Abstract
Objective
To investigate the longitudinal changes in principal readmission diagnoses within 30 days after hospitalization for acute exacerbation of COPD (AECOPD).
Study setting
Medicare claims data, 2010-2012.
Study design
Retrospective cohort study.
Data collection methods
We identified AECOPD hospitalizations aged ≥65 years, and examined the principal 30-day readmission diagnoses (respiratory-related, cardiac-related, and other conditions). We also constructed joinpoint regression models to test whether patients with each of the three major readmission conditions had a unique temporal pattern of readmission during the 30-day period.
Principal findings
Among 76,697 index hospitalizations with AECOPD, 14,090 (18.4%) were readmitted within 30 days. Respiratory-related conditions accounted for 55% of readmissions. The proportion of respiratory-related conditions as the readmission diagnosis decreased from post-discharge day 1 to day 8 (4.0% decrease), and then increased thereafter (13.2% increase; P=0.06). Cardiac-related conditions had a similar non-linear trend with an inflection point at day 6 (P=0.02), with a subsequent downward trend from day 22 (P=0.01). By contrast, the other conditions increased from day 1 to day 6 (15.0% increase), and then significantly decreased (28.8% decrease; P=0.04).
Conclusions
The proportions of principal discharge diagnosis of readmission changed significantly at around post-discharge day 7. Our findings advance research into identification of the underlying mechanisms and development of targeted interventions to prevent readmissions.
Keywords: COPD, 30-day readmission, age, hospitalization
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major public health problem, affecting at least 12 million US adults.1,2 Acute exacerbation of COPD (AECOPD) is responsible for 700,000 hospitalizations each year.3 Of these, one in five result in readmission within 30 days.4 To improve the quality of care and curb the healthcare burden of readmissions, the Affordable Care Act developed the Hospital Readmissions Reduction Program (HRRP) which penalizes hospitals for higher than expected 30-day readmission rate for the targeted conditions, including COPD.5
To develop effective strategies for reducing readmissions, it is important to understand why and when patients are readmitted after discharge.6 However, only few studies have investigated the HRRP targeted conditions with regard to differences in 30-day readmission diagnoses between the early (e.g., the first 7 days) and late periods following hospital discharge.6,7,8 Additionally, studies have demonstrated that patient care factors contribute to 30-day readmissions in a time-varying manner8,9 – e.g., the effects of inpatient management on the readmission risk diminishes rapidly after discharge.9 These studies suggest that 30-day readmission is not a homogeneous process and that it consists of different causes and time courses. Despite the clinical and policy relevance, no study has tested for the time-varying changes in the 30-day readmission diagnoses among the Medicare population, let alone patients hospitalized for AECOPD.
To address this knowledge gap, we analyzed the data of Medicare beneficiaries hospitalized for AECOPD to determine the principal discharge diagnoses of 30-day readmission and their different time courses following discharge. We hypothesized that the readmission diagnoses vary during the 30-day period and that their proportions change at around 7 days after discharge.
METHODS
Design and setting
We conducted a retrospective cohort study using a random sample of 5 million Medicare Beneficiaries from 2010 to 2012 Medicare Research Identifiable Files from the Centers for Medicare & Medicaid Services (CMS). Final action, fee-for-service, claims data, inpatient files were used to identify all Medicare beneficiaries who were hospitalized for AECOPD. To enable follow-up of specific patients, inpatient claims for individual patients were linked with the use of the Health Insurance Claim Number–Beneficiary Identification Code. The beneficiary identification code was then used to link beneficiary enrollment and demographics information from the Master Beneficiary Summary File, and the Chronic Conditions File that includes 27 chronic condition data warehouse flags. The institutional review board of Massachusetts General Hospital approved this study (2013P002545).
Study population
Based on the CMS publicly-reported readmission measures,10 we identified all hospitalizations made by patients (age ≥65 years) with a primary hospital discharge diagnosis of COPD using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes (ICD-9-CM diagnosis codes 491.21, 491.22, 491.8, 491.9, 492.8, 493.20, 493.21, 493.22, and 496), or those with a primary diagnosis of respiratory failure (ICD-9-CM diagnosis codes 518.81, 518.82, 518.84, and 799.1) and secondary diagnosis of COPD (e-Table 1). We excluded patients who left the hospital against medical advice, those who were transferred to another acute care facility, those who died during the index hospitalization, those without continuous enrollment in Medicare fee-for-service for one year prior to the date of the index hospitalization, and those without at least 30 days post-discharge enrollment in Medicare fee-for-service. Transfers were identified as consecutive claims on the same day with different provider (hospital) numbers for the same beneficiary based on CMS methodology.10 Additionally, according to the CMS recommendations,10 we considered only the first readmission within 30 days of discharge as a readmission while we did not count additional readmissions within the 30-day period as 30-day readmissions or index hospitalizations. We considered subsequent hospitalizations occurring after 30 days from discharge as index hospitalizations if they met the inclusion criteria.
Measurements
Medicare Master Beneficiary Summary Files included information on patient demographics (age, sex, race/ethnicity), quartiles for median household income, hospital admission and discharge dates, and enrollment status for Medicare beneficiaries. Median household income was determined by matching patients’ home ZIP codes and year of enrollment to ZIP code-linked estimates of annual median household incomes.11 Baseline health status was evaluated by the sum of the Chronic Condition Data Warehouse (CCW) defined chronic conditions that include 27 chronic condition indicator variables. These indicator variables have been developed from the claims data for chronic conditions that were deemed to be relevant to studies of Medicare-only beneficiaries.12 From the 27 CCW chronic conditions, we excluded COPD and asthma for calculating comorbidities.
Outcomes
The outcome was the primary discharge diagnosis at the readmission. A readmission was a hospital admission at any hospital for any reason occurring within 30 days of discharge from the index hospitalization for AECOPD.10 First, the principal diagnoses (>14,000 ICD-9-CM diagnosis codes) were consolidated into 285 mutually exclusive diagnostic categories using the Agency for Healthcare Research Quality’s Clinical Classifications Software (CCS),13 as done previously.7,14,15 Then, to make clinical interpretation more meaningful, and to address potential misclassifications, we further grouped the discharge diagnoses into 3 mutually-exclusive groups (e-Table 2): respiratory-related conditions (e.g., COPD, respiratory failure, pneumonia; CCS 122-134), cardiac-related conditions (e.g., heart failure; CCS 96-108), and other conditions (e.g., sepsis).7
Statistical analysis
To test whether patients with each of the three major readmission conditions (i.e., respiratory-related, cardiac-related, and other conditions) had a unique temporal pattern of readmission over 30-day period, we calculated the relative proportion of three conditions at each post-discharge day. Then, we constructed an unadjusted joinpoint regression model to investigate the change in the proportion of each condition during the 30-day period by using the Joinpoint Regression Program version 4.5.0.1 (Statistical Research and Applications Branch, National Cancer Institute).16 This analysis compares models by starting with no joinpoints and subsequently testing whether ≥1 joinpoints needed to be entered into the model to best fit the data. If there is no joinpoint, it would suggest no significant changes over time. If there are one or more joinpoints, it would suggest significant changes in the outcome of interest. With the use of Bayesian information criterion method, we identified optimal number of joinpoints in the trend.17,18 We also illustrated the change in the proportion of each grouped readmission diagnosis by using a LOWESS smoother with calculating 95%CI using a bootstrap method. In the sensitivity analysis, we stratified the analysis by age category (65-84 years vs. ≥85 years) according to a standard terminal age category in disease reporting in the US19 and the previous literature.6,20,21 We also used summary statistics to compare the proportion of each grouped diagnosis and five most frequent CCS-defined principal discharge diagnoses between age categories. As the temporal change was not examined between patients with different readmission diagnoses (i.e., no assumption of exchangeability was made), adjustment for covariates (e.g., demographics) was not conducted. We analyzed data using STATA 15.0 (StataCorp, College Station, TX) and R statistical software (version 3.4.0).
RESULTS
Baseline Characteristics
From 2010 to 2012, we identified 118,198 hospitalizations for AECOPD. Of these, 76,697 (65%) were determined as index hospitalizations at risk for 30-day readmissions (53,486 unique patients), based on the CMS-defined methodology. The index hospitalizations were made by patients with the median age of 76 years (IQR, 71-83 years); 60% were female, 85% were non-Hispanic white, and 73% had ≥5 comorbidities (Table 1).
Table 1.
Characteristics of index hospitalizations for acute exacerbation of chronic obstructive pulmonary disease, 2010-2012
| Variables | Overall (n=76,697) |
|---|---|
| Age (year), median (IQR) | 76 (71-83) |
| Age category | |
| 65-84 years | 62,257 (81) |
| ≥85 years | 14,440 (19) |
| Sex | |
| Male | 30,987 (40) |
| Female | 45,710 (60) |
| Race/ethnicity | |
| Non-Hispanic white | 65,257 (85) |
| Non-Hispanic black | 6,536 (9) |
| Hispanics | 3,303 (4) |
| Other/unknown | 1,601 (2) |
| Median household income | |
| 1st quartile (lowest) | 17,299 (23) |
| 2nd quartile | 17,860 (24) |
| 3rd quartile | 18,380 (25) |
| 4th quartile (highest) | 21,485 (29) |
| Number of comorbidities* | |
| 0-1 | 3,754 (5) |
| 2-4 | 17,325 (23) |
| ≥5 | 55,618 (73) |
| Discharge year | |
| 2010 | 27,037 (35) |
| 2011 | 27,046 (35) |
| 2012 | 22,614 (29) |
| Geographic region | |
| Northeast | 13,908 (18) |
| Midwest | 19,586 (26) |
| South | 34,056 (44) |
| West | 9,007 (12) |
| Guam, Puerto Rico and Virgin Islands | 140 (0.18) |
Data were shown as n (%) unless otherwise specified.
Abbreviation: IQR, interquartile range
Comorbidities were based on 27 chronic conditions (excluding chronic obstructive pulmonary disease) defined by the Centers for Medicare & Medicaid Services.
30-day Readmission Diagnosis
The crude 30-day readmission rate within 30 days after hospitalization for AECOPD was 18.4%. Overall, respiratory-related conditions accounted for 54.7% of readmission diagnoses, cardiac-related conditions for14.4%, and other conditions for 30.9% (eTable 3). By CCS category, COPD (30.0%) was the most frequent readmission diagnosis, followed by pneumonia (9.4%), respiratory failure (8.5%), heart failure (6.4%), and sepsis (4.6%). Among the other conditions, overall, septicemia was the most common readmission diagnosis (16% of the other conditions), followed by renal failure (6%), fluid and electrolyte disorders (5%), and gastrointestinal hemorrhage (5%). Compared to patients aged 65-84 years, those aged ≥85 years were more likely to be readmitted with cardiac-related or other conditions (both P<0.001).
Time-varying readmission diagnoses during the 30-day period
Figure 1 illustrates the changes in the proportion of respiratory-related, cardiac-related, and other conditions by day as the 30-day readmission diagnosis. For example, overall, the proportion of respiratory-related conditions decreased immediately after discharge, and the downward trend changed to an upward trend around one week after discharge; the other conditions had a different pattern. Specifically, the joinpoint model demonstrated that the proportion of respiratory-related conditions initially decreased by 4.0% until day 8 (0.5% decrease per day; Table 2) and then increased by 13.2% until day 30 (0.6% increase per day), reflecting an infliction point at day 8 (P=0.06). Likewise, the proportion of cardiac-related conditions decreased by 18.6% until day 6 (3.1% decrease per day), increased by 20.8% until day 22 (1.3% increase per day), and then again decreased by 16.8% (2.1% decrease per day), with significant infliction points at days 6 and 22 (both P<0.05). By contrast, the proportion of other conditions increased by 15.0% until day 6 (2.5% increase per day), and then decreased by 28.8% (1.2% decrease per day), reflecting a significant infliction point at day 6 (P=0.04).
Figure 1. Changes in the proportion of the three grouped 30-day readmission diagnoses, overall and stratified by age group.
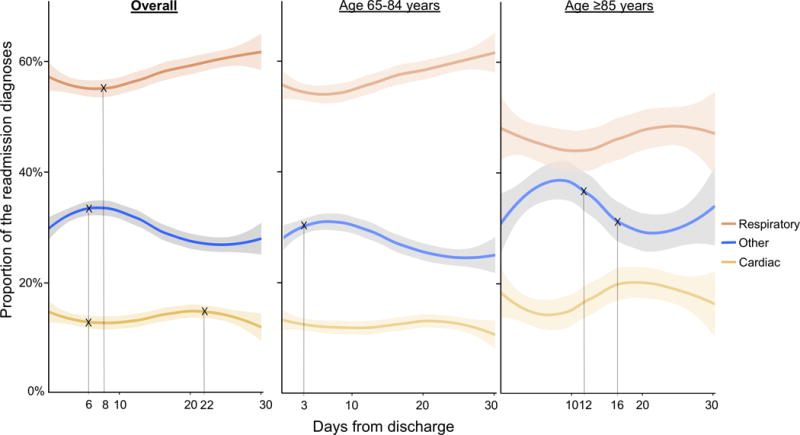
Overall, there was a non-linear U-shaped change in the proportion of respiratory-related conditions (red) as the readmission diagnosis with an inflection point at day 8 after discharge. The proportion of cardiac-related conditions (yellow) had a similar non-linear pattern with inflection points at day 6 and day 22. By contrast, the proportion of the other conditions (blue) had a different pattern with an inflection point at day 6. Compared to patients aged 65-84 years, those aged ≥85 years appeared to have a greater magnitude of changes, with delayed inflection points for the other conditions (at days 3 and 12).
Marks (×) indicate the infliction points.
Table 2.
Changes in the proportion of grouped 30-day readmission diagnoses by using joinpoint regression analysis
| Trend 1 | Change between trends 1 and 2 | Trend 2 | Change between trends 2 and 3 | Trend 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conditions as the readmission diagnosis | Total change, % | Change by day, % | Day of the change | P value for the trend change | Total change, % | Change by day, % | Day of the change | P value for the change | Total change, % | Change by day, % |
| Overall (n=14,090) | ||||||||||
| Respiratory-related | −4.0% | −0.5% | Day 8 | 0.06 | 13.2% | 0.6% | – | |||
| Cardiac-related | −18.6% | −3.1% | Day 6 | 0.02 | 20.8% | 1.3% | Day 22 | 0.01 | −16.8% | −2.1% |
| Other | 15.0% | 2.5% | Day 6 | 0.04 | −28.8% | −1.2% | – | |||
| Age category | ||||||||||
| Age 65-84 years (n=11,463) | ||||||||||
| Respiratory-related | 15.0% | 0.5% | – | |||||||
| Cardiac-related | 3.0% | 0.1% | – | |||||||
| Other | 29.1% | 9.7% | Day 3 | 0.29 | −29.7% | −1.1% | – | |||
| Age ≥85 years (n=2,627) | ||||||||||
| Respiratory-related | 21.0% | 0.7% | – | |||||||
| Cardiac-related | 6.0% | 0.2% | – | |||||||
| Other | 21.6% | 1.8% | Day 12 | 0.18 | −32.0% | −8.0% | Day 16 | 0.21 | 14.0% | 1.0% |
Stratification by age category showed similar non-linear patterns of the proportion of 3 readmission diagnoses during the 30-day period – with a potentially greater magnitude of changes in the older group (Figure 1). Although statistical power was limited in this subgroup analysis, the joinpoint models demonstrated that the proportion of the other conditions had an earlier infliction point (day 3) in patients aged 65-84 years while that had late infliction points (days 12 and 16) in patients aged ≥85 years (Table 2).
DISCUSSION
In nationally-representative data from 76,697 Medicare beneficiaries hospitalized for AECOPD, we found time-varying 30-day principal readmission diagnoses. Specifically, the proportions of respiratory- and cardiac-related conditions had a nadir in the early phase (day 7) of the 30-day period and increased thereafter, while the other conditions had a different pattern. Compared to patients (aged 65-84 years), older patients (aged ≥85 years) were more likely to be readmitted with cardiac-related or other conditions, and appeared to have more pronounced changes in the readmission diagnoses.
Consistent with our findings, a prior study using the 2006-2010 Medicare data reported that, after hospitalization for COPD, respiratory-related conditions accounted for approximately 50% of 30-day readmissions and that COPD was the leading diagnosis.7 In this previous study, the proportion of COPD as the readmission diagnosis was 25% in the early phase and 30% in the late phase of 30-day period following discharge.7 Additionally, in patients with the three other HRRP conditions (heart failure, myocardial infarction, and pneumonia), another study using Medicare data also demonstrated the differences in the proportion of readmissions for recurrent heart failure and pneumonia between different time periods (0-3 days, 4-7 days, 8-15 days, and 16-30 days).6 Our study corroborates these prior studies, and extends them by demonstrating, for the first time, significant changes in the proportion of three major readmission diagnoses (i.e., respiratory-related, cardiac-related, and other conditions) with unique inflection points after being hospitalized for AECOPD.
The underlying mechanisms of the time-varying readmission diagnoses are likely multifactorial. First, the timing of the changes (e.g., the nadir of the respiratory-related conditions at post-discharge day 8) corresponds to the timing of recovery from AECOPD – a function of acute illness severity and AECOPD management. A prospective cohort study reported that the median time to recover from AECOPD is 7 days and that peak expiratory flow improves by day 6 from the onset.22 Additionally, the subsequent increase in the respiratory conditions for readmission might be attributable to the diminished in-hospital treatment effects and to the transitions in care from hospital-level to community-level care.9,23 Indeed, a prior study using state-level administrative datasets reported that the contribution of hospital care to readmission risks declines after discharge, reaching a nadir at post-discharge day 7.9 Furthermore, in contrast to the intensified treatment for AECOPD and related respiratory conditions (e.g., pneumonia) during hospitalization, it is possible that comorbid conditions other than AECOPD (e.g., diabetes) are undermanaged during the hospitalization at discharge.24 Moreover, the literature has also shown that hospitalized patients (particularly older individuals) acquire vulnerability to myriad conditions that lead to a readmission – post-hospital syndrome.25,26 These mechanisms may explain, at least partially, the observed increase in the readmissions for other conditions in the early phase of 30-day period.
In conjunction with the previous literature,6–8,22,23,27,28 our findings suggest that, while readmission is one of the easy-to-measure metrics, its undying mechanisms are complex (e.g., patent’s clinical factors, quality of inpatient, transitional, and outpatient care, social support, transportation, health literacy and behavior). The quality of care during the index hospitalizations is solely a beginning part of readmission processes and its contribution rapidly diminishes after discharge. In contrast, the relative importance of other factors (e.g., chronic illness severity, outpatient care, social- and community-level factors) increases over time. In this context, this hypothesis generating study facilitates future investigations. To reduce readmissions and improve outcomes in patients with COPD, future studies should examine which of these readmissions are potentially preventable, identify their underlying mechanisms, and develop targeted preventive interventions based on the time-periods (e.g., 0-7 and 8-30 day periods). Indeed, studies have shown that well-executed communication among hospital providers, patients, and receiving providers at hospital discharge lowers readmissions in patients with chronic conditions, including COPD.23,24,28–30
Potential Limitations
Our study has several potential limitations. First, because this study is based on the claims data, misclassification of hospitalizations is possible. Nevertheless, we identified hospitalizations for COPD according to the CMS definitions11 which have a high specificity and positive predictive value (both ≥90%).31 Furthermore, Medicare data are considered accurate, are rigorously tested, and widely used to investigate readmissions.32,33 Second, the Medicare data do not contain some useful clinical and sociodemographic information which precluded us from evaluating the contribution of chronic severity of COPD and patient post-discharge environment. Therefore, our study does not completely address the complexity of mechanisms underlying readmissions (e.g., poor social support networks). Third, the current study used unadjusted joinpoint regression models because the temporal change was not compared between patients with different readmission diagnoses (i.e., no assumption of exchangeability was made). Yet, our findings might have been confounded by time-varying factors (e.g., comorbidities, socioeconomic status) even in a relatively short period (i.e., 30-day period after hospital discharge). Finally, the study population comprised Medicare beneficiaries with age ≥65 years, and, therefore, our inference may be not generalizable to younger individuals with COPD. Regardless, our study population accounts for 70% of hospitalizations for AECOPD,4 and is the one on which recent national improvement efforts have focused.5
CONCLUSIONS
Based on the nationally-representative 30-day readmission data of 76,697 Medicare beneficiaries hospitalized for AECOPD, we found time-varying principal readmission diagnoses, with important changes occurring at around post-discharge day 7. The respiratory- and cardiac-related conditions had their peak at the late phase while the other conditions had their peak at the early phase of the 30-day period following discharge. Additionally, older patients (aged ≥85 years) were more likely to be readmitted with cardiac-related or other conditions, and appeared to have more pronounced longitudinal changes in their readmission diagnoses. These findings lend additional support to the concept that the underlying mechanisms of 30-day readmissions are complex. For clinicians, the observed heterogeneity in readmission diagnoses underscore the importance of continued efforts to improve in-hospital and post-discharge care for COPD and comorbidities. For researchers and policymakers, our findings advance research into identification of the complex underlying mechanisms and development of targeted interventions to prevent readmissions.
Supplementary Material
Acknowledgments
We thank Drs. Atsushi Hirayama and Sarah Kyuragi Luthe (Massachusetts General Hospital) for critical revision of the manuscript.
Funding: This study was supported by the grant R01 HS-023305 (Camargo) from the Agency for Healthcare Research and Quality (Rockville, MD).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Notation of prior abstract publication/presentation: None
Conflict of Interest: Dr. Camargo has performed COPD-related consultation for AstraZeneca, GSK, and Mereo.
Other authors (Drs. Goto and Hasegawa, and Mr. Kamal) have no conflict of interest to declare.
Author contributions: T.G. takes responsibility for the paper as a whole. C.A.C. obtained research funding. T.G., C.A.C, and K.H. conceived the study. C.A.C. and K.H. supervised the conduct of the study. M.K.F., C.A.C. and K.H. provided statistical advice. T.G. and M.K.F. analyzed the data. T.G. drafted the manuscript, and all authors contributed substantially to its revision. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
References
- 1.Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(46):938–943. [PubMed] [Google Scholar]
- 2.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60(3):1–116. [PubMed] [Google Scholar]
- 3.HCUPnet. http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed November 7, 2015.
- 4.Goto T, Faridi MK, Gibo K, Toh S, Hanania NA, Camargo CA, Hasegawa K. Trends in 30-day readmission rates after COPD hospitalization, 2006–2012. Respir Med. 2017;130:92–97. doi: 10.1016/j.rmed.2017.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Readmissions Reduction Program (HRRP) https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed August 8, 2017.
- 6.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham KL, Wilker EH, Howell MD, Davis RB, Marcantonio ER. Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749. doi: 10.7326/AITC201506020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin DL, Bang H, Manickam RN, Romano PS. Rethinking Thirty-Day Hospital Readmissions: Shorter Intervals Might Be Better Indicators Of Quality Of Care. Health Aff (Millwood) 2016;35(10):1867–1875. doi: 10.1377/hlthaff.2016.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. 2014 Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Readmission Measures. 2014 http://altarum.org/sites/default/files/uploaded-publication-files/Rdmsn_Msr_Updts_HWR_0714_0.pdf. Accessed October 1, 2017.
- 11.Esri. Demographic, consumer, and business data. http://www.esri.com/data/esri_data. Accessed October 1, 2017.
- 12.Chronic Conditions Data Warehouse. https://www.ccwdata.org/web/guest/home. Accessed February 28, 2018.
- 13.Healthcare Cost and Utilization Project. Clinical Classifications Software for ICD-9-CM. 2015 https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed October 1, 2017.
- 14.Elixhauser A, Steiner C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2013. Readmissions to U.S. Hospitals by Diagnosis, 2010: Statistical Brief #153. [Google Scholar]
- 15.Hines AL, Barrett ML, Jiang HJ, Steiner CA. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2014. Conditions With the Largest Number of Adult Hospital Readmissions by Payer, 2011: Statistical Brief #172. [PubMed] [Google Scholar]
- 16.Joinpoint Trend Analysis Software. https://surveillance.cancer.gov/joinpoint/. Accessed September 28, 2017.
- 17.Yu B, Huang L, Tiwari RC, Feuer EJ, Johnson KA. Modelling population-based cancer survival trends using join point models for grouped survival data. J R Stat Soc Ser A Stat Soc. 2009;172(2):405–425. doi: 10.1111/j.1467-985X.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federico Rea EP, Compagnoni Matteo Monzio, Cantarutti Anna, Pugni Pietro, Bagnardi Vincenzo, Corrao Giovanni. Joinpoint regression analysis with time-on-study as time-scale. Application to three Italian population-based cohort studies. Epidemiology Biostatistics and Publich Health. 2017;14(3):e12616, 12611–12618. [Google Scholar]
- 19.Boscoe FP. Subdividing the age group of 85 years and older to improve US disease reporting. Am J Public Health. 2008;98(7):1167–1170. doi: 10.2105/AJPH.2008.133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Xu J. COPD-related Mortality by Sex and Race Among Adults Aged 25 and Over: United States, 2000–2014. NCHS Data Brief. 2016;(256):1–8. [PubMed] [Google Scholar]
- 21.Lodhi MK, Ansari R, Yao Y, Keenan GM, Wilkie D, Khokhar AA. Predicting Hospital Re-admissions from Nursing Care Data of Hospitalized Patients. Adv Data Min. 2017;2017:181–193. doi: 10.1007/978-3-319-62701-4_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 23.Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664–1670. doi: 10.1001/archinternmed.2010.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 25.Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804. doi: 10.1503/cmaj.150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 28.Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237. doi: 10.1001/archinternmed.2011.278. [DOI] [PubMed] [Google Scholar]
- 29.Hopkinson NS, Englebretsen C, Cooley N, et al. Designing and implementing a COPD discharge care bundle. Thorax. 2012;67(1):90–92. doi: 10.1136/thoraxjnl-2011-200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farkas J, Kadivec S, Kosnik M, Lainscak M. Effectiveness of discharge-coordinator intervention in patients with chronic obstructive pulmonary disease: study protocol of a randomized controlled clinical trial. Respir Med. 2011;105(Suppl 1):S26–30. doi: 10.1016/S0954-6111(11)70007-5. [DOI] [PubMed] [Google Scholar]
- 31.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, Observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543–1551. doi: 10.1056/NEJMsa1513024. [DOI] [PubMed] [Google Scholar]
- 33.Desai NR, Ross JS, Kwon JY, et al. Association Between Hospital Penalty Status Under the Hospital Readmission Reduction Program and Readmission Rates for Target and Nontarget Conditions. JAMA. 2016;316(24):2647–2656. doi: 10.1001/jama.2016.18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


