SUMMARY
Streptococcus mutans and Candida albicans are frequently co-isolated from dental plaque of children with early childhood caries (ECC), and only rarely found in non-ECC children, suggesting that these species interact in a manner that contribute to the pathogenesis of ECC. Previous studies have demonstrated that glucans produced by S. mutans are crucial for promoting biofilm formation and cariogenicity with C. albicans; however, it is unclear how non-glucan S. mutans biofilm factors contribute to increased biofilm formation in the presence of C. albicans. In this study, we examined the role of S. mutans antigen I/II in two-species biofilms with C. albicans, and determined that antigen I/II is important for the incorporation of C. albicans into the two-species biofilm, and is also required for increased acid production. The interaction is independent of Als1 and Als3 proteins, which are known streptococcal receptors of C. albicans. Moreover, antigen I/II is required for the colonization of both S. mutans and C. albicans during co-infection of Drosophila melanogaster in vivo. Taken together, these results demonstrate that antigen I/II mediates the increase of C. albicans numbers and acid production in the two-species biofilm, which represents new activities associated with this known S. mutans adhesin.
Keywords: Streptococcus mutans, Candida albicans, Antigen I/II, Early childhood caries
INTRODUCTION
Early childhood caries (ECC) remains one of the most common childhood diseases affecting underprivileged children in developed countries 1,2. Streptococcus mutans is one of the most important etiologic agents for ECC 3,4. S. mutans thrives in a cariogenic biofilm with multiple species of bacteria and fungi. The commensal fungus Candida albicans is frequently detected in high numbers with S. mutans recovered from ECC-affected children, but only sporadically from ECC-free children5–7. C. albicans is a common colonizer of the oral cavity and an opportunistic pathogen. Previous studies demonstrate that cross-kingdom interactions between C. albicans and S. mutans enhance co-species biofilms that promote caries development 8,9.
Streptococcus-derived glucosyltransferases (Gtfs) bind to C. albicans in an enzymatically active form, thereby facilitating the promotion of the two-species biofilm10,11 in a sucrose-dependent manner10,11. Although much is known about the contribution of exopolysaccharides from S. mutans and other streptococci in two-species biofilm development with C. albicans, the role of other S. mutans biofilm related factors is poorly understood.
The antigen I/II family of adhesins are cell wall-associated polypeptides that are widely distributed on the cell surface of many streptococci12. Antigen I/II is not only important for initial streptococcal adhesion to the host, but also for inter-bacterial adhesion and “secondary” colonization 12. Antigen I/II of S. gordonii has been shown to mediate co-aggregation with Agglutinin-like family (Als1 and Als3) proteins on C. albicans 13. However, the role of antigen I/II in the interaction between C. albicans and S. mutans under sucrose-dependent conditions is unknown. Given the fact that high sugar consumption is an important risk factor for the development of ECC, our goal was to determine how antigen I/II modulates the interaction between C. albicans and S. mutans in the presence of sucrose.
In this study, we demonstrate that S. mutans antigen I/II facilitates the attachment of C. albicans to S. mutans in the two-species biofilm and concurrently promotes acid production. This interaction is independent of known antigen I/II receptors, C. albicans Als1 and Als3 proteins. Moreover, antigen I/II is required for colonization of C. albicans during co-infection with S. mutans in a Drosophila melanogaster sucrose-dependent feeding model. Taken together, these results suggest that antigen I/II is critical for the interaction of S. mutans with C. albicans under sucrose conditions, which may contribute to heightened pathogenicity during co-infection with these two microbes.
METHODS
Bacterial strains and culture conditions
S. mutans UA159 and C. albicans strains used in this study (Table 1) were grown in Todd-Hewitt broth (THB) and yeast extract-peptone-dextrose plus uridine (YPDU) (2% dextrose, 2% bacto peptone, 1% yeast extract, and 80 mg/l uridine), respectively, in an atmosphere of 5% CO2 at 37°C.
Table 1.
Strains and plasmids used in this study
| Strain | Description | Source |
|---|---|---|
| UA159 | Wild-type Streptococcus mutans | 15 |
| ΔspaP | S. mutans antigen I/II mutant, ErmR | This study |
| ΔspaP::ΔspaP | Antigen I/II complemented, KanR, ErmR | This study |
| S. mutans-Gfp | Gfp-labeled S. mutans, KanR | 15,45 |
| ΔspaP-Gfp | Gfp-labeled UA159 antigen I/II mutant | This study |
| SC5314 | Wild-type Candida albicans | 46 |
| Δals1 | C. albicans als1 mutant | 47 |
| Δals3 | C. albicans als3 mutant | 47 |
| Δals1:Δals3 | C. albicans als1and als3 double mutant | 47 |
| Plasmids | ||
| pVPT | E.coli-Streptococci shuttle vetor, KanR | 48 |
Construction and complementation of the spaP mutant
S. mutans’ antigen I/II is encoded by spaP 14. PCR ligation mutagenesis was used to construct the spaP mutant in S. mutans UA159 or UA159-GFP 15,16. Primer pairs (Table 2) were used to amplify two flanking regions of spaP, and overlapping PCR was used to ligate an IFDC2 cassette into the flanking fragments17. The resulting PCR product was transformed into S. mutans UA159 or UA159-GFP. The spaP mutant transformants were selected on 10μg/ml erythromycin THB agar. To complement the spaP mutant, the full-length spaP gene was cloned into a shuttle vector pVPT-kan to produce spaP-pVPT-kan. A complemented strain was obtained by transforming this plasmid into the spaP mutant and selected on 1mg/mL kanamycin THB agar plates.
Table 2.
Primers used in this study
| spaP Wf-up | ACGGACAGGACGATATTATTTGGTA |
| spaP Wr-up | GAGTGTTATTGTTGCTCGGCATAAATCCTCCAAATCTGAATAAATC |
| spaP Wf-Down | GGTATACTACTGACAGCTTCTGACAGCATAGATATTACATTAGAATT |
| spaP Wr-Down | AACCACCATAAACCGAACGAACT |
| Complement-spaP -F-Sal | CATCAGTCGACGGCCAGAACATCTAGTCCAACT |
| Complement-spaP-R-Kpn | CATCAGGTACCTCAATCTTTCTTAGCCTTTAAGCCAAGC |
Biofilm formation assay
S. mutans and C. albicans single and two-species biofilms were grown in THB containing 1% sucrose. This condition was used to simulate ECC patients challenged with high dietary sugars. In brief, overnight cultures of S. mutans and C. albicans were sub-cultured into fresh THB and YPDU, grown to an OD470 of 0.6 and OD600 of 0.65, respectively, diluted 1:100, and aliquoted (200 μL) into a 96-well microtiter plate, and grown for 16 h at 5% CO2 at 37 °C under static conditions 18. Other growth conditions were also tested, whereby biofilms were grown in RPMI-1640 medium (Sigma) to promote biofilm formation of C. albicans. Biofilm biomass was measured at OD562 using crystal violet as described previously 19. Each experiment was performed in duplicate and replicated three times.
Quantification of Colony Forming Units (CFU) of S. mutans and C. albicans in co-culture
Biofilms were gently washed with PBS to remove planktonic cells. S. mutans and C. albicans cells from mono-species or two-species biofilms were scraped from the bottom of each well and vortexed with 200 uL of PBS. Samples were sonicated in a water bath for 15 seconds and 30 seconds, alternately, 5 times. This treatment ensured the disruption of bacterial aggregates. The biofilm suspensions were serially diluted with PBS, plated on THB agar (total bacteria), in addition to selective media for S. mutans (Gold’s media) and C. albicans (CHROMagar™), and incubated at 37°C with 5% CO2. CFUs were counted after 24 h.
Fluorescence microscopy and confocal laser scanning microscopy (CLSM) analysis
S. mutans-GFP and C. albicans single and two-species biofilms were grown in an 8 well microscope slide (ibidi) under 5% CO2 at 37°C for 16 h 15,20. The biofilms were gently washed with PBS three times to remove any unattached cells and stained with calcofluor white to label C. albicans (Sigma-Aldrich). Biofilms were grown with either 1μM dextran-conjugated pHRodo red or dextran-conjugated Cascade Blue (Molecular Probes, Invitrogen) to monitor pH changes or glucan production, respectively21. The stained biofilms were examined using fluorescence microscopy and CLSM as reported 22. Three independent experiments were performed, and the images displayed are representative of all studies. Bio-volume of biofilms were quantified by the program COMSTAT23,24. ImageJ was used to quantify fluorescence of acid (pHRodo red) and glucan (cascade blue) production.
Colonization of Drosophila melanogaster
Infection of Drosophila was performed as previously described18, 22, 25. This model was used to simulate sucrose-dependent bacterial colonization18, 22, 25. Briefly, mid-log phase wild-type S. mutans, spaP mutant, and C. albicans cells were harvested and re-suspended in 5% sucrose and adjusted to an OD600 of 2.5. The re-suspended cells (100 μL) were used to infect flies as previously described18,22,25. Flies were ground in an Eppendorf tube with 100 uL of PBS 2 days after infection with pipette tips and serial dilutions of the homogenates were plated onto THB agar for CFUs.
Statistical analysis
All data are expressed as mean±SD. Data were analyzed using the t-test. Differences were considered significant if P< 0.05.
RESULTS
Establishment of the in vitro S. mutans and C. albicans two species biofilm model
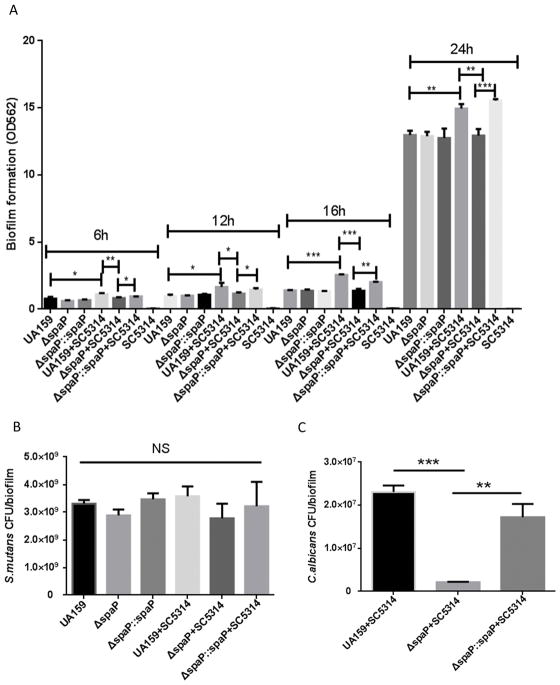
ECC is characterized as a carbohydrate-induced infectious disease, and high consumption of sucrose has been associated with an increased prevalence of ECC 26. Accordingly, a sucrose-dependent biofilm model was utilized to mimic the cariogenic conditions associated with the oral cavity of ECC children. First, we established the biofilm time course in THB media with 1% sucrose using time points of 6, 12, 16, and 24 hours. In addition, wild-type S. mutans and the spaP mutant were compared to examine the role of antigen I/II of S. mutans in the two-species biofilms with C. albicans. Single-species biofilms of S. mutans and C. albicans were used as controls. There was no significant difference in the single-species biofilms between the wild-type, spaP mutant, or spaP complemented S. mutans (Fig. 1A). Total biofilm biomass significantly increased in the two-species biofilm consisting of S. mutans UA159 and C. albicans SC5314; however, this increase was abolished by the loss of spaP in S. mutans, and partially restored in the spaP complemented strain (Fig 1A), suggesting antigen I/II encoded by spaP is required for promotion of the two species biofilm. Biofilms grown at 6, 12, 16, and 24 hours showed similar trends in biofilm biomass for each strain in either single or dual species biofilms (Fig 1A). However, 24 hour-biofilms exhibited extremely dense biofilms due to the rapid development and maturation of biofilms under the rich medium, which made it difficult to analyze. As a result of these preliminary studies, we selected 16 hour biofilms for further in vitro studies. Consistent with data from previous studies using similar growth conditions, C. albicans did not form single species biofilms (Fig 1A) 27. However, in RPMI-1640 medium, which supports the development of C. albicans biofilms, C. albicans formed robust single-species biofilms, but S. mutans biofilm formation was poor (Fig S1). This result does not represent the physiological conditions of ECC, since S. mutans is the primary colonizing microbe in cariogenic biofilms of ECC26. Clinical studies show a higher ratio of S. mutans than C. albicans in the dental plaque and saliva of children with severe early childhood caries (S-ECC)28. Therefore, we opted to use THB in order to form better S. mutans biofilms, which more closely represents the conditions of ECC.
Figure 1. Antigen I/II promotes the adherence of C. albicans in mixed species biofilms with S. mutans.
A. Crystal violet quantification of single and two-species biofilm biomass of wild-type S. mutans, spaP mutant, and C. albicans at 6, 12, 16, and 24 hours in THB and 1% sucrose B. CFU quantification of S. mutans, spaP mutant in single and two-species biofilms. C. CFU quantification of C. albicans. *(P<0.05), ** (P<0.005), *** (P<0.001),
S. mutans antigen I/II facilitates the incorporation of C. albicans in the two-species biofilm with S. mutans
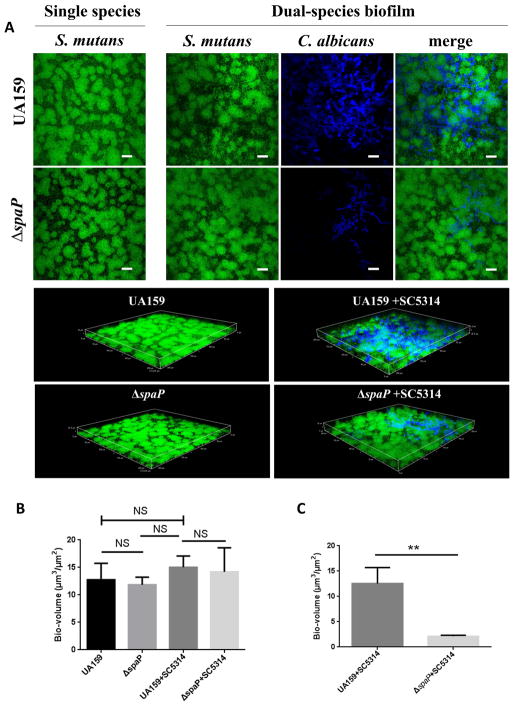
To further characterize the dual species biofilm, and evaluate the contribution of S. mutans and C. albicans in the dual biofilm, we quantified each organism by colony-forming-units (CFUs). Neither wild-type S. mutans, the spaP mutant, nor the complemented strain displayed increased CFUs with the addition of C. albicans (Fig 1B). In contrast, significant numbers of C. albicans (CFUs) were detected in wild-type S. mutans biofilms, but drastically decreased in the spaP mutant biofilms (Fig 1C). As shown by the CFU data, the number of S. mutans is 100 times more than C. albicans in the co-species biofilm, which is similar to the ratio of the two species detected in S-ECC children28. We furthered examined single and two-species biofilms using CLSM. Structural analysis revealed that S. mutans forms larger aggregates when co-cultured with C. albicans compared to the single S. mutans biofilm (Fig 2A). In addition, C. albicans cells were predominantly clustered together and interspersed throughout the two-species biofilm, but did not directly co-localize with S. mutans. Similar to wild-type S. mutans, the spaP mutant also formed larger aggregates in the two-species biofilm with C. albicans compared to a single species biofilm (Fig 2A). However, loss of spaP resulted in fewer C. albicans present throughout the biofilm (Fig 2C). These data suggest that C. albicans can alter the structure of the two-species biofilm and S. mutans antigen I/II supports the incorporation of C. albicans into the biofilm with S. mutans.
Figure 2. Confocal laser scanning microscopy studies of S. mutans and C. albicans single and co-species biofilms.
A. Confocal scanning laser microscopy images of wild-type S. mutans, spaP, and C. albicans single and two-species biofilms at 60X magnification. S. mutans was labeled with green fluorescent protein (GFP) and C. albicans was stained with calcofluor white. Scale bar: 20 μM. All biofilms were grown for 16 hours in THB and 1% sucrose. B. COMSTAT2 analysis of biovolume in confocal images of S. mutans. C. COMSTAT2 analysis of biovolume in confocal images of C. albicans. ** (P<0.005), NS, not significant.
Loss of C. albicans Als1 and Als3 does not affect dual-species biofilms with S. mutans
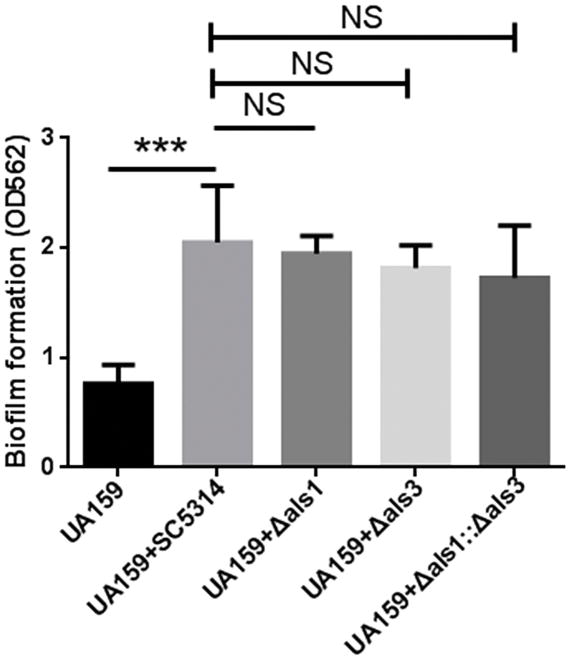
Als1 and Als3 proteins in C. albicans directly interact with antigen I/II-like protein, SspB, in S. gordonii, therefore, we tested whether single or double mutations in als1 and als3 would impact the two-species biofilm formation with S. mutans. As shown in Fig 3, loss of als1, als3, or both als1 and als3 did not affect biofilm formation with S. mutans, suggesting that Als1 and Als3 proteins are not required for C. albicans to interact with S. mutans in the presence of sucrose.
Figure 3. Als1 and Als3 do not play a role in the interaction between S. mutans and C. albicans.
Crystal violet quantification of single and two-species biofilms of wild-type S. mutans, wild-type C. albicans, als1 mutant, als3 mutant and double mutant. NS(P>0.05).
Acid production is promoted in the two-species biofilm
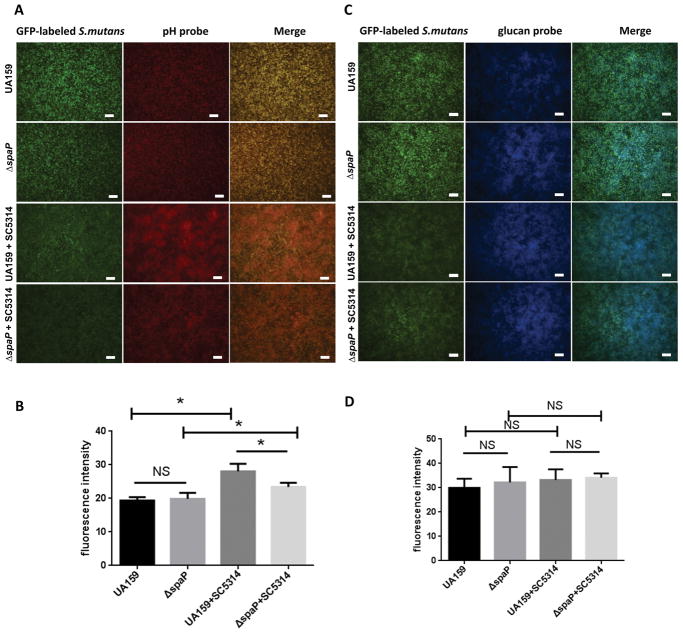
Acid production is critical for caries pathogenesis 29. To determine if the presence of antigen I/II influences the biofilm pH, we used a dextran-conjugated pH probe (red) to monitor pH changes in single and two-species biofilms of wild-type and spaP mutant S. mutans in the presence of C. albicans. Fluorescence microscopy showed an increase in fluorescent intensity in the S. mutans and C. albicans two-species biofilm, indicative of a more acidic condition (Fig 4A and 4B). However, the increase in acidity was reduced in the spaP mutant two-species biofilm. Notably, there was no significant difference in fluorescent intensity between the wild-type and spaP mutant single species biofilms, suggesting antigen I/II has no effect on acid production in S. mutans (Fig 4A and 4B). Unexpectedly, in the two-species biofilm, the fluorescence of S. mutans was weaker than observed in the single S. mutans biofilm despite the comparable numbers of bacteria detected by CFUs (Fig 1C and 1D). This phenomenon is likely due to the fact that S. mutans cells were clustered and partially masked by the dense C. albicans population. Glucan is another key factor that helps S. mutans form biofilms and promotes the development of caries30. Therefore, a specific dextran-conjugated probe for S. mutans glucan was used to monitor changes in glucan production in the single and two-species biofilms. There was no difference in the S. mutans glucan (blue) between single and two-species biofilms (Fig 4C and 4D). These results suggest that factors other than the presence of S. mutans glucans impact acid production within the two-species biofilms.
Figure 4. Acid production is promoted in the two-species biofilm of S. mutans and C. albicans.
A. Fluorescence microscopy images of wild-type S. mutans, spaP, and C. albicans single and mixed species biofilms at 10X magnification. S. mutans was labeled with green fluorescent protein (GFP) and acid production was monitored using a dextran-conjugated pHrhodo red probe. Scale bar: 200 μM. B. ImageJ analysis of fluorescence of pHrhodo red probe in ‘A’. C. S. mutans glucan was monitored with cascade blue. Scale bar: 200 μM. D. ImageJ analysis of fluorescence of cascade blue in ‘C’. *(P<0.05), NS, not significant.
Antigen I/II is critical for colonization of C. albicans during co-infection with S. mutans in Drosophila melanogaster
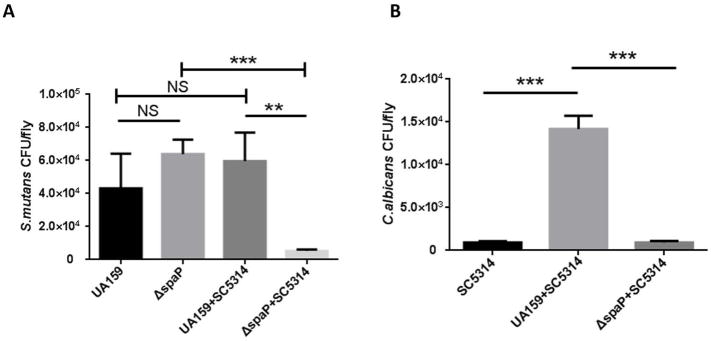
To determine if antigen I/II influences the colonization of S. mutans and C. albicans in vivo, we employed a widely used Drosophila sucrose-dependent colonization model. Flies were infected with mid-log phase cells of wild-type S. mutans, spaP mutant, C. albicans, and C. albicans with either wild type S. mutans or the spaP mutant, respectively. There was no difference in colonization between wild-type S. mutans and the spaP mutant in single species infection. The two-species infection did not enhance colonization of wild-type S. mutans compared to the single S. mutans infection. However, colonization by the spaP mutant in the co-infection was significantly inhibited compared to the single spaP infected group (Fig 5A), suggesting a potential synergistic effect between C. albicans and antigen I/II in vivo. C. albicans colonized Drosophila at a low level in the single species C. albicans infection and during co-infection with the spaP mutant. Importantly, colonization of C. albicans significantly increased approximately fifteen-fold during co-infection with S. mutans (Fig 5B). These data demonstrate that antigen I/II supports the colonization of both S. mutans and C. albicans during co-infection in Drosophila in vivo.
Figure 5. Antigen I/II is required for the colonization of both S. mutans and C. albicans during co-infection of Drosophila melanogaster.
Single or co-infection of Drosophila with S. mutans, spaP mutant, and C. albicans for 48 hours. A. CFU quantification of wild-type S. mutans, the spaP mutant in single and two-species infection of Drosophila. B. CFU quantification of C. albicans in single and two-species infection of Drosophila. Data are representative of three experiments performed in triplicate. ** (P<0.005), *** (P<0.001), NS, not significant.
DISCUSSION
C. albicans and S. mutans often coexist in pathogenic oral biofilms in ECC children; however, the underlying mechanism regarding their interaction is poorly understood. In our study, C. albicans and S. mutans formed enhanced two-species biofilms compared to a single S. mutans species biofilm in vitro and promoted the colonization of C. albicans in vivo. Interestingly, S. mutans cell number did not change between single and two-species biofilms in vitro, and only C. albicans numbers were increased in the two-species biofilms. However, the presence of C. albicans altered the S. mutans biofilm structure, which forms bigger aggregates, and may promote cariogenic potential. Prior studies have reported that C. albicans promotes the growth of S. mutans in the two-species biofilm, thus enhancing the biofilm formation8. In contrast, other studies reported that C. albicans and S. mutans display no synergistic effect, and some reports even document that S. mutans inhibits C. albicans growth and hyphae formation31–34. Different results may be due to the use of different biofilm conditions. In our model, we observed that many C. albicans cells were in yeast or pseudohyphae form (Fig 2), which is consistent with previous studies that demonstrate in low pH environments, C. albicans is typically in yeast or pseudohyphae form compared to hyphae form35. From a physiological standpoint, the yeast form of C. albicans may be more representative of C. albicans morphology under acidic, cariogenic conditions in the oral cavity. S. mutans dominating in co-species biofilms with fewer C. albicans is also consistent with clinical findings from ECC children28. Therefore, the model in our present study better mimics the conditions of cariogenic biofilms in ECC children. Overall, our studies demonstrate the dynamic interactions that exist between C. albicans and S. mutans. Our findings represent a previously unknown cooperative interaction between C. albicans and S. mutans.
Sucrose is an important factor in two-species biofilms of S. mutans and C. albicans29. In the presence of sucrose, Gtfs, particularly GtfB, bind to the surface of C. albicans to produce large amounts of glucan, which provide strong binding sites for S. mutans 36. Moreover, mannan of C. albicans was also involved in the binding to S. mutans GtfB27. However, additional factors important for the two-species interactions are unclear. In this study, we demonstrate that antigen I/II mediates the interaction between S. mutans and C. albicans. Loss of spaP coding for antigen I/II resulted in a significant reduction of C. albicans in the two-species biofilms both in vitro and in vivo. Interestingly, there was no change in the number of the spaP mutant cells recovered from the two-species biofilm in vitro, while the number of the spaP mutant cells were significantly decreased when co-infected with C. albicans in vivo. The reason for this inconsistency between our in vitro and in vivo data is unknown. It is plausible that the Drosophila immune response that facilitates clearance or reduced colonization of C. albicans in the absence of spaP, also simultaneously clears the spaP mutant during co-infection. Such clearance is protected by the two-species biofilm and the activity of antigen I/II. In fact, antigen I/II has been shown to be a potent immunomodulatory factor 12.
Antigen I/II family proteins are widely distributed on the cell wall of many streptococci and contain various adhesion epitopes associated with the interaction with other species 12. The antigen I/II-like protein of S. gordonii, SspB, mediates adhesion with C. albicans via binding to C. albicans Als3 proteins13,37. However, C. albicans Als1 and Als3 proteins were not required for two-species biofilm formation between C. albicans and S. mutans in our current study. Als3 is a hyphae-specific protein, and therefore, is expressed on C. albicans hyphae and pseudohyphae, but not on yeast form38,39. Most of Als1 is localized on the tube and hyphae of C. albicans40. In the co-species biofilm, C. albicans were generally yeast and pseudohyphae, which explains why Als1 and Als3 are not involved in the interaction between S. mutans and C. albicans in our biofilm model. Although Als1 and Als3 have been reported to be important for the interaction with S. gordonii, C. albicans harbors six additional Als proteins (Als2, Als4, Als5, Als6, Als7, and Als9). Therefore, we do not rule out the possibility that other Als proteins may play a role in the interaction with S. mutans41.
S. mutans glucan has been reported as a key factor for the interaction between S. mutans and C. albicans 27. Interestingly, we did not observe any changes in S. mutans glucan production, suggesting there is an unknown factor involved in our model system. It was noteworthy that the two-species biofilms with S. mutans and C. albicans exhibited a lower pH compared to single S. mutans biofilms. Acidogenicity is critical for the pathogenesis of S. mutans in ECC children 8. Increased production of acid in the S. mutans and C. albicans two-species biofilm would create a more cariogenic biofilm and promote the development of caries in ECC children. Both acid and glucan production are products of carbohydrate metabolism42. Glucan is the primary extracellular polysaccharide (EPS) of S. mutans and helps form the complex 3D architecture that allows the accumulation of acid30. However, in the present study, glucan production by S. mutans was not affected by C. albicans, suggesting that the change between wild-type S. mutans and the spaP mutant co-species biofilm is not dependent on the function of glucosyltransferases that synthesize glucan. Alternatively, C. albicans may contribute to acid production. It has been reported that C. albicans can produce and tolerate acid, which can support the development of caries in vivo 43–44. Of course, the hypothesis that C. albicans produces acid in a dual-species biofilm still needs further investigation.
In summary, our studies reveal a previously unknown dynamic interaction between S. mutans and C. albicans, and this activity is dependent on a well-known adhesin, antigen I/II. Our data support the notion that co-infection with S. mutans and C. albicans within the oral biofilm community may heighten pathogenicity by enhancing biofilm formation and acid production. Molecular mechanisms uncovered for the synergy exhibited between S. mutans and C. albicans would be beneficial for the design and development of targeted therapeutics that interrupt the pathogenesis of ECC.
Supplementary Material
Acknowledgments
We thank Drs. Mira Edgerton and Glen E. Palmer for the C. albicans strains. This work was supported by NIH/NIDCR grant R01DE022350 (to H.W.). C. Yang is supported by NIH/NIDCR training grant R90DE023056. J. Scoffield is supported by NIH/NIDCR grant K99DE025913. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. The Journal of the American Dental Association. 2009;140(6):650–657. doi: 10.14219/jada.archive.2009.0250. [DOI] [PubMed] [Google Scholar]
- 2.Yang R, Yang C, Liu Y, Hu Y, Zou J. Evaluate root and canal morphology of primary mandibular second molars in Chinese individuals by using cone-beam computed tomography. Journal of the Formosan Medical Association. 2013;112(7):390–395. doi: 10.1016/j.jfma.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Slayton RL. Reducing mutans streptococci levels in caregivers may reduce transmission to their children and lead to reduced caries prevalence. Journal of Evidence Based Dental Practice. 2011;11(1):27–28. doi: 10.1016/j.jebdp.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kanasi E, Johansson I, Lu SC, et al. Microbial risk markers for childhood caries in pediatricians’ offices. Journal of dental research. 2010;89(4):378–383. doi: 10.1177/0022034509360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja M, Hannan A, Ali K. Association of oral candidal carriage with dental caries in children. Caries research. 2010;44(3):272–276. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DMP. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Archives of oral biology. 2006;51(11):1024–1028. doi: 10.1016/j.archoralbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Beena M, Peedikayil FC, GufranAfmed M, Chandru T, Soni K, Dhanesh N. Comparison of Candida species isolated from children with and without early childhood caries: A descriptive cross-sectional study. Journal of Indian Society of Pedodontics and Preventive Dentistry. 2017;35(4):296. doi: 10.4103/JISPPD.JISPPD_160_17. [DOI] [PubMed] [Google Scholar]
- 8.Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infection and immunity. 2014;82(5):1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 2013;9(10):e1003616. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakubovics NS. Intermicrobial interactions as a driver for community composition and stratification of oral biofilms. Journal of molecular biology. 2015;427(23):3662–3675. doi: 10.1016/j.jmb.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Ricker A, Vickerman M, Dongari-Bagtzoglou A. Streptococcus gordonii glucosyltransferase promotes biofilm interactions with Candida albicans. Journal of oral microbiology. 2014;6(1):23419. doi: 10.3402/jom.v6.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady LJ, Maddocks SE, Larson MR, et al. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Molecular microbiology. 2010;77(2):276–286. doi: 10.1111/j.1365-2958.2010.07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infection and immunity. 2010;78(11):4644–4652. doi: 10.1128/IAI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Kelly C, Munro G, Whiley R, Lehner T. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infection and immunity. 1991;59(8):2686–2694. doi: 10.1128/iai.59.8.2686-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Worthington RJ, Melander C, Wu H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrobial agents and chemotherapy. 2011;55(6):2679–2687. doi: 10.1128/AAC.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X, Michalek S, Wu H. Effects of diadenylate cyclase deficiency on synthesis of extracellular polysaccharide matrix of Streptococcus mutans revisit. Environmental microbiology. 2016;18(11):3612–3619. doi: 10.1111/1462-2920.13440. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Okinaga T, Qi F, Zhang Z, Merritt J. Cloning-independent and counterselectable markerless mutagenesis system in Streptococcus mutans. Applied and environmental microbiology. 2011;77(22):8025–8033. doi: 10.1128/AEM.06362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng X, Zhang Y, Bai G, Zhou X, Wu H. Cyclic di-AMP mediates biofilm formation. Molecular microbiology. 2016;99(5):945–959. doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Zeng M, Fives-Taylor P. The glycan moieties and the N-terminal polypeptide backbone of a fimbria-associated adhesin, Fap1, play distinct roles in the biofilm development of Streptococcus parasanguinis. Infection and immunity. 2007;75(5):2181–2188. doi: 10.1128/IAI.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia S, Blackledge M, Michalek S, et al. Targeting of Streptococcus mutans Biofilms by a Novel Small Molecule Prevents Dental Caries and Preserves the Oral Microbiome. Journal of dental research. 2017 doi: 10.1177/0022034517698096. 0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia S, Du Q, Wu H. Streptococcus mutans copper chaperone, CopZ, is critical for biofilm formation and competitiveness. Molecular oral microbiology. 2016;31(6):515–525. doi: 10.1111/omi.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scoffield JA, Duan D, Zhu F, Wu H. A commensal streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS pathogens. 2017;13(4):e1006300. doi: 10.1371/journal.ppat.1006300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heydorn A, Nielsen AT, Hentzer M, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 24.Vorregaard M. Technical University of Denmark, DTU, DK-2800 Kgs. Lyngby, Denmark: 2008. Comstat2-a modern 3D image analysis environment for biofilms. [Google Scholar]
- 25.Scoffield JA, Wu H. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infection and immunity. 2015;83(1):101–107. doi: 10.1128/IAI.02396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz RJ. Causes, treatment and prevention of early childhood caries: a microbiologic perspective. Journal-Canadian Dental Association. 2003;69(5):304–307. [PubMed] [Google Scholar]
- 27.Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS pathogens. 2017;13(6):e1006407. doi: 10.1371/journal.ppat.1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao J, Moon Y, Li L, et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PloS one. 2016;11(10):e0164242. doi: 10.1371/journal.pone.0164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen WH, Burne RA, Wu H, Koo H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends in Microbiology. 2017 doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J, Klein MI, Falsetta ML, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS pathogens. 2012;8(4):e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryotic cell. 2009;8(11):1658–1664. doi: 10.1128/EC.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sztajer H, Szafranski SP, Tomasch J, et al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. The ISME journal. 2014;8(11):2256–2271. doi: 10.1038/ismej.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D, Sengupta A, Niepa TH, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Scientific Reports. 2017:7. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbosa JO, Rossoni RD, Vilela SFG, et al. Streptococcus mutans can modulate biofilm formation and attenuate the virulence of Candida albicans. PloS one. 2016;11(3):e0150457. doi: 10.1371/journal.pone.0150457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderone RA, Clancy CJ. Candida and candidiasis. American Society for Microbiology Press; 2011. [Google Scholar]
- 36.Gregoire S, Xiao J, Silva B, et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Applied and environmental microbiology. 2011;77(18):6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyer LL, Oh S-H, Jones R, Cota E. A proposed mechanism for the interaction between the Candida albicans Als3 adhesin and streptococcal cell wall proteins. Frontiers in microbiology. 2014;5:564. doi: 10.3389/fmicb.2014.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman DA, Oh S-H, Zhao X, et al. Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. Journal of microbiological methods. 2009;78(1):71–78. doi: 10.1016/j.mimet.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argimón S, Wishart JA, Leng R, et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryotic cell. 2007;6(4):682–692. doi: 10.1128/EC.00340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman DA, Oh S-H, Zhao X, Hoyer LL. Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody. Microbiology. 2010;156(12):3645–3659. doi: 10.1099/mic.0.043851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyer LL, Cota E. Candida albicans agglutinin-like sequence (Als) family vignettes: a review of Als protein structure and function. Frontiers in microbiology. 2016;7:280. doi: 10.3389/fmicb.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leme Paes, Koo H, Bellato C, Bedi G, Cury J. The role of sucrose in cariogenic dental biofilm formation—new insight. Journal of dental research. 2006;85(10):878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klinke T, Kneist S, de Soet JJ, et al. Acid production by oral strains of Candida albicans and lactobacilli. Caries research. 2009;43(2):83–91. doi: 10.1159/000204911. [DOI] [PubMed] [Google Scholar]
- 44.Klinke T, Guggenheim B, Klimm W, Thurnheer T. Dental caries in rats associated with Candida albicans. Caries research. 2011;45(2):100–106. doi: 10.1159/000324809. [DOI] [PubMed] [Google Scholar]
- 45.Wen ZT, Baker HV, Burne RA. Influence of BrpA on critical virulence attributes of Streptococcus mutans. Journal of bacteriology. 2006;188(8):2983–2992. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Molecular and General Genetics MGG. 1984;198(1):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 47.Nobile CJ, Schneider HA, Nett JE, et al. Complementary adhesin function in C. albicans biofilm formation. Current Biology. 2008;18(14):1017–1024. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou M, Fives-Taylor P, Wu H. The utility of affinity-tags for detection of a streptococcal protein from a variety of streptococcal species. Journal of microbiological methods. 2008;72(3):249–256. doi: 10.1016/j.mimet.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.