Abstract
INTRODUCTION
Sexual assault (SA) is alarmingly common and is associated with increased risk of psychiatric and medical conditions. However, many prior studies are limited to cross-sectional designs. Healthcare systems with electronic health records (EHRs) provide unique longitudinal data to examine whether SA is associated with changes in health and healthcare utilization.
METHODS
The sample included 1,350 Kaiser Permanente Northern California adult female patients with a SA diagnosis from 2009–2015 and 4,050 adult female patients without a SA diagnosis, matched on age, medical facility, and continuous enrollment during the study period. Using a retrospective cohort design, we tested whether an SA diagnosis was associated with twelve-month changes in psychiatric and medical comorbidities and healthcare utilization using difference-in-difference models. Analyses were conducted in 2017.
RESULTS
Patients with a SA diagnosis had a higher prevalence of psychiatric and medical comorbidities and greater healthcare utilization than matched patients without SA in the 12-months before the SA diagnosis, and greater increases in the prevalence of psychiatric disorders and stress-related somatic conditions and psychiatry and obstetrics/gynecology utilization (all p<0.001) 12-months after the SA diagnosis, relative to matched non-SA patients during this time.
DISCUSSION
SA is associated with increases in psychiatric disorders and stress-related somatic conditions as well as increases in utilization of psychiatry and obstetrics/gynecology. Clinicians should be trained in how to inquire about, respond to, and refer women who have experienced SA.
INTRODUCTION
Sexual assault (SA) is a critical public health concern. Up to 1 in 5 US women have been raped and an estimated 44% have experienced sexual violence other than rape in their lifetime.(1) Adolescents and young adults are at highest risk for SA, with nearly 4 in 5 victims of rape reporting that it first occurred before they were 25 years old.(1) In addition, low–income status, African American race, and previous SA are vulnerability factors for SA.(2)
SA is associated with multiple physical and mental health conditions. Women who experience SA are more likely to have chronic diseases (e.g., asthma, diabetes, chronic pain, hypertension, high cholesterol), gastrointestinal problems, gynecological and reproductive problems, sexually transmitted diseases, and poor self-reported health than women who do not experience SA.(2–6) SA is also associated with elevated mental health problems, including posttraumatic stress disorder (PTSD), depressive disorders, anxiety disorders, eating disorders, substance use disorders, cigarette smoking, difficulties sleeping and suicidal ideation.(2)
Despite elevated medical and psychiatric healthcare needs among SA patients, few studies have examined whether physical and mental health and healthcare utilization patterns change following SA. Existing studies have primarily relied on cross-sectional data and retrospective self-reported health and healthcare utilization, and longitudinal studies have typically not used adequate comparison groups of women without SA.(2) Thus, the extent to which these conditions are present before SA, and might represent risk factors for SA that should be screened for, remains unclear. While some studies indicate that use of healthcare services increases among women after SA,(7, 8) others have not found an association.(9) A recent retrospective cohort study found significantly higher incidence of somatic disorders and visits to a general practitioner in women with SA both before and after the assault relative to matched women without SA during the same time period.(10) However, this study did not include utilization of other health care services, including specialty treatment (e.g., psychiatry) and the emergency department, and analyses did not compare changes in diagnoses and healthcare utilization between SA and matched patients using multivariable models that control for secular trends.
Large integrated healthcare systems offer the opportunity to overcome these limitations by examining electronic health records (EHRs) to determine whether documented SA is associated with changes in health and healthcare utilization. This study addresses key gaps in the literature using a matched retrospective cohort design and difference-in-difference (DiD) framework to assess whether women with documented SA in the EHR had greater increases in psychiatric and medical conditions and healthcare utilization from the year before SA documentation (baseline) to the year after SA (follow-up), relative to matched women without a SA diagnosis during the same time period.
METHODS
Setting
Kaiser Permanente Northern California (KPNC) is a multi-specialty, non-profit healthcare delivery system providing comprehensive healthcare services to >4 million racially and socio-economically diverse patients representative of the Northern California population.(11),(12) Institutional review board approval for this study was obtained from KPNC.
Study Population
The initial study cohort comprised 2,650 women aged ≥18 with an adult SA diagnosis between January 1, 2009 and September 13, 2015 (when the transition to ICD10 codes occurred).
Patients without continuous enrollment in KPNC (defined as gaps <6 months) from 12-months prior through 12-months after documented SA, and those without an identified medical facility (N=1, 189) were excluded. After these exclusions, 1,461 eligible SA patients remained in the study.
Next, we randomly matched each woman with documented SA to 3 women without documented SA between January 1, 2009 and September 13, 2015, on continuous enrollment during the study, birth year, and medical facility (to account for potential differences in services, conditions, or unobservable differences by geographic region). The 111 SA patients who could not be matched to non-SA patients (on continuous enrollment, birth year and medical facility) did not differ significantly from those who were matched to non-SA patients on key demographics (age, race/ethnicity, median neighborhood family income) and were excluded, resulting in a final cohort of 1,350 patients with documented SA and 4,050 matched non-SA patients across 53 medical facilities.
Measures
Sexual Assault (SA)
Using ICD9 code searches and text searches among diagnoses, we identified the following three ICD9 codes associated with documented adult SA: V71.5 (Observation Following Alleged Rape or Seduction), E960.1 (Rape), and 995.83 (Adult Sexual Abuse). Patients were coded as having a documented instance of adult SA if they had an associated diagnosis code between January 1, 2009 and September 13, 2015. The first SA diagnosis during the study period was selected as the index date. As we were interested in SA specifically, other types of abuse (eg., psychological abuse, physical abuse, other adult abuse and neglect) were not included.
Demographics
Data on age group, race/ethnicity, and median neighborhood household income (geocoded from census data using patients’ addresses) were extracted from the EHR.
Comorbidities
We examined medical and psychiatric conditions at baseline and follow-up focusing on conditions that have been associated with SA in the literature.(2–6)
Diagnoses were extracted from the EHR using ICD9 and ICD10 codes and included, psychiatric disorders (anxiety disorder, depressive disorder, eating disorder, bipolar disorder, PTSD), substance use disorders (alcohol use disorder, drug use disorder), stress-related somatic conditions (stress, insomnia, palpitations, fatigue, urticaria), gastrointestinal conditions (dyspepsia, irritable bowel syndrome, peptic ulcer), pain-related diagnoses (pelvic pain, abdominal pain, headache, fibromyalgia, chronic, neck and back pain), and genitourinary system conditions (incontinence, vulvodynia, pain during intercourse, vaginitis, sexually transmitted infections) (Appendix 1).
Smoking status was obtained through routine screening by medical assistants during medical visits. Patients were considered smokers if they reported being a current smoker at any medical visit in the 12-month baseline or follow-up periods.
Body mass index (BMI) was calculated based on weight and height measurements recorded in the EHR. If there were multiple BMIs during the 12-month period, the largest BMI was selected. Patients with a BMI ≥30 were categorized as obese.
Healthcare Utilization
Healthcare utilization was extracted from the EHR and characterized into Psychiatry, Chemical Dependency, Primary Care, Obstetrics/Gynecology, and Emergency Department visits. Patients were coded as utilizing healthcare services at baseline or follow-up if they had ≥1 visit during the 12-month period. We also examined the number of healthcare visits at baseline and follow-up.
Statistical Analysis
Analyses were conducted in 2017 using SAS 9.3. We compared the distribution of demographic variables between SA and matched non-SA patients using chi-square tests for categorical variables and t-tests for continuous variables. We calculated the prevalence of each comorbidity at baseline and follow-up and tested whether the prevalence rates differed between SA and matched non-SA patients at each time point. We also tested whether the prevalence of comorbidities differed significantly between baseline and follow-up, separately among SA and matched non-SA patients. These analyses were repeated for healthcare utilization.
We next assessed whether SA was associated with changes in the prevalence of comorbidities and healthcare utilization by fitting models using a difference-in-difference (DiD) framework. This analytical approach estimates the difference in pre-post changes in an outcome (comorbidity, healthcare utilization) comparing an exposed group (SA) to an unexposed (non-SA) group,(13) while controlling for potential biases due to secular trends and confounding. That is, under the assumption that the trend in outcomes over time would have been the same between the two matched groups in the absence of SA exposure, the DiD methods would provide unbiased effect estimates of SA on the outcomes examined.
For each of the comorbid conditions examined, we fitted a Logistic regression model and reported the Odds Ratio (OR) along with 95% confidence interval derived from the coefficient of the interaction indicator of SA × time (baseline or follow-up). For each type of healthcare except Chemical Dependency, we fitted a Poisson regression model on counts of visits and reported the incidence rate ratio (IRR) along with 95% confidence interval derived from the coefficient of the interaction indicator of SA × time. Because only a small proportion of SA and non-SA patients had a Chemical Dependency visit, we examined it as a binary outcome by fitting a Logistic regression model. All models adjusted for race/ethnicity and median neighborhood household income. To adjust for multiple comparisons, we set our threshold for statistical significance at p<0.001 using a conservative Bonferroni correction.
RESULTS
Among patients with documented SA, 64% had documented adult SA, 22% had documented observation following alleged rape or seduction, 5% had documented rape, and 9% had more than one type of SA documentation. The SA sample was 48% Non-Hispanic White, 23% Hispanic, 15% Black, 8% Asian/Hawaiian Pacific Islander, and 6% Other, with a mean age of 34 (SD = 13.8), and average median neighborhood household income of $67,146. Compared to patients with SA who were included in the study, those excluded (see Methods) were more likely to be Non-Hispanic White (53%) or Black (19%) and less likely to be Hispanic (19%), Asian/Hawaiian Pacific Islander (6%) or Other (4%), p<0.0001. They were slightly younger (mean age = 32, p<0.0001) with a lower average median neighborhood household income ($59,009, p<0.0001). Compared to matched non-SA patients, SA patients were more likely to be African American and less likely to be Asian than matched patients (15% vs. 8% and 8% vs. 20% respectively, p< 0.001), and had lower average median neighborhood household income than matched patients ($61,389 vs. $69,375, p< 0.001) (not shown).
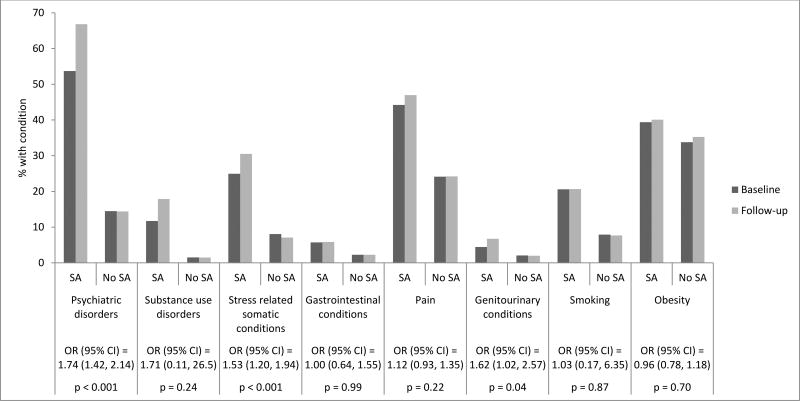
SA patients had a higher prevalence of nearly all comorbidities than matched non-SA patients at baseline and follow-up (Table 1). At baseline, among SA patients, the prevalence of psychiatric disorders was nearly fourfold that of matched patients (53.7% vs 14.5%, p<0.001), and substance use disorders were nearly eight times as common (11.7% vs 1.5%, p<0.001). SA patients were also more likely to have diagnosed stress-related somatic conditions (25.0% vs 8.1%, p<0.001), gastrointestinal conditions (5.7% vs 2.3%, p<0.001), pain diagnoses (44.2% vs 24.1%, p<0.001), and genitourinary conditions (4.4 vs 2.1%, p<0.001), and were more likely to be obese (39.4% vs 33.8%, p<0.001) and to report smoking (20.6% vs 7.9%, p<0.001) at baseline relative to matched non-SA patients. These differences remained significant between SA patients and matched patients at follow-up.
Table 1.
Comorbidities Diagnoses at Baseline and Follow-up among Patients with Documented Sexual Assault (SA) and Matched Non-SA Patients.
| Comorbidity | Baseline (N = 5,400) | Follow-Up (N = 5,400) | P-value comparing baseline vs. follow-up among |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| % of SA patients (N = 1,350) |
% of non-SA patients (N = 4,050) |
P-value | % of SA patients (N = 1,350) |
% of non-SA patients (N = 4,050) |
P-value | SA patients (N = 1,350) |
non-SA patients (N = 4,050) |
|
| Psychiatric Disorders | ||||||||
| Anxiety Disorder | 35.0 | 8.4 | <0.001 | 46.4 | 8.0 | <0.001 | <0.001 | 0.49 |
| Depressive Disorder | 37.2 | 8.4 | <0.001 | 47.8 | 8.3 | <0.001 | <0.001 | 0.97 |
| Eating Disorder | 1.9 | 0.4 | <0.001 | 2.3 | 0.3 | <0.001 | 0.42 | 0.43 |
| Bipolar Disorder | 11.3 | 1.5 | <0.001 | 14.2 | 1.5 | <0.001 | 0.02 | 1.00 |
| Posttraumatic Stress Disorder | 11.0 | 0.8 | <0.001 | 24.4 | 0.5 | <0.001 | <0.001 | 0.10 |
| Psychotic Disorders | 5.3 | 1.4 | <0.001 | 6.6 | 1.7 | <0.001 | 0.17 | 0.37 |
| Any | 53.7 | 14.5 | <0.001 | 66.8 | 14.4 | <0.001 | <0.001 | 0.90 |
| Substance Use Disorders | ||||||||
| Alcohol Use Disorder | 7.4 | 0.7 | <0.001 | 11.6 | 0.8 | <0.001 | <0.001 | 0.44 |
| Drug Use Disorder | 6.8 | 1.0 | <0.001 | 11.5 | 0.9 | <0.001 | <0.001 | 0.73 |
| Any | 11.7 | 1.5 | <0.001 | 17.9 | 1.5 | <0.001 | <0.001 | 0.85 |
| Stress-Related Somatic Conditions | ||||||||
| Stress | 11.1 | 1.9 | <0.001 | 16.3 | 1.6 | <0.001 | <0.001 | 0.31 |
| Insomnia | 8.5 | 1.8 | <0.001 | 8.3 | 1.6 | <0.001 | 0.84 | 0.50 |
| Palpitations | 2.4 | 1.4 | 0.01 | 3.0 | 0.9 | <0.001 | 0.34 | 0.05 |
| Fatigue | 6.4 | 3.0 | <0.001 | 7.6 | 3.0 | <0.001 | 0.26 | 0.90 |
| Urticaria | 1.4 | 0.8 | 0.07 | 2.2 | 0.9 | <0.001 | 0.11 | 0.90 |
| Any | 25.0 | 8.1 | <0.001 | 30.5 | 7.1 | <0.001 | 0.001 | 0.10 |
| Gastrointestinal Conditions | ||||||||
| Dyspepsia | 1.7 | 0.9 | 0.01 | 1.7 | 0.8 | 0.01 | 1.00 | 0.72 |
| Irritable Bowel Syndrome | 4.2 | 1.4 | <0.001 | 4.4 | 1.4 | <0.001 | 0.77 | 0.92 |
| Peptic Ulcer | 0.2 | 0.1 | 0.44 | 0.4 | 0.1 | 0.001 | 0.16 | 0.65 |
| Any | 5.7 | 2.3 | <0.001 | 5.9 | 2.3 | <0.001 | 0.87 | 1.00 |
| Pain | ||||||||
| Pelvic Pain | 9.3 | 3.2 | <0.001 | 10.6 | 3.4 | <0.001 | 0.27 | 0.62 |
| Abdominal Pain | 16.0 | 8.4 | <0.001 | 18.5 | 8.1 | <0.001 | 0.07 | 0.69 |
| Headache | 12.6 | 5.9 | <0.001 | 14.7 | 6.4 | <0.001 | 0.10 | 0.33 |
| Fibromyalgia | 6.2 | 0.8 | <0.001 | 5.9 | 1.8 | <0.001 | 0.81 | 0.38 |
| Chronic, neck, and back pain | 27.6 | 12.5 | <0.001 | 28.7 | 12.4 | <0.001 | 0.52 | 0.95 |
| Any | 44.2 | 24.1 | <0.001 | 47.0 | 24.2 | <0.001 | 0.15 | 0.94 |
| Genitourinary Conditions | ||||||||
| Incontinence | 1.0 | 0.8 | 0.34 | 1.3 | 0.6 | 0.03 | 0.59 | 0.51 |
| Vulvodynia | 0.2 | 0.2 | 0.56 | 0.3 | 0.1 | 0.02 | 0.71 | 0.16 |
| Pain during intercourse | 1.3 | 0.6 | 0.01 | 2.3 | 0.5 | <0.001 | 0.06 | 0.65 |
| Vaginitis | 0.0 | 0.1 | 0.41 | 0.6 | 0.2 | 0.01 | 0.005 | 0.16 |
| Sexually Transmitted Infection | 1.9 | 0.6 | <0.001 | 2.6 | 0.6 | <0.001 | 0.24 | 0.89 |
| Any | 4.4 | 2.1 | <0.001 | 6.7 | 2.0 | <0.001 | 0.01 | 0.75 |
| Other | ||||||||
| Smoking | 20.6 | 7.9 | <0.001 | 20.7 | 7.7 | <0.001 | 0.97 | 0.76 |
| Obesity (BMI>=30) | 39.4 | 33.8 | <0.001 | 40.1 | 35.2 | 0.004 | 0.72 | 0.23 |
Note. Boldface indicates statistical significance (p<0.001). Patients were matched on year of birth, sex, facility, and continuous enrollment (defined as enrollment gaps of less than 6 months) in the two years surrounding the SA. Baseline = 12 months prior to SA documentation or the same time period among matched non-SA patients. Follow-up = 12 months post SA documentation or the same time period among matched non-SA patients.
There were significant increases in psychiatric disorders (66.8% vs 53.7%, p<0.001) and substance use disorders (17.9% vs 11.7%, p<0.001) during the follow-up versus baseline period for SA patients. Of note, the prevalence of PTSD more than doubled in the year following an SA diagnosis, from 11% to 24%. Conversely, matched non-SA patients had no significant 12-month increases in comorbidities.
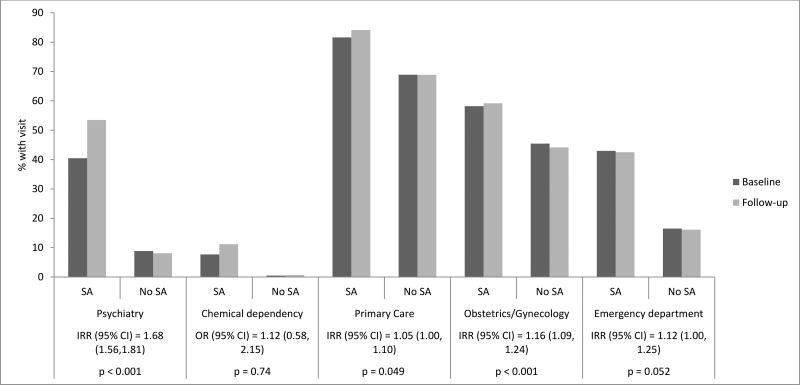
The mean number of healthcare visits for all utilization types was higher for SA patients versus matched non-SA patients at baseline and follow-up (all p< 0.001), with the exception of obstetrics/gynecology visits at baseline (Table 2). Among SA patients, the mean number of visits was higher during the follow-up versus baseline period for psychiatry. Healthcare utilization did not increase among matched non-SA patients.
Table 2.
Healthcare Utilization at Baseline and Follow-up among Patients with Documented Sexual Assault (SA) and Matched non-SA Patients.
| Healthcare Department | Baseline (N = 5,400) | Follow-Up (N = 5,400) | P-value comparing baseline
vs. follow-up among: |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| % of SA patients (N = 1,350) |
% of non-SA patients (N = 4,050) |
P-value | % of SA patients (N = 1,350) |
% of non-SA patients (N = 4,050) |
P-value | SA patients (N = 1,350) |
non-SA patients (N = 4,050) |
||
| Psychiatry | |||||||||
| 0 | 59.6 | 91.1 | 46.5 | 91.9 | |||||
| 1 | 7.3 | 2.5 | <0.001 | 7.4 | 3.0 | <0.001 | <0.001 | 0.12 | |
| 2 | 6.7 | 1.6 | 8.2 | 1.4 | |||||
| 3 | 3.4 | 1.2 | 4.8 | 0.7 | |||||
| 4 | 2.7 | 0.7 | 4.4 | 0.5 | |||||
| 5+ | 20.4 | 2.9 | 28.7 | 2.4 | |||||
| Mean (SD) | 4.5 (11.9) | 0.5 (3.2) | <0.001 | 6.3 (13.9) | 0.4 (2.6) | <0.001 | < 0.001 | 0.16 | |
| Chemical dependency | |||||||||
| 0 | 92.3 | 99.5 | 88.8 | 99.3 | |||||
| 1 | 1.6 | 0.1 | <0.001 | 1.5 | 0.1 | <0.001 | 0.03 | 0.09 | |
| 2 | 0.8 | 0.1 | 1.2 | 0.3 | |||||
| 3 | 0.5 | 0.1 | 0.8 | 0.1 | |||||
| 4 | 0.3 | 0.1 | 0.6 | 0.0 | |||||
| 5+ | 4.4 | 0.3 | 7.1 | 0.2 | |||||
| Mean (SD) | 2.7 (17.0) | 0.1 (2.8) | <0.001 | 6.1 (36.7) | 0.1 (1.4) | <0.001 | 0.002 | 0.24 | |
| Primary care | |||||||||
| 0 | 18.4 | 31.1 | 15.9 | 31.2 | |||||
| 1 | 15.8 | 22.9 | <0.001 | 16.3 | 23.6 | <0.001 | 0.36 | 0.93 | |
| 2 | 14.4 | 16.9 | 14.0 | 16.1 | |||||
| 3 | 10.4 | 9.9 | 12.4 | 9.7 | |||||
| 4 | 9.5 | 6.2 | 9.1 | 6.3 | |||||
| 5+ | 31.6 | 13.0 | 32.3 | 13.1 | |||||
| Mean (SD) | 4.4 (7.0) | 2.2 (3.1) | <0.001 | 4.5 (5.9) | 2.1 (3.1) | <0.001 | 0.57 | 0.57 | |
| Obstetrics/Gynecology | |||||||||
| 0 | 41.8 | 54.6 | 40.8 | 55.9 | |||||
| 1 | 24.1 | 24.0 | <0.001 | 23.0 | 23.5 | <0.001 | 0.71 | 0.89 | |
| 2 | 12.5 | 7.0 | 12.4 | 7.0 | |||||
| 3 | 7.7 | 3.5 | 7.6 | 3.3 | |||||
| 4 | 4.3 | 2.5 | 4.8 | 2.4 | |||||
| 5+ | 9.6 | 8.4 | 11.4 | 8.0 | |||||
| Mean (SD) | 1.7 (3.0) | 1.5 (3.3) | 0.01 | 2.0 (3.4) | 1.4 (3.3) | <0.001 | 0.08 | 0.42 | |
| Emergency department | |||||||||
| 0 | 57.0 | 83.5 | 57.5 | 83.9 | |||||
| 1 | 20.4 | 11.5 | <0.001 | 21.2 | 12.0 | <0.001 | 0.57 | 0.33 | |
| 2 | 9.2 | 3.1 | 9.6 | 2.3 | |||||
| 3 | 5.1 | 1.0 | 3.7 | 1.0 | |||||
| 4 | 2.2 | 0.4 | 1.9 | 0.4 | |||||
| 5+ | 6.1 | 0.5 | 6.2 | 0.6 | |||||
| Mean (SD) | 1.2 (2.6) | 0.3 (0.8) | <0.001 | 1.3 (3.6) | 0.2 (0.7) | <0.001 | 0.53 | 0.48 | |
Note. Boldface indicates statistical significance (p<0.001). Patients were matched on year of birth, sex, facility, and continuous enrollment (defined as enrollment gaps of less than 6 months) in the two years surrounding documented SA. Baseline = 12 months prior to SA documentation or the same time period among matched non-SA patients. Follow-up = 12 months post SA documentation or the same time period among matched non-SA patients.
DiD models indicated that after adjusting for race/ethnicity, income and secular trends, there were significantly higher relative odds of psychiatric disorders (OR (95%CI)=1.74 (1.42–2.14), p<0.001) and stress-related somatic conditions (OR (95%CI)=1.53 (1.20–1.94), p<0.001) in SA patients compared to matched non-SA patients during follow-up period relative to baseline (Figure 1). There was also an increase in the number of psychiatry (IRR (95%CI)=1.68 (1.56–1.81), p<0.001) and obstetrics/gynecology (IRR (95%CI)=1.16 (1.09–1.24), p<0.001) visits from baseline to follow-up in SA patients compared to matched non-SA patients (Figure 2).
Figure 1.
Unadjusted changes in proportions of patients with psychiatric and medical conditions from baseline to follow-up, and results from difference-in-differences (DiD) models comparing patients with documented sexual assault (SA) to matched non-SA patients.
Notes: p = P-value. Statistical significance based on p<0.001. Baseline = 12 months prior to SA documentation or the same time period among matched non-SA patients. Follow-up = 12 months post SA documentation or the same time period among matched non-SA patients. We conducted a Logistic regression on having any comorbidity diagnosis within the DiD framework and reported OR (95% CI). DiD models adjusted for median neighborhood household income and race/ethnicity. OR = Odds Ratio for the interaction of SA × time (baseline or follow-up). 95% CI = 95% Confidence Interval.
Figure 2.
Unadjusted changes in proportions of patients with at least one healthcare visit (by Department) from baseline to follow-up, and results from difference-in-differences (DiD) models comparing patients with documented sexual assault (SA) to matched non-SA patients.
Notes: p = P-value. Statistical significance based on p<0.001. Baseline = 12 months prior to SA documentation or the same time period among matched non-SA patients. Follow-up = 12 months post SA documentation or the same time period among matched non-SA patients. For chemical dependency visits, we conducted a Logistic regression on having any chemical dependency visit within the DiD framework and reported OR (95% CI); For the rest of the visit types (psychiatry, primary care, obstetrics/gynecology and emergency department), we conducted Poisson regressions on counts of visits within DiD framework and reported IRR (95% CI). DiD models adjusted for median neighborhood household income and race/ethnicity. OR = Odds Ratio for the interaction of SA × time (baseline or follow-up). 95% CI = 95% Confidence Interval. IRR = Incident Rate Ratio for the interaction of SA × time (baseline or follow-up).
DISCUSSION
This study assessed whether SA is associated with changes in psychiatric and medical conditions and healthcare utilization after accounting for changes in secular trends and controlling for both measured and unmeasured confounding. Women with a diagnosis of SA had a substantially higher prevalence of medical and psychiatric conditions and greater healthcare utilization in the year before and the year after the diagnosis of SA, relative to matched women without SA during the same time period. Results are consistent with a prior retrospective cohort study that found significantly higher incidence of several somatic disorders and visits to a general practitioner among women with SA relative to matched women without SA both before and after the assault.(10) The high percentage of patients experiencing psychiatric and medical conditions prior to a diagnosis of SA indicates that some of the association between SA and these conditions found in prior cross-sectional studies may be incorrectly attributed to SA, and speaks to the need for longitudinal studies with appropriate comparison groups.
There are several possible explanations for elevated psychiatric and medical needs and greater healthcare utilization among women in the year before a SA diagnosis. First, women with SA had significantly lower income and were more likely to be African American than matched women without an SA diagnosis, and racial/ethnic and income-related disparities in medical and psychiatric conditions may contribute in part to these baseline differences. Further, research indicates that low income status and African American race/ethnicity are vulnerability factors that may increase risk for SA.(2) For example, women with lower income may live in more dangerous neighborhoods where their risk for SA is higher.(14) Second, elevated medical and psychiatric conditions among women in the year before a diagnosis of SA may be due in part to the effects of cumulative trauma. For example, women exposed to adverse childhood experiences have greater risk for medical and psychiatric conditions(15) and future SA.(16) Further, SA is often recurrent(17) and women who disclose recent SA to a healthcare provider may have past adult experiences with SA that contribute to greater pre-existing comorbidities. Longitudinal studies that combine EHR data with data on social determinants of health, prior trauma exposure, and patient reported outcomes are needed to better understand these observed associations.
Of particular importance, the prevalence of psychiatric disorders and stress-related somatic conditions and utilization of psychiatry and obstetrics/gynecology increased significantly more among women in the year following the SA diagnosis compared to matched women without an SA diagnosis, after adjusting for income and race/ethnicity. Prior studies indicate higher prevalence of psychiatric conditions(2) and stress-related somatic conditions(18) among SA victims, and our findings suggest that SA may directly increase risk for these conditions. As outlined by Dworkin and colleagues, SA could contribute to increased risk for psychiatric disorders via its impact on cognitive distortions (e.g., overestimating the dangerousness of future situations), behavior changes (e.g., social withdrawal) and mood changes (e.g., increased sadness and anxiety).(19) Pre-existing mental health problems and previous use of mental health services are also associated with greater risk for psychopathology and treatment seeking after SA.(20–22) In our study, a large percentage of women with SA had a preexisting psychiatric disorder (54%) and many were already connected with psychiatric services (40%) in the year before the SA diagnosis. In some cases, SA may exacerbate existing conditions and contribute to increased symptomatology, leading to increased utilization. Further, increased healthcare utilization among women with SA versus matched women without SA likely provided additional opportunities to receive psychiatric and medical diagnoses.
Notably, there was a dramatic increase in PTSD diagnoses in the year after SA. Exposure to a traumatic event is required for a diagnosis of PTSD, a condition characterized by symptoms of re-experiencing the trauma, avoidance, and a state of hyperarousal. Prior studies have shown that rape is the most common cause of PTSD among women and nearly half of women exposed to trauma meet the criteria for PTSD during their lives.(23) Mental health following SA may be influenced by characteristics of the assault (duration, perceived life threat), trauma history and preexisting psychiatric disorders.(22, 24) There is growing evidence that early interventions can prevent the development of PTSD after SA,(25, 26) and clinicians and healthcare organizations can play a critical role in linking women with mental health services.(27, 28) Further, resiliency-based approaches to managing trauma may significantly reduce the symptom burden and possibly reduce the likelihood of progression to PTSD.(29)
There were no significant increases in substance use disorders, gastrointestinal disorders, pain, genitourinary conditions, smoking or obesity among patients with SA relative to matched patients without SA. Similarly, we did not see significantly increased utilization of chemical dependency, primary care, or emergency department use among SA versus matched non-SA patients using a conservative p-value adjustment for multiple comparisons. Most prior work has been cross-sectional and longitudinal studies have not used DiD models to compare changes in diagnoses and healthcare utilization among patients with versus without SA. Thus, some of the elevated risk for the conditions listed above found in prior studies may be, in part, due to differences in the sample of women with versus without SA and not necessarily differences directly attributable to the SA. Our study did in fact find significant increases in substance use disorders among women with a SA diagnosis; however, our multivariable modeling results indicated that the increases in these outcomes at 12 months were not significantly greater than increases in these outcomes in patients who did not receive an SA diagnosis after adjusting for multiple comparisons. Additional research is needed to better understand the mechanisms underlying the association of SA with specific types of medical conditions and healthcare utilization.
The short- and long-term consequences of SA may be preventable with early identification and follow-up and the American College of Obstetrics and Gynecology recommends that healthcare providers routinely screen all women for a history of SA.(30) Unfortunately, most women who experience SA do not seek medical treatment or disclose their experience to a healthcare provider.(27, 28) Reasons for not disclosing include distrust, fear of victim blaming and discrimination, embarrassment, believing SA is not relevant to care, and not being asked by a clinician.(31, 32) Healthcare systems and providers can play an important role in patients’ recovery by recognizing that various health procedures may trigger anxiety among women with SA(30) and providing sensitive and high quality care to SA victims. This includes creating an environment supportive of disclosure (e.g., posters/brochures in waiting and exam rooms), recognizing indicators and risk factors for SA (e.g., psychiatric disorders, stress-related somatic conditions), providing patient-centered and culturally competent prompt medical screening and evaluation, validating the disclosure, and coordinating follow-up referral for psychiatric and medical care.(27, 28, 31) In addition, repeated screening is often necessary, as the likelihood that patients disclose trauma may increase with subsequent screenings.
Limitations
Our study has several limitations. Our sample of women with SA is limited to those who disclosed SA to a healthcare provider; these women may represent a higher-risk subset of patients with SA.(31) Results may not be generalizable to women without healthcare access or to high trauma exposure populations (e.g., veterans). Further, patients with SA who were excluded (ie, those without an identified medical facility, no KPNC membership, or without continuous enrollment during the study period) had slightly different demographic characteristics than those included, which may impact generalizability of the findings. Our study matched patients on age and adjusted for race/ethnicity (we were unable to match patients on race/ethnicity due to a lack of patients without SA who met the matching criteria), and future studies are needed to examine whether changes in medical and mental health needs related to SA vary with demographic factors.(32) Since women with and without SA were different prior to the diagnosis of SA, we cannot say for certain that the DID approach produces completely unbiased treatment effects, and there may be some residual unobserved confounding. Details about SA (e.g., attempted versus completed rape, severity, duration, relationship to offender(s)) were not captured; future studies should examine whether certain aspects of SA are more strongly associated with changes in health and healthcare use. Finally, we did not have data on key factors related to SA and the legal system (e.g., law enforcement involvement, rape kit collection, subsequent trial) that might impact health and healthcare utilization.
The current study also has multiple strengths. The study takes place within a large, integrated healthcare delivery system serving >4 million patients. Our robust EHR allows for linkage of patient SA diagnoses with comorbidities and healthcare utilization over time, eliminating recall and non-responder biases. Further, use of a retrospective cohort design and DiD models allowed us to estimate longitudinal changes in comorbidities and healthcare utilization among patients with versus without documented SA.
CONCLUSIONS
SA is associated with significant increases in psychiatric disorder and stress-related somatic conditions as well as increases in utilization of psychiatry and obstetrics/gynecology. Previous research has identified several risk factors associated with SA, including low socioeconomic status and SA history. Future research should explore what role the pre-existing conditions we identified in this study among women diagnosed with SA may play in increasing SA risk. The American College of Obstetrics and Gynecology recommends routine screening for of all women for history of sexual assault,(30) and results suggest that clinicians should be trained in how to inquire about, respond to, and appropriately refer women who have experienced SA both recently and in the past.(27, 28) Further, studies are needed to identify best practices in clinician education, inquiry and interventions for SA that result in improved outcomes. Robust screening for trauma and trauma risk factors offers great potential for improving healthcare outcomes and patient quality of life. Research is needed to assess how layers of trauma accumulate and the longer-term implications of SA on health and healthcare utilization.
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (K01 Award DA043604). The study sponsor had no role in the study design, collection, analysis, interpretation of data, writing the manuscript or decision to submit the manuscript for publication.
Appendix
Appendix Table 1.
Comorbidity diagnosis codes, ICD9 and ICD10
| Diagnosis | ICD9 Codes | ICD10 Codes |
|---|---|---|
| Psychiatric Conditions | ||
| Anxiety disorder | 300.00–300.02,300.09,309.21,309.24,309.81 | F41.1,F41.3,F41.8,F41.9,F43.22,F43.1 |
| Depressive disorder | 296.2,296.3,296.82,298.0,300.4,301.12,309.0,309.1,309.28,311 | F32,F33,F34.1,F43.21,F43.23 |
| Eating disorder | 307.1,307.51 | F50 |
| Bipolar disorder | 296.0,296.1,296.4–296.8,296.81,296.89,296.69,301.13 | F30,F31,F34.0,F34.8,F34.9,F39 |
| Posttraumatic stress disorder | 30981 | F4311,F4312 |
| Psychotic disorders | 293.81,295,297,298,524 | F20,F21,F25,F29,F06.0,F06.2,F53 |
| Substance Use Disorders | ||
| Alcohol use disorders | 291,303,305.0,V69.8 | F10 |
| Drug use disorders | 292,304,305.2–305.9 | F11–F16.999,F18–F19.999 |
| Stress-Related Somatic Conditions | ||
| Stress | 308.3,308.4, 308.2, V62.89,308.9,308.1 | F43.8,R46.6, Z73.3,F43.9,Z63.79,F43.12,F43.0 |
| Insomnia | 327,327.01,327.02,327.09,780.52 | F51.04,F51.05,F51.09,G47.00,G47.09 |
| Palpitations | 785.1 | R00.2 |
| Fatigue | 780.79,780.71 | R53.82,R53.83,O26.811–O26.813,O26.819 |
| Urticaria | 708.0–708.5,708.8,708.9 | L50.0–L50.6,L50.8,L50.9,L56.3,O26.86 |
| Gastrointestinal Conditions | ||
| Dyspepsia | 536.8 | K30 |
| Irritable Bowel Syndrome | 564.1 | K58.0–K58.2 K58.8,K58.9 |
| Peptic Ulcer | 533.01, 533.11, 533.21, V12.71 | K27.0–K27.7, Z87.11 |
| Pain | ||
| Pelvic Pain | 625.9 | R10.2 |
| Abdominal Pain | 789.00–789.07 | R10.10,R10.30, R10.84,R10.9 |
| Headache | 307.81,339.02,339.05,339.09–339.12,339.20–339.22,339.42,339.44, 339.82,339.84,339.85,339.89,346.00–346.03, 346.10–346.13,346.20–346.23,346.30–346.33,346.40–346.43, 346.50,346.52,346.53,346.60–346.63,346.70–346.73,346.80–346.83,346.90–346.93,784.0 |
G43.001,G43.009,G43.011,G43.019,G43.101,G43.109,G43.111,G43.119,G43.401, G43.409,G43.419,G43.501,G43.511,G43.519,G43.601,G43.609,G43.611, G43.619,G43.701,G43.709,G43.711,G43.719,G43.801,G43.809,G43.811,G43.819,G43.821, G43.829,G43.831,G43.839,G43.901,G43.909,G43.911,G43.919,G43.B0,G43.B1,G43.C0,G43.C1, G43.D0,G43.D1,G44.001,G44.009,G44.029,G44.091,G44.099,G44.1,G44.201,G44.209,G44.211,G44.219, G44.221,G44.229,G44.309,G44.319,G44.321,G44.329,G44.52,G44.59,G44.81,G44.82,G44.85,G44.89,R51 |
| Fibromyalgia | 729.1 | M79.7 |
| Chronic, neck, and back pain | 723.1 | M54.2,M54.5,M54.89,M54.9 |
| Genitourinary Conditions | ||
| Incontinence | 625.6,788 | R39.81,N39.8 |
| Vulvodynia | 625.7 | N94.81 |
| Pain During Intercourse | 625.0 | N94.1 |
| Vaginitis | 625.1 | N76.0 |
| Sexually Transmitted Infections | 09,131,647.0–647.2,079.98,079.88,054.1 | A51 |
Footnotes
All other authors declare no conflict of interest.
References
- 1.Breiding MJ, Smith SG, Basile KC, Walters ML, Chen J, Merrick MT. Prevalence and characteristics of sexual violence, stalking, and intimate partner violence victimization--national intimate partner and sexual violence survey, United States, 2011. MMWR Surveill Summ. 2014;63(8):1–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Harrell MC, Castaneda LW, Adelson M, Gaillot S, Lynch C, Pomeroy A. A compendium of sexual assault research. Santa Monica, CA: RAND National Defense Research Institute; 2009. [Google Scholar]
- 3.Black MC, Basile KC, Breiding MJ, Smith SG, Walters ML, Merrick MT. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 summary report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 4.National Sexual Violence Resource Center. Research brief. Sexual Violence and Health. 2012 [Google Scholar]
- 5.Black MC. Intimate partner violence and adverse health consequences. American Journal of Lifestyle Medicine. 2011;5(5):428–439. [Google Scholar]
- 6.Santaularia J, Johnson M, Hart L, Haskett L, Welsh E, Faseru B. Relationships between sexual violence and chronic disease: a cross-sectional study. BMC Public Health. 2014;14:1286. doi: 10.1186/1471-2458-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golding JM, Stein JA, Siegel JM, Burnam MA, Sorenson SB. Sexual assault history and use of health and mental health services. Am J Community Psychol. 1988;16(5):625–44. doi: 10.1007/BF00930018. [DOI] [PubMed] [Google Scholar]
- 8.Koss MP. The impact of crime victimization on women's medical use. Journal of Women's Health. 2009;2(1):67–72. [Google Scholar]
- 9.Strike CJ, Ferris LE. Medical care use among women before and after sexual assault: a population study. J Obstet Gynaecol. 2001;21(3):285–91. doi: 10.1080/01443610120046431. [DOI] [PubMed] [Google Scholar]
- 10.Larsen ML, Hilden M, Skovlund CW, Lidegaard O. Somatic health of 2500 women examined at a sexual assault center over 10 years. Acta Obstet Gynecol Scand. 2016;95(8):872–8. doi: 10.1111/aogs.12903. [DOI] [PubMed] [Google Scholar]
- 11.Terhune C. Report: Kaiser tops state health insurance market with 40% share. Los Angeles Times: 2013. [Google Scholar]
- 12.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. 4. New York: Wiley; 2005. pp. 241–59. [Google Scholar]
- 13.Warton EM, Parker MM, Karter AJ. How D-I-D you do that? Basic difference-in-differences models in SAS. Oakland, CA: Kaiser Permanente Division of Research; [Google Scholar]
- 14.Greco D, Dawgert S. A Guide for Counselors and Advocates. Harrisburg, PA: Pennsylvania Coalition Against Rape; 2007. Poverty and Sexual Violence: Building Prevention and Intervention Responses. [Google Scholar]
- 15.ACEs: Best practices. Academy on Violence and Abuse (AVA); 2015. Academy on Violence and Abuse, National Health Collaborative on Violence and Abuse. [Google Scholar]
- 16.Ports KA, Ford DC, Merrick MT. Adverse childhood experiences and sexual victimization in adulthood. Child Abuse Negl. 2016;51:313–22. doi: 10.1016/j.chiabu.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arata CM. Repeated sexual victimization and mental disorders in women. Journal of Child Sexual Abuse. 1999;7(3):1–17. [Google Scholar]
- 18.Stein MB, Lang AJ, Laffaye C, Satz LE, Lenox RJ, Dresselhaus TR. Relationship of sexual assault history to somatic symptoms and health anxiety in women. Gen Hosp Psychiatry. 2004;26(3):178–83. doi: 10.1016/j.genhosppsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin ER, Menon SV, Bystrynski J, Allen NE. Sexual assault victimization and psychopathology: A review and meta-analysis. Clin Psychol Rev. 2017;56:65–81. doi: 10.1016/j.cpr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell D, Peterson R, Berliner L, Stewart T, Russo J, Whiteside L, et al. Factors associated with follow-up attendance among rape victims seen in acute medical care. Psychiatry. 2015;78(1):89–101. doi: 10.1080/00332747.2015.1015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price M, Davidson TM, Ruggiero KJ, Acierno R, Resnick HS. Predictors of using mental health services after sexual assault. J Trauma Stress. 2014;27(3):331–7. doi: 10.1002/jts.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell R, Dworkin E, Cabral G. An ecological model of the impact of sexual assault on women's mental health. Trauma Violence Abuse. 2009;10(3):225–46. doi: 10.1177/1524838009334456. [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, Breslau N. Epidemiological risk factors for trauma and PTSD. In: Yehuda R, editor. Risk Factors for Posttraumatic Stress Disorder. Arlington, VA: American Psychiatric Association; 1999. pp. 23–59. [Google Scholar]
- 24.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: a review. Depress Anxiety. 2012;29(10):833–42. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, et al. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry. 2012;72(11):957–63. doi: 10.1016/j.biopsych.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SL, Young SK, Billings DL, Bross CC. Health care-based interventions for women who have experienced sexual violence: a review of the literature. Trauma Violence Abuse. 2007;8(1):3–18. doi: 10.1177/1524838006296746. [DOI] [PubMed] [Google Scholar]
- 28.Vrees RA. Evaluation and management of female victims of sexual assault. Obstet Gynecol Surv. 2017;72(1):39–53. doi: 10.1097/OGX.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 29.Iacoviello BM, Charney DS. Psychosocial facets of resilience: implications for preventing posttrauma psychopathology, treating trauma survivors, and enhancing community resilience. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee on Health Care for Underserved Women. ACOG Committee Opinion no. 592: Sexual assault. Obstet Gynecol. 2014;123(4):905–9. doi: 10.1097/01.AOG.0000445581.43112.41. [DOI] [PubMed] [Google Scholar]
- 31.Lanthier S, Du Mont J, Mason R. Responding to delayed disclosure of sexual assault in health settings: A systematic review. Trauma Violence Abuse. 2016 doi: 10.1177/1524838016659484. [DOI] [PubMed] [Google Scholar]
- 32.Bryant-Davis T, Chung H, Tillman S, Belcourt A. From the margins to the center: ethnic minority women and the mental health effects of sexual assault. Trauma Violence Abuse. 2009;10(4):330–57. doi: 10.1177/1524838009339755. [DOI] [PubMed] [Google Scholar]