Abstract
Aim
The oral glucose tolerance test (OGTT), widely used as a gold standard for gestational diabetes mellitus (GDM) diagnosis, provides a broad view of glucose pathophysiology in response to a glucose challenge. We conducted the present study to evaluate metabolite changes before and after an oral glucose challenge in pregnancy; and to examine the extent to which metabolites may serve to predict GDM diagnosis in pregnant women.
Methods
Peruvian pregnant women (n = 100) attending prenatal clinics (mean gestation 25 weeks) participated in the study with 23% of them having GDM diagnosis. Serum samples were collected immediately prior to and 2-hours after administration of an 75-g OGTT. Targeted metabolic profiling was performed using a LC-MS based metabolomics platform. Changes in metabolite levels were evaluated using paired Student's t tests and the change patterns were examined at the level of pathways. Multivariate regression procedures were used to examine metabolite pairwise differences associated with subsequent GDM diagnosis.
Results
Of the 306 metabolites detected, the relative concentration of 127 metabolites were statistically significantly increased or decreased 2-hours after the oral glucose load (false discovery rate [FDR] corrected P-value < 0.001). We identified relative decreases in metabolites in acylcarnitines, fatty acids, and diacylglycerols while relative increases were noted among bile acids. In addition, we found that C58:10 triacylglycerol (β=-0.08, SE=0.04), C58:9 triacylglycerol (β=-0.07, SE=0.03), adenosine (β=0.70, SE=0.32), methionine sulfoxide (β=0.36, SE=0.13) were significantly associated with GDM diagnosis even after adjusting for age and body mass index.
Conclusions
We identified alterations in maternal serum metabolites, representing distinct cellular and metabolic pathways including fatty acid metabolism, in response to an oral glucose challenge. These findings offer novel perspectives on the pathophysiological mechanisms underlying GDM.
Keywords: GDM, Metabolomics, OGTT, Omics
Introduction
Gestational diabetes mellitus (GDM), or impaired glucose intolerance first diagnosed during pregnancy, is one of the most common medical complications of pregnancy [1]. Annually, it is estimated to affect approximately 8% of pregnancies in the US [2] and 5% globally [3, 4]. GDM is associated with increased risk of caesarean and operative vaginal delivery, macrosomia, intrauterine growth retardation, shoulder dystocia, neonatal hypoglycaemia, hypocalcaemia, and hyperbilirubinemia [5-7]. Recently, the International Diabetes Federation reported that approximately 16% of live births were complicated by hyperglycaemia during pregnancy and the frequency of GDM affected pregnancies is most likely to increase concurrently with the increase in risk factors like obesity and physical inactivity [3].
In uncomplicated pregnancies, maternal tissues have been shown to progressively become insensitive to insulin and insulin-mediated whole-body glucose disposal decreases by 50% and insulin secretion by 200%-250%, in order to maintain a euglycaemic state [8]. In pregnancies complicated by GDM, however, the increased demand for insulin is not met due to inadequate pancreatic β-cell function leading to inadequate compensation for the body's insulin needs [9]. Of note, a subgroup of women with the history of GDM are at increased risk of developing type 2 diabetes mellitus (T2DM) postpartum [10]. However, the underlying metabolic pathophysiology of GDM is not well understood [11].
Emerging metabolomics technologies, that enable systemic analysis of small molecules in biological specimens, have been successfully used to provide a novel and deeper insight in the etiopathogenesis of diseases [11]. Metabolomics provides an integrated profile of biological status by identifying biochemical changes before the onset of overt clinical disease [12-16]. Relatively few studies have been conducted to assess the underlying metabolic pathophysiology of GDM and no adequate predictive methods are available for GDM thus posing a clear need to develop non-invasive and objective diagnostic methods [11, 12, 17, 18].
The oral glucose tolerance test (OGTT) is widely used as a gold standard for GDM diagnosis and provides a broad view of glucose pathophysiology in response to a glucose challenge [19]. Therefore, we designed a study to systematically characterize acute maternal metabolic changes subsequent to a 75-g oral glucose challenge in late pregnancy. We reasoned that the application of metabolomics methods in the context of clinical obstetrics will yield insights into metabolic alternations underlying GDM pathogenesis and lead to the discovery of additional diagnostic or prognostic biomarkers [20].
Methods
Participants and Study Setting
This analysis used data initially gathered for the Screening, Treatment and Effective Management of Gestational Diabetes Mellitus (STEM-GDM) study, a cohort study designed to evaluate the prevalence of GDM using the new diagnostic criteria proposed by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) among Peruvian women attending perinatal care at Instituto Nacional Materno Perinatal (INMP) in Lima, Peru [21, 22]. The INMP, overseen by the Peruvian Ministry of Health, is the primary referral hospital for maternal and perinatal care. Recruitment period was between February 2013 and February 2014. Women who initiated prenatal care before 28 weeks' gestation were eligible to participate. Women were ineligible if they were younger than 18 years of age, did not speak and read Spanish, did not plan to carry the pregnancy to term or deliver at INMP, and/or were past 28 weeks' gestation.
Enrolled subjects were asked to participate in a structured interview that gathered information regarding sociodemographic, lifestyle, medical, and reproductive characteristics between 24-28-week gestation (25 weeks' gestation, on average). After an 8-hour, overnight fast, participants underwent a 75g, 2-hour OGTT. Fasting and 2-hour blood samples were collected, processed, and stored at −80°C until analysed. Following the blood sample collection, a brief physical examination was administered by a trained research nurse who took anthropometric measures including standing height and weight. All participants provided informed consent and the research protocol was approved by the Institutional Review Boards of the INMP, Lima, Peru and the Harvard T. H. Chan School of Public Health Office of Human Research Administration, Boston, MA, USA.
Analytical Population
The analytical population was derived from participants who enrolled in the STEM-GDM Cohort between February 2013 and February 2014. During this period, a total of 1,032 women participated in the study. A total of 100 women were randomly selected for the present metabolomics analysis. The 100 randomly selected women for this analysis did not differ in regards to sociodemographic and lifestyle characteristics as compared with the total cohort.
Metabolic Profiling
Fasting blood samples were collected at 25 weeks of gestation, on average, after an overnight fast, both immediately prior to and 2-hours after administration of 75-g OGTT. Samples were protected from ultraviolet light, kept on wet ice and centrifuged within 20 minutes of phlebotomy. Serum samples were stored at −80°C until assayed. Four liquid chromatography-tandem mass spectrometry (LC-MS) methods were used to profile serum polar metabolites and lipids. Negative ion mode, targeted MS analyses of polar metabolites were conducted as described previously [23]. Briefly, LC-MS samples were prepared from serum (30 μL) via protein precipitation with the addition of four volumes of 80% methanol containing inosine-15N4, thymine-d4 and glycocholate-d4 internal standards (Cambridge Isotope Laboratories; Andover, MA). The samples were centrifuged (10 min, 9,000 × g, 4°C) and the supernatants were analysed using an ACQUITY UPLC (Waters, Milford MA) coupled to a 5500 QTRAP triple quadrupole mass spectrometer (AB SCIEX, Framingham, MA). Extracts (10 μL) were injected directly onto a 150 × 2.0 mm Luna NH2 column (Phenomenex; Torrance, CA). The column was eluted at a flow rate of 400 μL/min with initial conditions of 10% mobile phase A (20 mM ammonium acetate and 20 mM ammonium hydroxide in water) and 90% mobile phase B (10 mM ammonium hydroxide in 75:25 v/v acetonitrile/methanol) followed by a 10 min linear gradient to 100% mobile phase. MS data were acquired using multiple reaction monitoring scans tuned for each compound using authentic reference standards. The ion spray voltage was -4.5 kV and the source temperature was 500°C. Raw data were processed using MultiQuant 2.1 software (SCIEX, Framingham MA). Nontargeted, positive ion mode analyses of polar metabolites and lipids were conducted using two separate methods as described previously [24]. Data for both methods were acquired using a Nexera X2 U-HPLC system (Shimadzu Scientific Instruments; Marlborough, MA) coupled to a Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific; Waltham, MA). Polar metabolites were extracted from body fluids and tissue homogenates (10 μL) using addition of nine volumes of 74.9:24.9:0.2 v/v/v acetonitrile/methanol/formic acid containing stable isotope-labelled internal standards (valine-d8, Isotec; and phenylalanine-d8, Cambridge Isotope Laboratories; Andover, MA). The extracts were centrifuged (10 min, 9,000 × g, 4°C), and the supernatants were injected onto a 150 × 2 mm Atlantis HILIC column (Waters; Milford, MA). The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mM ammonium formate and 0.1% formic acid in water) for 1 minute followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 minutes. Polar metabolite MS analyses were carried out using electrospray ionization in the positive ion mode using full scan analysis over m/z 70-800 at 70,000 resolution and 3 Hz data acquisition rate. Additional MS settings were: ion spray voltage, 3.5 kV; capillary temperature, 350°C; probe heater temperature, 300 °C; sheath gas, 40; auxiliary gas, 15; and S-lens RF level 40. Lipids were extracted from plasma and lung tissue homogenates (10 μL) using 190 μL of isopropanol containing 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphocholine as an internal standard (Avanti Polar Lipids; Alabaster, AL). After centrifugation (10 min, 9,000 × g, ambient temperature), supernatants (2 μL) were injected directly onto a 100 × 2.1 mm ACQUITY BEH C8 column (1.7 μm; Waters; Milford, MA). The column was eluted at a flow rate of 450 μL/min isocratically for 1 minute at 80% mobile phase A (95:5:0.1 vol/vol/vol 10 mM ammonium acetate/methanol/acetic acid), followed by a linear gradient to 80% mobile-phase B (99.9:0.1 vol/vol methanol/acetic acid) over 2 minutes, a linear gradient to 100% mobile phase B over 7 minutes, and then 3 minutes at 100% mobile-phase B. MS analyses were carried out using electrospray ionization in the positive ion mode using full scan analysis over m/z 200-1100 at 70,000 resolution and 3 Hz data acquisition rate. Additional MS settings were: ion spray voltage, 3.0 kV; capillary temperature, 320°C; probe heater temperature, 300 °C; sheath gas, 50; auxiliary gas, 15; and S-lens RF level 60.
Laboratory Analytical Procedures
Fasting blood samples were collected and processed in accordance with standard international procedures using the glucose oxidase method. The diagnosis of GDM was determined using the new IADPSG recommendations [25]. With this definition, the diagnosis of GDM was made when any of the following values from the 75-g OGTT is equaled or exceeded: fasting plasma glucose 5.1 mmol/L, 1-h plasma glucose 10.0 mmol/L, or 2-h plasma glucose 8.5 mmol/L.
Other Covariates
Maternal body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Categories of BMI were characterized as normal weight (<25 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2). Maternal age was categorized as follows: < 19, 20-29, 30-34, ≥ 35 years. Other social and demographic variables were categorized as: maternal education (≤ 6, 7-12, and > 12 completed years of schooling), nulliparous (yes vs. no), marital status (married or living with partner vs. single or living alone/divorced), difficulties to pay for necessities like food (very hard/hard, somewhat hard, and not hard), and difficulties to access medical care (very hard/hard, somewhat hard, and not hard).
Statistical Analysis
After excluding 19 metabolites with more than 20% missing or undetectable values, a total of 306 metabolites were analysed. Metabolites were transformed on the natural logarithm scale due to skewed distribution. Paired Student's t-tests were performed to examine the difference in metabolite levels between the two-time points (fasting and 2-hours post-glucose challenge). To account for multiple testing, a false discovery rate (FDR) procedure was applied [26]. For each metabolite, fold change was determined by taking the mean of the ratios of the log-transformed values between the two-time points for everyone. Then, we used two approaches to compute risk scores of metabolites associated with GDM. In the first approach, we used principal component analysis (PCA) and evaluated the association between the first principal component and GDM status using logistic regression as the first principal component is the one that explains the maximum amount of variance possible in the dataset. The risk score was defined as the product of the estimated regression coefficient and the value of the first principal component. For the second approach, we used logistic regression models to examine the relation between each metabolite within pathways and GDM status. Scores were computed by multiplying the delta value of each metabolite with its corresponding effect size then summing these products across all metabolites. A leave-one-out cross-validation procedure was implemented to protect against model over-fitting. Thus, successively one observation was left out from the sample (n), the remaining observations (n-1) constituted the training set and the left-out one the validation set. Each time, and for both approaches, regression coefficients were estimated on the training set. This process was repeated n times until all observations were validated. Scores were calculated for the validation set using estimated regression coefficients from the training set. Once all scores were computed a logistic regression model was fit to examine the association of the scores with GDM status. All statistical analyses were conducted using R [27].
Results
The socio-demographic characteristics of the participants are presented in Table I. A total of 100 pregnant women between the ages of 18 and 45 years (mean age = 28.6 years, standard deviation = 6.6 years) with mean gestational age of 25.6 weeks' (SD = 1.2 weeks) participated in the study. Most participants were married or living with their partner (84.0%) while 28.5% were employed during pregnancy. Approximately 20% of participants reported smoking before pregnancy while 29% reported alcohol consumption before index pregnancy. Only 1% reported smoking during index pregnancy while 4% reported alcohol consumption during pregnancy. Gestational diabetes (defined according to the international association for diabetes and pregnancy study group with fasting glucose ≥ 92 mg/dL) was present in 23% of study participants.
Table I. Characteristics of Study Population (n=100).
| Characteristics | n |
|---|---|
| Gestational age (weeks)* | 25.6 ± 1.2 |
| Maternal age (years) | 28.7 ± 6.6 |
| 18-19 | |
| 20-29 | 54 |
| 30-34 | 19 |
| ≥ 35 | 22 |
|
| |
| Marital status | |
| Married/living with partner | 84 |
| Other | 16 |
| Employed during pregnancy | 28 |
| Smoked before pregnancy | 20 |
| Smoked during pregnancy | 1 |
| Alcohol consumption before pregnancy | 29 |
| Alcohol consumption during pregnancy | 4 |
| Nulliparous | 34 |
| Body mass index (kg/m2)* | 28.2 ± 3.9 |
| Body mass index (kg/m2) | |
| < 18.5 | 23 |
| 18.5-24.9 | 48 |
| 25.0-29.9 | 23 |
| ≥ 30.0 | 6 |
| Diagnosed gestational diabetes** | 23 |
Data in mean ± SD or number (%);
Gestational diabetes (GDM) defined according to the International Association for Diabetes and Pregnancy Study Group with fasting glucose ≥ 92 mg/dl. [25]; BMI at the time of testing
Out of the 306 metabolites that were detected, the relative abundance of 127 metabolites had a statistically significantly increase or decrease after OGTT (FDR-corrected P-value < 0.001). Most metabolites decreased in response to OGTT (Table II-a and Supplemental Figure 1a; see supplementary materials associated with this article on line) while a few increased in response to OGTT (Table II-b and Supplemental Figure 1b; see supplementary materials associated with this article on line). The largest relative decrease after OGTT was seen in C8 carnitine which decreased by 20%, followed by C14:2 carnitine, C14:1 carnitine, C12 carnitine, and C4-OH carnitine which decreased by 13% each. The largest relative increases after OGTT were seen in adenosine and taurolithocholic acid, which increased by 25% and 12% respectively. Figure 1 shows a heat map of partial Pearson correlation coefficients of metabolite concentrations between before and after responses to OGTT, adjusted for gestational age and grouped by pathways. We found that baseline concentrations of metabolites in common biological pathways were correlated after OGTT. Higher positive correlations were noted for carnitines while higher negative correlations were noted for triacylglycerols.
Table II-a. Metabolites that decreased after glucose challenge (FDR adjusted paired t-test P-value < 0.001).
| Metabolite | Pathway group | Mean change | SD | Fold Change |
|---|---|---|---|---|
| C8 carnitine | Acylcarnitines | -1.45 | 0.95 | 1.20 |
| C14:2 carnitine | Acylcarnitines | -1.15 | 0.53 | 1.13 |
| C12 carnitine | Acylcarnitines | -1.07 | 0.56 | 1.13 |
| C4-OH carnitine | Acylcarnitines | -0.95 | 0.64 | 1.13 |
| C14:1 carnitine | Acylcarnitines | -1.09 | 0.53 | 1.13 |
| C10 carnitine | Acylcarnitines | -1.04 | 0.57 | 1.11 |
| 5-HETE | Fatty acid | -1.00 | 0.96 | 1.10 |
| C6 carnitine | Acylcarnitines | -0.76 | 0.40 | 1.09 |
| C12:1 carnitine | Acylcarnitines | -1.07 | 0.55 | 1.08 |
| C10:2 carnitine | Acylcarnitines | -0.60 | 0.38 | 1.08 |
| C14 carnitine | Acylcarnitines | -0.57 | 0.47 | 1.07 |
| C30:0 DAG | Diacylglycerols | -0.61 | 0.59 | 1.07 |
| Palmitoleic acid | Fatty acid | -1.26 | 0.43 | 1.07 |
| Gamma-Linolenic acid | Fatty acid | -1.18 | 0.47 | 1.06 |
| C32:2 DAG | Diacylglycerols | -0.56 | 0.69 | 1.06 |
| Aspartate | Amino acid | -0.54 | 0.40 | 1.06 |
| Methinonine sulfoxide | Amino acid | -0.44 | 0.56 | 1.06 |
| Glycine | Amino acid | -0.34 | 0.34 | 1.06 |
| Alpha-glycerophosphocholine | Glycerophospholipids | -0.70 | 0.35 | 1.06 |
| Allantoin | Purine | -0.43 | 0.61 | 1.05 |
| Docosapentaenoic acid | Fatty acid | -0.84 | 0.38 | 1.05 |
| C18:2 carnitine | Acylcarnitines | -0.46 | 0.38 | 1.05 |
| Adrenic acid | Fatty acid | -0.79 | 0.34 | 1.05 |
| C9 carnitine | Acylcarnitines | -0.60 | 0.54 | 1.05 |
| Eicosapentaenoic acid | Fatty acid | -0.77 | 0.36 | 1.05 |
| C18:1 carnitine | Acylcarnitines | -0.47 | 0.34 | 1.05 |
| Glutamate | Amino acid | -0.48 | 0.32 | 1.05 |
| Leucine | Amino acid | -0.56 | 0.26 | 1.04 |
| Isoleucine | Amino acid | -0.52 | 0.22 | 1.04 |
| 8.11.14-Eicosatrienoic acid | Fatty acid | -0.67 | 0.30 | 1.04 |
| Methionine | Amino acids | -0.42 | 0.23 | 1.04 |
| Citrulline | Amino acid | -0.38 | 0.20 | 1.04 |
| Serine | Amino acid | -0.37 | 0.22 | 1.04 |
| Linoleic acid | Amino acid | -0.70 | 0.28 | 1.04 |
| Phenylalanine | Amino acid | -0 47 | 0 27 | 1 04 |
| Oleic acid | Fatty acid | -0.67 | 0.26 | 1.03 |
| Thiamine | Amino acids | -0.46 | 0.37 | 1.03 |
| C18:0 LPE | Lysophosphatidyletha | -0.33 | 0.33 | 1.03 |
| Tyrosine | Amino acid | -0.39 | 0.23 | 1.03 |
| Docosahexaenoic acid | Fatty acid | -0.58 | 0.30 | 1.03 |
| C2 carnitine | Acyclarnitines | -0.43 | 0.30 | 1.03 |
| Asparagine | Amino acid | -0.29 | 0.19 | 1.03 |
| Niacinamide | Amino acid | -0.28 | 0.42 | 1.03 |
| Homocysteine | Amino acid | -0.29 | 0.64 | 1.03 |
| C16 carnitine | Acyclarnitines | -0.26 | 0.21 | 1.03 |
| Palmitic acid | Fatty acid | -0.51 | 0.18 | 1.03 |
| Taurine | Biogenic amines | -0.33 | 0.43 | 1.03 |
| Valine | Amino acids | -0.30 | 0.17 | 1.03 |
| Arachidonic acid | Fatty acid | -0.45 | 0.36 | 1.02 |
| C32:1 DAG | Diacylglycerols | -0.29 | 0.21 | 1.02 |
| Hydroxyproline | Amino Acid | -0.25 | 0.18 | 1.02 |
| C16:0 LPE | Lysophosphatidyletha | -0.24 | 0.33 | 1.02 |
| C42:0 TAG | Triacylglycerol | -0.25 | 0.33 | 1.02 |
| Ornithine | Biogenic amines | -0.23 | 0.25 | 1.02 |
| C18:1 LPE | Lysophosphatidyletha | -0.20 | 0.41 | 1.02 |
| Kynurenic acid | Amino Acid | -0.19 | 0.35 | 1.02 |
| Arginine | Amino acid | -0.31 | 0.20 | 1.02 |
| C32:0 DAG | Diacylglycerols | -0.25 | 0.19 | 1.02 |
| C34:0 DAG | Diacylglycerols | -0.23 | 0.17 | 1.02 |
| Myristic acid | Fatty acid | -0.38 | 0.16 | 1.02 |
| Stearic acid | Fatty acid | -0.37 | 0.18 | 1.02 |
| C18:0 LPC | Lysophosphatidylcholi | -0.28 | 0.24 | 1.02 |
| Threonine | Amino acids | -0.19 | 0.15 | 1.02 |
| C34:3 DAG | Diacylglycerols | -0.18 | 0.20 | 1.02 |
| Choline | Amino Acid | -0.22 | 0.24 | 1.02 |
Fold change represents mean of log-pre-OGTT/log- post-OGTT value.
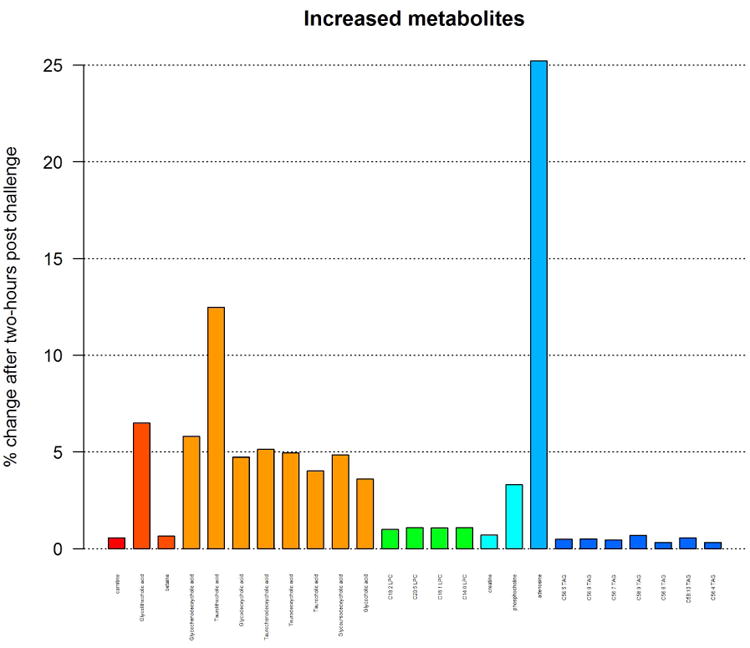
Table II-b. Metabolites that increased after glucose challenge (FDR adjusted paired t-test P-value < 0.001).
| Metabolite | Pathway group | Mean change | SD | Fold Change* |
|---|---|---|---|---|
| Adenosine | Purine | 1.64 | 1.32 | 1.25 |
| Taurolithocholic acid | Bile acid | 1.28 | 1.14 | 1.12 |
| Glycoli thocholic acid | Bile acid | 0.69 | 0.88 | 1.06 |
| Glycochenodeoxycholic acid | Bile acid | 0.87 | 0.85 | 1.06 |
| Taurochenodeoxycholic acid | Bile acid | 0.73 | 0.93 | 1.05 |
| Taurodeoxycholic acid | Bile acid | 0.68 | 0.89 | 1.05 |
| Glycoursodeoxycholic acid | Bile Acid | 0.56 | 0.96 | 1.05 |
| Glycodeoxycholic acid | Bile acid | 0.69 | 0.75 | 1.05 |
| Taurocholic acid | Bile acid | 0.53 | 0.95 | 1.04 |
| Glycocholic acid | Bile Acid | 0.50 | 0.97 | 1.04 |
| Phosphocholine | Organonitrogen | 0.42 | 0.70 | 1.03 |
Fold change represents mean of log-post-OGTT/log- pre-OGTT value.
Figure 1. Heat-map of partial Pearson correlation coefficients adjusted for gestational age for the 127 significantly changed metabolites ordered by pathway groups.
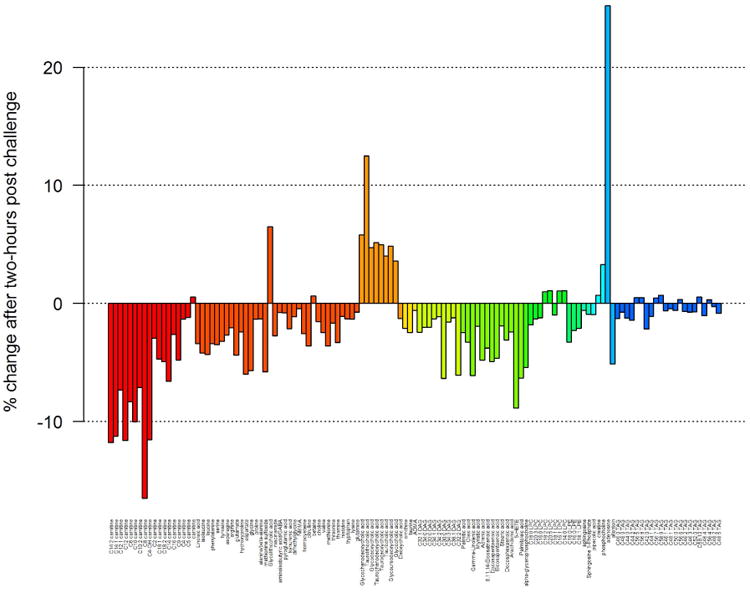
Figure 2 shows changes in metabolite levels in response to OGTT in groups of metabolic pathways. For example, major decreases in metabolite levels were noted in acylcarnitines, fatty acids and diacylglycerols pathways where more than 10% decrease in relative concentration was noted in the following metabolites: carnitines C12:2, C14:1, C12, C10:2 and C8. A major increase in concentration was noted in metabolites of bile acid pathway where adenosines increased by more than 20% and taurolithocholic acid increased by more than 10%.
Figure 2.
Changes in metabolite levels in response to two-hour post challenge are shown. Metabolites were ordered by pathway groups.
In analyses using linear regression procedures on each metabolite versus GDM status, we found that C58:10 triacylglycerol, C58:9 triacylglycerol, adenosine, and methionine sulfoxide were significantly associated with GDM status even after adjusting for age and BMI (Table III).
Table III-a. Differences in metabolite levels in response to OGTT between GDM and non GDM cases.
| Metabolite | Adjusted for age | Adjusted for age and BMI | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | SE | P value | Estimate | SE | P value | |
| C58:10 TAG | -0.085 | 0.037 | 0.030 | -0.082 | 0.037 | 0.031 |
| C58:9 TAG | -0.069 | 0.033 | 0.041 | -0.069 | 0.033 | 0.042 |
| Adenosine | 0.705 | 0.321 | 0.031 | 0.705 | 0.322 | 0.031 |
| Methionine sulfoxide | 0.356 | 0.131 | 0.008 | 0.357 | 0.131 | 0.008 |
| b. Effect of scores on GDM status evaluated by logistic regression | |||
|
| |||
| Estimate | SE | P value | |
|
|
|||
| Score based on pca | -29.3788 | 8.6236 | < 0.001 |
| Score based on univariate analysis | |||
| Normalized | -0.1714 | 0.05857 | 0.003 |
Next, to identify a linear combination of metabolites that might be associated with of GDM, we calculated risk scores using two approaches. In the first approach, we performed a PCA on normalized data. The association between the first principal component (representing 88% of variability) and GDM status was examined using a logistic regression model. The corresponding risk score computed by multiplying the first principal component by its effect size was found to be significantly associated with GDM risk (coefficient = -29.37, SE = 8.6, P < 0.001). The distribution of the scores by GDM status are shown in Supplemental Figures 2a and 2b (see supplementary materials associated with this article on line). Overall, the median scores for GDM women were lower in amino acids and glycerophospholipids while they are relatively higher for Lysophosphatidylcholines, as compared with non-GDM women. In the second approach, each metabolite's relation with GDM status was analysed using univariate logistic regression models and scores were computed by multiplying the delta value of each metabolite concentrations at baseline and post-OGTT with its corresponding regression coefficient and taking the sum over all metabolites. This score is also found to be associated with GDM status (β coefficient = -0.113, SE = 0.046, P = 0.0143).
Discussion
Using a targeted metabolomics approach, we found significant metabolic differences in various biological pathways in response to a glucose challenge during pregnancy. Specifically, we identified relative decreases in acylcarnitines, fatty acids, and diacylglycerols pathways while relative increases were noted in bile acids pathways. In addition, we found that C58:10 triacylglycerol, C58:9 triacylglycerol, adenosine, and methionine sulfoxide were significantly associated with GDM status even after adjusting for age and BMI.
Our findings showing decreases in acylcarnitines and diacylglycerols following glucose ingestion are not surprising [12]. Acylcarnitines, involved in β-oxidation of fatty acid or amino acid metabolism, are markers of mitochondrial dysfunction [28] and have been implicated in insulin resistance and energy homeostasis [29]. Moreover, acylcarnitines are synthesized by the enzyme carnitine palmitoyltransferase 1 (CPT 1) that is known to be responsible for the transport of long-chain fatty acids into the mitochondrial matrix [30]. Fatty acids can be metabolized via a mitochondrial FA oxidation (FAO) pathway which yields energy. The FAO competes with glucose oxidation in a process known as glucose-FA or Randle cycle [29]. Available evidence suggests that acylcarnitines play a significant role in energy metabolism and GDM pathogenesis [31]. Investigators have also postulated an alternative mechanism in which FAO rate outpaces the tricarboxylic acid cycle (TCA) thereby leading to the accumulation of intermediary metabolites such as acylcarnitines that may interfere with insulin sensitivity [29].
Lipotoxicity, excess lipid supply and accumulation, has been one of the theories proposed for the induction of insulin resistance in glucose and lipid metabolism [29, 32]. Diacylglycerols (DAG) are lipid intermediates that have been long implicated in insulin resistance and are signalling molecules and building blocks of cellular membranes, which harbour the insulin receptor. Animal studies have demonstrated that long-term ingestion of DAG prevents high fat-induced body weight gain and fat accumulation [33, 34]. Our study results showing decreases in DAG following glucose ingestion confirm prior observation in animal [35] and human studies [36] suggesting a key role for lipid intermediates such as DAG in the development of metabolic disorders such as GDM.
Our findings showing post-glucose increases in conjugated bile acid pathways are consistent with prior studies [12]. It has long been known that bile acids are amphipathic molecules that function as powerful detergents to facilitate absorption of lipids and nutrients and excretion of cholesterol and toxic metabolites [37]. In response to glucose ingestion, cholecystokinin stimulates the gallbladder to contract and release bile into the enterohepatic circulation [38]. Several lines of evidence have demonstrated that bile acids play important roles in glucose regulation and energy homeostasis [39, 40]. For example, studies from animal models have shown that the primary bile acids such as cholic acid synthesized from hepatic cholesterol increases energy expenditure and prevents the development of high-fat-induced obesity and insulin resistance [41]. These metabolic effects of bile acid are mediated by the G-protein-coupled receptor TGR5, leading to the induction of type 2 iodothyronine deiodinase. Recently, Vincent et al. have found that the postprandial bile acid response in obese patients with type 2 diabetes mellitus is greater than that in normo-glycaemic individuals [42]. In the past decade, there has been an increased recognition that bile acids are natural ligands for the farnesoid X receptor (FXR-α) and were found to activate specific nuclear receptors such as pregnane X receptor, vitamin D receptor, G protein-coupled receptors and cell signalling pathways [43]. A recent study by Fall et al found that increased concentrations of three 12α-hydroxylated bile acids (deoxycholic acid, glycocholic acid, and glycodeoxycholic acid) were associated with incident diabetes [44]. In addition, a genetic variant within the CYP7A1 locus, encoding the rate-limiting enzyme in bile acid synthesis, was found to be associated with lower type 2 diabetes risk [44]. Collectively these observations, coupled with our findings, provide additional insights into the key role bile acid pathways play in the pathogenesis of GDM and other metabolic disorders.
We found a sub-group of maternal metabolites (i.e., C58:10 triacylglycerol, C58:9 triacylglycerol, adenosine, and methionine sulfoxide) to be statistically significantly associated with GDM. These findings indicate the roles endothelial dysfunction and oxidative stress pathways play in the pathophysiology of GDM [45]. For instance, adenosine acts as a vasodilator in several vascular beds and acts as a stimulator of endothelial cell proliferation [46]. Adenosine has been implicated in physiological responses of different tissues [47, 48]. It contributes to endothelial dysfunction in endothelial cells from the umbilical veins of patients with GDM [49]. Of note, the transport and metabolism of adenosine is markedly impaired in foetal endothelial cells isolated from pregnancies complicated by GDM [47, 48]. In addition, increased concentration of adenosine upregulate expression of endothelial nitric oxide synthase via A2A adenosine receptors, which leads to increased nitric oxide synthesis, a potential cause of vascular dysfunction in GDM [50].
Investigators have reported that methionine sulfoxide can be considered a marker of oxidative stress and is associated with increased high sensitivity c-reactive protein [51, 52]. These findings, if replicated, should facilitate the identification of additional circulating biomarkers suitable for predicting the development or progression of GDM
Some strengths and limitations should be considered in interpreting our study findings. Using samples before and after glucose ingestion allowed subjects to serve as their own biological controls. This eliminated confounding by subject characteristics. However, there are several caveats that merit consideration. First, the cross-sectional design of our study does not allow for determination of the temporal relationship between perturbations of metabolites and GDM risk although the changes in metabolites were in agreement with known physiological actions of glucose. Longitudinal studies are needed following women earlier in their pregnancies. Second, the changes observed in metabolites were in relative concentrations. We were not able to provide absolute concentrations of metabolites. Absolute quantification of metabolites is a future area of investigation. Lastly, our study had a limited sample size and was conducted in women attending prenatal clinics in Peru and hence may not be generalizable to other populations.
In summary, we identified changes in several metabolites representing distinct biological pathways during an OGTT offering novel perspectives on the GDM pathogenesis. We found relative decreases in acylcarnitines, fatty acids, and diacylglycerols pathways while relative increases were noted in bile acids pathways. In addition, we found that C58:10 triacylglycerol, C58:9 triacylglycerol, adenosine, and methionine sulfoxide were significantly associated with GDM status even after adjusting for age and BMI.
Supplementary Material
Supplemental Figure 1a- Decreased metabolites in response to two-hour post challenge
Supplemental Figure 1b- Increased metabolites in response to two-hour post challenge
Supplemental Figure 2a- Distribution of scores by GDM status
Supplemental Figure 2b- Boxplots of the distribution of scores by GDM status
Acknowledgments
This research was supported by Roche Diagnostic Operations Inc. (project number 208617-5074547) and the National Institutes of Health (NIH), National Institute for Minority Health and Health Disparities (T37-MD0001449). The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors wish to thank the dedicated staff members of Asociacion Civil Proyectos en Salud (PROESA), Peru and Instituto Especializado Materno Perinatal, Peru for their expert technical assistance with this research.
Footnotes
Conflicts of Interest: None Declared
Appendix supplementary material: Supplementary materials (Supplemental Fig. 1a and 1b, and 2a and 2b) associated with this article can be found at http://www.scincedirect.com at doi …
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ADA. American Diabetes Association: Clinical Practice Recommendations. DiabetesCare. 2001;24(1):S1–133. [PubMed] [Google Scholar]
- 2.Jovanovic L, Liang Y, Weng W, Hamilton M, Chen L, Wintfeld N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes Metab Res Rev. 2015;31:707–16. doi: 10.1002/dmrr.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes. 2015;6:1065–72. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational diabetes mellitus: results from a survey of country prevalence and practices. J Matern Fetal Neonatal Med. 2012;25:600–10. doi: 10.3109/14767058.2011.587921. [DOI] [PubMed] [Google Scholar]
- 5.Illsley NP. Placental glucose transport in diabetic pregnancy. Clin Obstet Gynecol. 2000;43:116–26. doi: 10.1097/00003081-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 7.Group HSCR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet. 2002;78:69–77. doi: 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 8.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(2):S112–9. doi: 10.2337/dc07-s202. [DOI] [PubMed] [Google Scholar]
- 9.Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59:2625–30. doi: 10.2337/db10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dessi A, Marincola FC, Fanos V. Metabolomics and the great obstetrical syndromes--GDM, PET, and IUGR. Best Pract Res Clin Obstet Gynaecol. 2015;29:156–64. doi: 10.1016/j.bpobgyn.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Ho JE, Larson MG, Vasan RS, Ghorbani A, Cheng S, Rhee EP, et al. Metabolite profiles during oral glucose challenge. Diabetes. 2013;62:2689–98. doi: 10.2337/db12-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–11. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–17. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–8. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentley-Lewis R, Xiong G, Lee H, Yang A, Huynh J, Kim C. Metabolomic Analysis Reveals Amino Acid Responses to an Oral Glucose Tolerance Test in Women with Prior History of Gestational Diabetes Mellitus. J Clin Transl Endocrinol. 2014;1:38–43. doi: 10.1016/j.jcte.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enquobahrie DA, Denis M, Tadesse MG, Gelaye B, Ressom HW, Williams MA. Maternal Early Pregnancy Serum Metabolites and Risk of Gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2015;100:4348–56. doi: 10.1210/jc.2015-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8(11):639–49. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud. 2015;1:a000588. doi: 10.1101/mcs.a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelaye B, Larrabure-Torrealva GT, Qiu C, Luque-Fernandez MA, Peterlin BL, Sanchez SE, et al. Fasting lipid and lipoproteins concentrations in pregnant women with a history of migraine. Headache. 2015;55:646–57. doi: 10.1111/head.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice JR, Larrabure-Torrealva GT, Luque Fernandez MA, Grande M, Motta V, Barrios YV, et al. High risk for obstructive sleep apnea and other sleep disorders among overweight and obese pregnant women. BMC Pregnancy Childbirth. 2015;15:198. doi: 10.1186/s12884-015-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59:1657–67. doi: 10.1373/clinchem.2012.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–46. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ADA. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001:1165–88. [Google Scholar]
- 27.R Development core Team. R Foundation for Statistical Computing, 2008. Vienna, Austria: 2008. R: A language and environment for statistical computing. URL http://www.r-project.org/ [Google Scholar]
- 28.Lord R, Bralley J. Laboratory evaluations for integrative and functional medicine. 2nd. Duluth, GA: Metametrix Institution; 2008. [Google Scholar]
- 29.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indiveri C, Iacobazzi V, Tonazzi A, Giangregorio N, Infantino V, Convertini P, et al. The mitochondrial carnitine/acylcarnitine carrier: function, structure and physiopathology. Molecular aspects of medicine. 2011;32:223–33. doi: 10.1016/j.mam.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Qiu C, Enquobahrie DA, Frederick IO, Sorensen TK, Fernandez MA, David RM, et al. Early pregnancy urinary biomarkers of fatty acid and carbohydrate metabolism in pregnancies complicated by gestational diabetes. Diabetes research and clinical practice. 2014;104:393–400. doi: 10.1016/j.diabres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badin PM, Vila IK, Louche K, Mairal A, Marques MA, Bourlier V, et al. High-fat diet-mediated lipotoxicity and insulin resistance is related to impaired lipase expression in mouse skeletal muscle. Endocrinology. 2013;154:1444–53. doi: 10.1210/en.2012-2029. [DOI] [PubMed] [Google Scholar]
- 33.Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, et al. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–8. [PubMed] [Google Scholar]
- 34.Meng X, Zou D, Shi Z, Duan Z, Mao Z. Dietary diacylglycerol prevents high-fat diet-induced lipid accumulation in rat liver and abdominal adipose tissue. Lipids. 2004;39:37–41. doi: 10.1007/s11745-004-1199-1. [DOI] [PubMed] [Google Scholar]
- 35.Mori Y, Nakagiri H, Kondo H, Murase T, Tokimitsu I, Tajima N. Dietary diacylglycerol reduces postprandial hyperlipidemia and ameliorates glucose intolerance in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Nutrition. 2005;21:933–9. doi: 10.1016/j.nut.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Olsson J, Sundberg B, Viberg A, Haenni A. Effect of a vegetable-oil emulsion on body composition; a 12-week study in overweight women on a meal replacement therapy after an initial weight loss: a randomized controlled trial. Eur J Nutr. 2011;50:235–42. doi: 10.1007/s00394-010-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang JY. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G349–56. doi: 10.1152/ajpgi.00417.2002. [DOI] [PubMed] [Google Scholar]
- 38.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–52. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T, Aoyama J, Hashimoto M, Ohara M, Futami-Suda S, Suzuki K, et al. Correlation between postprandial bile acids and body fat mass in healthy normal-weight subjects. Clin Biochem. 2014;47:1128–31. doi: 10.1016/j.clinbiochem.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 42.Vincent RP, Omar S, Ghozlan S, Taylor DR, Cross G, Sherwood RA, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50(Pt 4):360–4. doi: 10.1177/0004563212473450. [DOI] [PubMed] [Google Scholar]
- 43.Carotti A, Marinozzi M, Custodi C, Cerra B, Pellicciari R, Gioiello A, et al. Beyond bile acids: targeting Farnesoid X Receptor (FXR) with natural and synthetic ligands. Curr Top Med Chem. 2014;14:2129–42. doi: 10.2174/1568026614666141112094058. [DOI] [PubMed] [Google Scholar]
- 44.Fall T, Salihovic S, Brandmaier S, Nowak C, Ganna A, Gustafsson S, et al. Non-targeted metabolomics combined with genetic analyses identifies bile acid synthesis and phospholipid metabolism as being associated with incident type 2 diabetes. Diabetologia. 2016;59:2114–24. doi: 10.1007/s00125-016-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enquobahrie DA, Qiu CF, Hevner K, Abetew D, Williams MA. Maternal plasma protein profiles in response to oral 50-gram glucose load in mid-pregnancy: a pilot study. Int J Mol Epidemiol Genet. 2011;2:292–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Plagemann PG, Wohlhueter RM, Woffendin C. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988;947:405–43. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 47.Sobrevia L, Mann GE. Dysfunction of the endothelial nitric oxide signalling pathway in diabetes and hyperglycaemia. Exp Physiol. 1997;82:423–52. doi: 10.1113/expphysiol.1997.sp004038. [DOI] [PubMed] [Google Scholar]
- 48.Sobrevia L, Jarvis SM, Yudilevich DL. Adenosine transport in cultured human umbilical vein endothelial cells is reduced in diabetes. Am J Physiol. 1994;267(1 Pt 1):C39–47. doi: 10.1152/ajpcell.1994.267.1.C39. [DOI] [PubMed] [Google Scholar]
- 49.Antonioli L, Blandizzi C, Csoka B, Pacher P, Hasko G. Adenosine signalling in diabetes mellitus--pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–41. doi: 10.1038/nrendo.2015.10. [DOI] [PubMed] [Google Scholar]
- 50.Vasquez G, Sanhueza F, Vasquez R, Gonzalez M, San Martin R, Casanello P, et al. Role of adenosine transport in gestational diabetes-induced L-arginine transport and nitric oxide synthesis in human umbilical vein endothelium. J Physiol. 2004;560(Pt 1):111–22. doi: 10.1113/jphysiol.2004.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teichert T, Hellwig A, Pessler A, Hellwig M, Vossoughi M, Sugiri D, et al. Association between Advanced Glycation End Products and Impaired Fasting Glucose: Results from the SALIA Study. PLoS One. 2015;10:e0128293. doi: 10.1371/journal.pone.0128293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–21. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1a- Decreased metabolites in response to two-hour post challenge
Supplemental Figure 1b- Increased metabolites in response to two-hour post challenge
Supplemental Figure 2a- Distribution of scores by GDM status
Supplemental Figure 2b- Boxplots of the distribution of scores by GDM status


