Abstract
Executive functions are complex both in the cognitive operations involved and in the neural structures and functions that support those operations. This complexity makes executive function highly vulnerable to the detrimental effects of aging, brain injury, and disease, but may also open paths to compensation. Neural compensation is often used to explain findings of additional or altered patterns of brain activations by older adults or patient populations compared to young adults or healthy controls, especially when associated with relatively preserved performance. Here we test the hypothesis of an alternative form of compensation, between different neuromodulator systems. 135 patients with Parkinson’s Disease (PD) completed a vesicular monoamine transporter type2 (VMAT2) and acetylcholinesterase PET scanning to assess the integrity of nigrostriatal dopaminergic, thalamic cholinergic, and cortical cholinergic pathways and a behavioral test (Stroop + task-switching) that put high demands on conflict processing, an important aspect of executive control. Supporting the compensatory hypothesis, regression models controlling for age and other covariates revealed an interaction between caudate dopamine and cortical cholinergic integrity: Cortical cholinergic integrity was a stronger predictor of conflict processing in patients with relatively low caudate dopaminergic function. These results suggest that although frontostriatal dopaminergic function plays a central role in executive control, cholinergic systems may also make an important contribution. The present results suggest potential pathways for remediation, and suggest that the appropriate interventions for each patient may depend on their particular profile of decline. Furthermore, they help to elucidate the brain systems that underlie executive control, which may be important for understanding other disorders as well as executive function in healthy adults.
Introduction
Brain injuries and neurodegenerative disorders can serve as “natural experiments” that provide insights into brain structure-function relations that would otherwise not be possible for technical or ethical reasons. Classic cases include patients with dramatic lesions, such as H.M. or Phineas Gage (Corkin and Scoville, 1984; Scoville and Milnder 1957; Harlow, 1868; Damasio et al., 1994). Neurodegenerative diseases provide another such opportunity, and cross-sectional or longitudinal analyses at different stages of severity fit especially well with the RDoC (Research Domain Criteria) mandate of understanding brain-behavior relationships throughout the entire range of function. The heterogeneity of neuromodulator system decline in Parkinson’s disease (PD) may make it particularly instructive. In addition to the dopaminergic declines that define the disorder, Parkinson’s patients often have degeneration along other neural pathways (cholinergic, noradrenergic, serotonergic, etc.; Dunois et al., 1983; Gaspar et al., 1991; Halliday et al., 1990; Kuhls et al., 1996; Rommelfanger and Weishenker 2007; Scatton et al., 1983; Stern et al., 1984). There are large individual differences in the degree of degeneration in these other pathways, and those differences can be relatively independent of each other and of the degree of dopaminergic decline (e.g., Bohnen and Albin, 2011; Dunois et al., 1983; Hall et al., 2014; Monchi et al., 2016; Müller and Bohnen, 2013; Perry et al., 1985; Pillon et al., 1989). These individual differences and their behavioral consequences can be leveraged to help illuminate the role that different neuromodulator pathways play in different aspects of cognition (see Calabresi et al., 2007; 2014; Frey, 2017 for reviews; see also Lopes et al., 2017 for functional connectivity measures).
We recently used this approach – using a PD patient sample with a particular neural degeneration as an natural experimental group – to demonstrate that thalamic cholinergic pathways play an important role in supporting the detection of targets that have strong “bottom-up” perceptual salience, whereas cortical cholinergic pathways support the ability to resist salient distractors (Kim et al., 2017; in press). Here we take a similar approach to examine how cholinergic and dopaminergic pathways contribute to executive functions, specifically the ability to overcome conflict from both long-term (Stroop) and short-term (task switching) response habits.
Executive function impairments are common in PD (Lees & Smith 1983; Dubois & Pillon 1996; McKinlay et al., 2010; Robbins & Cools, 2014), but the underlying neuropathology is not fully understood. The dual syndrome hypothesis (Kehagia et al., 2013), and the compensatory hypothesis (Bohnen et al., 2015; see also Bezard et al., 2003) take somewhat different views on the role of the cholinergic system. While the dual-syndrome hypothesis acknowledges some degree of overlap, it generally considers frontal-striatal dopaminergic declines as primarily responsible for executive control deficits in PD, and cholinergic degeneration as leading to memory, visuospatial, and psychiatric symptoms associated with PD dementia. In contrast, the compensatory hypothesis posits a more direct role for the cholinergic system. It proposes that frontostriatal dopamine declines may lead to compensatory reliance on (and possibly increased activity in) cortical cholinergic circuits.
By this view, patients who also have cortical cholinergic declines have reduced efficiency of cholinergically-mediated frontal-executive processes that might otherwise compensate for frontal-striatal dopaminergic losses. The compensatory hypothesis thus predicts that patients with both cholinergic and dopaminergic declines should have exacerbated executive function impairments, rather than the more global cognitive and psychiatric declines and prodromal dementia predicted to accompany cholinergic denervation by the dual-syndrome hypothesis.
The compensatory hypothesis is based in part on findings that a substantial proportion of patients with no apparent cognitive deficits, including on executive tasks, nonetheless had substantial denervation of caudate dopaminergic pathways (Bohnen et al., 2015). In contrast, cognitive impairment had a linear relationship with cortical cholinergic denervation. That is, even among nondemented patients, those with more severe cognitive deficits were more likely to have cholinergic denervation, usually accompanied by caudate dopaminergic denervation. Regression analyses indicated the caudate-dopaminergic and cortical-cholinergic pathways had both independent and interactive effects on cognitive outcomes. Furthermore, both the independent and interactive effects were stronger for executive-function tests than for verbal learning, psychomotor speed, or visuospatial measures.
The executive function measures examined in Bohnen et al. (2015) primarily tapped executive-control processes involved in planning and sequencing (Delis-Kaplan Executive Function System Sorting Test; WAIS III Picture Arrangement test). However, as noted earlier, executive control is a complex construct that incorporates a number of cognitive processes (see, e.g., Banich, 2009; Miyake et al., 2000), making it important to test whether the findings of Bohnen et al. are specific to planning and sequencing, or generalize to other aspects of executive control. Therefore, in the present study, we examine conflict processing, another important aspect of executive control.
Nondemented PD patients who had undergone PET assessments of cholinergic ([11C]PMP ligand) and dopaminergic ([11C]DTBZ ligand) function completed a modified version of the Stroop test that includes a rule-switching component (Bohnen et al., 1992). This task therefore places particularly high demands on executive-control processes, as on most trials participants must resist word-reading responses in order to name the ink color, but on occasional “switch” trials, they need to temporarily abandon the color-reading task set, return to word reading, and then immediately switch back to color-reading. In addition to the per se switching demands, this also makes it more difficult to maintain the “read the color” task set on the Stroop trials, increasing conflict (see discussions by Bugg et al., 2008; Kane & Engle, 2003, and others). The straightforward prediction from the dual-syndrome hypothesis is that measures of dopaminergic integrity should be the primary predictors of performance on both of these measures. In contrast, the compensatory hypothesis predicts an interaction between dopaminergic and cholinergic integrity, such that cortical-cholinergic integrity should be an especially important contributor to performance in patients with relatively low dopaminergic integrity.
Methods
Participants
All experimental procedures were approved by the University of Michigan’s Institutional Review Board, and were fully described to the participants before they consented to take part in the study. The data for the present study (PET and Stroop task) were collected in a combined session with other studies (Bohnen et al., 2015; Shah et al., 2016). Since the Stroop paradigm requires color discrimination, participation for the current study was limited to patients without color blindness. Thus, the patient sample of the current study was a subset of the Bohnen et al., 2015 study sample. Most of the participants underwent 2 PET imaging sessions, 1 MRI scanning session, and an entire day of motor/neuropsychological testing and received monetary compensation ($400) for their participation.
140 PD patients and 63 healthy controls (HC) participated in the study. Outliers whose overall behavioral performance (average Inverse Efficiency Score (IES) over 4 levels in the task, see Statistical Analysis) fell outside 3 standard deviations from the group means were excluded from the analyses (4 PD, 1 HC). One PD patient completed the dopaminergic and not the cholinergic scans due to technical problems. In order to keep a consistent dataset across analyses for the PD group, we omitted this person from the reported analyses. (Including this participant did not change any conclusions.) Thus, the final sample included a total of 135 PD patients (37 female, age range 50–84, mean age = 65.43) and 62 healthy older adults (37 female, age range 40–84, mean age = 65.17).
On average, motor symptom duration of the PD patients was 6.1 years (range, 5–19 years, standard deviation (SD) = 4.27) and the median Hoehn and Yahr severity score, assessed in the dopaminergic “off” state, was 2.5 (range, 1.0–5.0, SD = .58; Hoehn and Yahr, 1967). 128 patients were on dopaminergic treatment (average levodopa equivalent daily dose (LEDD; Tomlinson et al., 2010) was 744 mg, range 30–3180 mg). No patient was taking any cholinergic or anti-cholinergic medications.
Participants also completed neuropsychological tests evaluating affective, cognitive, and motor function. The measures included the Beck Depression Inventory II (BDI-II, Beck et al., 1961), the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), and the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS; copyright: Movement Disorder Society; Goetz et al., 2007). All participants had a minimum MoCA score of 19; as seen in Table 1 there were no differences between the HC and PD groups.
Table 1.
Demographic and behavioral performance of PD and HC. t & p corrected for violation of equal variances if applicable.
| HC (N = 62) | PD (N = 135) | t | p | Cohen's | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | d | |||
| Age (years) | 65.2 | 11.9 | 65.4 | 7.7 | −.2 | .879 | −.02 |
| Education (years) | 15.9 | 2.3 | 15.4 | 2.8 | 1.3 | .198 | .19 |
| Montreal Cognitive Assessment (MoCA) | 26.6 | 2.4 | 26.3 | 2.1 | .9 | .357 | .14 |
| Beck Depression Inventory (BDI) | 2.7 | 3.2 | 8.1 | 6.2 | −8.1 | ** | −1.00 |
|
| |||||||
| STR1 RT (s) | 49.2 | 9.4 | 54.2 | 11.6 | −3.0 | .003 | −.46 |
| STR2 RT (s) | 63.7 | 12.6 | 72.0 | 15.6 | −3.7 | ** | −.57 |
| STR3 RT (s) | 113.8 | 26.6 | 136.3 | 42.4 | −4.5 | ** | −.59 |
| STR4 RT (s) | 126.6 | 31.0 | 151.4 | 56.4 | −4.0 | ** | −.50 |
| STR1 error rate (%) | .02 | .13 | .13 | .74 | −1.7 | .096 | −.18 |
| STR2 error rate (%) | .23 | .66 | .61 | 1.26 | −2.9 | .005 | −.34 |
| STR3 error rate (%) | 1.47 | 2.62 | 3.02 | 4.49 | −3.1 | .003 | −.39 |
| STR4 error rate (%) | 1.82 | 3.28 | 3.60 | 5.45 | −2.8 | .005 | −.37 |
| STR1 IES | 49.23 | 9.45 | 54.34 | 11.84 | 3.0 | .003 | −.46 |
| STR2 IES | 63.86 | 12.63 | 72.51 | 16.04 | 3.8 | ** | −.58 |
| STR3 IES | 115.61 | 27.31 | 142.02 | 50.48 | 4.8 | ** | −.60 |
| STR4 IES | 129.43 | 33.61 | 159.70 | 69.60 | 4.1 | ** | −.50 |
M = mean, SD = standard deviation
indicates p < .0005.
Modified Stroop Task
All participants completed a modified version of the Stroop task (Bohnen et al., 1992) during laboratory testing (Figure 1). The task includes four levels that vary the stimulus presented (word or color patch) and the basis on which the participant is to respond (word meaning or ink color). Color-word meaning and ink colors were both drawn from a four-item set: red, yellow, green, and blue, with each used an equal number of times (25) within a level. For each level, the stimuli consisted of 100 items printed as a 10 by 10 array on a white letter size paper. At each level, the experimenter presented the stimulus set to the participants, gave the instruction, and recorded the total time participants took to complete the level and total number of errors in that level.
Figure 1. Task Procedure: Modified Stroop Task with rule-switching.
Participants were presented with 100 items in each level (in a 10 × 10 array), and asked to either read the color names (level I), name the ink color (level II, III), or switch between the rules (level IV). The total response time and accuracy were recorded for each level and converted into Inverse Efficiency Score (IES = RT / accuracy).
In level I (word-word), the stimuli were color words printed in black ink against a white background. Participants had to read the color words without stopping. In level II (patch-ink), the stimuli were color patches randomly intermixed, and participants were to say the ink color of each color patch without stopping. In level III (word-ink level), the stimuli were color words, each printed in a color incongruent with its meaning (i.e., the color word ‘red’ was never printed in red ink). Participants had to name the ink color and ignore word meaning. In level IV (word-ink-switch level), the stimulus set was similar as in level III, except that 20 items out of the total 100 were outlined with a box (in black ink). The boxes were randomly distributed over the 10 by 10 array. Participants were asked to say the ink color of the color words on most trials, but for the items outlined with the box they were to read the word. The difference score between levels III and II in this task indexes Stroop conflict effect, and the difference score between levels IV and II measures dual-conflict effect (Bohnen et al., 1992).
Positron Emission Tomography (PET)
PET data were collected using the same protocol as our previous studies (Kim et al., 2017; in press). To avoid potential confusion that might be introduced by different methodological descriptions across these papers, with permission of the editors we largely reproduce the text describing the PET data collection. Further details on the PET methodology can also be found in the Supplemental Methods and in Bohnen et al. (2012).
PD patients and HC underwent PET scanning that traces dopaminergic and cholinergic nerve terminal integrity. Patients came in for dopaminergic PET scanning in the dopaminergic off-state, i.e., after abstaining from dopaminergic drugs overnight. They resumed the dopaminergic medications immediately after the dopaminergic PET scanning before the 30-minute break before the cholinergic PET scanning. The PET scans were obtained close to the behavioral testing session (median 1 day; SD = 36.29; range 0 – 367 days; within 30 days for 184 out of 197 participants).
Although PD typically affects one side of the striatum more than the other, we report the values averaged over both hemispheres, rather than using separate predictors from the affected vs. unaffected brain side of the patients for the following reason: Unlike motor control functions, where the laterality of motor symptom severity is clearly associated with the (contra)lateral severity of striatal denervation, we did not have any concrete grounds on which to hypothesize on how the PD-effected side might influence the cognitive measures used here. To our knowledge, there is no evidence on the lateralization of striatal support of conflict or cognitive flexibility – particularly in association with PD - and our previous data did not show asymmetry in cholinergic denervation in PD (Bohnen et al., 2009). As a precaution, we did repeat our analyses using the measures from the clinically most affected side, and the conclusions were largely the same. Therefore, because averaged estimates typically provide more stable estimates than individual measures, we report the averaged measures for better quantification of our effects.
The integrity of dopaminergic nigrostriatal nerve terminals was measured with [11C]dihydrotetrabenazine (DTBZ), a vesicular mono- amine transporter type 2 analogue (VMAT2; see Bohnen et al., 2012 for details on DTBZ preparation, injection, and scanning parameters). The primary outcome parameter is DTBZ distribution volume ratio (DVR, Bohnen et al., 2009). Greater DVR indicates better dopaminergic terminal function. DTBZ DVR was measured for caudate and putamen. DTBZ PET scans were obtained for all PD and HC participants.
Cholinergic function was estimated using radio-labeled acetylcholine analogue [11C]methyl-4-piperidinyl propionate (PMP) PET, which measures acetylcholinesterase (AChE) activity. PMP PET scans were performed in the dopaminergic medication 'on' state. Details on PMP preparation, injection, and scanning parameters have been described previously (Bohnen et al., 2012). The primary outcome parameter is AChE hydrolysis rate (k3; min-1), with a higher k3 indicating higher cholinergic nerve terminal integrity. Although PMP PET is an indirect measure of cholinergic activity, it has been validated in both rodent and primate models (e.g, Kilbourn et al., 1996; Selden et al., 1998) as well as in humans (e.g., Kuhl et al., 1996; 1999). We are not aware of any evidence suggesting that dopaminergic therapy affects brain AChE hydrolysis rates. To the extent that dopaminergic therapy may affect cholinergic activity, its effects may be more likely to be on the availability of nicotinic or muscarinic receptors rather than AChE, given its long half-life of approximately 2.8 days (Wenthold et al., 1974).
AChE k3 was measured for cortex and thalamus, the target regions of two major cholinergic pathways, separately. Cortical measures are used to index cholinergic nerve terminal integrity of the basal forebrain (including the nucleus basalis of Meynert), whereas thalamic measures primarily (though not exclusively) reflect integrity of the brainstem pedunculopontine nucleus (Bohnen and Albin, 2011; Bohnen et al., 2012; see review by Varela, 2014).
Statistical Analysis
Response time (RT) and accuracy were recorded as the performance measures at each task level and then the RT was adjusted for accuracy (Inverse Efficiency Score (IES) = RT / correct (p); Townsend & Ashby, 1978; 1983). The IES difference between levels III and II was used as a measure of the Stroop conflict, and the IES difference between levels IV and level II was used as a measure of dual conflict (i.e., the Stroop and task-switching conflicts; Bohnen et al., 1992). Note that the inclusion of “switch” trials in the dual-conflict condition likely increases conflict on the Stroop trials (similar to manipulations of congruency; e.g., Bugg et al., 2008; Gonthier et al., 2016; Kane & Engle, 2003), so that the increase in demand from III to IV is nonlinear.
Independent-sample t-tests were used to compare the PD and HC groups on demographic measures and performance. First-level bivariate correlations were then used to characterize the relationships between the different measures, especially the neural predictors of the Stroop and dual-conflict effects. As described below, results for HC replicated previous effects of dopaminergic system status being negatively associated with age and positively associated with measures of executive function (e.g., Berry et al., 2016). A follow-up analysis controlled for age to test the assumption that age accounted for the dopamine-executive function correlation.
Our main hypotheses concerned the ability of cortical cholinergic integrity, caudate dopaminergic integrity, and their interaction, to predict the executive function measures. For ease of comparison with our prior studies examining the role of the thalamic cholinergic pathway in supporting bottom-up attention to salient signals and the cortical cholinergic pathway in supporting the ability to resist distraction from salient but irrelevant stimuli (Kim et al., 2017; in press) we first report analyses following the same series of hierarchical regression models used in those prior studies: The first step evaluated age as a predictor for the executive control functions (the Stroop and dual conflict measures separately). The second step added caudate DVR, cortical k3, and thalamic k3. The final step tested the critical interaction term (caudate-dopaminergic × cortical cholinergic). This final step in the hierarchical regression allows evaluation of whether the caudate-cortical interaction uniquely predicted performance over and above the shared variance with the other measures.
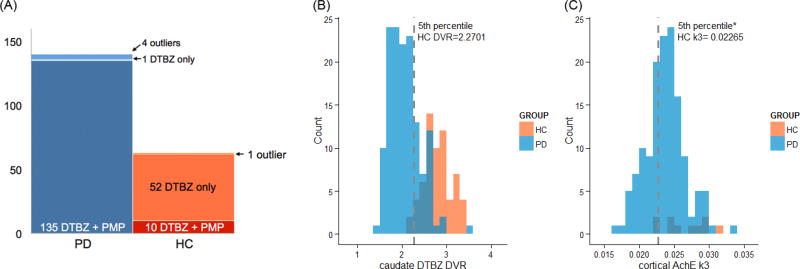
As an additional way to test and illustrate the hypothesized caudate-cortical interaction, we examined the correlation (and robust regression, see below) between each executive function measure and one of the PET measures (e.g., cortical AChE k3) in patient subgroups defined by the level of the other PET measure (e.g., low-, and normal- caudate DTBZ DVR). First, we tested the cortical cholinergic-executive function relationship in the low- and normal- dopaminergic groups. Next, we tested the caudate dopaminergic-executive function relationship in low- and normal- cortical cholinergic groups. The low-dopaminergic group was defined using the 5th percentile of DVR values in the healthy controls (n = 62). Due to the small number of HC with cholinergic PET measures in the present study (n = 10), the low-cholinergic group was defined using the cut-off score defined in a previous study from a larger HC sample (n = 29; Bohnen et al., 2012; using the 5th percentile of HC). Figure 2(B–C) shows the distribution of the DVR and k3 values in PD and HC overlaid with each other along with the cut-off values used to group patients into low- / normal- groups.
Figure 2. Sample size of the PET data and distributions of the caudate DVR and cortical k3 levels.
(A) Both types of PET scans were obtained for 135 out of total number of 140 PD patients. Dopaminergic PET was obtained for 62 HC, but cholinergic PET was limited to 10 HC. (B) The caudate DTBZ DVR measures of the PD patients are distributed in the lower range of the values obtained from the HC. 74.1% of the PD patients fall into the low caudate dopaminergic function group defined by the 5th percentile of the healthy controls. (C) Compared to the distribution of caudate DTBZ DVR measures, there is substantial overlap between the PD patient and HC distributions of the cortical AChE k3. About 30.4% of the PD patients fell into the low cortical cholinergic group defined by the 5th percentile of the healthy controls.
In each group of patients, bivariate first-level correlation analyses were first used to examine the relationship between the conflict effects and caudate dopaminergic function (in the low- and normal- cortical cholinergic groups) or cortical cholinergic function (in the low- and normal- caudate dopaminergic groups). Visual inspection of the initial scatterplot of this first-level correlation suggested potential influential cases, but formal outlier analyses did not identify these cases as unusual errors. As it is not extraordinary to have relatively large variance in patient data and we had no compelling reason to exclude these cases, we did not remove any case from the analyses. Instead, we additionally used robust regression (with Huber weighting) that weights observations differently depending on their residuals in linear regression (smaller weights for cases with larger residuals) to estimate the amount of variance in the executive functions explained by cortical cholinergic or caudate dopaminergic measures in each group. Age and thalamic k3 levels were controlled for in these correlation/robust regression analyses.
As effect sizes, we report Cohen’s d for t-tests, Pearson’s r for bivariate correlations, and standardized beta coefficient for multiple regression. G*power software (v 3.1., Faul et al., 2007; 2009) was used to estimate power for the critical multiple regression analyses.
Results
Demographic, neuropsychological tests, and overall performance data
Table 1 compares the HC (n = 62) and PD (n = 135) on demographic variables, neuropsychological test results, and behavioral performance in the task. PD and HC were comparable in age, education and general cognitive assessment (p > .1; absolute Cohen’s d < .20), but PD patients scored significantly higher in BDI (t = −8.1, p < .0005, Cohen’s d = −1.00). The higher BDI scores in PD are expected, as mild to moderate depressive symptoms occur in 40–50% of PD patients (Cummings, 1992; Tandberg et al., 1996). We therefore did not exclude participants on that basis. In all levels of the task, PD patients were slower and made more errors compared to HC (p < .01; Cohen’s d < −.33), except for the error rate in level I, where effects were marginal (p = .096; Cohen’s d = −.18).
Overall PET measures
Mean VMAT2 DTBZ DVR values for PD patients (n = 135) in the present study were mean (M) = 2.0710, SD = 0.3466 for caudate, M= 1.7743, SD = 0.2813 for putamen; M = 1.8732, SD=0.2881 for striatum (caudate and putamen together). For HC (n = 62), DVR values were M = 2.8188, SD = 0.3056 for caudate, M = 3.5763, SD = 0.3351 for putamen; M = 3.3238, SD = 0.3135 for striatum. For comparison, Bohnen et al. (2012) previously reported striatal DTBZ DVR values of M = 1.93, SD = 0.27 for PD (n=101) and M = 3.03, SD = 0.31 for HC (n = 29).
PMP PET was obtained from 135 PD and 10 HC. Mean PMP k3 values (min−1) in the present study were PD: cortical M = 0.0238, SD = 0.0028; thalamic M = 0.0546, SD = 0.0055; HC: cortical M = 0.0257, SD = 0.0031, thalamic M = 0.0608, SD = 0.0066. For comparison, the values reported from a larger sample in Bohnen et al. (2012) were PD (n = 101): cortical M=.0236, SD=.0027; thalamic M=.0542, SD=.0056); HC (n = 29): cortical M=.0263, SD=.0027; thalamic M=.0599, SD=.0074.
Stroop and dual-conflict effects correlate with caudate dopaminergic measures in both groups, and with cortical cholinergic measures in PD
Table 2 shows the first-level Pearson correlations between the performance measures, the PET measures of cholinergic and dopaminergic integrity, and individual difference variables (age and depression score (BDI)) that may contribute to variance on the performance and PET measures in the 62 HC (except for cholinergic PET measures, HC N = 10) and 135 PD patients with both the DTBZ and PMP PET measures. For the HC, correlations with the cholinergic PET measures represent data from only those 10 participants who completed the cholinergic scans. We report these for completeness, but do not interpret them given the small sample size.
Table 2.
Correlations between the behavioral measures, age, depression score (BDI), and the PET measures in HC (n=62, except for correlations with cholinergic PET measures, n =10) and PD (n = 135).
| Individual Characteristics |
Task Performance | Cholinergic PET |
Dopaminergic PET | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age | BDI | color naming |
Stroop conflict |
dual conflict |
thalamic k3 |
cortical k3 |
putamen DVR |
caudate DVR |
||||
| HC | Individual Characteristics | age | r | 1.00 | −.09 | .23 | .53 | .40 | .00 | .12 | −.28 | −.46 |
| p | .510 | .068 | ** | .001 | .999 | .746 | .030 | ** | ||||
| BDI | r | −.09 | 1.00 | −.01 | −.01 | −.04 | .23 | .28 | −.02 | .02 | ||
| p | .510 | .957 | .913 | .739 | .524 | .427 | .881 | .872 | ||||
| Task Performance | color naming | r | .23 | −.01 | 1.00 | .15 | .20 | −.20 | .05 | .03 | −.04 | |
| p | .068 | .957 | .233 | .114 | .583 | .894 | .798 | .784 | ||||
| Stroop conflict | r | .53 | −.01 | .15 | 1.00 | .72 | −.37 | −.23 | −.26 | −.32 | ||
| p | ** | .913 | .233 | ** | .290 | .529 | .039 | .011 | ||||
| dual conflict | r | .40 | −.04 | .20 | .72 | 1.00 | −.55 | −.47 | −.23 | −.30 | ||
| p | .001 | .739 | .114 | ** | .097 | .176 | .068 | .017 | ||||
| Cholinergic PET | thalamic k3 | r | .00 | .23 | −.20 | −.37 | −.55 | 1.00 | .82 | .10 | .09 | |
| p | .999 | .524 | .583 | .290 | .097 | .004 | .791 | .807 | ||||
| cortical k3 | r | .12 | .28 | .05 | −.23 | −.47 | .82 | 1.00 | .04 | .01 | ||
| p | .746 | .427 | .894 | .529 | .176 | .004 | .923 | .976 | ||||
| Dopaminergic PET | putamen DVR | r | −.28 | −.02 | .03 | −.26 | −.23 | .10 | .04 | 1.00 | .83 | |
| p | .030 | .881 | .798 | .039 | .068 | .791 | .923 | ** | ||||
| caudate DVR | r | −.46 | .02 | −.04 | −.32 | −.30 | .09 | .01 | .83 | 1.00 | ||
| p | ** | .872 | .784 | .011 | .017 | .807 | .976 | ** | ||||
| PD | Individual Characteristics | age | r | 1.00 | −.13 | .29 | .30 | .24 | −.25 | −.24 | .06 | −.19 |
| p | .148 | .001 | ** | .005 | .004 | .005 | .492 | .024 | ||||
| BDI | r | −.13 | 1.00 | .09 | .03 | .03 | .15 | .25 | −.15 | −.09 | ||
| p | .148 | .322 | .753 | .746 | .088 | .004 | .090 | .320 | ||||
| Task Performance | color naming | r | .29 | .09 | 1.00 | .48 | .56 | −.16 | −.17 | −.15 | −.34 | |
| p | .001 | .322 | ** | ** | .065 | .050 | .078 | ** | ||||
| Stroop conflict | r | .30 | .03 | .48 | 1.00 | .62 | −.11 | −.15 | −.13 | −.24 | ||
| p | ** | .753 | ** | ** | .212 | .076 | .124 | .005 | ||||
| dual conflict | r | .24 | .03 | .56 | .62 | 1.00 | −.17 | −.24 | −.14 | −.25 | ||
| p | .005 | .746 | ** | ** | .046 | .004 | .113 | .004 | ||||
| Cholinergic PET | thalamic k3 | r | −.25 | .15 | −.16 | −.11 | −.17 | 1.00 | .58 | .11 | .23 | |
| p | .004 | .088 | .065 | .212 | .046 | ** | .194 | .006 | ||||
| cortical k3 | r | −.24 | .25 | −.17 | −.15 | −.24 | .58 | 1.00 | .23 | .27 | ||
| p | .005 | .004 | .050 | .076 | .004 | ** | .008 | .002 | ||||
| Dopaminergic PET | putamen DVR | r | .06 | −.15 | −.15 | −.13 | −.14 | .11 | .23 | 1.00 | .80 | |
| p | .492 | .090 | .078 | .124 | .113 | .194 | .008 | ** | ||||
| caudate DVR | r | −.19 | −.09 | −.34 | −.24 | −.25 | .23 | .27 | .80 | 1.00 | ||
| p | .024 | .320 | ** | .005 | .004 | .006 | .002 | ** | ||||
indicates p < .0005
For the HC, neither caudate nor putamen dopamine measures predicted the speed of color naming used as a baseline measure for the executive-control tasks (p > .50), and the correlation between color-naming speed and age was only marginal (p = .07). Consistent with other findings linking increased age, reduced dopaminergic function, and executive control, the age, dopaminergic, and executive control (Stroop effect, dual-conflict effect) measures were moderately intercorrelated in the HC group. Controlling for age eliminated the ability of either dopaminergic measure to predict the performance measures (all r < .15, p > .25).
For the PD, color naming as well as the conflict effects showed significant correlations with age and caudate DVR (p < .01). Thalamic k3 showed significant correlations with age (p = .004) and dual conflict effects (p = .046). In addition, cortical k3 showed marginal to significant correlations with age (p = .005) and the behavioral measures (r = −.15 – −.24, p .08 – .004). Consistent with our previous findings (Kim et al., 2017, in press), age was correlated with the behavioral and the caudate and cortical PET measures (|r| ranges .19~.30, p < .05). Controlling for age reduced the correlation value by a small amount (approximately |r| .03–.06) for all measures (new values for caudate-DVR: color naming r = −.31, p < .0005, Stroop r = −.19, p = .32, dual-conflict r = −.21, p = .016; new values for corftical k3: color-naming r = −.09, p = .215, = .026, Stroop r = −.09, p = .32, dual conflict r = −.20, p = .023; compare to values in Table 2).
The caudate dopaminergic-cortical cholinergic interaction uniquely predicts Stroop and dual conflict effects in PD
A hierarchical regression model with three steps was used to test the compensatory hypothesis in the PD group. (Table 3 for the Stroop conflict effect; Table 4 for the dual conflict effect). In all of the regression models reported here, collinearity statistics were well within acceptable ranges (all tolerance values above .88; values above .10 are typically considered acceptable; all VIF values below 1.2; values below 10 are usually considered acceptable; Field, Miles, & Field 2012).
Table 3.
Hierarchical multiple linear regression model for the Stroop conflict effect in PD.
| coefficients | model statistics | power | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | T | p | R2 | Δ R2 | Δ F | sig. Δ F |
Model Fit F |
Model Fit p |
1−β | |
| step 1 model | .09 | .09 | 13.42 | ** | 13.42 | ** | .95 | ||||
| constant | .00 | .00 | 1.000 | ||||||||
| age | .30 | .30 | 3.66 | ** | |||||||
| step 2 model | .13 | .04 | 1.78 | .155 | 4.7 | .001 | .96 | ||||
| constant | .00 | .00 | 1.000 | ||||||||
| age | .26 | .26 | 3.05 | .003 | |||||||
| caudate DVR | 18 | −.18 | −2.07 | .040 | |||||||
| thalamic k3 | .03 | .03 | .33 | .739 | |||||||
| cortical k3 | 06 | −.06 | −.60 | .550 | |||||||
| step 3 model | .15 | .03 | 4.06 | .046 | 4.7 | .001 | .97 | ||||
| constant | 04 | −.53 | .601 | ||||||||
| age | .25 | .25 | 2.88 | .005 | |||||||
| caudate DVR | 24 | −.24 | −2.65 | .009 | |||||||
| thalamic k3 | .04 | .04 | .39 | .696 | |||||||
| cortical k3 | −.04 | −.04 | −.43 | .670 | |||||||
| caudate DVR * cortical k3 | .16 | .17 | 2.01 | .046 | |||||||
All variables standardized before being entered in the model. B, unstandardized coefficient; β, standardized coefficient.
indicates p < .0005
Table 4.
Hierarchical multiple linear regression model for the dual conflict effect in PD.
| coefficients | model statistics | power | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | R2 | Δ R2 |
Δ F | sig. Δ F |
Model Fit F |
Model Fit p |
1−β | |
| step 1 model | .06 | .06. | 8.28 | .005 | 8.28 | .005 | .83 | ||||
| constant | .00 | .00 | 1.000 | ||||||||
| age | .24 | .24 | 2.88 | .005 | |||||||
| step 2 model | .12 | .06 | 3.09 | .029 | 4.49 | .002 | .94 | ||||
| constant | .00 | .00 | 1.000 | ||||||||
| age | .17 | .17 | 1.99 | .049 | |||||||
| caudate DVR | −.17 | −.17 | −1.97 | .051 | |||||||
| thalamic k3 | .00 | .00 | .01 | .989 | |||||||
| cortical k3 | −.16 | −.16 | −1.52 | .131 | |||||||
| step 3 model | .15 | .03 | 4.69 | .032 | 4.63 | .001 | .97 | ||||
| constant | −.05 | −.56 | .574 | ||||||||
| age | .15 | .15 | 1.81 | .073 | |||||||
| caudate DVR | −.24 | −.24 | −2.60 | .010 | |||||||
| thalamic k3 | .01 | .01 | .07 | .943 | |||||||
| cortical k3 | −.14 | −.14 | −1.35 | .180 | |||||||
| caudate DVR * cortical k3 | .18 | .19 | 2.17 | .032 | |||||||
(N = 135, all PD patients with both the DTBZ and PMP PET measures). All variables standardized before being entered in the model. B, unstandardized coefficient; β, standardized coefficient.
indicates p < .0005
In the first model, age was entered as the only predictor, followed by the second model with caudate DVR, thalamic k3, and cortical k3 entered as additional predictors. In the final model, the caudate DVR-cortical k3 interaction term was added as a predictor. All three models reliably predicted the Stroop (Table 3, Model Fit p < .005) and dual conflict effects (Table 4, Model Fit p < .01). Importantly, adding the interaction term significantly increased the model fit for both the Stroop (Δ F = 4.06; p = .046) and dual conflict effects (Δ F = 4.69; p = .032), and the interaction term was a significant predictor over and above the effects of age or the individual PET measures (significant beta values for both Stroop and dual-conflict conditions; see Table 3).
Controlling for BDI and MoCA indices increased the model fit, suggesting that these variables accounted for additional variance in the conflict effects. Importantly, however, it made only miniscule changes to the b* values of the caudate-cortical interaction predictor (See Supplemental Materials). There was a slight change in significance associated with the interaction term due to the loss in degree of freedom that comes with adding additional variables in the model. We also conducted additional control analyses to confirm that our results were not confounded by the gap between PET and behavioral data collection or dopaminergic medication dosage (LEDD; See Supplemental Materials for details).
We next explored the interaction by dividing the patients into groups based on one measure (caudate DVR or cortical cholinergic) and then testing the effects of the other variable within each subgroup. That is, first, the cortical cholinergic-executive function relationship was examined in the low and normal dopaminergic groups separately. Then similarly, the caudate dopaminergic-executive control relationships were examined in the low and normal cholinergic groups separately. As described in the methods, the low and normal groups were defined using the 5th percentile value of the healthy control group (Figure 2 (B–C); Table 5 shows the sample size of each subgroup).
Table 5.
Resulting sample size of each PD subgroups
| cortical k3 | ||||
|---|---|---|---|---|
| low | normal | total | ||
| caudate DVR | Low | 36 | 64 | 100 |
| normal | 5 | 30 | 35 | |
| total | 41 | 94 | 135 | |
(low: 5th percentile of the normal controls)
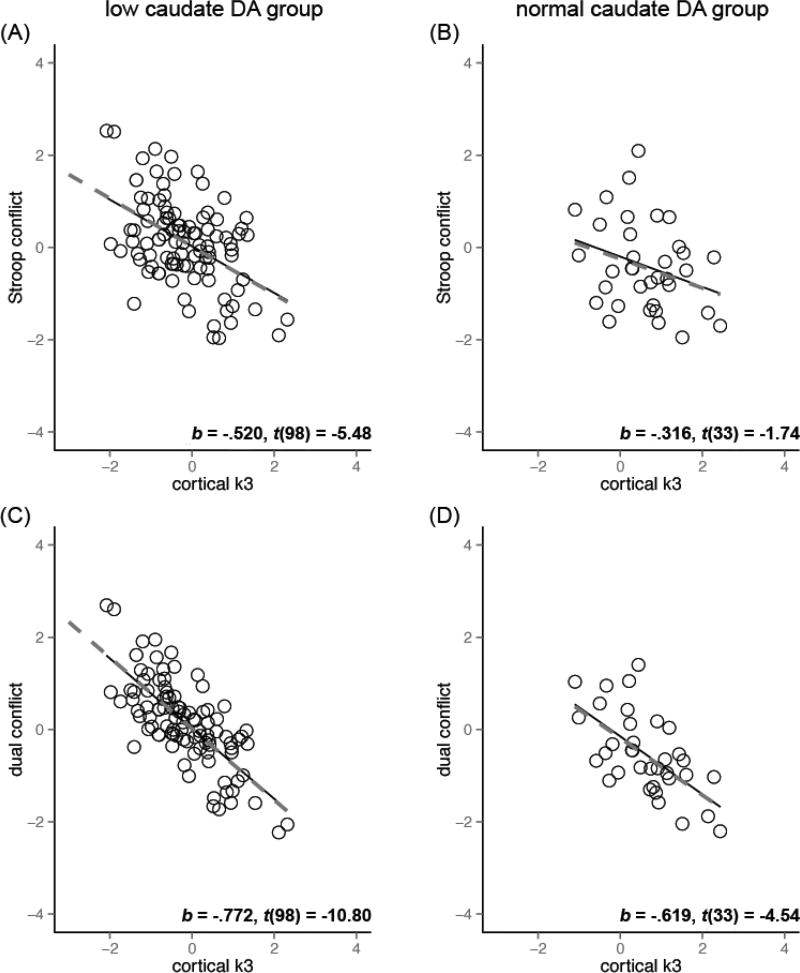
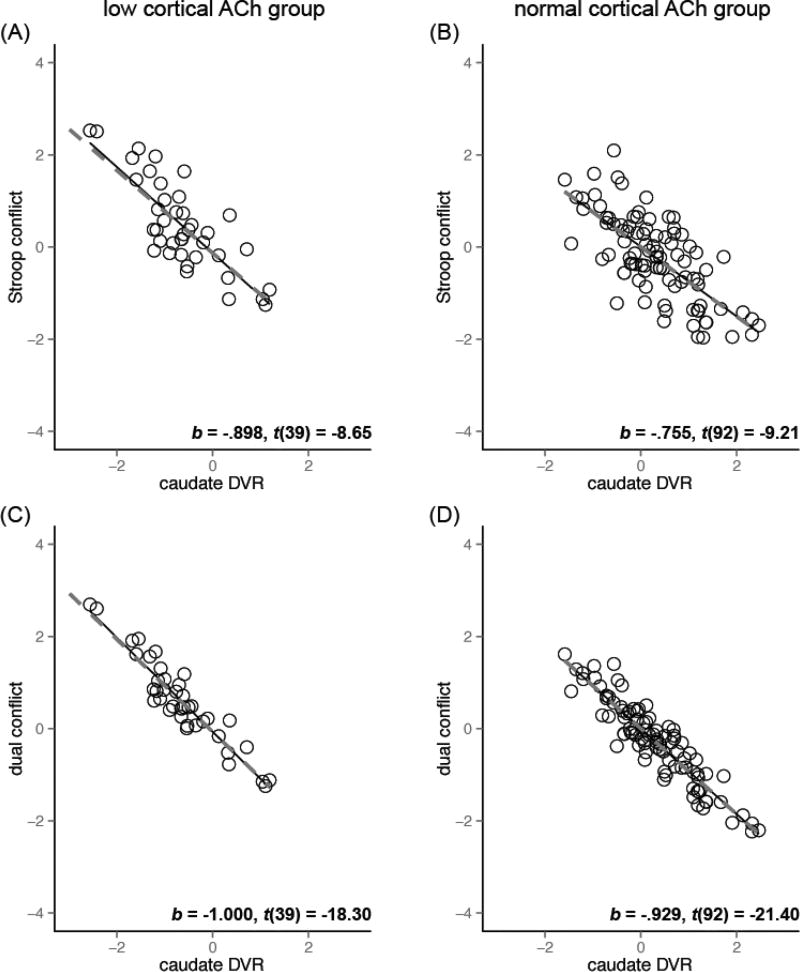
Figure 3 illustrates the relationship between cortical cholinergic function and the executive measures in the groups with low (A, C) and normal (B, D) caudate dopaminergic level, with the least square (solid lines) and robust regression (dashed lines) fit lines. Robust linear regression revealed cortical k3 as a stronger predictor of conflict effects in the low caudate dopaminergic group compared to the normal dopaminergic group (b* = −.52 as opposed to b* = −.32 for Stroop conflict, b* = −.77 as opposed to b* = −.62 for dual conflict). In contrast, caudate DVR was a strong predictor of conflict effects regardless of the cortical cholinergic groups (Figure 4, all | b* | > .75).
Figure 3. Cortical cholinergic function and conflict effects the in low and normal caudate dopaminergic group after controlling for age and thalamic k3.
Data shown are for the conflict effects residualized on age and thalamic k3). Left column: low caudate DA group. Right column: normal caudate DA group. (A, C) Cortical k3 was a significant predictor of conflict effects in all cases except for the Stroop conflict effect in the normal caudate DVR group (p = .06, other p’s < .0001). This association between cortical k3 and conflict effects were stronger in low caudate DVR group (A, C) than normal caudate DVR (B, D) group. The fit lines are based on the least squares (solid) and robust regression (dashed) results. The b*, t-value, and DF: t-test results are for the robust regression coefficients.
Figure 4. Caudate dopaminergic function and conflict effects the in low and normal cortical cholinergic group after controlling for age and thalamic k3.
Data shown are for the conflict effects residualized on age and thalamic k3. Left column: low cortical ACh group. Right column: normal cortical ACh group. High levels of caudate dopaminergic function were associated with smaller conflict effects in both low and normal cortical cholinergic groups (all p < .0001). The fit lines are based on the least squares (solid) and robust regression (dashed). The b*, t-value, and DF: t-test results are for the robust regression coefficients.
Discussion
Executive functions are complex, both in terms of the cognitive operations involved and in the brain structure and functions that support them. They are therefore among the last cognitive functions to fully develop, and often the first and most impaired by aging, brain injury, and disease (see, e.g., Casey et al., 2005; Cepeda & Kramer, 2001; Craik & Bialystock, 2006; Craik et al., 2006; Glisky, 2007; Luszxz, 2011; Paus 2005). These impairments often impact real-life functions including the management of finances and medication, job performance, and even the ability to participate in social interactions and understand jokes (e.g., Uekermann et al., 2007). However, a potential silver lining to this complexity is that it opens paths to compensation at both the behavioral and neural level. At the behavioral level, this often includes the increased use of environmental support, and modifying activities so that they do not exceed one’s level of ability (Craik & Byrd, 1982; Baltes, 1997). At the neural level, increased or altered patterns of activation, such as those measured by fMRI, are often associated with better performance and thus interpreted as compensation in many populations, including both patients and healthy adults (e.g., Cabeza et al., 2002; Eberling et al., 1997; Helmich et al., 2007; see reviews by Appel-Cresswell et al., 2010l Barulli & Stern, 2013; Bezard et al., 2003; Grady, 2008; Navntoft & Dreyer, 2016; Rajah & D’Esposito, 2005; Reuter-Lorenz & Lustig, 2005;). Here we used data from patients with PD to examine the potential for a different form of compensation, between the cholinergic and dopaminergic neuromodulator systems.
Our results are consistent with the hypothesis that the cholinergic and dopaminergic systems both play a role in executive function, and that each may compensate to some degree for declines in the other. Both the cortical-cholinergic and caudate-dopamine measures predicted conflict effects at the level of zero-order correlations, although only the caudate-dopamine term remained a significant independent predictor in the regression model. However, the cortical-cholinergic × caudate-dopaminergic interaction term explained a significant amount of variance in both Stroop and dual-conflict measures, beyond the effects of the individual predictors.
Furthermore, when the interaction term was entered into the model, the strength of the relation (beta value) between the caudate dopaminergic measure and the executive function measures increased, rather than decreased. This indicates that the cholinergic-dopaminergic interaction had a suppressor effect (MacKinnon, Krull, & Lockwood, 2000) on the link between caudate dopamine and executive function – that is, only after controlling for this interaction was the extent of impairment associated with caudate dopamine denervation revealed.
We cannot fully rule out the possibility of prodromal dementia in either the HC or PD, but our results generally do not support the idea that the effects found here are related to nonspecific neurocognitive decline. Neither the putamen-dopaminergic nor thalamic-cholinergic measures were related to either conflict measure. Furthermore, PD patients and HC had equivalent scores on the dementia screen(MoCA), and controlling for scores on this measure did not impact the conclusions.1
It is also important to note that other neuromodulator systems that were not measured here (e.g., serotonergic, noradrenergic) likely also make a contribution to executive control. However, studies in rodents, where specific neuromodulator systems can be targeted, provide supporting evidence for specifically cholinergic-dopaminergic interactions. For example, in a dual-lesion (cortical-cholinergic and striatal-dopaminergic) model of PD, the cholinergic lesion significantly affected rodents’ vulnerability to distraction and falls only in combination with caudate dopaminergic lesions and vice versa – neither cholinergic nor dopaminergic lesions had much impact on these measures by themselves (Kucinski et al., 2013). Pharmacologic enhancement of cholinergic function (donepezil and idalopirdine) improved the ability to recover from distractor effects, especially the ability to re-instate correct performance after a short disruption (Kucinski et al., 2017). In contrast, large dopaminergic lesions (without a cholinergic lesion) led to low vigor for and control over movement, but without apparent effects on attention-motor interactions (Kucinski et al., 2015).
This leads to the next obvious question about the current results: What does “compensation” between the dopaminergic and cortical cholinergic systems – and perhaps others - entail? Do they make equivalent contributions to executive control quantitatively (in terms of their relative importance and degree of variance accounted for), and/or qualitatively (do they support the same cognitive operations, or different ones)? Our ability to answer these questions is limited by the particulars of this study (including the use of a paper-and-pencil behavioral measure rather than a computerized one that might have allowed more fine-grained analyses of different trial types and of within-subject response-time variation). It is also important to note that we measured the striatal dopaminergic and cortical (and thalamic) cholinergic status of participants at a relatively broad level, not online activity of these systems during the tasks within specific (e.g., frontal-parietal) circuits.2 However, our results offer some promising clues, especially when considered in combination with other findings.
First, our results do support a primary role for caudate dopaminergic function in executive function. This of course comes with the caveat that dopaminergic denervation is a major feature of PD, but similar zero-order correlations between the caudate dopamine measure and the executive-control measures were also found for the healthy controls. Second, decomposition of the interaction (Figures 3 and 4) suggests that at least in the conditions tested here, cortical-cholinergic compensation for dopaminergic deficits is more prominent than the reverse. That is, especially for Stroop conflict, the relationship between cortical k3 and performance was stronger for the low-caudate group than for the normal-caudate group (Figure 3), whereas the relationship between caudate dopamine and performance was quite high for both low and normal cortical cholinergic groups (Figure 4).
Third, the cholinergic contribution was generally larger for the dual-conflict condition than for the Stroop conflict condition (Figure 4 A&B vs C&D), whereas the difference in the size of the cholinergic contribution for the low versus normal caudate-dopamine groups (i.e., the compensatory effect) was more prominent for the Stroop conflict measure than for the dual-conflict measure (Figure 4 A&C vs B&D). On the one hand, this might simply reflect the higher demands associated with dual conflict (Stroop + task-switching) than for Stroop alone. However, another possibility is that this reflects qualitative differences in the demands made by these conditions and the operations supported by striatal dopaminergic and cortical cholinergic function, respectively.
Caudate dopaminergic function has been linked to cognitive flexibility via updating and reinforcing stimulus-response associations with this function further modulated by reward (e.g., Cools et al., 2001; O’Doherty et al., 2004). This can also lead to attentional biasing towards previously-reinforced stimulus-response associations even when they are not appropriate (Anderson et al., in press). It has also been suggested that dopaminergic function may be involved in representations of task context (e.g., Braver & Barch, 2002), but it is not clear whether these context effects are caudate-specific. Some animal data suggest that dopaminergic modulation of top-down control depends at least in part in cooperation with cortical cholinergic circuits (St. Peters et al., 2011).
On the basis of this and other findings, we have suggested that cortical cholinergic function, particularly in fronto-parietal circuits, may operate at multiple timescales to support top-down control: a sustained (on the order of seconds to minutes) neuromodulatory effect helping to support the maintenance of relevant task sets when challenged by distractors (e.g., Berry et al., 2015; 2017), and a transient (millisecond scale) response involved in breaking out of the ongoing task and reactivating dormant task sets in response to an external cue (e.g., Howe et al., 2013; Parikh et al., 2007; Gritton et al., 2016; see Sarter, Parikh, & Howe, 2009 and Lustig & Sarter, 2015 for reviews of the human and animal literature; Sarter, Albin, Kucinski, & Lustig, 2014 for relevance to PD). Although in most cases they would work together to support goal-driven behavior, the hypothesized cholinergic contribution may be distinguished from the dopaminergic one in that the emphasis is on the more abstract level of task-set representations, rather than specific stimulus-response associations. (See also Kehagia et al., 2010, 2014 for rodent and patient evidence on the role of different frontal circuits and neuromodulators at different levels of task representation and implementation).
The PET data in the present study do not allow us to assess the temporal features of cholinergic activity, but the demands of each task suggest that the longer-term neuromodulatory component may be especially important for the Stroop task, whereas the dual-conflict task may also require the transient component’s involvement. The Stroop task requires overcoming habitual stimulus-response (i.e., word-reading) associations, and updating them to reflect the current task context and new stimulus-response association of color-reading. This stimulus-response updating function is consistent with ideas of dopaminergically-mediated processes.
However, Stroop performance can also be supported by (potentially cholinergically-modulated) top-down maintenance of the goal of focusing on the color information and preventing the currently-irrelevant word-information dimensions of the stimulus from entering the focus of attention (e.g., Bugg et al., 2008; Kane & Engle, 2003; Gonthier et al., 2016). For low-caudate dopamine patients, who may have particular difficulty in updating to the color-reading rather than word-reading response, the ability to maintain the task set of filtering out currently-irrelevant word information may be especially important. We previously showed that cortical cholinergic integrity, but not caudate dopaminergic integrity, predicted the ability to resist distraction from salient but irrelevant external stimuli (Kim et al., in press). Notably, the performance of low-cholinergic PD patients was similar to that of healthy individuals with a genetic polymorphism that reduces the ability to sustain cholinergic activity (Berry et al., 2014).
In contrast, the dual-conflict condition requires not only sustaining the task set of responding to the color information on most trials, but also the occasional re-activation of the dormant word-reading task set. The reactivation of dormant (after relatively long periods of disuse) task sets has been linked to transient basal forebrain-right prefrontal cholinergic activity in rodents (Parikh et al., 2007), and a series of cross-species experiments suggests that a similar transient right prefrontal activation observed using fMRI in humans during the re-activation of dormant task sets is cholinergically-mediated (Howe et al., 2013). Notably, optogenetic stimulation of these cholinergic transients can trigger responses associated with the previously-dormant response set even in the absence of the relevant stimulus (Gritton et al., 2016).
A potentially important difference between the current study and many studies of task-switching is that most task-switching studies use frequent switches, with both tasks active about 50% of the time; whereas in the current study the alternative word naming task was rare and occurred on only 20% of trials. The transient cholinergic response associated with re-activation of dormant task sets appears to be primarily active in the case of rare switches to the alternate rule, and to not be as critical when task-switching involves dynamically switching between rules that are both in a relatively active state due to recent use (Parikh et al., 2007; Howe et al., 2013).
The differences between (re)activating a relatively dormant task rule in response to rare switch demands versus dynamic switching between recently-used, and presumably highly-activated, conflicting stimulus-response associations may also explain why some aspects of our results differ from those observed by Beste et al. (2012). They tested healthy subjects and individuals with pre-symptomatic Huntington’s disease (“pre-HDs”) in a paradigm that has some similarities to our dual-conflict condition, but with both congruent and incongruent trials, and frequent (50% of trials) task switches. Pre-HDs had equivalent performance to controls on all trials except incongruent (e.g., BLUE printed in green ink) trials that required a task switch (word vs color reading, or vice versa), consistent with a specific role of dopaminergic modulation of frontal-striatal circuits in flexibly alternating between conflicting stimulus-response associations. Further, caudate volume correlated with electrocorticography (EEG) measures (N2 event-related potential amplitude and power in the delta band) associated with the selecting the correct response and inhibiting the incorrect one.
At a reviewer’s suggestion, we examined whether caudate volume was related to our PET measures or behavioral effects, but did not find any evidence for such. The different patterns between the two studies are likely due to both the differences in the physiological measures used (caudate volume and N2 are both likely to reflect the combined effects of several factors, whereas the PET measures assess more restricted aspects of functional activity that may precede measurable volume declines), and the tasks. In particular, the more frequent rule-switching used in the Beste et al. study is more consistent with the hypothesized contribution of caudate dopamine function to dynamic stimulus-response updating, as opposed to the relatively infrequent rule re-activation required in our study.
Compensation for reduced dopaminergic function may also rely more heavily on different systems in these more dynamic updating situations. Vazey et al. (2014, personal communication) found evidence for a compensatory role of norepinephrine in these cases: They tested task-switching (with equivalent rule-use) in a dual-lesion model similar to that used by Kucinsinki and colleagues, but in this case adding norepinephrine rather than cholinergic lesions to the striatal dopamine lesions. Only the dual-lesioned animals exhibited robustly impaired cognitive flexibility. This suggests that in the dopaminergic single lesion rodents, the intact norepinephrine system may have compensated for the functional impairment that would otherwise be induced by dopaminergic depletion (See also Berry et al., 2017 and Kehagia et al., 2010 for discussion of the potentially complementary and interacting roles of different neuromodulator systems in supporting cognitive control).
Together these findings suggest a heuristic framework in which frontostriatal dopaminergic function supports executive function through the reinforcement and updating of the appropriate stimulus-response associations for the current task context, cortical cholinergic modulation supports the (re)activation of more abstract task-goal representations after disuse or in the face of challenge, and norepinephrine helps support switching between task rules that are in relatively activated states. This conceptualization is considerably more complex than those that focus almost exclusively on the role of dopamine in executive function, but in the end will itself most likely prove to be overly simplistic. However, it may provide a useful guide for manipulating parameters (e.g., the level of abstraction and frequency of switches in task-switching) in both healthy and clinical populations to establish the relative contributions, and interactions, of these systems. Meanwhile, the present results, support the hypothesis that cortical cholinergic function can help compensate for dopaminergic declines to support executive function. Although the multi-system, interactive nature of executive function may make things more difficult for us as scientists, it may also provide avenues for compensation and remediation in patient populations and even healthy adults.
Supplementary Material
Acknowledgments
The authors thank all patients and volunteers for their time commitment. This work was supported by the Department of Veterans Affairs [grant number I01 RX000317]; the Michael J. Fox Foundation; and the NIH [grant numbers P01 NS015655 and RO1 NS070856 with additional support from P50 NS091856]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests.
As an additional check, we also examined the correlations between MoCA score and the PET measures for the PD patients. Both before and after controlling for age, the caudate DVR measure was if anything the strongest predictor of MoCA (before controlling for age: r = .17, p = .049; after controlling for age: r = .17, p = .057; the slight change in p value is most likely due to the loss of degrees of freedom entailed by the partial correlation). There was no relationship between MoCA and the cholinergic measures (thalamic or cortical) either before or after controlling for age, all r < .15, p > .20. Although the MoCA is an imperfect instrument, these results do not provide evidence for the hypothesis of prodromal dementia linked to cholinergic declines in this sample.
At a reviewer’s suggestion, we examined the possibility of differential effects across subregions. However, the subregion results were highly intercorrelated and co-linear, and factor analysis also returned them as a single factor, arguing against such separation. Despite this, to address the reviewer’s query we conducted separate models for each subregion (see Supplementary Materials). In general the results for the different subregions were in a similar direction to, but weaker than, the results found for the whole-cortical measure, and typically did not meet standard criteria for statistical significance. One exception was that the occipital cortex k3 × caudate DVR interaction for the dual-conflict condition was approximately as strong as the whole-cortex measure, possibly reflecting differential cholinergic declines in this region in PD (see, e.g., Shimada et al., 2009). However, given the relatively large number of comparisons when one considers each region separately, and the lack of an a priori reason to predict a particular relationship to the dual-conflict condition, we do not interpret this strongly but only report it for completeness.
References
- Anderson BA, Kuwabara H, Wong DF, Roberts J, Rahmim A, Brašić JR, Courtney SM. Linking Dopaminergic Reward Signals to the Development of Attentional Bias: A Positron Emission Tomographic Study. NeuroImage. 2017 May 30; doi: 10.1016/j.neuroimage.2017.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson's disease. Current opinion in neurology. 2010 Aug 1;23(4):407–12. doi: 10.1097/WCO.0b013e32833b6019. [DOI] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American psychologist. 1997 Apr;52(4):366. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Banich M. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in cognitive sciences. 2013 Oct 31;17(10):502–9. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961 Jun 1;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berry AS, Blakely RD, Sarter M, Lustig C. Cholinergic capacity mediates prefrontal engagement during challenges to attention: evidence from imaging genetics. Neuroimage. 2015 Mar 31;108:386–95. doi: 10.1016/j.neuroimage.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M, Lustig C. Disposed to distraction: Genetic variation in the cholinergic system influences distractibility but not time-on-task effects. Journal of cognitive neuroscience. 2014 Sep;26(9):1981–91. doi: 10.1162/jocn_a_00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Sarter M, Lustig C. Distinct Frontoparietal Networks Underlying Attentional Effort and Cognitive Control. Journal of Cognitive Neuroscience. 2017 Mar 2; doi: 10.1162/jocn_a_01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Baker SL, Vogel JW, O'Neil JP, Janabi M, Schwimmer HD, Marks SM, Jagust WJ. Aging affects dopaminergic neural mechanisms of cognitive flexibility. Journal of Neuroscience. 2016 Dec 14;36(50):12559–69. doi: 10.1523/JNEUROSCI.0626-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Ness V, Lukas C, Hoffmann R, Stüwe S, Falkenstein M, Saft C. Mechanisms mediating parallel action monitoring in fronto-striatal circuits. Neuroimage. 2012 Aug 1;62(1):137–46. doi: 10.1016/j.neuroimage.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends in neurosciences. 2003 Apr 30;26(4):215–21. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Müller ML, Petrou M, Kotagal V, Koeppe RA, Scott PJ, Frey KA. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA neurology. 2015 Feb 1;72(2):194–200. doi: 10.1001/jamaneurol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behavioural brain research. 2011 Aug 10;221(2):564–73. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Jolles J, Twijnstra A. Modification of the Stroop Color Word Test improves differentiation between patients with mild head injury and matched controls. The clinical neuropsychologist. 1992 Apr 1;6(2):178–84. doi: 10.1080/13854049208401854. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009 Nov 17;73(20):1670–6. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain. 2010 Apr 22;133(6):1747–54. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. Journal of Cerebral Blood Flow & Metabolism. 2012 Aug;32(8):1609–17. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience & Biobehavioral Reviews. 2002 Nov 30;26(7):809–17. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Jacoby LL, Toth JP. Multiple levels of control in the Stroop task. Memory & Cognition. 2008;36(8):1484–1494. doi: 10.3758/MC.36.8.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002 Nov 30;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Galletti F, Saggese E, Ghiglieri V, Picconi B. Neuronal networks and synaptic plasticity in Parkinson's disease: beyond motor deficits. Parkinsonism & related disorders. 2007 Dec 31;13:S259–62. doi: 10.1016/S1353-8020(08)70013-0. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nature neuroscience. 2014 Aug 1;17(8):1022–30. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in cognitive sciences. 2005 Mar 31;9(3):104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather J. Changes in executive control across the life span: examination of task-switching performance. Developmental psychology. 2001 Sep;37(5):715. [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001 Dec 1;124(12):2503–12. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Corkin HMS, Scoville WB. Lasting Consequences of Bilateral Medial Temporal Lobectomy: Clinical Course and Experimental Findings. Semin IN Neurol. 1984;4 [Google Scholar]
- Craik FI, Byrd M. Aging and cognitive processes. Springer; Boston, MA: 1982. Aging and cognitive deficits; pp. 191–211. [Google Scholar]
- Craik FI, Byrd M. Aging and cognitive processes. Springer US; 1982. Aging and cognitive deficits; pp. 191–211. [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends in cognitive sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok E, Bialystock E. On structure and process in lifespan cognitive development. Lifespan cognition: Mechanisms of change. 2006:3–14. [Google Scholar]
- Cummings JL. Depression and Parkinson's disease: a review. The American journal of psychiatry. 1992 Apr 1;149(4):443. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Depression and Parkinson’s disease: A review. The American Journal of Psychiatry. 1992;149(4):443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. Journal of neurology. 1996 Nov 1;244(1):2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Dunois B, Ruberg M, Javoy-Agid F, Ploska A, Agid Y. A subcortico-cortical cholinergic system is affected in Parkinson's disease. Brain research. 1983 Dec 12;288(1):213–8. doi: 10.1016/0006-8993(83)90096-3. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Bankiewicz KS, Jordan S, VanBrocklin HF, Jagust WJ. PET studies of functional compensation in a primate model of Parkinson's disease. Neuroreport. 1997 Aug 18;8(12):2727–33. doi: 10.1097/00001756-199708180-00017. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007 May 1;39(2):175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Field A, Miles J, Field Z. Discovering statistics: Using R. Washington, DC: Sage Publication Ltd; 2012. [Google Scholar]
- Frey KA. Seminars in nuclear medicine. 1. Vol. 47. Elsevier; 2017. Jan 1, Updates in Molecular Brain Imaging; pp. 2–4. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson's disease. Annals of neurology. 1991 Sep 1;30(3):365–74. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. Brain aging: models, methods, and mechanisms. 2007:3–20. [Google Scholar]
- Goetz CG, Fahn S, MartinezMartin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Movement Disorders. 2007 Jan 1;22(1):41–7. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Gonthier C, Braver TS, Bugg JM. Dissociating proactive and reactive control in the Stroop task. Memory & Cognition. 2016;44(5):778–788. doi: 10.3758/s13421-016-0591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Knodt AR, Hariri AR. Basal forebrain moderates the magnitude of task-dependent amygdala activity. Social Cognitive and Affective Neuroscience. 2015;10(4):501–507. doi: 10.1093/scan/nsu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008 Mar 1;1124(1):127–44. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proceedings of the National Academy of Sciences. 2016 Feb 23;113(8):E1089–97. doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014 Jul 24;137(9):2493–508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB. Loss of brainstem serotonin-and substance P-containing neurons in Parkinson's disease. Brain research. 1990 Feb 26;510(1):104–7. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery after passage of an iron bar through the head. Publ Massachusetts Med Soc [Google Scholar]
- Helmich RC, de Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia. 2007 Dec 31;45(10):2201–15. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism onset, progression, and mortality. Neurology. 1967 May 1;17(5):427-. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. Journal of Neuroscience. 2013 May 15;33(20):8742–52. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DM, Kilbourn MR, Lee LC. A simple synthesis of [11 C] dihydrotetrabenazine (DTBZ) Nuclear medicine and biology. 1997 Feb 28;24(2):197–9. doi: 10.1016/s0969-8051(96)00213-2. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegenerative diseases. 2013;11(2):79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Revisiting the effects of Parkinson's disease and frontal lobe lesions on task switching: the role of rule reconfiguration. Journal of neuropsychology. 2014 Mar 1;8(1):53–74. doi: 10.1111/jnp.12004. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Current opinion in neurobiology. 2010 Apr 30;20(2):199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Snyder SE, Sherman PS, Kuhl DE. In vivo studies of acetylcholinesterase activity using a labeled substrate, N-[11 C] methylpiperdin-4-yl propionate ([11 C] PMP) 1996 doi: 10.1002/(SICI)1098-2396(199602)22:2<123::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kim K, Müller ML, Bohnen NI, Sarter M, Lustig C. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson’s disease patients with defined cholinergic losses. NeuroImage. 2017 Apr 1;149:295–304. doi: 10.1016/j.neuroimage.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Müller ML, Bohnen NI, Sarter M, Lustig C. The cortical cholinergic system contributes to the top-down control of distraction: Evidence from patients with Parkinson's disease. NeuroImage. 2017 Dec 19; doi: 10.1016/j.neuroimage.2017.12.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Jong IE, Sarter M. Reducing falls in Parkinson's disease: interactions between donepezil and the 5-HT6 receptor antagonist idalopirdine on falls in a rat model of impaired cognitive control of complex movements. European journal of neuroscience. 2017 Jan 1;45(2):217–31. doi: 10.1111/ejn.13354. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M. Modeling Fall Propensity in Parkinson's Disease: Deficits in the Attentional Control of Complex Movements in Rats with Cortical-Cholinergic and Striatal–Dopaminergic Deafferentation. Journal of Neuroscience. 2013 Oct 16;33(42):16522–39. doi: 10.1523/JNEUROSCI.2545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Sarter M. Modeling Parkinson’s disease falls associated with brainstem cholinergic systems decline. Behavioral neuroscience. 2015 Apr;129(2):96. doi: 10.1037/bne0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA, Kilbourn MR. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology. 1999 Mar 1;52(4):691–691. doi: 10.1212/wnl.52.4.691. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, Ficaro EP, Wieland DM, Koeppe RA, Frey KA, Foster NL. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Annals of neurology. 1996 Sep 1;40(3):399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain. 1983 Jun 1;106(2):257–70. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Lopes R, Delmaire C, Defebvre L, Moonen AJ, Duits AA, Hofman P, Leentjens AF, Dujardin K. Cognitive phenotypes in parkinson's disease differ in terms of brain-network organization and connectivity. Human brain mapping. 2017 Mar 1;38(3):1604–21. doi: 10.1002/hbm.23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Sarter M. Translational Neuropsychopharmacology. Springer, Cham; 2015. Attention and the cholinergic system: relevance to schizophrenia; pp. 327–362. [DOI] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. Handbook of the psychology of aging. 2011;7:59–72. [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention science. 2000 Dec 1;1(4):173–81. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Characteristics of executive function impairment in Parkinson’s disease patients without dementia. Journal of the International Neuropsychological Society. 2010 Mar;16(2):268–77. doi: 10.1017/S1355617709991299. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki A, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cognitive Psychology, 2000. 2000;41(1):49–199. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Hanganu A, Bellec P. Markers of cognitive decline in PD: the case for heterogeneity. Parkinsonism & related disorders. 2016 Mar 31;24:8–14. doi: 10.1016/j.parkreldis.2016.01.002. Monchi. [DOI] [PubMed] [Google Scholar]
- Müller ML, Bohnen NI. Cholinergic dysfunction in Parkinson’s disease. Current neurology and neuroscience reports. 2013 Sep 1;13(9):377. doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005 Apr 1;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Navntoft CA, Dreyer JK. How compensation breaks down in Parkinson's disease: Insights from modeling of denervated striatum. Movement Disorders. 2016 Mar 1;31(3):280–9. doi: 10.1002/mds.26579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann SA, Sarah M, Ferrell RE, Flory Jd, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biological Psychiatry. 2006;60(10):1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- O'doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. science. 2004 Apr 16;304(5669):452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. Journal of Neuroscience. 2013;33(19):8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007 Oct 4;56(1):141–54. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in cognitive sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, Blessed G, Fairbairn A, Tomlinson BE, Perry RH. Cholinergic correlates of cognitive impairment in Parkinson9s disease: comparisons with Alzheimer9s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1985 May 1;48(5):413–21. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MS, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic–corticopetal cholinergic circuitry. Journal of Neuroscience. 2011 Jun 29;31(26):9760–71. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Cusimano G, Bonnet AM, Lhermitte F, Agid Y. Does cognitive impairment in Parkinson's disease result from non-dopaminergic lesions? Journal of Neurology, Neurosurgery & Psychiatry. 1989 Feb 1;52(2):201–6. doi: 10.1136/jnnp.52.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005 Jul 27;128(9):1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Current opinion in neurobiology. 2005 Apr 30;15(2):245–51. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cools R. Cognitive deficits in Parkinson's disease: a cognitive neuroscience perspective. Movement Disorders. 2014 Apr 15;29(5):597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochemical pharmacology. 2007 Jul 15;74(2):177–90. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Experimental neurology. 2014 Jul 31;257:120–9. doi: 10.1016/j.expneurol.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature Reviews Neuroscience. 2009 May 1;10(5):383–90. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain research. 1983 Sep 26;275(2):321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milnder B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/JNNP.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain: a journal of neurology. 1998 Dec 1;121(12):2249–57. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shah N, Frey KA, LTM Müller M, Petrou M, Kotagal V, Koeppe RA, Scott PJ, Albin RL, Bohnen NI. Striatal and Cortical β-Amyloidopathy and Cognition in Parkinson's Disease. Movement Disorders. 2016 Jan 1;31(1):111–7. doi: 10.1002/mds.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Hirano S, Shonotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Tluczek L, Jewett DM, Nguyen TB, Kuhl DE, Kilbourn MR. Synthesis of 1-[11 C] methylpiperidin-4-yl propionate ([11 C] PMP) for in vivo measurements of acetylcholinesterase activity. Nuclear medicine and biology. 1998 Nov 30;25(8):751–4. doi: 10.1016/s0969-8051(98)00045-6. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Côté L. Reaction time and vigilance in Parkinson's disease: possible role of altered norepinephrine metabolism. Archives of Neurology. 1984 Jan Oct;41(10):1086–9. doi: 10.1001/archneur.1984.04050210084021. [DOI] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease: a community-based study. Archives of neurology. 1996 Feb 1;53(2):175–9. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement disorders. 2010 Nov 15;25(15):2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. Methods of modeling capacity in simple processing systems. Cognitive theory. 1978;3:200–39. [Google Scholar]
- Townsend JT, Ashby FG. Stochastic modeling of elementary psychological processes. CUP Archive. 1983 [Google Scholar]
- Uekermann J, Channon S, Daum I. Humor processing, mentalizing, and executive function in normal aging. Journal of the International Neuropsychological Society. 2006 Mar;12(2):184–91. doi: 10.1017/S1355617706060280. [DOI] [PubMed] [Google Scholar]
- Varela C. Thalamic neuromodulation and its implications for executive networks. Frontiers in neural circuits. 2014;8 doi: 10.3389/fncir.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C. Thalamic neuromodulation and its implications for executive networks. Front. Neural Circuit. 2014;8:69. doi: 10.3389/fncir.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Mahler HR, Moore WJ. The half-life of Acetylcholinesterase in mature rat brain. Journal of neurochemistry. 1974 Jun 1;22(6):941–3. doi: 10.1111/j.1471-4159.1974.tb04319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.