Google Scholar]
- 34. Sickles E, D'orsi C, Bassett L, et al. Mammography. Reston, VA: American College of Radiology, 2013. [Google Scholar]
- 35. Goebelsmann U, Bernstein G, Gale J, et al. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy In: Lepow I, Crozier R, eds. Vasectomy: immunologic and pathophysiologic effects in animals and man. New York: Academic Press, 1979. 165. [Google Scholar]
- 36. Goebelsmann U, Horton R, Mestman J, et al. Male pseudohermaphroditism due to testicular 17‐hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 1973;36:867–79. [DOI] [PubMed] [Google Scholar]
- 37. Probst‐Hensch N, Ingles S, Diep A, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer 1999;6:165–73. [DOI] [PubMed] [Google Scholar]
- 38. Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol‐17beta to human plasma protein at body temperature. J Steroid Biochem Mol Biol 1982;16:801–10. [DOI] [PubMed] [Google Scholar]
- 39. Vermeulen A, Verdonck L, Kaufman J. A critical evaluation of simple methods for estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. [DOI] [PubMed] [Google Scholar]
- 40. Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determina‐tions in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11:1065–107. [PubMed] [Google Scholar]
- 41. Box G, Cox D. An analysis of transformations. J R Stat Soc 1964;26:211–52. [Google Scholar]
- 42. Shapiro S, Wilk M. An analysis of variance test for normality (complete samples). Biometrika 1965;52:591–611. [Google Scholar]
- 43. Boyd NF, Dite GS, Stone J, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 2002;347:886–94. [DOI] [PubMed] [Google Scholar]
- 44. Aiello EJ, Tworoger SS, Yasui Y, et al. Associations among circulating sex hormones, insulin‐like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2005;14:1411–7. [DOI] [PubMed] [Google Scholar]


