Abstract
Background
Current practice methods are unclear as to the most safe and effective prophylactic pharmacotherapy and method of delivery to reduce postoperative endophthalmitis occurrence.
Methods
A systematic review and meta-analysis using Meta-analysis of Observational Studies in Epidemiology guidelines was performed to compare the efficacy of intracameral cefuroxime, moxifloxacin and vancomycin in preventing postphacoemulsification cataract surgery endophthalmitis. A safety analysis of intracameral antibiotics was concurrently performed.
Data sources
BIOSIS Previews, CINAHL, ClinicalTrials.gov, Cochrane Library, Dissertations & Theses, EMBASE, PubMed, ScienceDirect and Scopus were searched from inception to January 2017. Data were pooled using a random effects model. All articles were individually reviewed and data were extracted by two independent reviewers. Funnel plot, risk of bias and quality of evidence analyses were performed.
Results
Seventeen studies with over 900 000 eyes were included, which favoured the use of intracameral antibiotics at the end of cataract surgery (OR 0.20; 95% CI 0.13 to 0.32; P<0.00001). The average weighted postoperative endophthalmitis incidence rates with intracameral cefuroxime, moxifloxacin and vancomycin were 0.0332%, 0.0153% and 0.0106%, respectively. Secondary analyses showed no difference in efficacy between intracameral plus topical antibiotics versus intracameral alone (P>0.3). Most studies had low to moderate risk of bias. The safety analysis showed minimal toxicity for moxifloxacin. Dosing errors led to the majority of toxicities with cefuroxime. Although rare, vancomycin was associated with toxic retinal events.
Conclusion
Intracameral cefuroxime and moxifloxacin reduced endophthalmitis rates compared with controls with minimal or no toxicity events at standard doses. Additionally, intracameral antibiotics alone may be as effective as intracameral plus topical antibiotics.
Introduction
Endophthalmitis is a sight-threatening inflammation of the eye. For patients and surgeons alike, one of the most feared complications of cataract surgery is acute postoperative endophthalmitis (POE).1 With over 10 million cataract surgeries performed worldwide every year,1,2 effective POE prophylaxis is necessary. Povidone-iodine solution has historically been the standard for POE prophylaxis, 3 but other modalities include intracameral (IC), topical, subconjunctival and oral antibiotics. Of the identified risk factors for POE, many authors state that both the route of administration and the type of antibiotic are important factors for risk mitigation, 4–6 which has led to an increased number of IC antibiotic studies. Currently, although IC administration is widely accepted, there is no consensus on the best prophylactic therapy or route of administration for POE prevention.6,7 However, antibiotics including cephalosporins, fluoroquinolones and vancomycin have been tested for effective POE prevention.
The aim of this study was to evaluate the safety and efficacy of intracameral cefuroxime (ICC) intracameral moxifloxacin (ICM) and intracameral vancomycin (ICV) as prophylactic pharmaco-therapy for prevention of POE.
Methods
Eligibility criteria for considering studies for this review
We conducted a systematic review and meta-analysis of relevant literature using the Meta-analysis of Observational Studies in Epidemiology guidelines.8 The methods are described in detail in online supplementary eMethods. We considered randomised controlled trials (RCT) and observational studies that evaluated patients undergoing phacoemulsification cataract surgery with a minimum sample size of 500 eyes. Interventions included IC antibiotics (ie, cefuroxime, moxifloxacin or vancomycin) at the end of cataract surgery. Comparisons included non-IC antibiotics (topical, subconjunctival or non-specified) at the end of cataract surgery. The primary outcome was the incidence of postcataract surgery endophthalmitis. Secondary analyses examined the effects of geographic location, and the addition of topical antibiotics on POE incidence. Studies were excluded if they included extracapsular cataract extraction (ECCE) surgeries that could not be separated from phacoemulsification surgery data. Culture results from POE cases were reviewed to determine the spectrum of microorganisms causing endophthalmitis in this population.
We also reviewed studies within our literature search that reported safety or toxicity data with ICC, ICM and ICV. Eligible studies included animal models or postoperative humans who underwent phacoemulsification cataract surgery. Toxicity to the cornea, anterior chamber (AC), or retina, or a change in intraocular pressure (IOP) or visual acuity (VA) were analysed.
search methods for identifying studies
We identified published studies from BIOSIS Previews (ISI Web of Knowledge), CINAHL (EBSCOhost), ClinicalTrials. gov, Cochrane Library (Wiley Interscience), Dissertations & Theses Global (ProQuest), EMBASE (Embase. com), PubMed (National Library of Medicine), ScienceDirect (Elsevier) and Scopus (Elsevier) from inception to January 2017. There were no language restrictions. To optimise search criteria, we developed a detailed and comprehensive search strategy with an information specialist (MM) for each electronic database (online supplementary eMethods). EndNote V.X7 was used for deduplication (EndNote, Thomson Reuters).
study selection
Each article was independently reviewed by two reviewers. The titles and abstracts (if available) were screened. Full-text copies were obtained for all potentially relevant articles and reviewed for inclusion and data collection. Disagreements in selection were reconciled by a separate reviewer. Language interpreters assisted in reviewing non-English articles, resulting in a single person reviewing these articles. References in full-text articles were screened for relevance and added if they met inclusion criteria. We contacted authors as needed for additional study details to assist in the data analysis.
Data collection and risk of bias assessment
We extracted the following: type of study, IC antibiotic used, country of origin, incidence of POE with and without IC antibiotics, dose of antibiotic, use of topical antibiotics, location of toxicity and microorganisms isolated in POE. Two risk of bias tools were used. For the efficacy analysis, we used a Cochrane Risk of Bias Assessment Tool: For Non-Randomized Studies of Interventions. For the safety analysis, we used the Office of Health Assessment and Translation Risk of Bias Rating Tool for Human and Animal Studies. Funnel plots were evaluated using Review Manager V.5.3 (RevMan V.5.3).9 Additionally, we used the GRADEprofiler (V.3.6.1) to assess the quality of evidence.
Data synthesis and analysis
We used RevMan V.5.39 for the statistical analysis. Studies were stratified by the antibiotic used post surgery. As the primary outcome was dichotomous, OR estimates and corresponding 95% CIs were calculated for each study. OR estimates were combined using the random effects Mantel-Haenszel method. Summary of OR estimates was given for each stratum and collection of studies. ORs compared IC versus non-IC antibiotics. Heterogeneity was assessed by the Q and I2 statistics, calculated for each stratum and for the full collection of studies. Results were displayed using forest plots. Funnel plots enabled evaluation of publication bias.9 Secondary analyses examined the effect of geographic location (Europe vs non-Europe) on the risk of POE while stratifying by antibiotic type. Similarly, the effect on POE of topical antibiotics in conjunction with the primary IC antibiotic was examined and stratified by antibiotic type. The number and percent of microorganisms identified in POE cases as well as the safety analysis were tabulated as descriptive statistics.
Results
Results for the efficacy of IC antibiotics
We reviewed 4849 titles and abstracts; for 70 of these, the full text was evaluated (figure 1). Seventeen articles met the inclusion criteria.4,7,10–25 The European Society of Cataract & Refractive Surgeons (ESCRS) study was the only RCT4; 16 were observational studies (15 retrospective cohort studies10–13,15–23,25 and 1 case–control study7). Within the 16 observational studies, 9 compared ICC 1 mg/0.1 mL (4 ICC only and 5 ICC with topical antibiotics),10–13,15–18,24 6 compared ICM 100–500 mcg/0.1 mL (with 1 study ranging from 5 to 50 mcg/0.1 mL)19 (2 ICM only and 4 ICM with topical antibiotics),7,11,19,20,24,25 and 5 compared ICV 1 mg/0.1 mL (1 ICV only and 4 ICV with topical antibiotics)7,11,21–23 against their corresponding controls (ie, postoperative topical, subconjunctival or oral antibiotics). One study did not define the antibiotic doses administered.7 The 16 observational studies enrolled 909 582 eyes and the 1 RCT enrolled 16 211 eyes (online supplementary eTable 1).
Figure 1.
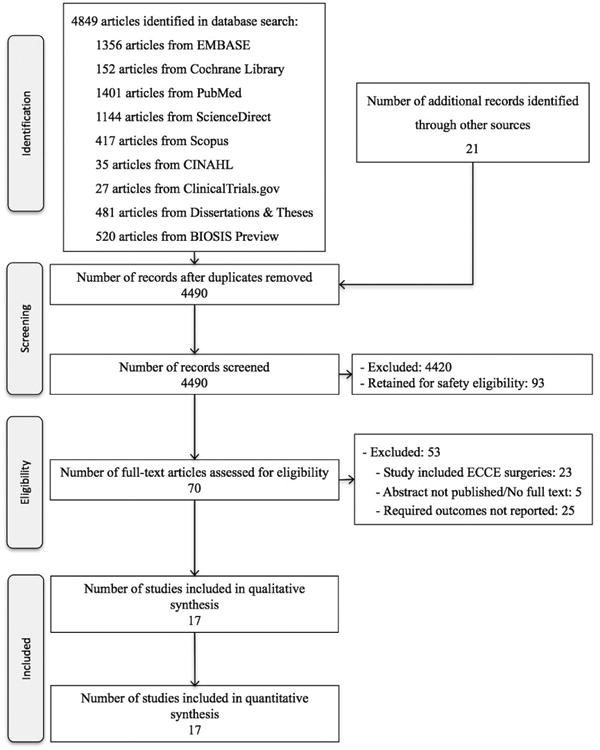
Flow chart of study selection. Flow diagram of study selection for the efficacy of intracameral antibiotics at the end of cataract surgery in reducing postoperative endophthalmitis incidence. ECCE, extracapsular cataract extraction.
Common reasons for excluding studies included the lack of a control or comparison group, and the inability to separate ECCE from phacoemulsification data. Of the 17 studies, 8 were based in Europe (including the RCT),4,10,12,13,15–17,21 2 were based in Canada,7,11 2 in the USA,22,24 2 in India,18,25 and 1 each was based in Japan,19 Australia23 and Colombia.20 The Matsuura et al's study used a bag and chamber flushing technique.19 All other studies used a small volume injection at the end of surgery. Rudnisky et al were contacted and calculation of values from their published study was performed for the groups who received ICM and ICV.7
The 17 included studies had mild to moderate risk of bias (online supplementary eFigures 1 and 2). Confounding variables were most common as some observational studies did not control for preoperative antibiotic regimen, use of steroid drops, surgical incision site, phacoemulsification method, surgical complications or patient comorbidities. However, studies that recognised these variables controlled their risk of bias through matching and analytical tests. The remaining sources of bias were determined to be low risk for most studies, which included selection of participants, departure from intended interventions, missing data, measurement of outcomes and reporting. Random effect analysis funnel plots of the 17 included studies reflected only minimal bias (online supplementary eFigure 3).
Using GRADEprofiler, the overall quality of evidence for the included observational studies was moderate (online supplementary eTable 2). Quality was downgraded by one level to account for the risk of bias due to confounding in multiple studies. Quality was upgraded due to the large effect found in the pooled data for observational studies. The overall quality of the RCT was graded as high due to the study design, low risk of bias, large measure of effect and direct comparisons.
The overall pooled data favoured the use of IC antibiotics at the end of phacoemulsification cataract surgery (OR, 0.20; 95% CI 0.13 to 0.32; P<0.00001). Within ICC groups, a lower incidence of endophthalmitis in the treatment group was observed (OR, 0.26; 95% CI 0.15 to 0.45; P<0.00001). These data are similar to the RCT (OR, 0.21; 95% CI 0.08 to 0.54; P=0.001).4 A lower incidence of endophthalmitis in treatment groups was also observed for ICM (OR, 0.30; 95% CI 0.13 to 0.67; P=0.004) and ICV (OR, 0.09; 95% CI 0.02 to 0.42; P=0.002) (figure 2).
Figure 2.
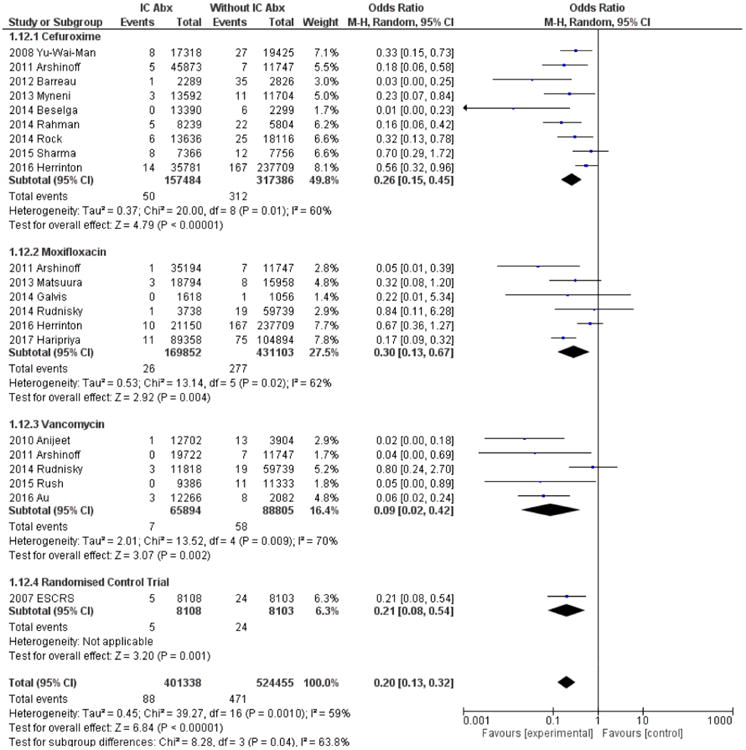
Forest plot of postoperative endophthalmitis incidence with and without intracameral antibiotics. Pooled data comparing incidence of postphacoemulsification cataract surgery endophthalmitis rates with and without IC antibiotics (ie, cefuroxime, moxifloxacin and vancomycin). Abx, antibiotic; ESCRS, European Society of Cataract & Refractive Surgeons; IC, intracameral.
In secondary analyses, there was no statistically significant difference in POE rates between patients treated with IC antibiotics plus topical antibiotics and patients treated with IC antibiotics alone within the cefuroxime (χ2=0.04; df=1; P=0.85), vancomycin (χ2=0.31; df=1; P=0.58) and moxifloxacin groups (χ2=0.78; df=1; P=0.38) (online supplementary eFigure 4).
Geographic forest plot analysis showed statistical significance in favour of IC antibiotics regardless of location. The average weighted POE incidence of ICC in Europe was 0.0366% compared with 0.0303% in non-European countries. For ICV, the incidence was 0.0079% compared with 0.0113%, respectively. There were no moxifloxacin studies performed in Europe for comparison (online supplementary eFigure 5).
The average weighted incidence rates of POE with ICC,4,10–18,24 ICM7,11,19,20,24,25 and ICV7,11,21–23 from the 16 observational studies and 1 RCT were 0.0332%, 0.0153% and 0.0106%, respectively.
Results for the safety analysis of IC antibiotics
Thirty-three studies met the inclusion criteria for the safety and toxicity analysis. Of these studies, there were 7 animal studies, 7 case series, 15 cohort studies, 2 cohort and animal studies, and 2 RCTs. Animal studies included rabbit and rat eyes. Eleven studies discussed the safety of ICC,26–36 3 discussed ICV safety37–39 and 15 discussed ICM safety.19,40–52 Three studies compared ICC versus ICV53–55 and one study compared ICV versus ICM.56 Cefuroxime doses ranged from 1 to 10 mg/0.1 mL, vancomycin doses ranged from 0.0375 to 1 mg/0.1 mL, and moxifloxacin doses ranged from 15 to 500 mcg/0.1 mL. Postoperative follow-up ranged from 1 day to 12 months.
Of the 33 studies analysed for risk of bias, the principal causes of moderate to high bias were confounding variables and protocol deviations. Selection, attrition and reporting bias were determined to be low to moderate risk of bias (online supplementary eFigures 6–8). Lack of homogeneity between studies (eg, differences in study methods, species and antibiotic concentrations) was the greatest challenge in comparing studies.
In the cefuroxime group, a total of 503 eyes were analysed for safety and toxicity of ICC (table 1). Of these, 69 (14%) eyes were reported to have toxic effects from the antibiotic; 23 had corneal oedema (CE),29,32,53 6 had endothelial cell death,57 17 developed toxic anterior segment syndrome (TASS),33 13 had cell or fibrin formation in the AC,32,34 14 had elevated IOP,29,32 18 had macular oedema31,32,36 and 15 had poor VA.29,32,36
Table 1. Postoperative safety profile of intracameral cefuroxime.
| structure | study | Dose of IC cefuroxime | (−) no toxicity, (+) toxicity | Additional details | |
|---|---|---|---|---|---|
|
| |||||
| Eyes (−) | Eyes (+) | ||||
| Cornea | Çakır et al33 | 1 mg/0.1 mL | 7* | 10* | Cohort. Toxic anterior segment syndrome after cataract surgery. |
| Lam et al 28 | 1 mg/0.1 mL | 34* | 0* | Cohort. No significant effect on ECD (P=0.74) compared with normal saline. | |
| Montan et al5 | 1 mg/0.1 mL | 45* | 0* | Cohort. No significant ECL with ICC (P>0.05). | |
| Ozlem et al53 | 1 mg/0.1 mL | 8† | 2† | Animal study. ICC versus ICV versus BSS. No corneal thickening at 3 and 6 hours. The levels of oxidative stress products were higher in the ICC group (P<0.001). | |
| Pérez-Canales et al54 | 1 mg/0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. CCT thickening resolved by 1-month follow-up. ECD reduced in both groups at 1 week after phacoemulsification. No significant reduction in the percentage of hexagonal cells at 1 or 3 months postoperatively compared with preoperatively. | |
| Sakarya and Sakarya 30 | 3 mg/0.1 mL | 6* | 0* | Case series. Accidental dilution error. No significant change in ocular findings was noted at 6 months, including macular oedema, VA loss or IOP elevation. | |
| Olavi29 | 5 mg/0.1 mL | 1* | 15* | Case series. Accidental dilution error. Fifteen eyes were noted to have CE. | |
| Delyfer et al32 | 10 mg/0.1 mL | 4* | 2* | Case series. Accidental dilution error. Two eyes had CE. | |
|
| |||||
| AC | Çakır et al33 | 1 mg/0.1 mL | 0* | 17* | Cohort. Toxic anterior segment syndrome after cataract surgery. |
| Montan et al5 | 1 mg/0.1 mL | 45* | 0* | Cohort. No significant induced laser fare intensity (P>0.05). | |
| Pérez-Canales et al54 | 1 mg/0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. AC cell was higher on day 1 with ICV, but resolved by day 7. | |
| Gradin and Mundia34 | 1 mg/0.1 mL | 28* | 7* | Cohort. No significant AC inflammation between control and ICC (P=0.857). | |
| Delyfer et al32 | 10 mg/0.1 mL | 0* | 6* | Case series. Accidental dilution error. All six cases with significant AC inflammation. | |
|
| |||||
| IOP | Pérez-Canales et al54 | 1 mg/0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. No significant effect on IOP. |
| Olavi29 | 5 mg/0.1 mL | 8* | 8* | Case series. Accidental dilution error. Eight eyes had elevated IOP. | |
| Delyfer et al32 | 10 mg/0.1 mL | 4* | 2* | Case series. Accidental dilution error. Two eyes had elevated IOP. | |
|
| |||||
| Retina | Gupta et al27 | 1 mg/0.1 mL | 34* | 0* | RCT. No macular thickening (P=0.34) at 5 weeks postoperatively. |
| Lam et al28 | 1 mg/0.1 mL | 34* | 0* | Cohort. No difference in central macular thickness (P=0.32) postoperatively. | |
| Le Dû and Pierre-Kahn31 | 1 mg/0.1 mL | 0* | 6* | Case series. Macular oedema reported per OCT. Possible dilution error. | |
| Pérez-Canales et al55 | 1 mg/0.1 mL | 0* | 30* | Case series. ICC versus ICV. Macular thickness increased from baseline (P=0.501 at 1 week and P=0.005 at 3 months). Macular thickness was comparable in both groups, suggesting postoperative inflammation rather than antibiotic choice. | |
| Giménez-de-la-Linde et al35 | 1 mg/0.1 mL | 221* | 0* | Cohort. No evidence of cystoid macular oedema in 221 cases. | |
| Wong et al36 | 9 mg/0.1 mL | 7* | 6* | Cohort. Macular oedema developed, however, by 1 week symptoms had resolved. | |
| Delyfer et al32 | 10 mg/0.1 mL | 0* | 6* | Case series. Accidental dilution error. All developed macular oedema. | |
|
| |||||
| VA | Lam et al28 | 1 mg/0.1 mL | 34* | 0* | Cohort. No significant effect on VA compared with those treated with normal saline. |
| Pérez-Canales et al55 | 1 mg/0.1 mL | 30* | 0* | Case series. No difference in VA between ICC and ICV at 1, 4 and 12 week(s) (P=>0.5). | |
| Olavi29 | 5 mg/0.1 mL | 8* | 8* | Case series. Accidental dilution error; eight eyes had reduced VA. | |
| Wong et al36 | 9 mg/0.1 mL | 7* | 6* | Cohort. Accidental dilution error. Six patients had visual acuity of 20/70 or worse. | |
| Delyfer et al32 | 10 mg/0.1 mL8 | 5* | 1* | Case series. Dilution error. One patient had a persistent halo with reduced VA. | |
The commercially available product Aprokam (cefuroxime) has a concentration of 1 mg/0.1 mL.
Human eyes.
Rabbit eyes.
AC, anterior chamber; BSS, balanced salt solution; CCT, central corneal thickness; CE, corneal oedema; ECD, endothelial cell density; ECL, endothelial cell loss; IC, intracameral; ICC, intracameral cefuroxime; ICV, intracameral vancomycin; IOP, intraocular pressure; OCT, optical coherence tomography; RCT, randomised controlled trial; VA, visual acuity.
In the vancomycin group, 171 eyes were analysed for safety and toxicity of ICV (table 2). None of the studies comparing vancomycin with control groups found any significant changes in IOP, endothelial cell density, AC inflammation, CE or macular oedema.37,38,53–56 However, a case series showed 36 eyes with vancomycin-associated haemorrhagic occlusive retinal vasculitis (HORV) resulting in VA worse than 20/200 in 22 of the eyes.39
Table 2. Postoperative safety profile of intracameral vancomycin.
| structure | study | Dose of IC vancomycin | (−) no to toxicity, (+) toxicity | Additional details | |
|---|---|---|---|---|---|
|
| |||||
| Eyes(−) | Eyes (+) | ||||
| Cornea | Lindquist and Robinson 37 | 0.0375 mg/0.1 mL | 3† | 0† | Animal study. No significant difference in ECD between experimental (right eye ICV) and control (left eye BSS) groups (P=0.13) |
| 0.075 mg/0.1 mL | 3† | 0† | |||
| 0.1875 mg/0.1 mL | 3† | 0† | |||
| 0.750 mg/0.1 mL | 3† | 0† | |||
| Kowalski et al56 | 0.100 mg/0.1 mL | 3† | 0† | Animal study. ICM compared with ICV. The corneal thickness increased on day 1; however, thickness returned to normal on day 7. No difference in CCT was found between groups. | |
| Gimbel et al38 | 1 mg/0.1 mL | 50* | 0* | Cohort study. No adverse effects on the corneal endothelium were demonstrated with the use of ICV with gentamicin. No P value reported. | |
| Ozlem et al53 | 1 mg/0.1 mL | 10† | 0† | Animal study. ICC versus ICV versus BSS. Neither caused corneal thickening or oedema at 3 and 6 hours. The levels of oxidative stress products were significantly higher in the ICC group (P<0.001) but not significantly changed in the ICV group (P>0.05). | |
| Pérez-Canales et al 54 | 1 mg / 0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. CCT elevated at 1 week, but resolved by 1 month follow up. ECD reduced in bothgroups at 1 week post phacoemulsifcation, but no changes at 1-month and 3-month follow up. No significant reduction in percentage of hexagonal cells at 1 or 3-month postoperative compared to preoperative. | |
|
| |||||
| AC | Pérez-Canales et al54 | 1 mg/0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. AC cell was higher on day 1 with ICV, but resolved by day 7 for both groups. |
|
| |||||
| IOP | Pérez-Canales et al 54 | 1 mg/0.1 mL | 30* | 0* | Case series. ICC versus ICV groups. No significant difference in IOP between two groups. |
|
| |||||
| Retina | Pérez-Canales et al55 | 1 mg/0.1 mL | 0* | 30* | Case series. ICC versus ICV groups. Average macular thickness increased compared with baseline (P=0.017 at 1 week; P=0.000 at 1 month; P=0.000 at 3 months). Macular thickness changes were comparable in both groups, suggesting that retinal thickening could be a consequence of postoperative inflammation rather than antibiotic choice. |
| Witkin et al 39 | 1 mg / 0.1 mL | 0* | 36* | Case series. Authors characterised presenting signs of haemorrhagic occlusive retinal vasculitis associated with ICV. | |
|
| |||||
| VA | Witkin et al39 | 1 mg/0.1 mL | 6* | 22* | Case series. Only 6 eyes had 20/40 or better VA; 22 of 36 eyes (61%) had 20/200 or worse and 8 of 36 eyes (22%) had no light perception. |
Human eyes.
Rabbit eyes.
AC, anterior chamber; BSS, balanced salt solution; CCT, central corneal thickness; ECD, endothelial cell density; IC, intracameral; ICC, intracameral cefuroxime; ICM, intracameral moxifoxacin; ICV, intracameral vancomycin; IOP, intraocular pressure; VA, visual acuity.
In the moxifloxacin group, 1243 eyes were analysed for safety and toxicity of ICM (table 3). Fifty-five eyes treated previously with penetrating keratoplasty had increased central corneal thickness (P<0.05) and decreased endothelial cell density (P<0.05).43 At 500 mcg/0.1 mL, Akal et al50 found 8 of 10 rat eyes with elevated caspase-3 and 9 of 10 eyes with elevated caspase-8 indicating increased apoptotic activity (P>0.05). Matsuura et al reported that 15 mcg/0.1 mL was safe and provided concentrations above MIC90 (minimum inhibitory concentration to inhibit 90% of organisms) for 2 hours for most of the resistant pathogens.45
Table 3. Postoperative safety profile of intracameral moxifloxacin.
| structure | study | Dose of IC moxifoxacin | (−) no toxicity, (+) toxicity | study details | |
|---|---|---|---|---|---|
|
| |||||
| Eyes (−) | Eyes (+) | ||||
| Cornea | Matsuura et al40 | 15 mcg/0.1 mL | 36† | 0† | Cohort. There was no difference in ECC and CCT in 15 mcg/0.1 mL and control as well as in comparison of 50 mcg/0.1 mL. Matsuura et al used the bag and chamber flushing method for ICM administration in their studies. This method irrigates the anterior chamber and the area behind the intraocular lens. |
| 15 mcg/0.1 mL versus | 66† | 0† | |||
| 50 mcg/0.1 mL | |||||
| Matsuura et al19 | 20 mcg/0.1 mL | 555* | 0* | Cohort. No difference was observed in ECL between treatment (ICM) and control. | |
| Kowalski et al56 | 25 mcg/0.1 mL | 3† | 0† | Animal study. ICM compared with ICV. The corneal thickness increased on day 1; however, thickness returned to normal on day 7. No difference in CCT was found between groups. | |
| 50 mcg/0.1 mL | 3† | 0† | |||
| Matsuura et al45 | 50 mcg/0.1 mL | 6* | 0* | Cohort. No toxic finding reported. | |
| Arbisser41 | 100 mcg/0.1 mL | 200* | 0* | Cohort. No stromal oedema was observed. | |
| Akal et al50 | 500 mcg/0.1 mL | 2‡ | 8‡ | Animal study. Staining for apoptotic activity with caspase-3 and caspase-8 was higher in the moxifloxacin group versus control and sham injection (P>0.05). | |
| Arslan et al43 | 500 mcg/0.1 mL | 0* | 55* | Cohort. All patients had a history of PK. ECC was reduced (P<0.001) and CCT increased (P<0.001). However, results are similar to other reported phacoemulsification cases. | |
| Asena et al49 | 500 mcg/0.1 mL | 48† | 0† | Animal study. No differences in the corneal findings. | |
| Espiritu et al42 | 500 mcg/0.1 mL | 65* | 0* | Cohort. No difference in ECC (P=0.737) and pachymetry (P=0.65). | |
| Kim et al 46 | 500 mcg/0.1 mL | 9† | 0† | Animal study. No statistical difference in CCT (P=0.06). | |
| Kobayakawa et al4 | 7 500 mcg/0.1 mL | 6† | 0† | Animal study. Corneal damage rate was found to be 0% with moxifloxacin. | |
| Ekinci Koktekir and Aslan 44 | 500 mcg/0.1 mL | 30* | 0* | Cohort. Postoperative pachymetry in ICM versus control was not significantly different (P=0.345). | |
| Lane et al 48 | 500 mcg/0.1 mL | 26* | 0* | RCT. ICM versus BSS. No difference in ECC or CCT at 3 months (P>0.05). | |
| Zhou et al51 | 500 mcg/0.1 mL | 91* | 0* | Cohort. No difference between patients receiving ICM and topical moxifloxacin drops. | |
| Cetinkaya et al52 | 500 mcg/0.1 mL | 33* | 0* | Cohort. No difference in corneal oedema compared with control. | |
|
| |||||
| AC | Arbisser41 | 100 mcg/0.1 mL | 200* | 0* | Cohort. Postoperative day 1 AC cell was lower in the ICM group (P=0.0007). |
| Espiritu et al42 | 500 mcg/0.1 mL | 65* | 0* | Cohort. AC cell noted on postoperative day 1 which resolved on next exam. | |
| Lane et al 48 | 500 mcg/0.1 mL | 17* | 9* | RCT. Not significantly different from controls (P>0.05). | |
| Zhou et al 51 | 500 mcg/0.1 mL | 91* | 0* | Cohort. No difference between patients receiving ICM and topical moxifloxacin drops. | |
| Cetinkaya et al52 | 500 mcg/0.1 mL16 | 33* | 0* | Cohort. No difference between patients receiving ICM and topical moxifloxacin drops. | |
|
| |||||
| IOP | Matsuura et al40 | 15 mcg/0.1 mL | 36† | 0† | Cohort. No difference in IOP (P>0.5) at 3 months for both comparisons. |
| 15 mcg/0.1 mL versus | 33† | 0† | |||
| 50 mcg/0.1 mL | |||||
| Ekinci Koktekir and Aslan44 | 500 mcg/0.1 mL | 30* | 0* | Cohort. No difference in IOP versus control (P=0.15). | |
| Lane et al 48 | 500 mcg/0.1 mL | 26* | 0* | RCT. No difference in IOP versus control (P>0.05). | |
| Cetinkaya et al52 | 500 mcg/0.1 mL16 | 33* | 0* | Cohort. No difference in IOP versus control (P>0.05). | |
|
| |||||
| Retina | Matsuura et al40 | 15 mcg/0.1 mL | 36† | 0† | Cohort. No difference in FT at 1 and 3 months for both comparisons (P>0.05). |
| 15 mcg/0.1 mL versus | 33† | 0† | |||
| 50 mcg/0.1 mL | |||||
| Arbisser41 | 100 mcg/0.1 mL | 31* | 0* | Cohort. Macular thickness and volume showed mean increases of less than 3% and 4% in all sectors, respectively, compared with preoperative readings. | |
| Ekinci Koktekir and Aslan44 | 500 mcg/0.1 mL | 30* | 0* | Cohort. No difference in macular thickness versus control groups (P=0.107). | |
|
| |||||
| VA | Espiritu et al 42 | 500 mcg/0.1 mL | 65* | 0* | Cohort. All eyes had VA 20/30 or better. |
| Lane et al 48 | 500 mcg/0.1 mL | 26* | 0* | RCT. All eyes had VA 20/30 or better. | |
| Zhou et al 51 | 500 mcg/0.1 mL | 91* | 0* | Cohort. No difference between patients receiving ICM and topical moxifloxacin drops. | |
| Cetinkaya et al52 | 500 mcg/0.1 mL | 33* | 0* | Cohort. No significant difference in VA compared with control. | |
Human eyes.
Rabbit eyes.
Rat eyes.
AC, anterior chamber; BSS, balanced salt solution; CCT, central corneal thickness; ECC, endothelial cell count; ECL, endothelial cell loss; F T, foveal thickness; IC, intracameral; ICM, intracameral moxifloxacin; ICV, intracameral vancomycin; IOP, intraocular pressure; PK, penetrating keratoplasty; RCT, randomised controlled trial; VA, visual acuity.
Analysis of microorganisms identified in Poe cases
The data for causative infectious agents of POE were extracted from the 17 included studies. Ten studies provided data,4,12,13,16–25 which included 145 endophthalmitis cases. The predominant microorganisms causing POE in postphacoemulsification cataract surgeries were coagulase-negative Staphylococcus (S. epidermatis, S. hominis, S. saprophyticus, S. warneri) (33 of 145; 22.8%). The second most common group was unspecified gram-negative rods (15 of 145; 10.3%). Other common organisms were Staphylococcus aureus (7 of 145; 4.8%), gram-positive organisms (unspecified) (7 of 145; 4.8%) and Streptococcus pneumoniae (8 of 145; 5.5%). In addition, one case of Aspergillus fumagatus (0.7%), four cases of Pseudomonas aeruginosa (2.8%) and three cases of methicillin-resistant Staphylococcus aureus (MRSA) (2.1%) were also reported. A large fraction of cases yielded no growth (56 of 145; 38.6%) (online supplementary eFigure 9).
Discussion
An effective and safe prophylactic treatment at the end of cataract surgery is needed to prevent serious sight-threatening endophthalmitis. In this meta-analysis, we identified nearly two decades of POE data from 909 582 eyes (observational studies) and 16 211 eyes (RCT), giving this analysis sufficient power to detect very small differences in rare outcomes such as endophthalmitis. Overall pooled data favoured the use of IC antibiotics to reduce POE rates when compared with controls (figure 2). ICC findings were consistent with the ESCRS findings.4 Additionally, χ2 analysis showed no difference between IC plus topical antibiotics compared with IC antibiotics alone, which suggests that postoperative topical antibiotics may provide no additional benefit.14,24,58 Pooled weighted averages for POE incidence favour ICM or ICV with incidences of 0.0153% and 0.0106%, respectively, compared with ICC (0.0332%). Quality of studies was graded at moderate to high with predominately low to moderate risk of bias.
Our systematic review of IC antibiotic safety suggests that ICC is relatively safe, but has had more complications with contamination, dilution errors and TASS along with macular toxicity compared with vancomycin and moxifloxacin (tables 1–3). ICV at 1 mg/0.1 mL showed no significant corneal or AC toxicities.37,38,53–55 However, ICV has more recently been associated with rare cases of HORV.39 Two studies with ICM 500 mcg/0.1 mL suggest decreased corneal cell density and increased apoptotic markers of the cornea. However, the majority of studies suggest no significant toxicities to the cornea, AC, retina or VA.41,42,44–49,52,56,58
IC antibiotic selection
ICC is the only IC antibiotic that has been analysed for efficacy by an RCT and has the most observational studies of the three antibiotics. From a phone survey of 250 ESCRS members, over 90% of surgeons would use cefuroxime if an approved product were commercially available.59 Although Aprokam is approved in Europe, a product which likely overcomes the risks associated with dilution error preparations, it remains unavailable in some nations and has not been approved by the Food and Drug Administration. Challenges to cefuroxime use include the potential for allergic reactions in patients with beta-lactam allergies,60,61 overdosing risks associated with preparation in areas where Aprokam is not available, and poor coverage of methicillin-resistant, penicillin-resistant gram-positive bacteria or multiresistant enterococci and some gram-negative species such as Pseudomonas.62
In our study, the incidence and OR of POE for ICV were the lowest; however the total population size was the smallest of the three groups. Vancomycin has superior MRSA coverage but does not cover gram-negative bacteria. This may be meaningful as only 2% of POE cases in this study were caused by MRSA versus 10% caused by gram-negative bacteria. The risk of vancomycin-resistant bacteria is also an important consideration.63
There are currently no RCTs that have evaluated the efficacy of ICM for POE prevention. In our study, the average weighted incidence of POE with ICM was lower than ICC but higher than ICV. The predominant dose concentration used in included studies was 100 mcg/0.1 mL vs undiluted 500 mcg/0.1 mL.7,20,25,64 Analysis suggests 500 mcg/0.1 mL may be more effective than 100 mcg/0.1 mL; however, this is based on only four studies.7,20,25,64 Literature review showed a low postoperative toxicity profile for ICM, possibly due to its self-sterilising properties which negates addition of potentially harmful preservatives in solution.65,66 Moxifloxacin also provides broader antimicrobial coverage of bacteria that have been isolated in POE compared with cefuroxime and vancomycin.67,68
study strengths and limitations
Other reviews and meta-analyses on this topic leave the question as to the added value of this study.69,70 Given that ECCE is known to have increased POE rates,71 this study aims to represent the most current practice methods with only phacoemulsification cases. Additionally, this study compared IC alone to IC plus topical postoperative antibiotics to assess the impact on POE incidence. Most importantly, this study provides a novel systematic review comparing the safety and toxicity of ICC, ICM and ICV. Limitations of this analysis include the lack of RCTs for ICM and ICV. Only one RCT has been performed analysing IC antibiotics.4 All other studies included were observational studies, which have a higher risk of bias. As study cohorts were not evaluated concurrently, this can lead to bias as surgical techniques improve over time. Furthermore, variability in techniques such as lens type,4 incision type and location,72 complications,73 and experience and age of the surgeon4,73 could not be adjusted in this analysis. However, we attempted to reduce risk of bias by only including phacoemulsification cataract surgeries, which we believe makes the data more robust as evidence suggests ECCE has a higher rate of endophthalmitis.71 Consequently, many large studies that could not separate ECCE or meet other inclusion criteria were excluded from this study, specifically, several studies from France, Sweden, Israel and Iran.72,74–79 These studies reported an ICC POE incidence range from 0.05% to 0.023%, which approximates our study finding of 0.0332%. Lastly, this study targeted IC antibiotics and therefore leaves the question of whether IC antibiotics are superior to postoperative, topical antibiotics alone. It has been suggested that the primary POE reducing element is the antibiotic type (eg, fourth-generation fluoroquinolones), despite the route of administration.7 Conversely, the ESCRS study strongly favoured IC use of cefuroxime over topical third-generation fluoroquinolones.4 A future analysis with focused search criteria for this question is needed.
Conclusion
Our study assessed two decades of POE incidence from over 900 000 eyes reported in 17 studies. The average weighted incidence rates of POE were 0.0332% (ICC), 0.0153% (ICM) and 0.0106% (ICV). Additionally, IC antibiotics alone may be as effective as IC plus postoperative topical antibiotics; however, the lack of direct comparison and the variety of topical antibiotics could suggest an alternative interpretation. These data showed that although very rare, ICV has been associated with HORV. ICC had minimal toxicity events at standard doses. ICM was the most studied antibiotic for safety and found to have a low toxicity profile at all studied concentrations. Future direct comparison studies of IC antibiotics as well as an RCT for ICM efficacy and tolerance would add to the current literature.
Supplementary Material
Acknowledgments
We thank Melissa L Rethlefsen, MSLS, who provided guidance in using the MOOSE guidelines for the meta-analysis and supervised the data presentation for the systematic review.
Funding: This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764), National Institutes of Health (EY014800), and an unrestricted grant from Research to Prevent Blindness, New York, to the Department of Ophthalmology and Visual Sciences, University of Utah.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bjophthalmol-2017-311051).
Contributors: All authors contributed to the design or acquisition of data, analysis or interpretation of data, and contributed to drafting or critically revising the article for important intellectual content and final approval of the version to be published.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aaberg TM, Flynn HW, Schiffman J, et al. Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology. 1998;105:1004–10. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 2.Foster A. Cataract and “Vision 2020-the right to sight” initiative. Br J Ophthalmol. 2001;85:635–7. doi: 10.1136/bjo.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769–75. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 4.Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Montan PG, Wejde G, Koranyi G, et al. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28:977–81. doi: 10.1016/s0886-3350(01)01269-x. [DOI] [PubMed] [Google Scholar]
- 6.Ng JQ, Morlet N, Bulsara MK, et al. Reducing the risk for endophthalmitis after cataract surgery: population-based nested case-control study: endophthalmitis population study of Western Australia sixth report. J Cataract Refract Surg. 2007;33:269–80. doi: 10.1016/j.jcrs.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 7.Rudnisky CJ, Wan D, Weis E. Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. Ophthalmology. 2014;121:835–41. doi: 10.1016/j.ophtha.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.The Cochran Collaboration. (RevMan) RM Review Manager (RevMan) 5.3. Copenhagen: The Nordic Cochran Centre; 2014. [Google Scholar]
- 10.Yu-Wai-Man P, Morgan SJ, Hildreth AJ, et al. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:447–51. doi: 10.1016/j.jcrs.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37:2105–14. doi: 10.1016/j.jcrs.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Barreau G, Mounier M, Marin B, et al. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38:1370–5. doi: 10.1016/j.jcrs.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Myneni J, Desai SP, Jayamanne DG. Reduction in postoperative endophthalmitis with intracameral cefuroxime. J Hosp Infect. 2013;84:326–8. doi: 10.1016/j.jhin.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39:8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Beselga D, Campos A, Castro M, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur J Ophthalmol. 2014;24:516–9. doi: 10.5301/ejo.5000417. [DOI] [PubMed] [Google Scholar]
- 16.Rahman N, Murphy CC. Impact of intracameral cefuroxime on the incidence of postoperative endophthalmitis following cataract surgery in Ireland. Ir J Med Sci. 2015;184 doi: 10.1007/s11845-014-1127-y. [DOI] [PubMed] [Google Scholar]
- 17.Röck T, Bramkamp M, Bartz-Schmidt KU, et al. Using intracameral cefuroxime reduces postoperative endophthalmitis rate: 5 years experience at the University Eye Hospital Tübingen. Klin Monbl Augenheilkd. 2014;231:1023–8. doi: 10.1055/s-0034-1383013. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Sahu SK, Dhillon V, et al. Reevaluating intracameral cefuroxime as a prophylaxis against endophthalmitis after cataract surgery in India. J Cataract Refract Surg. 2015;41:393–9. doi: 10.1016/j.jcrs.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura K, Miyoshi T, Suto C, et al. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg. 2013;39:1702–6. doi: 10.1016/j.jcrs.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Galvis V, Tello A, Sánchez MA, et al. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol Eye Dis. 2014;6:OED.S13102–4. doi: 10.4137/OED.S13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anijeet DR, Palimar P, Peckar CO. Intracameral vancomycin following cataract surgery: An eleven-year study. Clin Ophthalmol. 2010;4:321–6. doi: 10.2147/opth.s9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush SW, Vu D, Rush RB. The safety and efficacy of routine administration of intracameral vancomycin during cataract surgery. J Ophthalmol. 2015;2015:1–5. doi: 10.1155/2015/813697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au CP, White AJ, Healey PR. Efficacy and cost-effectiveness of intracameral vancomycin in reducing postoperative endophthalmitis incidence in Australia. Clin Exp Ophthalmol. 2016;44:803–11. doi: 10.1111/ceo.12789. [DOI] [PubMed] [Google Scholar]
- 24.Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123:287–94. doi: 10.1016/j.ophtha.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: analysis of 600 000 surgeries. Ophthalmology. 2017;124:768–75. doi: 10.1016/j.ophtha.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Montan PG, Wejde G, Setterquist H, et al. Prophylactic intracameral cefuroxime. Evaluation of safety and kinetics in cataract surgery. J Cataract Refract Surg. 2002;28:982–7. doi: 10.1016/s0886-3350(01)01270-6. [DOI] [PubMed] [Google Scholar]
- 27.Gupta MS, McKee HD, Saldaña M, et al. Macular thickness after cataract surgery with intracameral cefuroxime. J Cataract Refract Surg. 2005;31:1163–6. doi: 10.1016/j.jcrs.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Lam PT, Young AL, Cheng LL, et al. Randomized controlled trial on the safety of intracameral cephalosporins in cataract surgery. Clin Ophthalmol. 2010;4:1499–504. doi: 10.2147/OPTH.S15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmol. 2012;90:e153–e154. doi: 10.1111/j.1755-3768.2010.02103.x. [DOI] [PubMed] [Google Scholar]
- 30.Sakarya Y, Sakarya R. Cefuroxime dilution error. Eur J Ophthalmol. 2010;20:460–1. doi: 10.1177/112067211002000232. [DOI] [PubMed] [Google Scholar]
- 31.Le Dû B, Pierre-Kahn V. Early macular edema after phacoemulsification and suspected overdose of cefuroxime: report of six cases. J Fr Ophtalmol. 2014;37:202–10. doi: 10.1016/j.jfo.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Delyfer MN, Rougier MB, Leoni S, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011;37:271–8. doi: 10.1016/j.jcrs.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Çakır B, Celik E, Aksoy NÖ, et al. Toxic anterior segment syndrome after uncomplicated cataract surgery possibly associated with intracamaral use of cefuroxime. Clin Ophthalmol. 2015;9:493–7. doi: 10.2147/OPTH.S74249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gradin D, Mundia D. Effect of intracameral cefuroxime on fibrinous uveitis after pediatric cataract surgery. J Pediatr Ophthalmol Strabismus. 2011;48:45–9. doi: 10.3928/01913913-20100420-03. [DOI] [PubMed] [Google Scholar]
- 35.Giménez-de-la-Linde M, Giménez-Alcántara B, Barañano-Alcaide R, et al. Macular oedema after uncomplicated cataract surgery. Possible relationship with the volume of intracameral cefuroxime. Arch Soc Esp Oftalmol. 2017;92:49–50. doi: 10.1016/j.oftal.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Wong DC, Waxman MD, Herrinton LJ, et al. Transient macular edema after intracameral injection of a moderately elevated dose of cefuroxime during phacoemulsification surgery. JAMA Ophthalmol. 2015;133:1194–7. doi: 10.1001/jamaophthalmol.2015.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindquist TD, Robinson LD. The effect of vancomycin on the corneal endothelium. Cornea. 1996;15:41–5. [PubMed] [Google Scholar]
- 38.Gimbel HV, Sun R, DeBrof BM. Prophylactic intracameral antibiotics during cataract surgery: The incidence of endophthalmitis and corneal endothelial cell loss. Eur J Cataract Refract Surg. 1994;6:280–5. [Google Scholar]
- 39.Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. 2017;124:583–95. doi: 10.1016/j.ophtha.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura K, Suto C, Inoue Y, et al. Safety of intracameral injection of moxifloxacin using total replacement technique (bag and chamber flushing) J Ocul Pharmacol Ther. 2014;30:771–6. doi: 10.1089/jop.2014.0029. [DOI] [PubMed] [Google Scholar]
- 41.Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:1114–20. doi: 10.1016/j.jcrs.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Espiritu CR, Caparas VL, Bolinao JG. Safety of prophylactic intracameral moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg. 2007;33:63–8. doi: 10.1016/j.jcrs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Arslan OS, Arici C, Unal M, et al. Safety of prophylactic intracameral moxifloxacin ophthalmic solution after cataract surgery in patients with penetrating keratoplasty. Int J Ophthalmol. 2014;7:795–9. doi: 10.3980/j.issn.2222-3959.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekinci Koktekir B, Aslan BS. Safety of prophylactic intracameral moxifloxacin use in cataract surgery. J Ocul Pharmacol Ther. 2012;28:278–82. doi: 10.1089/jop.2011.0132. [DOI] [PubMed] [Google Scholar]
- 45.Matsuura K, Suto C, Akura J, et al. Comparison between intracameral moxifloxacin administration methods by assessing intraocular concentrations and drug kinetics. Graefes Arch Clin Exp Ophthalmol. 2013;251:1955–9. doi: 10.1007/s00417-013-2294-7. [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Park YH, Lee YC. Comparison of the effect of intracameral moxifloxacin, levofloxacin and cefazolin on rabbit corneal endothelial cells. Clin Exp Ophthalmol. 2008;36:367–70. doi: 10.1111/j.1442-9071.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 47.Kobayakawa S, Hiratsuka Y, Watabe Y, et al. Comparison of the influence of intracameral gentamicin, gatifloxacin, and moxifloxacin on the corneal endothelium in a rabbit model. Jpn J Ophthalmol. 2010;54:481–5. doi: 10.1007/s10384-010-0838-5. [DOI] [PubMed] [Google Scholar]
- 48.Lane SS, Osher RH, Masket S, et al. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg. 2008;34:1451–9. doi: 10.1016/j.jcrs.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 49.Asena L, Akova YA, Goktaş MT, et al. Ocular pharmacokinetics, safety and efficacy of intracameral moxifloxacin 0.5% solution in a rabbit model. Curr Eye Res. 2013;38:472–9. doi: 10.3109/02713683.2012.763101. [DOI] [PubMed] [Google Scholar]
- 50.Akal A, Ulas T, Goncu T, et al. Does moxifloxacin alter oxidant status in the cornea? An experimental study. Cutan Ocul Toxicol. 2015;34:139–43. doi: 10.3109/15569527.2014.918138. [DOI] [PubMed] [Google Scholar]
- 51.Zhou AX, Messenger WB, Sargent S, et al. Safety of undiluted intracameral moxifloxacin without postoperative topical antibiotics in cataract surgery. Int Ophthalmol. 2016;36:493–8. doi: 10.1007/s10792-015-0151-x. [DOI] [PubMed] [Google Scholar]
- 52.Cetinkaya S, Cetinkaya YF, Acir NO, et al. Application of intracameral moxifloxacin to prevent endophthalmitis in cataract surgery. International Eye Science. 2015;15:1680–3. [Google Scholar]
- 53.Ozlem TY, Necati DM, Fatma YM, et al. Are cefuroxime and vancomycin really safe on the corneal endothelial cells? Graefes Arch Clin Exp Ophthalmol. 2010;248:415–20. doi: 10.1007/s00417-009-1267-3. [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Canales JL, Pérez-Santonja JJ, Campos-Mollo E. Corneal endothelial changes after intracameral vancomycin injection in cataract surgery. J Cataract Refract Surg. 2015;41:126–34. doi: 10.1016/j.jcrs.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Canales JL, Pérez-Santonja JJ, Campos-Mollo E. Evaluation of macular thickness changes after intracameral vancomycin in cataract surgery. Int Ophthalmol. 2015;35:49–57. doi: 10.1007/s10792-014-0017-7. [DOI] [PubMed] [Google Scholar]
- 56.Kowalski RP, Romanowski EG, Mah FS, et al. Intracameral Vigamox (moxifoxacin 0.5%) is non-toxic and effective in preventing endophthalmitis in a rabbit model. Am J Ophthalmol. 2005;140:497.e1–497.e11. doi: 10.1016/j.ajo.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Yoeruek E, Spitzer MS, Saygili O, et al. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34:2139–45. doi: 10.1016/j.jcrs.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Zhou AX, Messenger WB, Sargent S, et al. Safety of undiluted intracameral moxifloxacin without postoperative topical antibiotics in cataract surgery. Int Ophthalmol. 2016;36 doi: 10.1007/s10792-015-0151-x. [DOI] [PubMed] [Google Scholar]
- 59.Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40:138–42. doi: 10.1016/j.jcrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Villada JR, Vicente U, Javaloy J, et al. Severe anaphylactic reaction after intracameral antibiotic administration during cataract surgery. J Cataract Refract Surg. 2005;31:620–1. doi: 10.1016/j.jcrs.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 61.Moisseiev E, Levinger E. Anaphylactic reaction following intracameral cefuroxime injection during cataract surgery. J Cataract Refract Surg. 2013;39:1432–4. doi: 10.1016/j.jcrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Lim CH, Williams SC, Yun ST, et al. Persistent concerns regarding intracameral cefuroxime. J Cataract Refract Surg. 2014;40:1236–7. doi: 10.1016/j.jcrs.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Hospital Infection Control Practices Advisory Committee (HICPAC) Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–13. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen ET, Shorstein NH. Preparation of intracameral antibiotics for injection. J Cataract Refract Surg. 2013;39:1778–9. doi: 10.1016/j.jcrs.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tripathi BJ, Tripathi RC. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res. 1989;6:395–403. [PubMed] [Google Scholar]
- 66.Haas MG, Yung CW, Chaluvadi U, et al. Vigamox: how good is its self-preservation? J Cataract Refract Surg. 2006;32:899–900. doi: 10.1016/j.jcrs.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 67.Behndig A, Cochener B, Güell JL, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39:1421–31. doi: 10.1016/j.jcrs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert DN, Robert ECC, Eliopoulos GM, et al. The sanford guide to antimicrobial therapy. 41. Sperryville, VA: Antimicrobial Therapy, Inc; 2011. [Google Scholar]
- 69.Kessel L, Flesner P, Andresen J, et al. Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis. Acta Ophthalmol. 2015;93:303–17. doi: 10.1111/aos.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Wang X, Chen X, et al. Perioperative antibiotics to prevent acute endophthalmitis after ophthalmic surgery: a systematic review and meta-analysis. PLoS One. 2016;11:e0166141. doi: 10.1371/journal.pone.0166141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ravindran RD, Venkatesh R, Chang DF, et al. Incidence of post-cataract endophthalmitis at Aravind Eye Hospital: outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg. 2009;35:629–36. doi: 10.1016/j.jcrs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Lundström M, Wejde G, Stenevi U, et al. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114:866–70. doi: 10.1016/j.ophtha.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 73.Friling E, Lundström M, Stenevi U, et al. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15–21. doi: 10.1016/j.jcrs.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 74.Daien V, Papinaud L, Gillies MC, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmol. 2016;134:810–6. doi: 10.1001/jamaophthalmol.2016.1351. [DOI] [PubMed] [Google Scholar]
- 75.Creuzot-Garcher C, Benzenine E, Mariet AS, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: a nationwide study in france from 2005 to 2014. Ophthalmology. 2016;123:1414–20. doi: 10.1016/j.ophtha.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 76.Behndig A, Montan P, Stenevi U, et al. One million cataract surgeries: Swedish National Cataract Register 1992-2009. J Cataract Refract Surg. 2011;37:1539–45. doi: 10.1016/j.jcrs.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 77.Lundström M, Friling E, Montan P. Risk factors for endophthalmitis after cataract surgery: Predictors for causative organisms and visual outcomes. J Cataract Refract Surg. 2015;41:2410–6. doi: 10.1016/j.jcrs.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 78.Katz G, Blum S, Leeva O, et al. Intracameral cefuroxime and the incidence of post-cataract endophthalmitis: an Israeli experience. Graefes Arch Clin Exp Ophthalmol. 2015;253:1729–33. doi: 10.1007/s00417-015-3009-z. [DOI] [PubMed] [Google Scholar]
- 79.Jabbarvand M, Hashemian H, Khodaparast M, et al. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123:295–301. doi: 10.1016/j.ophtha.2015.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


