Abstract
Brain photobiomodulation (PBM) therapy using red to near-infrared (NIR) light is an innovative treatment for a wide range of neurological and psychological conditions. Red/NIR light is able to stimulate complex IV of the mitochondrial respiratory chain (cytochrome c oxidase) and increase ATP synthesis. Moreover, light absorption by ion channels results in release of Ca2+ and leads to activation of transcription factors and gene expression. Brain PBM therapy enhances the metabolic capacity of neurons and stimulates anti-inflammatory, anti-apoptotic, and antioxidant responses, as well as neurogenesis and synaptogenesis. Its therapeutic role in disorders such as dementia and Parkinson’s disease, as well as to treat stroke, brain trauma, and depression has gained increasing interest. In the transcranial PBM approach, delivering a sufficient dose to achieve optimal stimulation is challenging due to exponential attenuation of light penetration in tissue. Alternative approaches such as intracranial and intranasal light delivery methods have been suggested to overcome this limitation. This article reviews the state-of-the-art preclinical and clinical evidence regarding the efficacy of brain PBM therapy.
Keywords: photobiomodulation therapy, low-level laser therapy, brain function, cortical neurons, traumatic brain injury, stroke, dementia, depression
1. Introduction
Over the past two decades, brain photobiomodulation (PBM) therapy has been introduced as an innovative modality for the stimulation of neural activity in order to improve brain function. This light-based technique involves exposure of neural tissue to a low fluence of light (ranging from < 1 to >20 J/cm2, and at wavelengths ranging from red to near-infrared (NIR) (600 to 1100 nm) via various light delivery methods [1]. The safety and optimal treatment parameters of brain PBM therapy such as wavelength, fluence, power density, number of repetitions, duration of treatment, and the mode of light delivery (continuous or pulsed) have been variously investigated in preclinical studies [2–5]. The first in vivo evidence of the neurotherapeutic effects of PBM therapy were achieved in the rabbit embolic stroke model to test its ability to prevent damage or repair damage to the brain occurring after a stroke [6]. The neuroprotective effects of laser and light emitting diodes (LED) in diverse neurological conditions such as traumatic brain injury (TBI) [7], ischemic stroke (IS) [8], Alzheimer’s disease (AD) [9], Parkinson’s disease (PD) [10], and psychological disorders such as depression and anxiety [11,12], as well as age-related cognitive decline [13,14] have been also shown.
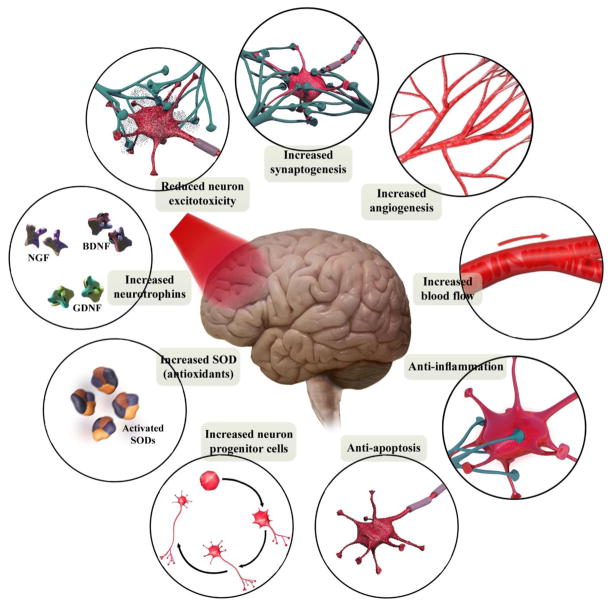
The beneficial effects of PBM are mainly thought to result from the photostimulation of the mitochondrial electron transfer chain (ETC). When PBM is applied at optimum fluences (energy densities) and wavelengths, it produces therapeutic effects in the target organs without causing any adverse effects [15,16]. PBM therapy increases cerebral blood flow (CBF) [17–19], augments brain energy metabolism [17,20,21] and increases antioxidant defenses [20]. Moreover, its ability to promote neuronal protection and survival is mediated through modulation of anti-apoptotic and pro-apoptotic mediators [22,23] and inflammatory signaling molecules [24,25] as well as the stimulation of neurotrophic factors [4,26,27]. Besides these therapeutic effects at the molecular level, there is also considerable evidence of changes occurring at the behavioral level such as cognitive-enhancement, antidepressant effects and improved sleep [7,28–30].
By focusing on the mechanistic and neurobiological basis as well as biophysical aspects, the present review aims to provide an overview of the cerebral response to PBM in different preclinical and clinical studies.
2. Mechanism of action of photobiomodulation therapy
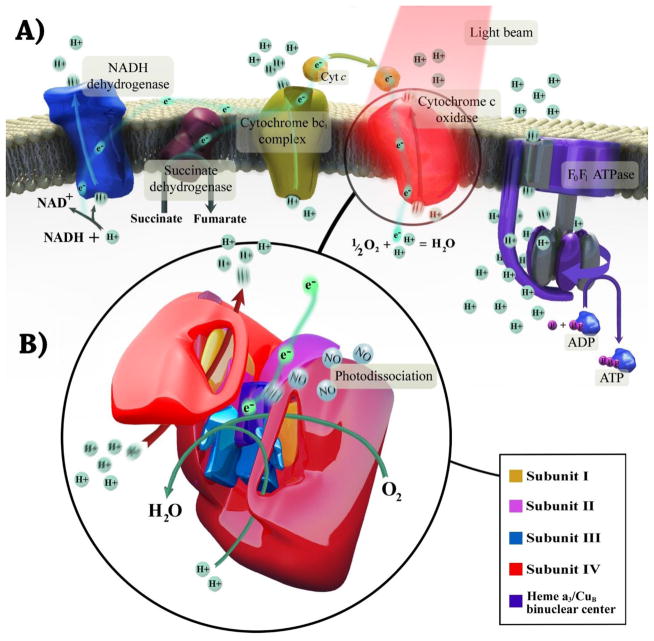
The different wavelengths of light have different degrees of absorption, scattering, and reflection by biological media and tissues. Generally, biological tissues contain a range of chromophores such as water, oxyhemoglobin (HbO2), deoxyhemoglobin (Hb), myoglobin, melanin, cytochromes, and flavins. Water molecules significantly absorb light energy at wavelengths greater than 970 nm, while wavelengths shorter than 600 nm are absorbed by flavins, hemoglobin and melanin. For these reasons, there is an optical window for PBM therapy in the range of the red to NIR spectrum [31] and the therapeutic efficacy of this wavelength range has been determined in several studies [17,32,33]. The mechanisms underlying the interaction between light and tissue is very complex due to the various chromophore components present inside the cells. Now it is clear that excitation of mitochondrial cytochrome c oxidase (CCO) serves as a primary photoacceptor in the red to NIR region and probably plays an essential role in action mechanism of PBM therapy [34]. CCO is the terminal enzyme in the mitochondrial ETC and consists of 13 protein subunits containing two heme centers (Heme a and Heme a3) as well as two copper centers (CuA and CuB). This enzyme transfers electrons from cytochrome c to O2 via CuA and from heme a to the heme a3/CuB binuclear center [35]. CCO has absorption peaks in the red (Heme a, 605 nm; CuA reduced, 620 nm; heme a3/CuB, 655 nm; CuB oxidized, 680 nm) and the NIR spectral regions (CuB reduced, 760 nm; CuA oxidized, 825 nm) [36,37]. When light is shone on CCO, photon energy is absorbed by the various metal centers of CCO and their electrons are excited from the ground state to upper excited states [38]. It is proposed that PBM in the red and NIR spectrum causes photodissociation of nitric oxide (NO) from the binuclear center (a3/CuB) of CCO. Since it is known that NO inhibits electron transport in the ETC, dissociation of NO can increase the mitochondrial membrane potential (MMP), increase oxygen consumption and hence the proton gradient ultimately leading to an increase in ATP production [39] (Fig. 1). These events are followed by production of reactive oxygen species (ROS) and release of Ca2+ as versatile second messengers, in turn leading to activation of transcription factors and signaling mediators such as NF-κB and resulting in long-lasting effects on cells [31,40–42] (Fig. 2). Although action mechanisms of light in the region of 600–850 nm have been based on the excitation of mitochondrial CCO, recent results of a study Wang et al. [43] have revealed a new cellular mechanism for NIR light at 980 nm based on activation of heat or light sensitive calcium ion channels (Fig. 2).
Fig. 1. Mechanism of photobiomodulation therapy in mitochondria.
(A) The flow of electrons and protons through the mitochondrial respiratory chain. Photobiomodulation stimulates cytochrome c oxidase, improves its catalytic activity, and elevates ATP synthesis. All these results in enhancement of neuronal respiration and metabolic capacity. (B) The structure of cytochrome c oxidase and electrons path through its subunits. The complex contains two copper centers as well as two heme prosthetic groups. Photobiomodulation may dissociate nitric oxide from binuclear center (a3/CuB), allowing oxygen to return, and facilitates electron transfer and increases proton gradient.
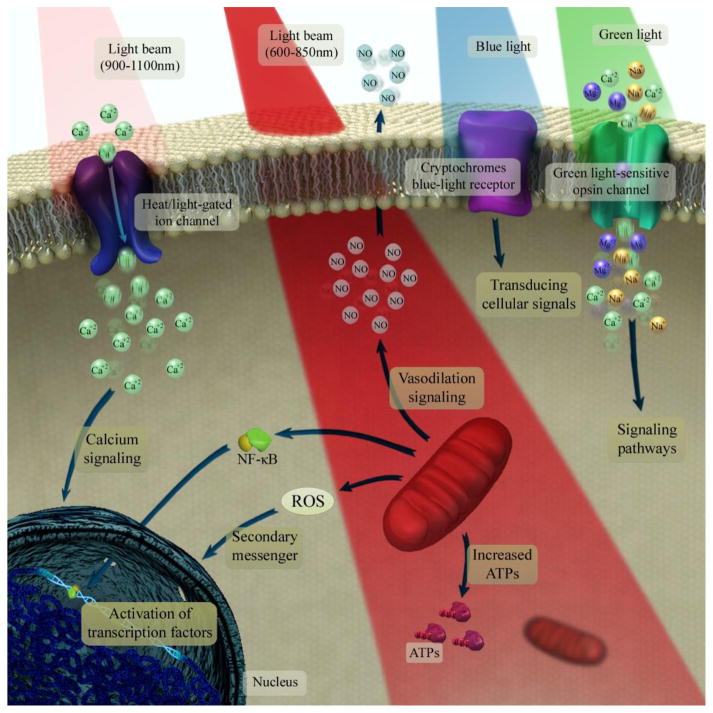
Fig. 2. Photobiomodulation underlying mechanisms at the cellular and molecular levels.
Light at 600–850 nm is absorbed by the mitochondrial electron transfer chain and leads to upregulation of the neuronal respiratory capacity. The near-infrared light at range of 900–1100 nm is absorbed by structured water clusters formed in or on a heat/light-gated ion channels. An increase in vibrational energy of water cluster leads to perturb the protein structure and opening the channel which ultimately allows modulation of intracellular Ca2+ levels. The absorption of green light by neuronal opsin photoreceptors (OPN2–5) activates transient receptor potential channels which causes non-selective permeabilization to Ca2+, Na+ and Mg2+. The cryptochromes (a class of flavoprotein blue-light signaling receptors) absorb blue light and seems to activate the transducing cellular signals via part of the optic nerve to the suprachiasmatic nucleus in the brain, which is important in regulation of the circadian clock.
Furthermore, beneficial effects of wavelengths such as 1064 [18,44–46], 1068 [47], and 1072 nm [48,49] have been reported in various studies. Since the light scattering is somewhat higher at 800–850 nm (the peak absorption of CCO), photons may not penetrate to deeper tissues compared to longer wavelengths. However, at 1064–1072 nm light absorption of CCO is less than 800–850 nm, but owing to the low hemoglobin absorption and low light scattering, light at this spectrum readily travels through tissues and can stimulate either CCO or ion channels located in deeper structures [50–52]. Another proposed mechanism is that absorption of light in this spectrum activates vibrational dynamics in nanostructured water complexes and this may affect the tertiary structure of cellular pumps and motors [38,53]. Furthermore, modulation of inducible NO synthase (NOS) and heat-shock proteins resulting in a reduction of apoptosis have been proposed for stimulatory effects of this spectral region [49,52]. Other important mechanisms that have been proposed to play a role in PBM applications include the photooxidative activation of latent extracellular transforming growth factor-beta (TGF-β) [258], and activation of calcium ion channels depending on the chromophore proteins, opsin-3 and opsin-4 [259].
3. Current approaches for light delivery to different parts of the brain
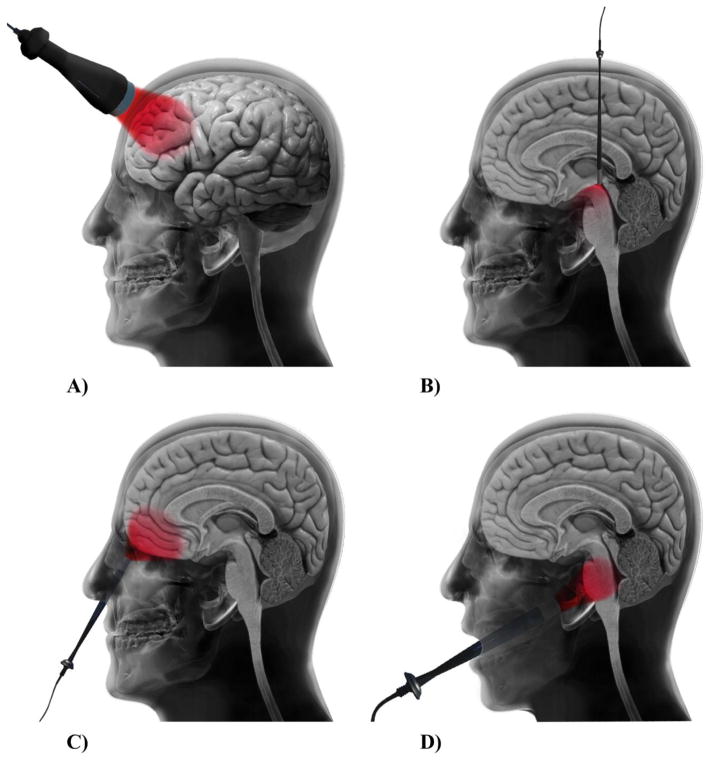
Non-invasive delivery of light from an external light source (laser or LED) to the head and then into the brain is commonly referred to as transcranial PBM (Fig. 3A). In this method, light passes through a series of layers including the scalp, periosteum, skull bone, meninges, and dura, and will partly reach the cortical surface of the brain [54]. Owing to exponential attenuation of light during the traveling through the skull and brain tissues, the maximum dose (a small fraction of the incident light) will be delivered to those neurons located in the outermost layers of the cortex. Thereafter, there is a further gradient of light penetration within the cortex, so that only a very limited number of neurons will absorb an adequate dose in this irradiation method [55]. Some novel technologies such as an optical focusing technique called “near-infrared time-reversed ultrasonically encoded (TRUE)’ [56], and some combination treatment approaches based on nanoparticles and light [57,58] have been suggested to maximize the penetration of light into the brain and partly solve the dose delivery problem. Furthermore, a recent Monte Carlo simulation study suggested that a transcranial multi-directional irradiation approach using multiple-LED arrays (181 point sources over the entire scalp) enhances cerebral photon flux and uniformity of photon distribution, without causing a rise in temperature [59]. Application of a high-power laser (10–15 W, Class 4) instead of a low-power (<0.5 W, Class 3) laser has also been suggested in order to deliver a higher fluence to deeper layers of the neocortex without any thermal damage [60,61].
Fig. 3. Different approaches for light delivery to achieve brain photobiomodulation therapy.
(A) Transcranial, (B) intracranial, (C) intranasal photobiomodulation therapy, (D) brain photobiomodulation via oral cavity.
Dysregulation of neurons and circuits in the subthalamic nucleus (STN) and the globus pallidus internus (GPi) (two deeper regions of the brain) have a role in PD pathology [62]. In fact, the limited ability of light to penetrate to deeper brain structures restricts the delivery of a sufficient dose to midbrain neurons. In recent years there have been attempts to develop an effective technique for delivering light to deeper brain tissues such as the substantia nigra pars compacta (SNc), using the same intracranial approach as is used in the deep brain stimulation technique [63] (Fig. 3B). Intracranial delivery of light via an implanted optical fiber has been described as a novel technology used both for PBM [64] as well as for optogenetic studies [65]. A Monte Carlo study showed that when a fiber optic source is located in the third ventricular region, it has a 20-fold higher efficiency in delivering light to the SNc region compared to the non-invasive transcranial method [66]. To support this finding, only 1% of 670 nm LED light reached the mouse SNc at a depth of 5 mm from the light source in the transcranial method [67]. Besides, a preliminary study regarding the neuroprotective effects of intracranial PBM showed no toxic adverse effects around the implant sites in the midbrain [68].
Although the majority of researchers have focused on the application of transcranial and intracranial illumination methods [69,70], light irradiation via the nasal cavity (intranasal method) and/or the oral cavity have also resulted in improvement in dementia and PD symptoms [71–73] (Fig. 3C and D). In the intranasal PBM method, the light source is located inside the nostril at the back of the nose and due to a thinner thickness of the ethmoid plate, it can directly irradiate subcortical (hypothalamus, thalamus, amygdala, hippocampus) and cortical (orbitofrontal cortex) structures of the limbic system in the brain which are related to AD and PD pathologies [74]. In order to achieve an adequate fluence at the human SNc, a combination of light irradiation via the sphenoid sinus and oral cavity has also been suggested [66]. Moreover, in a recent case series report, combination treatment with intranasal and transcranial LED devices improved cognition and functional abilities in 4 patients with dementia [71].
Meanwhile, bright LED light (448 nm) applied through the ear canal can penetrate the skull and reach the temporal lobes of the human brain [75]. PBM therapy to treat the brain via the ear canal has also been suggested, in order to benefit seasonal affective disorder [76,77] and modulation of brain function [75].
4. Light penetration through the scalp, skull, and brain tissues
Knowledge of the optical properties (absorption and scattering coefficients) of the different tissues of the head allows identification of the optimum wavelength range where light transmission is maximal [78]. To date, several reports including studies conducted ex vivo (animals and human), in vivo (animals), and using Monte Carlo simulation have been published reporting light penetration through the different tissues composing the head (such as the scalp, skull, white and gray brain matter, etc.) [79]. Light penetration in tissues depends on several optical parameters such as wavelength, irradiance, exposure time, exposed area, coherence, and pulse structure [60]. A variety of anatomical and physiological factors contribute to light penetration through the head, including individual head geometry and tissue composition. Furthermore, variability in scalp-brain distance (SBD) among the different regions of the brain (for example, frontal regions have shorter SBD than medial parietal regions) could affect the light penetration depth [80–82].
The human calvarial bone contains minerals (58%), protein (24.6%), water (12.2%), and carbohydrate (5.2%), and these components are responsible for high optical absorption and scattering of the skull [83]. A study on porcine skull showed lower absorption values for light at wavelengths between 700 to 850 nm [84]. Moreover, approximately 54% of NIR light at 810 nm passed through the prefrontal area of the rat skull (0.58 mm thickness) [85], and up to 39% of 808 nm laser light penetrated the rat skull (0.82 mm thickness) [86]. Data from light transmittance through the human skull showed that 2% of laser light at 1064 nm [44], and 3.7% of LED light at 810 nm [11] passed through the frontal bone. Jagdeo et al. [87] also using 830 nm LED light, reported a penetration depth of 0.9% at the temporal, 2.1% at the frontal, and 11.7% at the occipital bones of the human skull measured with intact soft tissue. Since the thickness of the temporal bones is less than the thickness of the frontal and occipital bones, the amount of soft tissue is correspondingly larger and accounts for the lower light transmission, since soft tissue has lower transmission than bone. A positive correlation between decreased NIR laser light penetration and increased human skull thickness (but not density) has also been reported [88].
NIR light at 808 nm penetrated to a depth of 25–30 mm through rabbit brain tissue [6]. In addition, about 12% of 808 nm laser light could reach the rat midbrain [10]. Pig tissue is considered to best resemble human tissue in terms of dimensions and components. Aulakh et al. [89] used a freshly deceased pig head to measure penetration of 808 nm pulsed wave (PW) light, and found that 9.2% of light could reach a depth 5 mm into the brain. They also found that the use of higher output power and a longer pulse duty cycle could deliver a higher dose to deeper tissue [89]. Continuous wave (CW) laser light at 808 nm penetrated to a depth of roughly 8 mm in human brain tissue below the cortical surface [90]. Furthermore, 1.23% of 980 nm and 2.9% of 810 nm light from high-power laser devices could penetrate across 30 mm of skin, skull, and brain tissue [60]. In terms of gray and white matter transmittance, Yaroslavsky et al. [91] reported penetration depths of 0.79 mm (630 nm), 0.83 mm (670 nm), 0.9 mm (850 nm), and 1 mm (1064 nm) for white matter, and 4.06 mm (630 nm), 4.4 mm (670 nm), and 3.28 mm (1064 nm) for gray matter.
In addition, a comprehensive study conducted by Hart and Fitzgerald [92] determined the transmission of light over the range of 450–880 nm for human scalp, skull, and brain tissue. A value of 24% was reported for light transmittance at 740 nm for the scalp. Approximately 12% of light at 790 nm could penetrate through the temporal bone (6 mm thickness). The caudal region of the skull (6 mm thickness) showed maximum transmission of 7.5% at 770 nm, and the central crown region (10 mm thickness) showed peak transmission of 4.5% at 820 nm. Transmission of ~1% at 830 nm was also reported by the authors for fresh brain tissue (12 mm thickness).
There is an ongoing debate about whether the penetration of laser light in tissue might actually be larger than LED light. Some researchers put forward the idea that, instead of laser devices which produces a long narrow beam of penetrating coherent light, while non-coherent LED devices have spatial divergence and only provide penetration through a thin volume of tissue surface [60,93,94]. On the other hand, no significant difference has been shown between light penetration through the human skull for LED (830 nm) and laser (810 nm) light [95]. Further simulations and laboratory studies are required to reveal exact coherence-dependency of light transmission through the human head tissues.
5. Biphasic dose-response in photobiomodulation therapy
A biphasic or inverted U-shaped dose-response curve has been shown in several studies conducted in the PBM field, and this phenomenon is known as the Arndt-Schulz law [2,4,28,96]. According to this relationship, light at very low doses has no significant stimulatory effects (below threshold), whereas small (but still larger) doses above this threshold produce beneficial effects. On the other hand, much higher doses of PBM can have inhibitory and even harmful effects. A study in cultured cortical neurons showed that, with a constant irradiance (25 mW/cm2), the peak efficiency was obtained at 3 J/cm2 for ATP production, along with an increase in MMP as well as calcium levels. Whereas both low (0.03 and 0.3 J/cm2) and high (10 J/cm2) doses showed a minor stimulatory effects, and even inhibitory effects due to mitochondrial damage occurred at higher fluence (30 J/cm2) [2].
As noted previously, various wavelengths have been reported to have their own particular biological effects and mechanisms, so the determination of the optimal dose for each range of wavelengths in the red to NIR region is of great importance. Study of the proliferation of human adipose-derived stem cells following PBM showed a peak dose response for 810 nm at a fluence of 3 J/cm2, whereas, the peak dose response for 980 nm was observed at fluences of 0.03 or 0.3 J/cm2 [43]. However, a study in a transcranial mouse TBI model showed superior beneficial effects of 810 nm compared with 980 nm wavelengths at the same fluence (36 J/cm2) [97]. It seems that, due to different dose-response, PBM using much lower doses of 980 nm wavelengths could be required, compared to the doses needed of 810 nm light.
6. Neurobiological impacts
6.1. Neuronal bioenergetics functions
It has long been established that mitochondrial dysfunction has a pivotal role in the etiology of many (if not most) neurological and psychological disorders [98,99]. Under pathologic conditions, mitochondria may undergo major changes including reduced respiratory chain complex activity and lower ATP synthesis, overproduction of ROS, and the loss of MMP, inner mitochondrial permeability transition, and the release of cytochrome c into the cytosol [100]. The beneficial effect of PBM on energy metabolism of various cell types has been investigated [101]. Neural tissue is very rich in mitochondria [102] hence exposure to light can readily interact with CCO as a mediator of neuronal energy metabolism. This is of great importance, because it is accepted that absorption of far-red to NIR light (600–850 nm) by neuronal CCO is the main initiating event in the brain PBM.
The early studies by Wong-Riley et al. in cultured rat visual cortical neurons revealed that irradiation using LED light (4 J/cm2) at wavelengths of 670 nm and 830 nm was more effective than 770 nm and 880 nm in the up-regulation of CCO activity [103], while 670 nm light significantly reversed the down-regulation of CCO activity induced by tetrodotoxin [104]. LED light irradiation at 633 nm resulted in augmentation of CCO activity in the prefrontal cortex (PFC) of naïve rats by 14% (10.9 J/cm2) [17], in the superior colliculus by 26% and in the whole brain by 60% (3.6 J/cm2) in a rat model of rotenone-induced neurotoxicity [105]. Recent studies conducted by Zhang and his research team show that transcranial LED therapy (808 nm) significantly increased the CCO activity in the PFC in a mouse stress model (41 J/cm2) [29], as well as in the hippocampus of a murine Aβ-induced Alzheimer’s disease (AD) model (3 J/cm2) [20]. In the study using a transgenic mouse model of AD, Purushothuman et al. [21] also demonstrated a significant restoration of CCO expression patterns in the neocortex and hippocampus following 4 weeks of transcranial LED therapy (670 nm).
Neural tissues have a high dependence on mitochondrial-produced ATP. Transcranial PBM therapy using 808 nm laser increased cerebral ATP levels in amyloid protein precursor (APP) transgenic mice (6 J/cm2) [9], Aβ-induced AD mice (3 J/cm2) [20], as well as a mouse model of major depression (41 J/cm2) [29]. Although a single-session of LED treatment (670 nm, 4 J/cm2) increased the ATP content of 1-methyl-4-phenylpyridinium (MPP+) exposed striatal neurons [106], a single session of laser irradiation did not enhance ATP levels either in Aβ treated PC12 cells (670 nm, 1 J/cm2) [107] or in the PD cybrid cell lines (at 810 nm, 2 J/cm2) [108]. Differences in applied light fluences could be a reasonable explanation for these disparate findings.
Direct irradiation of the parietal cortex of normal rats with 830 nm laser light also resulted in an increase in the ATP/ADP ratio [109]. Interesting studies by Lapchak and his research team in a rabbit embolic stroke model revealed that one session of transcranial laser treatment (808 nm) in CW mode (0.9 J/cm2) [110] and 100-Hz PW mode (4.5 and 31.5 J/cm2) [111] significantly increased the cortical ATP content. Furthermore, the effectiveness of 10-Hz PW laser light (810 nm) in increasing brain ATP production have been shown in a mouse TBI model [7,112].
It should be noted that, studies looking at the peak response of cellular ATP production in cells exposed to PBM therapy, could provide information leading to better treatment planning. Studies in human neuronal cells (808 nm, 0.05 J/cm2) [113] and mouse muscle cells (630+850 nm, 2.5 J/cm2) [114] revealed that the maximum ATP production occurred at 10 min and 3–6 h post-irradiation, respectively. Although these in vitro studies demonstrated transient bio-stimulatory effects of PBM, more recently, Mintzopoulos et al. [115] using phosphorus magnetic resonance spectroscopy (31P-MRS) evaluated the cortical levels of phosphocreatine (PCr) and PCr/β-nucleoside triphosphate (β-NTP) ratios following acute and chronic transcranial laser therapy (808 nm) in dogs. No significant change in the PCr/β-NTP ratios and PCr levels were observed immediately after a single irradiation. While, repeated irradiation over 2 weeks showed prolonged beneficial effects and improved cerebral bioenergetics.
6.2. Cerebral blood flow (CBF)
It is believed that impaired cerebral vascular perfusion is the one of the first manifestations of most brain disorders [116–119]. NO is a powerful vasodilator which could be released by photodissociation process from its binding sites in the respiratory chain during PBM. According to preclinical findings, PBM can increase the neuronal NO content, increase the vessel diameter, and improve CBF [120–122]. Therefore, it could be considered that PBM therapy of specific areas of the brain potentially affects regional CBF [1].
Studies on mouse brain mitochondria showed that, although the LED light at 590 nm significantly increased NO synthesis by modulating CCO/NO activity, light at wavelengths of 627 and 660 nm did not have significant stimulatory effects [37]. Uozumi et al. [121] suggested that a transient increase in CBF during brain PBM therapy depends on NOS activity and NO concentration. They showed that transcranial NIR PBM (808 nm) increased CBF in the illuminated hemisphere (by 30%) and the opposite hemisphere (by 19%), as well as cortical NO concentration (by 50%) during a 45 min irradiation. Pre-treatment with red LED light (610 nm) also resulted in an increase in CBF at 30 min after reperfusion in a mouse cerebral ischemia model [122].
Moreover, some clinical studies have shown the impact of transcranial LED therapy on CBF. Nawashiro et al. [19] reported that LED treatment delivered bilaterally to the forehead (850 nm) increased CBF (by 20%) in the left anterior frontal lobe of a patient in a vegetative state. Besides, Salgado et al. [123] showed that transcranial LED irradiation (627 nm) significantly improved blood flow velocity in the left middle cerebral artery (by 30 %) and the basilar artery (by 25 %) in healthy subjects. According to Schiffer et al. [11], one session of LED treatment to the forehead (810 nm) could significantly increase the prefrontal CBF of patients with major depression and anxiety immediately after light, but not at 2 to 4 weeks post-irradiation. Differences in these results could be attributed to variations in the optical and treatment parameters employed such as wavelengths, irradiation area, and number of sessions.
To date, various animal and human studies have shown enhancement of cerebral energy production and improved O2 consumption following transcranial PBM. Transcranial PBM at red and NIR wavelengths induced an increase in cerebral O2 consumption in naïve rats [17] as well as AβPP transgenic mice [9]. Furthermore, in the most recent clinical investigations by Hanli Liu and her colleagues, improvement in cerebral oxygenation and hemodynamics was found both during and following transcranial laser irradiation at 1064 nm [18,50].
6.3. Oxidative stress
It is accepted that mitochondria are the main source of oxidative stress (ROS), and excessive ROS generation affects neurons in part by damaging their mitochondrial function [124]. A growing body of literature has shown correlations between various brain conditions, such as AD [125], TBI [126], stroke [127] and major depression disorder (MDD) [128], and vulnerability to oxidative stress. The beneficial or harmful effects of PBM are in part linked to mitochondrial ROS production [129]. As already mentioned, low levels of mitochondrial ROS induced by PBM at low doses are involved in modulation of cellular signaling pathways [130–132]. However, PBM delivered at higher doses (for example 120 J/cm2) can produce excessive amounts of ROS, and can result in activation of cellular apoptotic pathways [133]. The neuroprotective effects of laser and LEDs against oxidative stress have been reported in Aβ [134] and other in vitro models using neurotoxins [23,32,112,135,136] (Table 1).
Table 1.
Published in vitro studies on the neuroprotection effects of photobiomodulation in neuronal activities
| Study/Year | Cell types | Light Source | Wavelengths | Irradiation parameters | Findings |
|---|---|---|---|---|---|
| Oron et al. 2007 [113] | Cultured human neuronal cells | Laser, GaAs, Photothera, Inc. (Carlsbad, CA, USA) | 808 nm | 600 mW, 50 mW/cm2, 0.05 J/cm2, 1 sec | Increased ATP content at 10 min post-irradiation |
| Sharma et al. 2011 [2] | Cultured mouse cortical neurons | Laser, Photothera, Inc. (Carlsbad, CA, USA) | 810 nm | 25 mW/cm2; 0.03, 0.3, 3, 10, or 30 J/cm2, single irradiation, CW | Highest increase in mitochondrial ROS (at 3 and 30 J/cm2); increased intracellular NO (at 0.3 J/cm2); increased MMP (at 0.3 and 3 J/cm2); increased intracellular Ca2+ (at 3 J/cm2); increased intracellular ATP (at 3 J/cm2) |
| Fukuzaki et al. 2013 [216] | Human-derived glioblastoma cells | Laser, SUWTECH, LDC-2500 (China) | 532 nm | 60 mW, 845 mW/cm2, 10.1, 20.3, or 30.4 × 102 J/cm2; with corresponding duration of 20, 40 or 60 min; CW | Increased cell proliferation at 48 h post-irradiation through elevation of Akt expression mediated by suppression of PTEN production (at 20.3 and 30.4 × 102 J/cm2) |
| Fukuzaki et al. 2015 [215] | Neural stem/progenitor cell derived | Laser, SUWTECH, LDC-2500 (China) | 532 nm | 60 mW, 845 mW/cm2, 10.1, 20.3, or 30.4 × 102 J/cm2; with corresponding duration of 20, 40 or 60 min; CW | Increased cell proliferation (at 30.4 × 102 J/cm2); promoted migration of NSPCs through increased Akt expression |
| Yan et al. 2017 [27] | Dorsal root ganglion neurons | Laser, HN-1000, Laser Technology Application Research Institute (Guangzhou, China) | 632.8 nm | 12.74 mW/cm2; 0.5, 1, 1.9, and 3.8 J/cm2; with corresponding duration of 0.7, 1.25, 2.5, and 5 min in the dark, respectively; single irradiation, CW | Enhanced cell viability and neuritogenesis through induction of BDNF mRNA expression by increasing of Ca2+ influx, phosphorylated levels of CREB and ERK proteins |
| Duan et al. 2003 [156] | PC12 cell (Aβ25–35-induced neurotoxicity) |
LEDs, self-made GaAlAs | 640 nm | 0.05–1 mW/cm2, 30–60 min, single irradiation, CW | At 0.09 mW/cm2 and 60 min diminished apoptosis and attenuated DNA fragmentation |
| Yang et al. 2010 [134] | Primary astrocytes (Aβ1–42-induced neurotoxicity) |
Laser, He-Ne | 632.8 nm | 1.5 mW/cm2, 16.2 J/cm2, 3 h, single irradiation, CW | Decreased oxidative stress burden via suppression of superoxide anion production, NADPH oxidase; and phosphorylation of cPLA2; inhibited pro-inflammatory markers including IL-1β and iNOS |
| Sommer et al. 2012 [107] | SH-EP and PC12 cells (Aβ42-induced neurotoxicity) |
Laser | 670 nm | 17.36 mW/cm2, 1 J/cm2, 1 min, single irradiation, PW at 1-Hz | Increased ATP levels in Aβ42-free SH-EP cells SH-EP cells: reduced intracellular Aβ42 aggregate amounts; increased cell proliferation PC12 cells: small decrease in ATP levels in Aβ42-challenged |
| Liang et al. 2012 [165] | SH-SY5Y, PC12, and HEK293T cells (Aβ25–35-induced neurotoxicity) |
Laser, HN-1000 (Guangzhou, China) | 632.8 nm | 12.74 mW/cm2, 2 J/cm2, single irradiation, CW | In all cell types: decreased apoptosis via Akt/GSK3b/b-catenin pathway |
| Meng et al. 2013 [168] | SH-SY5Y cell and mice hippocampal primary neuron (Aβ25–35 and Aβ1–42-induced neurotoxicity) |
Laser, HN-1000, Laser Technology Application Research Institute (Guangzhou, China) | 632.8 nm | 12.74 mW/cm2; 0.5, 1, 2, or 4 J/cm2; with corresponding duration of 0.7, 1.25, 2.5, and 5 min in the dark, respectively; single irradiation, CW | At 2 J/cm2: promoted cell survival and improved dendrite growth atrophy through up-regulation of BDNF mediated by activation of ERK/CREB signaling pathway |
| Duggett and Chazot 2014 [47] | Cath.a-differentiated cells (Aβ1–42-induced neurotoxicity) |
LEDs, Virulite Distribution Ltd (UK) | 1068 nm | 5 mW/cm2, 5 sets of 3 min irradiation (with 30 min interval) for 3 days, PW at 600-Hz, with DC of 300 μ sec | Decreased cell death (3.5–25 μM of Aβ42) |
| Trimmer et al. 2009 [108] | PD cybrid cells | Laser, Acculaser, PhotoThera, Inc. (Carlsbad, CA, USA) | 810 nm | 50 mW/cm2, 2 J/cm2, 40 sec, single irradiation, CW | Increased total distance traveled and velocity of mitochondria at 2 h post-irradiation |
| Wong-Riley et al. 2001 [104] | Cultured rat cortical neurons (TTX-induced neurotoxicity) |
LEDs, GaAlAs | 670 nm | 50 mW/cm2, 4 J/cm2, 80 sec, CW | Increased CCO activity in all three metabolic categories of neurons (daily irradiation for 5 days); increased CCO activity in darkly reactive cell type (a single irradiation) |
| Wong-Riley et al. 2005 [103] | Cultured rat visual cortical neurons (KCN-induced neurotoxicity) |
LEDs, Quantum Devices, Inc. (Barnaveld, WI, USA) | 670, 728, 770, 830, or 880 nm | 50 mW/cm2, 4 to 30 J/cm2, 80 to 600 sec, CW | 670 and 830 nm significantly increased CCO activity and ATP content back to control levels compared to 728, 880, and 770 nm (each at 4 J/cm2) 670 nm: pre-irradiation at 30 J/cm2 reduced cell death |
| Liang et al. 2006 [23] | Cultured rat visual cortical neurons (KCN-induced neurotoxicity) |
LEDs, Quantum Devices, Inc. (Barnaveld, WI, USA) | 670 nm | 50 mW/cm2, 30 J/cm2, single irradiation, CW | Pre-irradiation reduced cell death (100 μM of KCN) and (300 μM of KCN); reduced number of ssDNA-positive neurons (100 μM of KCN) and (300 μM of KCN); reduced caspase-3 and Bax levels, and increased Bcl-2 levels (both 100 and 300 μM of KCN); reduced ROS production (300 μM of KCN) |
| Liang et al. 2008 [135] | Cultured rat occipital cortical and striatal neurons (KCN- or MMP+- or rotenone-induced neurotoxicity) |
LEDs, Quantum Devices, Inc. (Barnaveld, WI, USA) | 670 nm | 50 mW/cm2, 4 J/cm2, 80 sec, 1 to 4×/day, CW | KCN: reduced apoptosis (1 irradiation) and (2 irradiations); reduced ROS production (2, 3, and 4 irradiations); reduced NO production (2 and 3 irradiations); reduced nitrotyrosine expression (2 irradiations); highest increase in CCO activity and ATP level (2 irradiations) MPP+: twice a day irradiation suppressed ROS and NO generation, increased ATP level and attenuated apoptosis in both types of neurons Rotenone: twice a day irradiation reduced apoptosis, ROS and NO levels, and increased ATP level in both types of neurons |
| Ying et al. 2008 [106] | Cultured rat visual cortical and striatal neurons (Rotenone- or MPP+-induced neurotoxicity) |
LEDs, Quantum Devices, Inc. (Barnaveld, WI, USA) | 670 nm | 50 mW/cm2, 4 J/cm2, 80 sec, CW | Rotenone: LED irradiation and pre-irradiation decreased apoptosis in both types of neurons; MPP+: LED irradiation and pre-irradiation decreased apoptosis in both types of neurons; LED irradiation and pre-irradiation increased ATP content in striatal neurons |
| Giuliani et al. 2009 [235] | PC12 cell (H2O2-induced neurotoxicity) |
Laser, SANYO DL3149-055A, (RGM, Genoa, Italy) | 670 nm | 0.005 or 0.011 mW/cm2; 0.11, 0.22, 5.06 or 10.12 J/cm2; 20 or 900 sec, single irradiation, PW at 100-Hz with DC of 1% or 50% | Enhanced axonal protection via stimulation of NGF-induced neurite outgrowth; rescued MMP (at all fluencies); increased cell viability (at 0.11 and 0.22 J/cm2) |
| Huang et al. 2013 [32] | Cultured mouse cortical neurons (H2O2- or CoCl2- or rotenone-induced neurotoxicity) |
Laser, Photothera, Inc. (Carlsbad, CA, USA) | 810 nm | 20 mW/cm2; 3 J/cm2, 150 sec, single irradiation, CW | Increased cell viability (at 10 and 20μM of H2O2, 0.2, 0.5, 1, and 2 mM of CoCl2, and 0.2, 2, and 5 μM of rotenone); decreased mitochondrial and cytoplasmic ROS production, and increased MMP (at 500 μM of CoCl2, 20 μM of H2O2, and 200 nM of rotenone) |
| Dong et al. 2015 [112] | Cultured SH-SY5Y cells (CoCl2-induced neurotoxicity) |
LEDs, PhotoMedex (Horsham, PA, USA) | 830 nm | 0.1, 0.5, 1, 3, or 10 J/cm2, CW | Increased cell viability and ATP production (at 3 and 10 J/cm2); decreased lactate production at 18 h post-toxin treatment (at 3 J/cm2); decreased ROS production and increased MMP; reduced cytochrome c leakage and diminished caspase-3 activation; suppressed apoptosis (at 3 J/cm2) |
| Choi et al. 2012 [213] | Cultured rat cortical neurons (OGD-induced neurotoxicity) |
LEDs, QRAY, Inc. (Seoul, Korea) | 710 nm | 50 mW/cm2, 4 J/cm2, 4 min, 1 to 4× within 8 h at 2 h intervals for 7 days, CW | Enhanced cell protection; promoted neurite outgrowth and synaptogenesis mediated by MAPK activation |
| Yu et al. 2015 [136] | Cultured mouse cortical neurons (OGD-induced neurotoxicity) |
Laser, Photothera, Inc. (Carlsbad, CA, USA) | 810 nm | 25 mW/cm2, 0.3 J/cm2, 2 min, single irradiation, CW | Decreased NO production and nNOS activity (at 5 and 30 min post-irradiation); decreased NO donor SNAP-induced neuron death; promoted Akt and Bcl-2 expression (at 1 and 2 h); ameliorated Bax and BAD expression (at 1 and 2 h); suppressed caspase-3 and cleaved caspase-3 expression (at 2 h) |
Note: Aβ, amyloid beta; Akt, Protein kinase B; ATP, adenosine triphosphate; BAD, Bcl-2-associated death promoter; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; BDNF, brain-derived neurotrophic; CCO, cytochrome c oxidase; cPLA2, cytosolic phospholipase A2; CREB, cAMP responsive element binding; CW, continuous wave; DC, duty cycle; DNA, deoxyribonucleic acid; ERK, extracellular signal-regulated kinase; GaAlAs, gallium aluminum arsenide; GaAs, gallium arsenide; GSK3b, glycogen synthase kinase-3β gene; He-Ne, helium–neon; IL, interleukin; iNOS, inducible nitric oxide; KCN, potassium cyanide; LEDs, light emitting diodes; MAPK, mitogen-activated protein kinase; MMP, mitochondrial membrane potential; MMP+, 1-methyl-4-phenylpyridinium ion; mRNA, messenger ribonucleic acid; NADPH, nicotinamide adenine dinucleotide phosphate; NGF, nerve growth factor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NSPCs, neural stem/progenitor cells; OGD, oxygen-glucose deprivation; PD, Parkinson’s disease; PTEN, phosphatase and tensin homolog deleted on chromosome ten; PW, pulsed wave; ROS, reactive oxygen species; SNAP, S-nitro-N-acetylpenicillamine; ssDNA, single-stranded DNA; TTX, tetrodotoxin
Prolonged and elevated production of NO has neurotoxic effects and potentially contributes to tissue damage. On the other hand, suppression of nitric oxide synthase (NOS) activity has been shown after red light irradiation (660 nm) at 4 days post-ischemic event in rats [137]. Suppression of NOS isoforms (endothelial, neuronal, and inducible NOS) by 660 nm laser [137] and improvement of total antioxidant capacity by 808 nm laser [20] have also been put forward as mechanisms responsible for PBM regulation of oxidative stress. Besides, irradiation of blue laser (405 nm) to the HT7 acupuncture point (forepaw) surprisingly elevated superoxide dismutase and catalase, and suppressed acetylcholinesterase activities in the rat hippocampus [138].
Nevertheless, since the mitochondria are the primary site for red/NIR light-cell interactions, it seems that brain PBM could be the first step towards restoration of oxidative stress-induced mitochondrial dysfunction.
6.4. Neuroinflammation
Neuroinflammation is one of the crucial pathophysiological findings in brain disorders, which is chiefly mediated by activated microglial cells [139]. In response to different kinds of neuronal damage, microglia undergoes a series of morphological and proliferative alterations leading to the release of pro-inflammatory markers, including chemokines, cytokines, NO and ROS [140,141]. The overproduction of ROS activates translocation of the transcription factor NF-κB into the nucleus, which ultimately triggers expression of pro-inflammatory cytokines [142]. PBM reduces pro-inflammatory cytokines via inhibition of NF-κB signaling pathways, resulting in attenuation of inflammatory reactions [143,144]. Among the numerous cytokines, tumor necrosis factor-a (TNF-α) as well as interleukins (IL) such as IL-1β, IL-6, IL-10 and IL-18 have been the most studied examples related to brain PBM therapy [9,24,25,145–147]. In an early study, Moreira et al. [25] assessed the anti-inflammatory effects of NIR lasers on the alteration of cerebral interleukins in rat model of cryogenic brain injury and found a decreased level of IL-1β at 24 h compared to 6 h. Transcranial laser also prevented the occurrence of secondary brain injury in a mouse closed-head TBI model and suppressed expression of IL-1β and IL-6 at 6 h post-injury induction [146]. Lee and co-workers used transcranial LED in a mouse photothrombotic stroke model to demonstrate a reduction of IL-1β and IL-18 levels at 72 h post-ischemia [147]. In another study, they also showed beneficial pre-conditioning effects of PBM therapy (2 consecutive days prior to ischemic insult) for reduction of cortical TNF-α and IL-1β expression at 24 h post-ischemia [24]. Moreover, 710 nm PBM therapy activated cellular immunity via increase in the expression of IL-10 in peripheral blood mononuclear cells at 20 days post-stroke [145]. The NIR laser (810 nm) also suppressed inflammation via IL-1β, TNF-α and TGF-β suppression in the brain of AD mouse [9]. Besides these reports, an excessive number of sessions of laser therapy (daily for 14 days) surprisingly increased glial fibrillary acidic protein (GFAP) expression, leading to temporary inhibition of the brain repair process in the SVZ region, whereas the beneficial effects of PBM resumed over the long-term [28]. This piece of evidence supports the idea that the anti-inflammatory effects of brain PBM may at least partly be due to its ability to modulate microglial activity and a subsequent decrease in inflammatory mediators.
6.5. Neuronal apoptosis
Apoptosis is one of the contributing pathophysiological mechanisms in normal brain aging [148] and also in neurodegenerative conditions such as AD [149] and PD [150]. Among the various apoptotic pathways, the intrinsic pathway also known as the mitochondrial pathway plays a key role in programmed cell death. Apoptosis is initiated by a decline in MMP and release of the pro-apoptotic factor, cytochrome c, from the mitochondria into the cytoplasm leading to activation of caspase-3 activity [151]. The pro-apoptotic and anti-apoptotic Bcl-2 family of proteins are also believed to be essential regulators of apoptosis [152,153]. Over-expression of Bax or an increased Bax/Bcl-2 ratio triggers activation of the caspase cascade and results in apoptosis [154]. The first evidence for the anti-apoptotic effects of PBM was observed by Shefer et al. [155] in skeletal muscle satellite cells. The authors reported that laser irradiation (632.8 nm) protected skeletal muscle satellite cells from apoptosis by decreasing p53, p21 as well as Bax, and increasing Bcl-2 levels at 24 h post-irradiation. LED light (640 nm) significantly prevented apoptosis in PC12 cells caused by Aβ25–35 toxicity at 24 h post-irradiation [156]. In addition, it has been reported that LED treatment twice a day (670 nm) significantly decreased the number of striatal and cortical neurons undergoing apoptosis induced by exposure to rotenone and MPP+ [135]. LED pre-treatment with 670 nm light at fluences of 4 J/cm2 [106,157], and 30 J/cm2 [23] resulted in the significant rescue of primary neurons from apoptosis induced by different neurotoxins. Beneficial effects of PBM at wavelengths of ~810 nm on mitochondrial structure and MMP collapse have been shown in various in vitro neurotoxicity models [20,32,136]. Light at red (LED, 670 nm) [158] and NIR wavelengths (laser, 810 nm) [136] also significantly ameliorated neuronal apoptosis via reduction of pro-apoptotic factors such as Bax, BAD, and inhibition of caspase-3 activity. The initial mechanism of light absorption by mitochondrial inner membrane enzyme chromophores improving MMP would be one conceivable explanation for this neuroprotective effect [159].
The protein kinase C (PKC) family is composed of serine/threonine kinases that have pivotal roles in apoptosis. PKC activation can influence cellular Bax and Bcl-xl expression and ultimately inhibits cell apoptosis [160,161]. Zhang et al. [162] showed that laser irradiation (632.8 nm) at low doses (0.156, 0.312, and 0.624 J/cm2) significantly reversed PC12 cell apoptosis by decreasing the Bax/Bcl-xl ratio of mRNA via the PKC activation pathway.
Beside these findings, other anti-apoptotic mechanisms for PBM have also been proposed. Laser irradiation (632.8 nm) significantly inhibited the activation of glycogen synthase kinase (GSK-3b), Bax, and caspase-3, and thereby prevented staurosporine-induced cell apoptosis via inactivation of the GSK-3b/Bax pathway [163]. In addition, it was suggested that PBM (632.8 nm) was able to inhibit PC12 cell apoptosis via the activation of the Akt/YAP/p73 [164] and/or the Akt/GSK3b/b-catenin pathways [165]. The anti-apoptotic effects of PBM have been also reported in in vivo models of transient cerebral ischemia [8,121], Aβ-induced AD [20], and TBI [22,166].
6.6. Neurotrophic factors and neurogenesis
Among the different members of the family of neurotrophic factors (neurotrophins), the most attention has been focused on the stimulatory effect of PBM on brain derived neurotrophic factor (BDNF), neuronal growth factor (NGF), and glial cell-derived neurotrophic factor (GDNF). Increased expression of neurotrophins such as BDNF and NGF may account for observations of stimulation of neurogenesis and synaptogenesis [167]. Augmentation of BDNF expression could lead to reduced atrophy of cortical dendrites in the central nervous system (CNS) during the progression of AD [168]. In this respect, the rescue of dendritic atrophy following PBM (632.8 nm) through activation of the ERK/CREB/BDNF pathway has been suggested [168]. In similar studies using the same laser (632.8 nm), PBM induced intracellular IP3 receptor activation resulting in intracellular Ca2+ increases and consequent activation of ERK/CREB pathway, which eventually improved BDNF expression [27]. In in vivo studies, coherent laser light (670 nm) remarkably improved BDNF expression in the occipital cortex of rats [158]. A recent report in a primate PD model also revealed an increase in GDNF expression in the striatum accompanied by behavioral improvements following intracranial PBM therapy using non-coherent LED light (670 nm) [169].
To date, the neuroprotection effects of PBM in terms of neurogenesis have been shown only in ischemic stroke [170,171] and TBI models [26,28,96,166]. The first in vivo evidence for PBM-induced neurogenesis and migration of neuroprogenitor cells came from the work of Oron et al. [170]. They showed that PBM (808 nm) in the rat brain significantly increased the number of proliferating cells (incorporating BrdU) in the subventricular zone (SVZ) of the hemisphere ipsilateral to the occlusion at 28 days post-stroke. Laser irradiation (650 nm) to the acupuncture points of GV20 (head) and HT7 (right forepaw) significantly upregulated gene expression of CREB and BDNF in the hippocampus and improved cognitive impairment in rat ischemic model [171]. In a remarkable series of studies, Xuan et al. determined the optimal regimen of transcranial PBM (810 nm) for neuroprotection in TBI mice, and reported that laser irradiations for 1 or 3 consecutive days notably stimulated neurogenesis, and up-regulated migrating neuroprogenitor cells, BDNF in the DG and SVZ and a marker for synaptogenesis and neuroplasticity (synapsin-1) in the cortex [26,96,166]. Hippocampal atrophy and neurogenesis deficits in the dentate gyrus (DG) have been shown in MDD and AD [172,173]. Given this, it might be considered that these conditions should be benefited by PBM, but due to lack of data, further evaluation of PBM effects on the neurogenesis process is required in both the AD and depression animal models.
6.7. Effects on intrinsic brain networks
In the brain, a collection of distant but integrated structures provide widespread neuronal connections, which are called “intrinsic brain networks”. The default mode network (DMN), salience network (SN) and central executive network (CEN) are the most important examples of these formations. These networks are not only activated upon stimulation by neural inputs, but also their activities are detectable even in the resting state [174]. This may indicate that cerebral networks through their dynamic activities and anatomical connectivity work together to regulate intrinsic brain activity [175]. Besides, these intrinsic networks are able to modulate higher levels of cognitive and emotional functions [176]. Both chronic neurodegenerative disease and acute brain insults, cause an imbalance in the activity of these networks [177,178]. For instance, in TBI patients, abnormalities in higher level cognitive activities are associated with weak connections within and between the DMN, SN and CEN nodes, resulting in impaired dynamic interactions of these networks [179,180]. There is a hypothesis that the geographical matching of light irradiation sites on the head, with the corresponding anatomical regions of intrinsic networks within the brain may allow re-establishment of these functions, and may have enhanced therapeutic benefit [174]. In this respect, Naeser et al. [30] reported findings in TBI patients who received transcranial LED therapy over the DMN, SN, and CEN nodes and displayed enhanced cognitive functions, likely through the augmentation of metabolic capacity in these intrinsic networks. Moreover, Naeser et al proposed that the ability of PBM to reduce PTSD symptoms might stem from the modulation of DMN and SN activities [30]. On the other hand, application of PBM in stroke patients with aphasia demonstrated neurotherapeutic efficacy through the stimulation of cortical nodes within the CEN network [181].
7. Systemic effects
Although carrying out brain PBM therapy via direct irradiation methods (Fig. 3) is considered to be the main treatment approach, the neuroprotective benefits of irradiation to specific areas of the body other than the brain have been also reported. It has been proposed that the brain might benefit remotely from light stimulation of different organs, in a systemic manner (indirect or abscopal effects) [182]. Clinical studies have demonstrated that LED irradiation (660 and 850 nm) to the back and thighs alleviated depression symptoms of patients with low back pain [183]. Laser irradiation (514 and 632.8 nm) to auricular acupoints and to the neck of patients with alcohol addiction also decreased depression and ameliorated the symptoms accompanying alcohol withdrawal [184]. Additionally, results from a study in a mouse PD model revealed an abscopal neuroprotective benefit by delivering PBM (670 nm) to remote tissues (whole body excluding the head) by rescuing the loss of midbrain dopaminergic neurons [67]. The exact mechanisms of the systemic response to PBM therapy have remained obscure, but up-regulation of immune cell function [185], modulation of pro- and anti-inflammatory cytokines [186], and a potential increase of ATP levels in platelet mitochondria [187] have been suggested. It is also very likely that the stimulated migration of mesenchymal stem cells to the damaged sites in the brain could exert an neuroprotective abscopal effect [188,189]. In this way, PBM of the bone marrow (tibia) has been suggested to stimulate and mobilize mesenchymal stem cells, and consequently allow their migration to the brain, where they could restore cognitive function in the progressive stages of AD [190,191]. Since the calvarial bone marrow of the skull has large numbers of stem cells, and there is also considerable blood flow in the scalp and the skull, light absorption by other tissues before the light actually reaches the brain (in a similar fashion to the remote action described above) might make a contribution to the neuroprotective effects [1]. Besides remote irradiation, full-body PBM therapy using laser or LED devices has also provided neuroprotective benefits in some animal studies [158]. LED light (710 nm) when applied directly to the top of the animal’s cage significantly activated cellular immunity, reduced microglial activation and lessened brain infarction size as well as produced improvement in neurological scores in a rat stroke model [145]. It is worthwhile to mention that long-term irradiation of white fluorescent light to the whole-body induced a reduction in dopaminergic neurons in the mouse SNc, whereas 710 nm LED did not [192]. Full-body LED PBM therapy (1072 nm) also caused an improvement in working memory in middle-aged mice [14] as well as reduced Aβ plaque deposition in transgenic-AD mice [49].
Recently, the NovoThor LED whole body “light-pod” (660 and 850 nm) has been introduced by Thor Photomedicine (Chesham, Bucks, UK) for the full-body irradiation in humans. The application of this whole-body light pod could benefit muscle performance and reduce muscle fatigue and pain, as well as help with weight loss in combination with exercise [193,194]. It is possible to imagine that use of this non-invasive technique could also be efficacious for preconditioning and post-conditioning to benefit a wide variety of brain disorders.
8. Clinical application
Medically speaking a wide range of neurological and psychological disorders affects various cerebral structures. Recent clinical brain PBM therapy studies have been focused on conditions such as AD, PD, TBI and ischemic stroke as well as MDD. However there is also a growing interest for application of this non-invasive modality in perfectly healthy individuals to improve their cognitive abilities (cognitive enhancement) (Table 3).
Table 3.
Published clinical studies on the neuroprotection effects of photobiomodulation in neuronal activities
| Study/Year | Subjects (n) | Light Source | Wavelengths | Irradiation parameters | Irradiation approach/sites | Findings |
|---|---|---|---|---|---|---|
| Wu et al. 2012 [253] | Healthy volunteers (40) | Laser, 6 diodes, Advanced Chips & Products Corp. (Hillside, NJ, USA) | 830 nm | 7 mW per diode, 20 J/cm2, 10 min, PW at 10-Hz with DC of 50% | Remote tissue irradiation; 1 site, left palm |
Increased amplitude power of alpha rhythms and theta waves mainly in posterior head regions; decreased amplitude power of beta activities in anterior head regions |
| Barrett and Gonzalez-Lima 2013 [44] | Healthy volunteers (40) | Laser, CG-5000, HD Laser Center (Dallas, TX, USA) | 1064 nm | 250 mW/cm2, 60 J/cm2, 4 min, one irradiation session, CW | Transcranially; 2 sites, unilateral (right frontal pole on 4 cm medial and lateral) |
Improved reaction time in Psychomotor Vigilance Task and performance in a delayed match-to-sample memory task; appeared sustained positive emotional states 2 weeks post-irradiation |
| Konstantinovi et al. 2013 [220] | Healthy volunteers (14) | Laser, Endolaser 476, Enraf Nonius (Rotterdam, Netherlands) | 905 nm | 50 mW/cm2, 3 J/cm2 per site, 60 sec, PW at 3000-Hz | Transcranially; 5 sites, over the primary motor cortex (M1) area, centered at the hot-spot for the FDI muscle |
Transitory reduction of the excitability in the motor cortex |
| Blanco et al. 2015 [45] | Healthy volunteers (30) | Laser, Cell Gen Therapeutics, LLC (Dallas, TX, USA) | 1064 nm | 250 mW/cm2, 60 J/cm2, 8 min, one irradiation session, CW | Transcranially; 2 sites, (lower and upper portion of right lateral forehead at EEG map sites: FP2, F4) |
Improved executive function assessed by Wisconsin Card Sorting Task |
| Chaieb et al. 2015 [255] | Healthy volunteers (55) | Laser, coupled with Weber Medical acupuncture | 810 nm | 500 mW/cm2 at scalp via 4 laser needles, 10 min, one irradiation session, CW | Transcranially; 4 sites, over the primary motor cortex (M1) area |
Decreased amplitude of motor-evoked-potentials; increased short interval cortical inhibition and decreased facilitation |
| Salgado et al. 2015 [123] | Healthy elderly women (25) | LEDs | 627 nm | 70 mW/cm2, 10 J/cm2, 30 sec for each site (total of 4 sites), 2×/week for 4 weeks | Transcranially; 4 sites, frontal and parietal regions of scalp |
Increased systolic and diastolic velocity of the left middle cerebral artery |
| Hwang et al. 2016 [46] | Healthy volunteers (60) | Laser, CG-5000, Cell Gen Therapeutics, LLC (Dallas, TX, USA) |
1064 nm | 250 mW/cm2, 60 J/cm2, 8 min, one irradiation session, CW | Transcranially; 2 sites, medial and lateral right forehead |
Improved sustained attention in the Psychomotor Vigilance Task and working memory in the delayed match-to-sample task |
| Tian et al. 2016 [18] | Healthy volunteers (12) | Laser, CG-5000, Cell Gen Therapeutics (Dallas, TX, USA) |
1064 nm | 250 mW/cm2, 13.75 J/cm2 per 1 min for 10 min, one irradiation session, CW | Transcranially; 2 sites, center of forehead (aimed at medial frontal lobes bilaterally) or right side of forehead (aimed at right lateral frontal lobe) |
Increased oxygenated hemoglobin concentration and decreased deoxygenated hemoglobin concentration in both cerebral hemispheres over time during irradiation (10 min) and post-irradiation (6 min) |
| Blanco et al. 2017 [210] | Healthy volunteers (118) | Laser, CG-5000, Cell Gen Therapeutics, LLC (Dallas, TX, USA) |
1064 nm | 250 mW/cm2, 60 J/cm2, 8 min, one irradiation session, CW | Transcranially; 2 sites, lower and upper portion of right lateral forehead at EEG map sites: FP2, F4, and F8 sites |
Improved prefrontal rule-based learning; no significant effects on information-integration learning |
| Grover et al. 2017 [221] | Healthy volunteers (31) | LEDs, LumiWave Infrared Light Therapy Device, BioCare Systems, Inc. (Parker, CO, USA) | 903 nm | 16.67 mW/cm2, 20 J/cm2 at skull, 20 min, CW | Transcranially; Multiple areas in the occipital, left temporal, and right temporal lobes above the ear line, as well as the frontal and parietal lobes |
Improved reaction time in qEEG event-related response test |
| Moghadam et al. 2017 [209] | Healthy volunteers (39) | LEDs, multi-LED array source with 20 cells, Iranbargh (Tehran, Iran) | 850 nm | 285 mW/cm2, 60 J/cm2, 2.5 min, CW | Transcranially; 1 site, right frontal pole of the cortex (at EEG map site: FP2) |
Improved attentional performance in Level-1 of parametric Go/No-task |
| Wang et al. 2017 [50] | Healthy volunteers (11) | Laser, CG-5000, Cell Gen Therapeutics, LLC (Dallas, TX, USA) |
1064 nm | 250 mW/cm2, 13.75 J/cm2 per 1 min for 8 min, one irradiation session, CW | Transcranially; 1 site, right forehead |
Increased cerebral concentrations of oxidized CCO, oxygenated and total hemoglobin during and post-irradiation |
| Naeser et al. 2011 [33] | Chronic TBI (1 with depression) (2) | LEDs, three cluster heads | 633 and 870 nm | 19.39 mW/cm2 and 22.48 mW/cm2, 13.3 J/cm2, 10 min per site, 1×/week for 6 years or 1×day for 4 months, CW | Transcranially; Bilateral left and right forehead (and multiple other areas) |
Improved executive function and memory; decreased post-traumatic stress disorder symptoms |
| Naeser et al. 2014 [30] | Chronic TBI (2 with depression) (11) | LEDs, MedX Health Model 1100 (Toronto, Canada) |
633 and 870 nm | 500 mW, 22.48 mW/cm2, 13 J/cm2, 10 min per site, 3×/week for 6 weeks, CW | Transcranially; 11 sites, midline and bilateral forehead |
Improved sleep quality; decreased post-traumatic stress disorder symptoms; improved performance in social, interpersonal, and occupational functions |
| Morries et al. 2015 [61] | Chronic TBI (6 with MDD) (10) | Laser, LiteCure LT1000, (Newark, DE, USA) Diowave 810 (Diowave, Riviera Beach, FL, USA) | 810 and 980 nm | 10 and 15 W, 14.8–28.3 J/cm2, 8–12 min per site, 2–3×/week for 8 weeks, PW at 10-Hz | Transcranially; 2 sites, bilateral (forehead) 3 bilateral (prefrontal and temporal) |
Improved symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability |
| Hesse et al. 2015 [198] | TBI with disorders of consciousness (5) | Laser, Power Twin 21 by MKW Laser system | 785 nm | 10 mW/cm2, 10 min, 5×/week for 6 weeks, CW | Transcranially; 5 sites, on the level of the superior crest of the fossa sphenoidale on the forehead |
Improved alertness and awareness; occurred epileptic fits as a side effect |
| Lampl et al. 2007 [199] | Acute stroke (120) | Laser, Neurothera PhotoThera Inc. (Carlsbad, CA, USA) | 808 nm | 10 mW/cm2, 1.2 J/cm2 at cortex, 2 min for each site, CW | Transcranially; 20 sites, multiple areas over the entire shaved scalp |
Positive effects of irradiation within 24 h of stroke onset evaluated by National Institutes of Health Stroke Scale, modified Rankin Scale, Barthel Index, and Glasgow Outcome Scale |
| Zivin et al. 2009 [90] | Acute stroke (660) | Laser, Neurothera PhotoThera Inc. (Carlsbad, CA, USA) | 808 nm | 10 mW/cm2, 1.2 J/cm2 at cortex, 2 min for each site, CW | Transcranially; 20 sites, multiple areas over the entire shaved scalp |
No significant positive effects of irradiation within 24 h of stroke onset |
| Hacke et al. 2014 [200] | Acute stroke (630) | Laser, Neurothera PhotoThera Inc. (Carlsbad, CA, USA) | 808 nm | 10 mW/cm2, 1.2 J/cm2 at cortex, 2 min for each site, CW | Transcranially; 20 sites, multiple areas over the entire shaved scalp |
No measurable neuroprotective effect when irradiation applied within 24 h of stroke onset |
| Boonswang et al. 2012 [201] | Chronic stroke (1) | LEDs, XR3T-1 device (THOR, London, UK) | 660 and 850 nm | 1400 mW, 2.95 J/cm2 delivered to 32 sites, 1 min for each site, spot size of 0.196 cm2, 1/week for 8 weeks | Multiple areas; 32 sites, including cerebral cortices, brainstem and cervical spine (8 sites) and core musculature and lymphatics (24 sites) |
Positive change in every area of deficits and improved physical clinical signs |
| Maksimovich 2015 [196] | Alzheimer’s disease (89) | Laser, coupled with fiber-optic light guided instrument | visible region of spectrum | 20 mw, fiber diameter of 25–100 μm, 20–40 min, CW, or PW, or combined modes | Transcatheterly; Threading a fiber optic through a catheter in femoral artery (advancing fiber optic to distal site of anterior and middle cerebral arteries) |
Improved cerebral microcirculation and cognitive recovery; decreased permanent dementia |
| Berman et al. 2017 [195] | Dementia (11) | LEDs, 15 arrays of 70 LEDs/array (total of 1100 LEDs set) |
1060–1080 nm | 6 min/day for 28 consecutive days, PW at 10-Hz with DC of 50% | Transcranially; whole brain irradiation with LED helmet |
Improved executive functioning including clock drawing, immediate recall, praxis memory, visual attention and task switching; improved EEG amplitude and connectivity measures |
| Saltmarche et al. 2017 [71] | Dementia (5) | LEDs, “810” and “Neuro” devices, Vielight, Inc. (Toronto, Canada) | 810 nm | 14.2 or 41+23 mW/cm2 per LED, 10.65 or 24.6+13.8 J/cm2, 25 or 20 min, PW at 10-Hz, for 12 weeks | Transcranially + Intranasally; Multiple areas, bilateral mesial prefrontal cortex, precuneus/posterior cingulate cortex, angular gyrus, and hippocampus |
Improved function and sleep quality; decreased angry outbursts, anxiety, and wandering |
| Vargas et al. 2017 [206] | Older adults at risk for cognitive decline (12) | Laser, CG-5000, Cell Gen Therapeutics, LLC (Dallas, TX, USA) |
1064 nm | 3.4 W, 250 mW/cm2, 120 or 137.5 J/cm2 per session, CW | Transcranially; 2 sites, forehead at 4.2-cm diameter medial site and 4.2-cm diameter lateral site (EEG map site: FP2 point) |
Improved reaction time and lapses in psychomotor vigilance task and correct responses in delayed match to sample task; increased resting-state EEG alpha, beta, and gamma power; promoted more efficient prefrontal blood-oxygen-level dependent-fMRI response |
| Maloney et al. 2010 [197] | Parkinson’s disease (8) | Laser, PL5000, Erchonia Medical Inc. (Melbourne, FL, USA) | NR | Daily for 2 weeks | Transcranially; Multiple areas (bilateral occipital, parietal, temporal, frontal lobes and along sagittal sutures) |
Improved balance, gait, freezing, cognitive function, rolling in bed, and difficulties in speech assessed by Visual Analog Scale |
| Schiffer et al. 2009 [11] | Major depressive disorder (10) | LEDs, Marubeni America Corp. (Santa Clara, CA, USA) | 810 nm | 250 mW/cm2, 60 J/cm2, 4 min, one irradiation session, CW | Transcranially; 2 sites, bilateral (right and left forehead at EEG map sites: F3, F4) |
Decreased depression and anxiety rates at 2-week post-irradiation assessed by Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale; no significant effects on cerebral blood flow |
| Cassano et al. 2015 [203] | Major depressive disorder (4) | Laser, Neurothera PhotoThera Inc. (Carlsbad, CA, USA) | 808 nm | 5 W, 700 mW/cm2, 84 J/cm2, 2 min per site, 2×/week for 3 weeks, CW | Transcranially; 4 sites, bilateral (right and left forehead center at 20 and 40 mm from sagittal line) |
Decreased depression rate at 6 to 7 weeks post-irradiation assessed by Hamilton Depression Rating Scale-17 items |
| Disner et al. 2016 [202] | Patients with elevated depression symptoms (51) | Laser, CG-5000, Cell Gen Therapeutics (Dallas, TX, USA) |
1064 nm | 250 mW/cm2, 60 J/cm2, 4 min per site, for 2 sessions, CW | Transcranially; 2 sites, medial and lateral parts of the left or right side of the forehead |
Right prefrontal irradiation improved attention bias modification intervention effects and depression symptoms |
| Petrie et al. 2016 [183] | Patients with low back pain (10) | LEDs, Thor-UK:DDII, LED-104 instrument (UK) | 660 and 850 nm | 100 mW/cm2, 3 J/cm2, 6 min per session (30 sec/site), 3× over 12 days | Remote tissue irradiation; 12 sites, symmetrical bilateral: 8 thoracic and 4 posterior-thigh sites |
Decreased depression rate assessed by one-question depression-scale; improved functional status assessed by Advise-Rehab Global Scale |
| Zalewska-Kaszubska and Obzejta 2004 [184] | Alcoholics (53) | Laser | 514 and 632.8 nm | 514 nm: 100 mW, 10 sec for each site 632.8 nm: 25 mW, 5 min |
Multiple areas on head and neck; 514 nm (auricular concha, bilaterally to 5 points) 632.8 nm (spot on the neck vessel projection) |
Decreased depression rate assessed by Beck Depression Inventory–Fast Screen; increased α-endorphin level |
| Naeser et al. 2012 [181] | Patients with aphasia (3) | LEDs, 9 red + 52 NIR diodes | NR | 22.2 mW/cm2, 13 or 13+39 J/cm2, 3×/week for 6 weeks, PW at 146-Hz | Transcranially; | Improved comprehension and interfaced assessed by picture naming tasks |
| Nawashiro et al. 2012 [19] | Patient in a persistent (1) vegetative state | LEDs, SUN-MECHATRONICS (Tokyo, Japan) | 850 nm | 11.4 mW/cm2, 20.5 J/cm2 at scalp, 30 min, 2×/day for 73 days | Transcranially; 2 sites, left and right forehead areas superior to the eyebrows |
Increased regional cerebral blood flow; improved neurological outcomes |
| Werner et al. 2016 [256] | Patients with disorders of consciousness (16) | Laser, Power Twin 21 by MKW Laser system | 785 nm | 10 mW/cm2, 10 min, 5×/week for 4 weeks, CW | Transcranially; 5 sites, along with horizontal line at the level of the upper edge of the two fossa sphenoidalis |
Improved alertness and awareness over time assessed by Revised Coma Recovery Scale |
Note: AD, Alzheimer’s disease; CCO, cytochrome c oxidase; CW, continues wave; DC, duty cycle; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; LEDs, light emitting diodes; MDD, major depressive disorder; PD, Parkinson’s disease; PW, pulsed wave; qEEG, quantitative electroencephalography; TBI, traumatic brain injury
8.1. Alzheimer’s disease
Despite the existence of several animal studies, there have only been a few studies on the efficacy of PBM therapy in AD and dementia patients. Regarding these human studies, significant improvements in sleep quality, mood states, EEG patterns as well as improved cognitive function including memory and attention, have been obtained as a consequence of NIR PBM therapy [71,195]. Besides, red laser delivered via an arterial catheter leading into the brain gave improvement of CBF in AD patient, and also resulted in a remarkable reduction of dementia scores [196].
8.2. Parkinson’s disease
To date, the majority of the clinical investigations revealed positive impacts of transcranial PBM therapy in conditions such as TBI, stroke and depression, in which the target area was in the cortical regions of the brain. On the other hand, PD pathogenesis is linked to abnormalities in the SNc, a midbrain structure that is located at a depth 80–100 mm from the coronal suture, below the dura. Studies have suggested that light in the NIR region may not penetrate the human brain deeper than 20 mm from the cortical surface [68]. This is considered to be a clear limitation in the application of transcranial PBM therapy in human PD. However, in the only (non-controlled, non-randomized) study in PD patients, improved motor and cognitive functions has been reported following 2 weeks of transcranial PBM therapy [197].
8.3. Traumatic brain injury
So far, although the majority of animal studies have been conducted on acute TBI models, by contrast the majority of clinical studies have been conducted on chronic TBI patients. It is quite common for humans who recover from a moderate or severe head injury to suffer from a wide variety of long-lasting symptoms including cognitive impairment (eg, poor memory, impaired executive function, and difficulties concentrating), headaches, disturbed sleep, and depression. In the early open studies in TBI, transcranial LED therapy (633/870 nm) improved self-awareness, self-regulation in social functioning and sleep quality [30,33]. The higher fluence of NIR laser resulted in greater clinical efficacy such as diminished signs of headache and improved sleep quality as well as improved cognitive and mood states in TBI patients [61]. In addition, improving the alertness and awareness in TBI patients with severe disorders of consciousness was achieved following irradiation at 785 nm, a somewhat uncommon wavelength for transcranial PBM therapy [198].
8.4. Stroke
To date, three clinical trials, called “Neurothera Effectiveness and Safety Trials” (NEST-1 [199], NEST-2 [90], and NEST-3 [200]) have been carried out in acute stroke patients. Although the phase I and II studies showed both the safety and effectiveness of PBM therapy using 808 nm laser (applied within 24 h of stroke onset), phase III trials were disappointing and were terminated for futility at an interim analysis stage. Besides these, an effort has been made in occasional studies to show neuroprotective or neuroreparative effects of PBM therapy in chronic stroke patients via transcranial [181] and multiple area [201] irradiation methods.
8.5. Depression
The development of effective and sustainable treatment modalities for major depression has been a global aim for decades. To date, studies on antidepressant effects of PBM therapy have had relatively short follow-up periods and could be divided into two types of studies, patients with MDD [11,202,203] and TBI patients with comorbid depression [30,33,61]. The first study in MDD patients showed that a single-session of LED therapy alleviated depression and anxiety symptoms (Hamilton scales) at 2 weeks post-irradiation [11]. Using transcranial LED therapy, a significant decrease in PTSD scores and depression levels has been also reported after 1 week of treatment, while result of 2 months post-treatment did not show an overall linear trend response [30]. Besides LED light, the use of transcranial high-power lasers ameliorated depression symptoms at 6–8 weeks post-irradiation in TBI cases with comorbid depression [61] and in MDD patients [203]. A study by Disner et al. [202] also revealed that transcranial laser therapy delivered to the right forehead was more effective for alleviation of depression symptoms than PBM to the left forehead, and this observation may suggest a brain area-dependent effect of PBM in MDD patients.
8.6. Healthy subjects
In the real-world, mental activities require complex cognitive processes such as short-term and long-term memory, decision making, sustained attention, problem solving and planning, and executive function [204,205]. On the other hand, the enhancement or maintenance of cognitive functions in older adults who commonly experience some decline with age, and who are also at risk of dementia is now becoming of great interest [206]. In this regard, many researchers believe that PBM therapy will become one of the promising cognitive enhancing agents in the upcoming years [207–209]. In a fascinating series of studies using 1064 nm laser, Gonzalez-Lima and his research team showed enhancement of higher-order cortical functions such as prefrontal rule-based learning, sustained attention, and short-term memory as well as executive functions in healthy young subjects [44–46,210]. A further study by this group provided evidence that PBM therapy to the prefrontal region could improve cerebral oxygenation and enhance brain hemodynamic processes that are necessary in higher-level cognitive functioning [50].
9. Influence of irradiation parameters in brain PBM therapy
Generally, among the various treatment parameters [211], the five most important factors that affect the outcome of brain PBM therapy are: wavelength, fluence and irradiance, operation mode (CW or PW), coherence, and treatment duration and repetition regimen.
9.1. Wavelengths
Light in the spectral bands at 650–680 nm and 800–870 nm matches the absorption of relatively oxidized CCO, while the band at 750–770 nm matches reduced CCO [212]. Likewise the action spectra determined for cellular responses such as DNA synthesis has direct correlation with the absorption peaks of oxidized CCO [103]. Given this, a large body of studies on the neural applications of PBM have employed specific wavelengths in the red including 606, 627, 630, 632.8, 640, 660, and 670 nm and in the NIR regions including 785, 800, 804, 808, 810, 830, and 850 nm (see Tables 1–3), but most effective overall benefits have come from using NIR wavelengths. On the other hand, NIR light around 728 nm, known as the least effective wavelength in terms of CCO absorption [103], has been tested in only a few studies [97,145,214,213]. Moreover, notable enhancements in brain functions have been achieved using 1064 nm Nd:YAG laser light which has high penetration ability through the skull [210,202]. Increases in neuronal proliferation and ATP production have been found following green laser irradiation (532 nm) [215,216], and promotion of differentiation and proliferation of stem cells following irradiation with blue LED (420 nm) and green light (540 nm) [217] have been also shown. The activation of transducing cellular signals through the several intermediates following the absorption of blue and green light by cryptochromes and opsin photoreceptors, respectively, has been explained as mechanism involved in photostimulatory effects of this range of wavelengths (Fig. 2) [144,218]. The transmittance of blue-green light at 400–540 nm is less than 0.1% through the human skull [219] so these wavelengths are considered not to be applicable for transcranial irradiation methods, however, the development of intracranial PBM (fiber-based) using this spectrum of light needs to be evaluated. All together, matching the wavelengths to the absorption peaks of cellular photoacceptors such as CCO, Ca2+ ion channels, as well as water molecules, while also considering the penetration depth of the light and the molar concentration of the chromophores in the tissue, make optimizing brain PBM therapy a fascinating challenge [79].
9.2. Fluence and irradiance
Fluence or energy density is calculated by multiplying irradiance (W/cm2) by time (sec), which is defined as the amount of energy per unit of area (J/cm2). The in vitro data from neuronal cell cultures have revealed a threshold for stimulation of CCO activity and ATP production between 0.3 to 3 J/cm2 [2,103]. Concerning in vivo and clinical transcranial PBM studies, delivered fluence is commonly reported on the scalp, skull, or on the cortical surface. Although the optimal fluences for treatment of various animal models of brain disorders have yet to be clearly identified, the average fluences ranging from 0.1 to 15 J/cm2 were found to be enough for effective PBM of neural cells [6,9,17]. In the human transcranial PBM studies fluences on the scalp have ranged from 10 to 30 J/cm2 for neurological disorders [61,71], 13 to 84 J/cm2 for psychological disorders [11,30,203], and 15 to 60 J/cm2 for normal subjects [210,220]. Irradiation sites have been chosen in various parts of the head, but the forehead has been most often used due to the lack of hair which can serve as a formidable barrier to light penetration depending on its color and density. Likewise, in order to improve brain function, different fluence from 10 to 137.5 J/cm2 have been applied on the scalp or skull of healthy subjects [18,123,210,221]. The overall results from clinical reports indicated that a cortical fluence of approximately 1 to 2 J/cm2 per site may be sufficient to achieve positive effects [44,200]. However, as mentioned earlier, using 1.2 J/cm2 cortical fluence, phase III of acute ischemic stroke trial failed to show benefit [200]. The various thicknesses of different patient’s skulls, a single irradiation session (rather than daily repetitions), and irradiation areas which were not optimized for the location of that particular patient’s stroke are possible factors which may have influenced this failure [79,88].
The irradiance or power density used in the NEST trials with an 810 nm laser was 700 mW/cm2, and active cooling was required in the laser applicator that was applied to 20 regions of the shaved head. Henderson and Morries [60] have propounded the use of even higher laser powers on the principal that these high power densities are necessary for the light to reach deeper brain structures. However, the laser probe needs to keep in constant movement over the forehead to prevent unacceptable heating of the skin [18,61]. 10–70 mW/cm2 is the typical LED irradiance used in the studies [123,203]. Due to a high amount of delivered laser light, the potential temperature gain is more concerned, while compared with LED light.
Although there is no comprehensive data to correlate the improvement in the severity of brain disorders and effective PBM therapy protocols, studies on the use of this therapy for treatment of MDD patients suggest that the successful outcomes largely depend on the delivered dosage, number of sessions, treatment intervals, as well as the total length of the treatment program [203,222]. These criteria have been confirmed by other studies [223,224] that reported the effectiveness of the PBM therapy was not only affected by dosage, but also the initial severity of the damage affected the treatment outcomes. It should be noted that there is a real risk of overtreatment if too much light is delivered either too quickly or too often [257].
9.3. Operation mode
In the literature, brain PBM therapy has been applied in CW mode or in PW mode at repetition rates from 1 to 3000-Hz [107,220]. However, it should be added that PW mode at specific frequencies of 10-Hz [7,61,71,85], 40-Hz [225], and 100-Hz [9,226–228] has demonstrated significant benefits to the brain. The superior safety and better biostimulatory effects of PW mode in comparison to CW mode have been explained by some reasonable hypotheses [239,230]. Owing to the existence of ON and OFF times, laser in PW mode produces less heat and consequent less histopathological damage [3]. According to some researchers, light in PW mode has better penetration depth (due to the higher peak power density in short pulses) than light in CW mode, which might reach the deeper structures of the brain [60,230]. Besides these justifications, possible resonance between different brain waves (frequency ranging from 1–100 Hz) [231] and the frequency of PW mode light could occur during brain PBM therapy [7]. Additionally, there might be resonance between a pulse duration of 100 msec (equal to a frequency of 10-Hz) and the half-life of opening and closing of mitochondrial membrane ion channels (typically measured in tens of msec), PW light could have more effects on the brain [232,233].
9.4. Coherence
Many different types of laser and LED devices have used for PBM therapy. The laser devices generate coherent light with various coherence lengths depending on the band-width of the specific laser. Coherence lengths of lasers can range from many meters for the He-He laser to only a few mm for diode lasers, while LED devices emit non-coherent light. Lasers are also much more monochromatic (band-widths vary from pm to a few nm), while LEDs have a broader band-width (typically 30 nm full width half maximum). Overall LED therapy appears to be similarly effective as laser therapy for superficial tissue, but for transcranial brain PBM, comparisons between these sources seem to be in favor of lasers for deeper penetration. On the other hand, LED arrays are less expensive, and can irradiate much larger surface areas of tissue (particularly useful for the head e.g. frontal region).
9.5 Treatment duration and repetition regimen
Although there have been no studies specifically looking at the effect of irradiation time on brain PBM therapy, it is likely that this parameter will indeed be important. A study carried out using 810 nm PBM on zymosan-induced arthritis in the rat knee compared a low and a high fluence (3 J/cm2 and 30 J/cm2) delivered at a low and a high irradiance (5 mW/cm2 and 50 mW/cm2). The low fluence/low irradiance group (taking 10 min) and the high fluence/high irradiance group (also taking 10 min) together with the high fluence/low irradiance (taking 100 min) were all equally effective in reducing inflammation in the knee. The only combination that was not effective was the low fluence at the high irradiance (only taking 1 min). The conclusion was that the PBM needs to continue for a long enough period in order for the tissue to respond, and that 10 min is a reasonable choice [234].
Many of the earlier animal studies for stroke and TBI used a single session of PBM for instance delivered within 24 hours of a stroke or a TBI. The excellent results obtained by this regimen encouraged human clinical trials for acute stroke using a single session of PBM. Even though NEST-1 and NEST-2 trials showed success with a single session, NEST-3 did not. Would NEST-3 have been successful if it had used several PBM sessions repeated in the days following onset of the stroke? Even though the trial of PBM for depression and anxiety only used a single session, and positive results were obtained at examination two weeks later, the benefit had largely disappeared by 4 weeks. In our opinion, it is unreasonable to expect a single session of PBM in humans to have the best effects, and treatments should probably be repeated for several days in case of acute events such as TBI or stroke, and for more extended periods in the case of chronic diseases such as AD, PD, MDD, and PTSD. However it is worth pointing out that it might be possible to overdo the number of sessions of PBM, since a study in mouse TBI found that 14 daily sessions was indeed too much, while 7 daily sessions was fine [28].
10. Conclusion
Because neural tissues contain large amounts of mitochondrial CCO, application of red to NIR lights (600–850) for brain PBM therapy is highly attractive. The main problem so far has been getting enough light into the brain to accomplish the beneficial effects. In recent years, irradiation in the wavelength range between 980 nm and 1100 nm has been growing rapidly, and its different mechanisms of action including stimulation of ion channels and water molecules suggest it might even be combined with red/NIR. Improving cerebral metabolic function, stimulating neurogenesis and synaptogenesis, regulating neurotransmitters, and providing neuroprotection via anti-inflammatory and antioxidant biological signaling are the most important effects of brain PBM therapy (Fig. 4). The overall results from extensive preclinical and clinical studies in the brain PBM field suggest that modest levels of red and NIR light show biostimulatory effects without any thermal damage, and could improve neurobehavioral deficits associated with many brain disorders. Nevertheless, it is still not completely clear whether chronic repetition of brain PBM will be necessary for sustained clinical benefit, especially in psychological and neurodegenerative disorders. Owing to the beneficial impacts of brain PBM therapy in depression and anxiety, new trials for other psychiatric disorders such as schizophrenia, autism, bipolar, attention-deficit hyperactivity and obsessive–compulsive disorders might well emerge in the future. Development of new techniques for effective light delivery to deeper structures of the brain is crucial, because of involvement of the limbic system and midbrain abnormalities seen in some brain disorders. In this respect, intracranial and intranasal irradiation methods, as well as the oral cavity route, even via the ear canal could be options. Although therapeutic influences of intracranial PBM therapy has been focused on PD researches, it is postulated that developing this technique also potentially effective for those conditions that are associated with limbic system dysfunctions such as anhedonia, anxiety as well as impaired emotional processing. Preliminary evidence of benefit has been obtained in autism spectrum disorders. There is an epidemic of AD that is expected to hit the Western world as the overall population ages, and there has been a noticeable lack of any effective pharmacological therapies that have been approved for AD. Although the evidence for the effectiveness of PBM in the treatment of AD is still very preliminary, it is possible that PBM will play an even larger role in society in years to come if clinical trials now being conducted are successful. The authors conclude that clinic or home-based PBM therapy using laser or LED devices will become one of the most promising strategies for neurorehabilitation in upcoming years.
Fig. 4. Brain tissue-specific functional processes that occur after brain photobiomodulation therapy.
On a cellular level, photobiomodulation can reduce apoptosis and excitoxicity, increase antioxidants, neurotrophins and stimulate neuroprogenitor cells. On a tissue level, photobiomodulation therapy can increase blood flow and angiogenesis, reduce inflammation and help neurons form new connections. BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor; NGF, nerve growth factor; SOD, superoxide dismutase.
Table 2.
Published in vivo studies on the neuroprotection effects of photobiomodulation in neuronal activities
| Study/Year | Relevance | Light Source | Wavelengths | Irradiation parameters | Irradiation approach/sites | Findings |
|---|---|---|---|---|---|---|
| Michalikova et al. 2008 [14] | Naïve middle-age mouse | Laser | 1072 nm | 6 min/day for 10 days | Full body irradiation | Enhanced acquisition of working memory for spatial navigation; no effects on exploratory activity or anxiety responses |
| Uozumi et al. 2010 [121] | Naïve mouse | Laser, B&W Tek, Inc. (Newark, DE, USA) | 808 nm | 0.8, 1.6, or 3.2 W/cm2, spot diameter of 3 mm, 45 min, one irradiation session, CW | Transcranially; At the left hemisphere, 2 mm posterior to and 3 mm left of the bregma |
At 1.6 W/cm2: Increased CBF in the irradiated and opposite hemisphere over the 45 min irradiation; increased cortical NO concentration; decreased neuronal apoptosis induced by transient cerebral ischemia in cerebral cortex and CA1 subfield of dorsal hippocampus |
| Rojas et al. 2012 [17] | Naïve rat | LEDs, LEDtronics, Inc. (Torrance, CA, USA) | 660 nm | 9 mW/cm2, 1, 5, 5.4, 10.9, 21.6, or 32.9 J/cm2; with corresponding duration of 111, 565, 600, 1200, 2400, or 4800 sec, respectively; CW | Transcranially; Holding probe at the 1 cm from the dorsal head surface |
Enhanced O2 consumption in PFC (at 5.4 J/cm2); improved extinction of fear-conditioned memories (at 10.9 J/cm2); decreased renewal of conditioned-fear (at 5.4 J/cm2); increased CCO activity in PFC (at 10.9 J/cm2) |
| Mintzopoulos et al. 2017 [115] | Naïve dog | Laser, PhotoThera, Inc. (Carlsbad, CA, USA) | 808 nm | 4–10 mW/cm2 on the dura mater, 0.48–1.2 J/cm2 per site (total of 2 sites); spot diameter of 22 mm, 2 min, 1 session or 3×/week for 2 weeks, CW | Transcranially; At the anterior and posterior cranium midline locations |
Improved cerebral bioenergetics via increase of PCr/β-NTP ratios and PCr level (at 2 weeks post-irradiation) |
| De Taboada et al. 2011 [9] | AD (mouse APP transgenic model) |
Laser, Photothera, Inc. (Carlsbad, CA, USA) | 808 nm | 1.2, 6, or 12 J/cm2 at cortex; fiber diameter of 3 mm, 2 min, 3×/week for 6 months, PW at 100-Hz with 2 msec pulse duration, or CW | Transcranially; At a point in sagittal suture, 4 mm caudal to coronal suture |
Improved learning and memory in MWM test (by all regimens); decreased CSF (by PW regimens), plasma (by all regimens), and brain (by all regimens) Aβ level; decreased brain inflammatory markers such as IL-1β, TNF-α, and TGF-β (by all regimens); increased ATP level and O2 consumption (by PW at 6 J/cm2) |
| Grillo et al. 2013 [49] | AD (mouse TASTPM model) |
LEDs, Virulite Distribution Limited | 1072 nm | 5 mW/cm2, 1.8 J/cm2, 6 min/day for 2 days, biweekly for 5 months, PW at 600-Hz with DC of 300 μs | Full body irradiation | Increased HSP27, 60, 70, 105 and P-HSP27, and decreased PS1, αB-crystallin, APP, Aβ1–40, Aβ1–42, and phosphorylated tau proteins expression; decreased small Aβ1–40/42 and Aβ1–42plaque deposition in Dentate Gyrus and cerebral cortex |
| Sutalangka et al. 2013 [138] | AD (rat AF64A model) |
Laser, Xinland International Limited (Xi’an, Shaanxi, China) | 405 nm | 52.63 J/cm2, spot diameter of 500 μm, 10 min/day for 2 weeks, CW | Remote tissue irradiation; At the HT7 point or a point 2–4 mm lateral to the HT7 acupoint |
Improved learning and memory in MWM test; increased catalase and SOD activities and decreased AChE activity in hippocampus |
| Purushothu man et al. 2014 [21] | AD (mouse K3 and APP/PS1 transgenic models) |
LEDs, WARP 10; Quantum Devices (Barneveld, WI, USA) | 670 nm | 4 J/cm2, 90 sec, 5×/week for 4 weeks, CW | Transcranially; Holding probe 1 to 2 cm above the head |
K3 mouse: decreased phosphorylated tau and NFTs in neocortex and hippocampus; decreased oxidative stress markers such as 8-OHDG and 4-HNE in neocortex; enhanced CCO expression patterns in neocortex and hippocampus APP/PS1 mouse: decreased number, size, and burden of Aβ plaques in neocortex and hippocampus |
| Farfara et al. 2015 [191] | AD (mouse 5XFAD transgenic model) |
NR | NR | 400 mW, 1 J/cm2, spot diameter of 0.3 cm, 6× (at 10-day intervals, for 2 months), CW | Remote tissue irradiation; At the middle portion of the medial part of the tibia |
Improved memory in object recognition and fear-conditioning tests; decreased Aβ burden in hippocampus |
| Purushothu man et al. 2015 [236] | AD (mouse K3 and APP/PS1 transgenic models) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 4 J/cm2, 90 sec, 5×/week for 4 weeks, CW | Transcranially; Holding probe 1 to 2 cm above the head |
APP/PS1 mouse: reduced number, size and deposition of Aβ plaques in cerebellar cortex K3 mouse: decreased formation of NFTs, phosphorylated tau, oxidative stress, and increased CCO expression in cerebellar cortex |
| Da Luz Eltchechem et al. 2017 [237] | AD (rat Aβ25–35 model) |
LEDs, AlGalnP, RL5-R12008 | 627 nm | 70 mW, 7 J/cm2, 100 sec/day for 21 days | Transcranially; Holding probe at 1 cm from the frontal region of scalp |
Improved motor skills in OFT at days 14 and 21; improved spatial memory in MWM test at day 14; reduced amount of Aβ |
| Lu et al. 2017 [20] | AD (rat Aβ1–42 model) |
Laser, 808M100, Dragon Lasers (Changchun, China) |
808 nm | 25 mW/cm2, 3 J/cm2 at cerebral cortex, spot area of 1 cm2, 2 min/day for 5 consecutive days, CW | Transcranially; At the 3 mm posterior to eye and 2 mm anterior to ear |
Suppressed neuronal degeneration; suppressed expression of mitochondrial fission and preserved mitochondrial fusion proteins; recovered changes in mitochondrial dynamics; improved mitochondrial function through reduction of Bax/Bcl-2 ratio and increase of MMP, CCO activity and ATP levels; inhibited G6PDH and NADPH oxidase activities; enhanced total antioxidant capacity; inhibited glial activation, proinflammatory cytokines production and tau hyperphosphorylation; attenuated cytosolic level of cytochrome c, caspase-9 and-3 activities; improved spatial learning and memory in Barnes maze task and long-term recognition memory in NOR test |
| Shaw et al. 2010 [238] | PD (mouse MPTP model) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 40 mW/cm2 at scalp, 5.3 mW/cm2 inside skull, 0.47 J/cm2 per irradiation (total of 4 irradiations over 30 h), 90 sec, irradiation area of 10 cm2, CW | Transcranially; Holding probe at 1 cm from the head |
Increased TH+ cell numbers in SNc (at 50 and 100 mg/kg MPTP) |
| Peoples et al. 2012 [239] | PD (mouse MPTP model) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 5 J/cm2 over 10 sessions, 90 sec/session, CW | Transcranially; Holding probe at 1–2 cm from the head |
Increased TH+ cell numbers in SNc (at both simultaneous irradiation and post-irradiation regimens) |
| Shaw et al. 2012 [240] | PD (mouse MPTP model) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 0.5 J/cm2, 90 sec, CW | Transcranially; Holding probe at 1–2 cm from the head |
Decreased Fos+ cell numbers in STN and ZI after acute (~1 day) and chronic (5 weeks) MPTP insult |
| Moro et al. 2013 [241] | PD (MPTP Balb/c and C57BL/6 mouse models) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 0.47 J/cm2 per session (total of 4 sessions over 30 h), 90 sec, CW | Transcranially; Holding probe at 1–2 cm from the head |
Balb/c mouse: increased TH+ cell numbers in SNc; improved locomotor activities in OFT including velocity, high mobility and immobility times |
| Johnstone et al. 2014 [67] | PD (mouse MPTP models) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 40 mW/cm2, 4 J/cm2, 90 sec, CW | Head or body irradiation | At both head or body irradiations: increased TH+ cell numbers in SNc (at 50 mg/kg MPTP); increased glial cell numbers in SNc (at 75 mg/kg MPTP) |
| Moro et al. 2014 [68] | PD (mouse MPTP models) |
LEDs, (SMT 670, Epitex) coupled with an optical fiber (FT300EMT, Thorlabs) | 670 nm | 1.5 mW/cm2 (PW) or 14.5 mW/cm2 (CW), fiber diameter of 300 μm, continuous irradiation for 6 days | Intracranially; Implant site: lateral ventricle |
Increased TH+ cell numbers in SNc (at PW mode); no observable behavioral and tissue deficits |
| Oueslati et al. 2015 [10] | PD (rat AAV-based genetic model) |
Laser, RLTMDL-808–2W, Roithner Lasertechnik GmbH (Vienna, Austria) | 808 nm | 2.5 or 5 mW/cm2 at midbrain, 2 irradiation spots of about 1 cm2, 100 sec/day for 4 weeks | Transcranially; Irradiation of both sides of the head |
Improved motor performance in cylinder test (at 2.5 or 5 mW/cm2); decreased dopaminergic neuronal loss in the substantia nigra and preserved dopaminergic fibers in the ipsilateral striatum (at 5 mW/cm2) |
| Reinhart et al. 2015 [242] | PD (mouse MPTP models) |
LEDs, LED EPITEX SMT810, EPITEX Inc. (Kyoto, Japan) | 810 nm | 160 mW, 57.6 mJ (at skull for total of 4 irradiations), 90 sec/session | Transcranially; Full head irradiation |
Increased TH+ cell numbers in SNc; improved locomotor activity in OFT |
| Darlot et al. 2016 [64] | PD (monkey MPTP model) |
Laser, coupled with an optical fiber (HCP-MO200T) | 670 nm | 10 mW; 25 or 35 J over 5 or 7 days, respectively; with 5 seconds ON/60 seconds OFF | Intracranially; Implant site: 4 mm rostral to posterior commissure, 3 mm below anterior commissure-posterior commissure line |
Improved PD signs and locomotor activities including movement and velocity; increased number of nigral Nissl-stained and TH+ cells and striatal TH+ terminals; no observable behavioral and tissue deficits |
| Massri et al. 2016 [243] | PD (monkey MPTP model) |
Laser, coupled with an optical fiber (HCP-MO200T) | 670 nm | 10 mW; 25 or 35 J over 7 days; with 5 seconds ON/60 seconds OFF | Intracranially; Implant site: near the SNc of both sides |
Decreased number and cell size of astrocytes in both the SNc and striatum; decreased cell size of microglia in both the SNc and striatum |
| Moro et al. 2016 [244] | PD (monkey MPTP model) |
Laser, coupled with an optical fiber (HCP-MO200T) | 670 nm | 10 mW, 125 J for 25 days continuous irradiation, with 5 seconds ON/60 seconds OFF | Intracranially; Implant site: a region close to midline in the midbrain, encompassing the ventral tegmental area |
Improved PD signs; increased number of nigral Nissl-stained cells and density of striatal TH+ terminals |
| Reinhart et al. 2016 [245] | PD (rat 6-OHDA model) |
LEDs, (SMT 670; Epitex) coupled with an optical fiber (FT300EMT; Thorlabs) | 670 nm | 333 nW or 0.16 mW, 634 mJ or 304 J, fiber diameter of 300 μm, for 23 consecutive days, PW (2×/day for 90 sec) or CW | Intracranially; Implant site: a region near the SNc (including red nucleus and ventral tegmental area) |
Decreased rotational behavior at days 14 (at CW of 304 J) and 21 (at CW of 304 J, and PW of 634 mJ); increased TH+ cell numbers in SNc (at PW of 634 mJ) |
| Reinhart et al. 2016 [246] | PD (mouse MPTP model) |
LEDs, WARP 10, Quantum Devices (Barneveld, WI, USA) | 670 nm | 5.3 mW/cm2, ~0.5 J/cm2 at midbrain, 90 sec, 2×/day for 2, 4, or 6 days, CW | Transcranially; Holding probe at 1–2 cm from the head |
Improved locomotor activity in OFT (at all regimens); increased TH+ cell numbers in SNc (at all regimens) |
| Reinhart et al. 2016 [247] | PD (mouse MPTP model) |
LEDs, Epitex devices (models 670-66-60 and 810-66-60; epoxy lens infrared illuminators) | 670 and/or 810 nm | 15 or 30 mW, total dosage of 11 or 22 J, 45 or 90 sec, 2×/day for 2 days, CW | Transcranially; Full head irradiation |
Improved locomotor activity in OFT and increased TH+ cell numbers in SNc (at all regimens, especially in concurrent and sequential irradiation regimens) |
| Massri et al. 2017 [169] | PD (mouse and monkey MPTP model, and rat 6-OHDA model) |
Laser, coupled with an optical fiber | 670 nm | 0.16 mW for mouse and rat, and 10 mW for monkey, fiber diameter of 300 μm, continuous irradiation for 2 (mouse), 23 (rat) and 5 (monkey) days | Intracranially; Implant sites: lateral ventricle in mouse, a midline region of midbrain in rat and monkey |
Increased number of TH+ cells, TH+ terminal density, and GDNF expression patterns in striatum of monkey |
| Leung et al. 2002 [137] | Stroke (rat cerebral ischemia model) |
Laser, Omega Excel Laser (London, UK) |
660 nm | 8.8 mW, 2.64, 13.2, or 26.4 J/cm2; with corresponding duration of 1, 5, or 10 min, respectively; one irradiation session, PW at 10-Hz | Transcutaneously; Directly through a burr hole 5 mm from the cerebrum |
Decreased specific activity of NOS and increased expression of TGF-β1 at 4 days post-injury (at all regimens); down-regulated expression of three NOS isoforms including eNOS, nNOS, and iNOS at 4 days post-injury (at all regimens) |
| Lapchak et al. 2004 [6] | Stroke (rabbit embolic model) |
Laser, Acculaser (PhotoThera, Inc) coupled with OZ Optics Ltd fiber | 808 nm | 7 mW/cm2 for 2 min (0.84 J/cm2) or 25 mW/cm2 for 10 min (15 J/cm2), one irradiation session, CW | Transcranially; Holding probe in direct contact with the skin |
Improved behavioral performance and decreased effective clot dose for stroke 3 h after clot injection (at cortical fluence of 15 J/cm2) |
| De Taboada et al. 2006 [248] | Stroke (rat atherothrombotic model) |
Laser, GaAs, Photothera, Inc. (San Diego, CA, USA) | 808 nm | 7.5 mW/cm2 at brain tissue level, 0.9 J/cm2 per site (total of 2 sites), fiber diameter of 4 mm, 2 min, one irradiation session | Transcranially; At two locations on head (3 mm dorsal to eye and 2 mm anterior to ear) either ipsilateral, contralateral, or to both sides of stroke |
Improved modified neurological score at 14, 21, and 28 days post-stroke (by all irradiated locations in the skull) |
| Oron et al. 2006 [170] | Stroke (rat atherothrombotic model) |
Laser, GaAs (PhotoThera, Inc) | 808 nm | 7.5 mW/cm2 at brain tissue level, 0.9 J/cm2 per site (total of 2 sites), fiber diameter of 4 mm, 2 min, PW at 70-Hz or CW | Transcranially; At two locations on head (3 mm dorsal to eye and 2 mm anterior to ear) and on contralateral hemisphere to stroke |
Improved neurological scores at 14 and 21 days post-stroke (CW mode when applied 24 h post-stroke); increased SVZ cell proliferation and migration (CW mode) |
| Lapchak et al. 2007 [228] | Stroke (rabbit embolic model) |
Laser, Acculaser coupled with OZ Optics Ltd fiber optic (Berkeley, CA, USA) | 808 nm | 7.5 mW/cm2, 0.9–1.2 J, 2 min, PW at 100-Hz (2 msec pulse duration with DC of 20%) or 1000-Hz (0.3 msec pulse duration with DC of 30%), or CW | Transcranially; Holding probe in direct contact with shaved scalp |
Improved behavioral performance and decreased effective clot dose for stroke 6 h after clot injection (at both PW mode regimens) |
| Lapchak et al. 2008 [249] | Stroke (rabbit embolic model) |
Laser, Acculaser coupled with OZ Optics Ltd fiber optic | 808 nm | 10 mW/cm2, 2 min, one irradiation session, CW | Transcranially; Holding probe in direct contact with scalp |
No significant effects on hemorrhage incidence, volume or survival rate |
| Lapchak et al. 2010 [111] | Stroke (rabbit embolic model) |
Laser, Acculaser coupled with OZ Optics Ltd fiber optic | 808 nm | 7.5, 37.5, or 262.5 mW/cm2; 0.9, 4.5, or 31.5 J/cm2 at cortex, spot diameter of 5 mm, 2 min, PW at 100-Hz or CW | Transcranially; Holding probe in direct contact with scalp |
Increased cortical ATP content (at PW mode, both of 4.5 and 31.5 J/cm2) |
| Yip et al. 2011 [8] | Stroke (rat cerebral ischemia model) |
Laser, GaAlAs, Omega Excel Laser (London, UK) | 606 nm | 8.8 mW, 2.64, 13.20, or 26.40 J/cm2; with corresponding duration of 1, 5, or 10 min, respectively; PW at 10-Hz | Transcutaneously; Directly through a burr hole 5 mm from the cerebrum |
Increased expression of Akt, phosphorylated-Akt, Bcl-2 and pBAD (at all regimens); decreased expression of caspase-3 (at all regimens) and caspase-9 (at 2.64 and 13.20 J/cm2) at 4 days post-stroke |
| Choi et al. 2012 [145] | Stroke (rat focal cerebral ischemia and reperfusion model) |
LEDs, Qray Inc. (Seongnam, Korea) | 710 nm | 0.042 mW/cm2, 1.796 J/cm2, irradiation area of 1.13 cm2, 12 h/day continuous irradiation for 20 days | Full body irradiation; Randomly over the whole skin |
Increased CD4+ (after 2 and 10 days post-stroke) and CD8+ T (after 10 and 20 days post-stroke) cell populations; decreased infarct volume (at day 21); increased expression of IL-10 (at day 20); decreased microglial activation in striatum and cortex (at day 20); increased number of CD4+CD25+ Treg cells in ischemic core and penumbra; improved neurological severity score and step fault scores |
| Huisa et al. 2013 [250] | Stroke (rabbit small clot embolic model) |
Laser, Acculaser coupled with OZ Optics Ltd fiber optic | 808.5 nm | 7.5, 10.8, or 20 mW/cm2, 2 min, CW | Transcranially; Holding probe in direct contact with scalp |
Improved behavioral performance and decreased effective clot dose for stroke (at double and triple irradiation regimens) |
| Fukuzaki et al. 2015 [215] | Stroke (mouse transient mild ischemia model) |
Laser, Nd:YVO4, SUWTECH, LDC-2500 (China) | 532 nm | 845 mW/cm2, 30.4 × 102 J/cm2 at cortex, 60 min, CW | Transcranially; At the surface of temporal skull over left auditory cortex |
Promoted the migration of NSPCs into deeper layers of the neocortex; increased phosphorylated-Akt and Akt expression levels at 4 h post-irradiation |
| Lapchak and Boitano 2016 [110] | Stroke (rabbit small clot embolic model) |
Laser, GaAIAs, coupled with OZ Optics Ltd fiber optic cable | 808 nm | 7.5 mW/cm2, 0.9 J/cm2 at cortex, 2 min, CW | Transcranially; Holding probe in direct contact with scalp |
Improved behavioral performance at 2 day post-stroke; increased cortical ATP levels at 6 h post-stroke |
| Lee et al. 2016 [24] | Stroke (mouse photothrombotic cerebral focal ischemia model) |
LEDs, Color Seven Co. (Seoul, Korea) | 610 nm | 1.7 mW/cm2, 2 J/cm2, spot diameter of 4 mm, 20 min, twice a day for 2 days prior to ischemic event, CW | Transcranially; At the skin via double-sided tape at the right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra |
At 24 h post-stroke: decreased infarct size and edema; improved neurological and motor function; decreased astrocyte and microglia cells; decreased transcription of iNOS, COX-2, TNF-α, IL-1β, TLR-2, CCL2 and CXCL10 in the ischemic cortex; decrease in protein levels of COX-2 and TLR2; inhibited p38, JNK and ERK-1/2 MAPK activation; attenuated translocation of the NF-κB p65 protein subunit from cytosol to nucleus; attenuated MPO protein levels; improved rearrangement of tight junction proteins and attenuated blood-brain barrier disruption |
| Meyer et al. 2016 [226] | Stroke (rabbit small clot embolic model) |
Laser, coupled with OZ Optics Ltd fiber optic cable (PhotoThera, SanDiego, CA, USA) | 808.5 nm | 7.5–333 mW/cm2 at cortex, 2 min, 1 or 3 irradiation sessions, CW or PW at 10- or 100-Hz with DC of 20% | Transcranially; Holding probe in direct contact with shaved skull |
Improved behavioral performance at 2 h post-stroke (at 1,111 mW/cm2 with 100-Hz PW); no observable tissue necrosis or microscopic neural damage |
| Lee et al. 2017 [147] | Stroke (mouse photothrombotic cerebral focal ischemia model) |
LEDs, Color Seven Co. (Seoul, Korea) | 610 nm | 1.7 mW/cm2, 2 J/cm2, spot diameter of 4 mm, 20 min, twice a day for 3 days.. commencing at 4 h post-ischemia, CW | Transcranially; At the skin via double-sided tape at the right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra |
At 72 h post-stroke: decreased infarct volume; improved neurological function; decreased MPO protein levels as a marker for neutrophil infiltration; decreased microglial activation via decrease of Iba-1(+)/CD68(+) cells; decreased cell death and reduced NLRP3, cleaved caspase-1 and -11, IL-1β and IL-18 levels; decreased TLR-2 protein levels; suppressed phospho-JNK and phospho-ERK; decreased translocation of NF-κB p65 protein subunit into nucleus |
| Lee et al. 2017 [122] | Stroke (mouse middle cerebral artery occlusion/reperfusion model) |
LEDs, Color Seven Co. (Seoul, Korea) | 610 nm | 1.7 mW/cm2, 2 J/cm2, spot diameter of 4 mm, 20 min, twice a day for 2 days prior to ischemic event, CW | Transcranially; At the skin via double-sided tape at the right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra |
At 24 h post-stroke: decreased infarct size and edema; improved neurological function; improved vestibular-motor dysfunction in WGT; increased CBF during 30 min after reperfusion; increased phosphorylated eNOS and decreased phosphorylated Akt expression levels |
| Yun et al. 2017 [171] | Stroke (rat middle cerebral artery occlusion model) |
Laser, Ellise-005, Ver. 1.0.1, Wontech (Daejeon, South Korea) | 650 nm | 30 mW, fiber diameter of 125 μm, 5 min, once every 2 days for 2 weeks, PW at 100-Hz | Laser acupuncture points; At the GV20 (head) and HT7 (right forepaw) points |
Improved learning and memory in MWM test; decreased cholinergic neuronal cell loss in the hippocampal CA1 region; upregulated gene expression of CREB, BDNF, and Bcl-2 and downregulated gene expression of Bax. |
| Oron et al. 2007 [251] | TBI (mouse) |
Laser, PhotoThera, Inc. (Carlsbad, CA, USA) | 808 nm | 10 or 20 mW/cm2, 1.2 or 2.4 J/cm2, fiber diameter of 3 mm, 2 min, CW | Transcranially; At the sagittal suture located 4 mm caudal to coronal suture |
Improved neurological severity score at 5–28 days post-TBI (at both regimens); decreased lesion volume at 28 days post-TBI (at both regimens) |
| Moreira et al. 2009 [25] | TBI (rat) |
Laser, MM Optics Ltda (São Carlos, SP, Brazil) | 660 or 780 nm | 40 mW, 3 or 5 J/cm2 per site (total of 2 sites); irradiation area of 0.04 cm2, 3 or 5 sec, CW | Direct irradiation; At the injury site |
Brain: decreased IL-1β at 24 h post-TBI compared to 6 h (at 5 J/cm2 660 nm, or 3 J/cm2 780 nm) Blood: increased TNF-α at 24 h vs. 6 h (at 3 J/cm2 660 nm, 5 J/cm2 660 nm, or 5 J/cm2 780 nm); increased IL-6 at 24 h vs. 6 h (at 3 J/cm2 660 nm, 5 J/cm2 660 nm, or 5 J/cm2 780 nm); decreased IL-6 at 6 or 24 h vs. control (at 3 J/cm2 660 nm) |
| Ando et al. 2011 [7] | TBI/post TBI depression (mouse model) |
Laser, DioDent Micro 810, HOYA ConBio (Fremont, CA, USA) | 810 nm | 50 mW/cm2, 36 J/cm2, 12 min, one irradiation session, CW or PW at 10- or 100-Hz with DC of 50% | Transcutaneously; On the left front-parietal cortex (3 mm anterior to lambda and 2.5 mm left of midline) |
Improved neurological severity score and increased body weight (at all regimens); improved depressive-like behaviors at 28 days post-TBI in FST (at 10-Hz) and TST (at 10- and 100-Hz); decreased lesion size at 15 and 28 days post–TBI (at 10-Hz) |
| Khuman et al. 2012 [252] | TBI (mouse) |
Laser, Thor Photomedicine Ltd. (Chesham, Buckinghamshire, UK) |
800 nm | 250–1000 mW/cm2, 60–210 J/cm2, spot area of 1.32 cm2, 2 or 7 min, CW | A) via an open craniotomy; Holding probe at 1 cm above head B) Transcranially; At the right and left parieto-temporal region |
Improved cognitive performance in MWM test (at 60 J/cm2 by transcranially or via an open craniotomy); decreased microglial activation at 48 h post-TBI |
| Quirk et al. 2012 [22] | TBI (rat) |
LEDs | 670 nm | 50 mW/cm2, 15 J/cm2, 5 min, 2 sessions/day for 72 h or 10 days, CW | Transcranially; Holding probe at 0.5 cm above the head |
Improved locomotor activity in OFT (at 10 days post-irradiation); increased Bcl-2 and GSH, and decreased Bax expression levels |
| Wu et al. 2012 [97] | TBI (mouse) |
Laser, BWF-665-1, B&W-Tek; 730/6, Diomed Inc.; DioDent Micro 810 and V-Raser, ConBio (USA) |
665, 730, 810, or 980 nm | 150 mW/cm2, 36 J/cm2, spot diameter of 1 cm, 4 min, one irradiation session, CW | Transcranially; Over the sutured incision |
Improved neurological severity score and decreased small deficits in brain at 4 weeks post-irradiation (at 665 or 810 nm) |
| Xuan et al. 2013 [96] | TBI (mouse) |
Laser, DioDent Micro 810, HOYA ConBio (Fremont, CA, USA) | 810 nm | 25 mW/cm2, 18 J/cm2, spot diameter of 1 cm, 12 min, for 1, 3, or 14 days, CW | Transcranially; Centrally on top of the head |
By 1 or 3×day: improved neurological severity scores and WGT scores; decreased brain lesions sizes at 14 and 28 days post-TBI By 3×day: decreased degeneration at 14 and increased neurogenesis at 28 days post-TBI |
| Xuan et al. 2014 [166] | TBI (mouse) |
Laser, DioDent Micro 810, HOYA ConBio (Fremont, CA, USA) | 810 nm | 25 mW/cm2, 18 J/cm2, spot diameter of 1 cm, 12 min, for 1 or 3 consecutive days, CW | Transcranially; Entire head irradiation |
At both 1 and 3×day: improved motor functions in WGT (at days 21, and 28 post-TBI); improved learning and memory in MWM (at day 28); decreased caspase-3 expression in lesion region (at day 4); increased neurogenesis in Dentate Gyrus and SVZ (at days 7 and 28); up-regulated migrating neuroprogenitor cells and neuronal differentiation in Dentate Gyrus and SVZ (at days 7 and 28) |
| Zhang et al. 2014 [146] | TBI (mouse) |
Laser, Acculaser, PhotoThera (Carlsbad, CA, USA) | 810 nm | 150 mW/cm2, 36 J/cm2, spot diameter of 1 cm, 4 min, PW at 10-Hz (50 msec pulse duration) | Transcranially; Holding probe in direct contact with scalp at contusion site |
Improved neurological severity score and increased body weight gain (at 3–28 days post-TBI); decreased IL-1β (at 6 h) and IL-6, CCL2, and CXCL10 (at 6 h and 28 days); upregulated TNF-α; decreased morphological deficits such as necrotic and apoptotic cells in neocortex and hippocampus (at 28 days); increased cortical ATP (at 6 h and 28 days) |
| Dong et al. 2015 [112] | TBI (mouse) |
Laser, Acculaser, PhotoThera (Carlsbad, CA, USA) | 810 nm | 150 mW/cm2, 36 J/cm2, 4 min, PW at 10-Hz (50 msec pulse duration) | Transcranially; Holding probe in direct contact with scalp at contusion site |
Prevented the loss of hippocampal tissues; protected the hippocampus from secondary damage; increased ATP and deceased ROS production in injured cortex (at 5 h post-TBI); decreased lesion size (at 3 and 7 days) |
| Xuan et al. 2015 [26] | TBI (mouse) |
Laser, PhotoThera, Inc. (Carlsbad, CA, USA) | 810 nm | 50 mW/cm2, 36 J/cm2 at scalp, spot diameter of 1 cm, 12 min, for 1 or 3 days, CW | Transcranially | At 1×day: improved neurological severity score (at 21 and 28 days post-TBI); increased expression of BDNF in Dentate Gyrus and SVZ By 3×day: improved neurological severity score (at 14, 21 and 28 days); increased expression of BDNF in Dentate Gyrus and SVZ area, and synapsin-1 in SVZ and lesion area (at 28 days) |
| Xuan et al. 2016 [28] | TBI (mouse) |
Laser, DioDent Micro 810, HOYA ConBio (Fremont, CA, USA) | 810 nm | 25 mW/cm2, 18 J/cm2, spot diameter of 1 cm, 12 min, for 3 or 14 days, CW | Transcranially; Covered the entire skull |
By 3×day: improved neurological severity score; improved cognitive performance in MWM test; decreased lesion size (at 2–8 weeks post-TBI); increased expression of GFAP in perilesional cortex, Dentate Gyrus and SVZ (at 8 weeks) By 14×day: decreased lesion size (at 8 weeks); increased expression of GFAP in perilesional cortex, Dentate Gyrus and SVZ (at 8 weeks) |
| Wu et al. 2012 [227] | Depression (rat chronic stress model) |
Laser, PhotoThera, Inc. (Carlsbad, CA, USA) | 810 nm | 350 mW, 120 J/cm2, probe diameter of 3 mm, 2 min, 3×/week for 3 weeks, PW at 100-Hz with DC of 20% | Transcranially; At the midline of the dorsal surface of the shaved head in region between eyes and ears |
decreased depressive-like behaviors in FST |
| Mohammed 2016 [253] | Depression (rat pharmacological model) | Laser, Lasotronic Inc. (Zug, Switzerland) | 804 nm | 640 mW/cm2, 38 J/cm2 per point, spot diameter of 4 mm, 1 min per point, for 7 consecutive days, CW | Transcranially; At six points arranged symmetrically, three on each side of the skull |
Decreased depressive-like behaviors in FST; regulated EEG in all wave frequency bands such as Delta, Alpha, Beta-1, and Beta-2 except for theta wave |
| Salehpour et al. 2016 [85] | Depression (rat chronic stress model) |
Laser, Mustang 2000+ (Moscow, Russia) | 630 or 810 nm | 89 or 562 mW/cm2; 6 or 10.7 J/cm2; probe diameter of 3 mm, 4×/week for 3 weeks, PW at 10-Hz with DC of 50% | Transcranially; At the midline of the dorsal surface of the shaved scalp in prefrontal region |
810 nm: decreased depressive-like behaviors in FST; increased body weight; decreased blood glucose levels 630 nm: decreased serum cortisol and blood glucose levels |
| Xu et al. 2016 [29] | Depression (mouse space restriction or Ahi1 KO model) |
Laser, quartz-silica fiber, Shenzhen Fuzhe Technology Co., Ltd. (Shenzhen, China) | 808 nm | 23 mW/cm2, 41.4 J/cm2, spot diameter of 1 cm, 30 min/day for 28 consecutive days, CW | Transcranially | Stress mouse: decreased depressive-like behaviors in FST and TST (at days 14, 21, and 28); increased ATP synthesis in PFC, increased CCO levels and activity in PFC Ahi1 KO mouse: decreased depressive-like behaviors in FST and TST (at day 28) |
| Salehpour et al. 2017 [13] | Aging (mouse D-galactose model) | Laser, Thor Photomedicine (Chesham, UK) | 660 or 810 nm | 200 mW, 4.75 W/cm2, 4 or 8 J/cm2, 3×/week for 6 weeks, PW at 10-Hz with DC of 88% | Transcranially; Covered the entire brain |
660 and 810 nm: improved spatial memory in Barnes maze test and episodic-like memory in What-Where-Which test (at 8 J/cm2); increased number of vital mitochondria, ATP content, MMP and CCO activity (at 8 J/cm2); decreased ROS levels (at 4 and 8 J/cm2); attenuated apoptosis via decrease of Bax/Bcl-2 and caspase-3 levels (at 8 J/cm2) |
Note: 4-HNE, 4-Hydroxynonenal; PS1, presenilin-1; 6-OHDA, 6-hydroxydopamine; 8-OHDG, 8-hydroxydeoxyguanosine; Aβ, amyloid beta; AChE, acetylcholinesterase; AD, Alzheimer’s disease; Akt, Protein kinase B; APP, amyloid precursor protein; ATP, adenosine triphosphate; β-nucleoside triphosphate; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; BDNF, brain-derived neurotrophic; CBF, cerebral blood flow; CCL2, C-C motif chemokine Ligand 2; CCO, cytochrome c oxidase; CD, cluster of differentiation; COX-2, cyclooxygenase 2; CREB, cAMP responsive element binding; CSF, cerebrospinal fluid; CW, continuous wave; CXCL10, C-X-C motif chemokine 10; DC, duty cycle; EEG, electroencephalography; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinases; FST, forced swimming test; G6PDH, glucose-6-phosphate dehydrogenase; GaAs, gallium arsenide; GaAlAs, gallium aluminum arsenide; GDNF, glial-cell-line-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; GSH, glutathione; HSP, heat shock protein; Iba-1, ionized calcium binding adaptor molecule 1; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, Jun kinases; KO, knockout; LEDs, light emitting diodes; MAPK, mitogen-activated protein kinases; MMP, mitochondrial membrane potential; MPO, myeloperoxidase; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MWM, Morris water maze; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen; NF-κB, nuclear factor kappa B; NFTs, neurofibrillary tangles; NLRP3, pyrin domain-containing 3; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NOR, novel object recognition; NOS, nitric oxide synthase; NR, not reported; NSPCs, neural stem/precursor cells; OFT, open field test; pBAD, phosphorylated Bcl-2-associated death promoter; PCr, phosphocreatine; β-NTP; PD, Parkinson’s disease; PFC, prefrontal cortex; P-HSP, phosphorylated heat shock protein; PW, pulsed wave; ROS, reactive oxygen species; SNc, substantia nigra pars compacta; SOD, superoxide dismutase; SVZ, subventricular zone; TBI, traumatic brain injury; TGF-β, Transforming growth factor–β; TH, tyrosine hydroxylase; TLR-2, Toll-like receptor 2; TNF-α, tumor necrosis factor-α; TST, tail suspension test; WGT, wire-grip test
Acknowledgments
Michael R Hamblin was supported by US NIH grants R01AI050875 and R21AI121700, Air Force Office of Scientific Research grant FA9550-13-1-0068, US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Opt. 2016;19(1):013003. doi: 10.1088/2040-8986/19/1/013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med. 2011;43(8):851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomed Laser Ther. 2006;24(4):458–466. doi: 10.1089/pho.2006.24.458. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y-Y, Chen AC-H, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose-Response. 2009;7(4):9–27. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy TJ, De Taboada L, Hildebrandt PK, Ziemer EL, Richieri SP, Streeter J. Long-term safety of single and multiple infrared transcranial laser treatments in Sprague–Dawley rats. Photomed Laser Surg. 2010;28(5):663–667. doi: 10.1089/pho.2009.2581. [DOI] [PubMed] [Google Scholar]
- 6.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35(8):1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 7.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang Y-Y, Wu Q, Whalen MJ, Sato S. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6(10):e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yip K, Lo S, Leung M, So K, Tang C, Poon D. The effect of low-energy laser irradiation on apoptotic factors following experimentally induced transient cerebral ischemia. Neuroscience. 2011;190:301–306. doi: 10.1016/j.neuroscience.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 9.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 10.Oueslati A, Lovisa B, Perrin J, Wagnières G, van den Bergh H, Tardy Y, Lashuel HA. Photobiomodulation suppresses alpha-synuclein-induced toxicity in an AAV-based rat genetic model of Parkinson’s disease. PloS One. 2015;10(10):e0140880. doi: 10.1371/journal.pone.0140880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5(1):46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salehpour F, Rasta SH. The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder. Rev Neurosci. 2017;28(4):441–453. doi: 10.1515/revneuro-2016-0087. [DOI] [PubMed] [Google Scholar]
- 13.Salehpour F, Ahmadian N, Rasta SH, Farhoudi M, Karimi P, Sadigh-Eteghad S. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose–induced aging mice. Neurobiol Aging. 2017;58:140–150. doi: 10.1016/j.neurobiolaging.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Michalikova S, Ennaceur A, van Rensburg R, Chazot P. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol Learn Mem. 2008;89(4):480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Santana-Blank L, Rodríguez-Santana E, Santana-Rodriguez KE, Reyes H. “Quantum Leap” in Photobiomodulation Therapy Ushers in a New Generation of Light-Based Treatments for Cancer and Other Complex Diseases: Perspective and Mini-Review. Photomed Laser Surg. 2016;34(3):93–101. doi: 10.1089/pho.2015.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, De Taboada L, O’Connor M, Delapp S, Zivin JA. Thermal effects of transcranial near-infrared laser irradiation on rabbit cortex. Neurosci Lett. 2013;553:99–103. doi: 10.1016/j.neulet.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis. 2012;32(3):741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- 18.Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. 2016;48(4):343–349. doi: 10.1002/lsm.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg. 2012;30(4):231–233. doi: 10.1089/pho.2011.3044. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC-Y, Cohen RM, Zhang Q. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex–evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6(1):2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirk BJ, Torbey M, Buchmann E, Verma S, Whelan HT. Near-infrared photobiomodulation in an animal model of traumatic brain injury: improvements at the behavioral and biochemical levels. Photomed Laser Surg. 2012;30(9):523–529. doi: 10.1089/pho.2012.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang H, Whelan H, Eells J, Meng H, Buchmann E, Lerch-Gaggl A, Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139(2):639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 24.Lee HI, Park JH, Park MY, Kim NG, Park K-J, Choi BT, Shin Y-I, Shin HK. Pre-conditioning with transcranial low-level light therapy reduces neuroinflammation and protects blood-brain barrier after focal cerebral ischemia in mice. Restor Neurol Neurosci. 2016;34(2):201–214. doi: 10.3233/RNN-150559. [DOI] [PubMed] [Google Scholar]
- 25.Moreira MS, Velasco IT, Ferreira LS, Ariga SKK, Barbeiro DF, Meneguzzo DT, Abatepaulo F, Marques MM. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B, Biol. 2009;97(3):145–151. doi: 10.1016/j.jphotobiol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics. 2015;8(6):502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan X, Liu J, Zhang Z, Li W, Sun S, Zhao J, Dong X, Qian J, Sun H. Low-level laser irradiation modulates brain-derived neurotrophic factor mRNA transcription through calcium-dependent activation of the ERK/CREB pathway. Lasers Med Sci. 2017;32(1):169–180. doi: 10.1007/s10103-016-2099-0. [DOI] [PubMed] [Google Scholar]
- 28.Xuan W, Huang L, Hamblin MR. Repeated transcranial low-level laser therapy for traumatic brain injury in mice: biphasic dose response and long-term treatment outcome. J Biophotonics. 2016;9(11–12):1263–1272. doi: 10.1002/jbio.201500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, Zhang G, Yang L, Li J, Du X, Zhang Q, Xu X. Low-Level Laser Irradiation Improves Depression-Like Behaviors in Mice. Mol Neurobiol. 2016;54(6):4551–4559. doi: 10.1007/s12035-016-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP, III, Baker EH. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31(11):1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamblin MR, Demidova TN. Biomed Opt. 61001. Vol. 6140. International Society for Optics and Photonics; 2006. Mechanisms of low level light therapy; pp. 1–12. [Google Scholar]
- 32.Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2013;6(10):829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2011;29(5):351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karu T. Molecular mechanism of the therapeutic effect of low-intensity laser radiation. Lasers Life Sci. 1988;2(1):53–74. [Google Scholar]
- 35.Hill BC. Modeling the sequence of electron transfer reactions in the single turnover of reduced, mammalian cytochrome c oxidase with oxygen. J Biol Chem. 1994;269(4):2419–2425. [PubMed] [Google Scholar]
- 36.Karu T, Kolyakov S. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Ther. 2005;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 37.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. J Photochem Photobiol B, Biol. 2011;102(3):182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Santana-Blank L, Rodríguez-Santana E, Santana-Rodríguez K. Theoretic, experimental, clinical bases of the water oscillator hypothesis in near-infrared photobiomodulation. Photomed Laser Surg. 2010;28(S1):41–52. doi: 10.1089/pho.2009.2647. [DOI] [PubMed] [Google Scholar]
- 39.Hamblin MR. Biomedical Optics (BiOS) Vol. 6846. International Society for Optics and Photonics; 2008. The role of nitric oxide in low level light therapy; pp. 684602–1. [Google Scholar]
- 40.Karu TI. EOS/SPIE European Biomedical Optics Week. International Society for Optics and Photonics; 2000. Mechanisms of low-power laser light action on cellular level; pp. 1–17. [Google Scholar]
- 41.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):348–364. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16(1):4. doi: 10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Huang Y-Y, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Sub. 2017;1861(2):441–449. doi: 10.1016/j.bbagen.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett D, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. 2015;11(1):14–25. doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang J, Castelli DM, Gonzalez-Lima F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci. 2016;31(6):1151–1160. doi: 10.1007/s10103-016-1962-3. [DOI] [PubMed] [Google Scholar]
- 47.Duggett NA, Chazot PL. Low-Intensity Light Therapy (1068 nm) Protects CAD Neuroblastoma Cells from [Beta]-Amyloid-Mediated Cell Death. Biol Med. 2014;6(3):1. [Google Scholar]
- 48.Dougal G, Lee S. Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin Exp Dermatol. 2013;38(7):713–718. doi: 10.1111/ced.12069. [DOI] [PubMed] [Google Scholar]
- 49.Grillo S, Duggett N, Ennaceur A, Chazot P. Non-invasive infra-red therapy (1072nm) reduces β-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J Photochem Photobiol B, Biol. 2013;123:13–22. doi: 10.1016/j.jphotobiol.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Tian F, Reddy DD, Nalawade SS, Barrett DW, Gonzalez-Lima F, Liu H. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab. 2017;37(12):3789–3802. doi: 10.1177/0271678X17691783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep. 2016;6:30540. doi: 10.1038/srep30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford A, Barlow A, Chazot PL. Probing the differential effects of infrared light sources IR1072 and IR880 on human lymphocytes: evidence of selective cytoprotection by IR1072. J Photochem Photobiol B, Biol. 2005;81(1):9–14. doi: 10.1016/j.jphotobiol.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Tsai S-R, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B, Biol. 2017;170:197–207. doi: 10.1016/j.jphotobiol.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol. 2013;86(4):447–457. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Lapchak PA. Taking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational science. Ann Med. 2010;42(8):576–586. doi: 10.3109/07853890.2010.532811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Lai P, Ma C, Xu X, Grabar AA, Wang LV. Optical focusing deep inside dynamic scattering media with near-infrared time-reversed ultrasonically encoded (TRUE) light. Nat Commun. 2015;6:5904. doi: 10.1038/ncomms6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sagar V, Atluri V, Tomitaka A, Shah P, Nagasetti A, Pilakka-Kanthikeel S, El-Hage N, McGoron A, Takemura Y, Nair M. Coupling of transient near infrared photonic with magnetic nanoparticle for potential dissipation-free biomedical application in brain. Sci Rep. 2016;6:29792. doi: 10.1038/srep29792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Zhang Y, Takle K, Bilsel O, Li Z, Lee H, Zhang Z, Li D, Fan W, Duan C. Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS nano. 2016;10(1):1060–1066. doi: 10.1021/acsnano.5b06383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yue L, Humayun MS. Monte Carlo analysis of the enhanced transcranial penetration using distributed near-infrared emitter array. J Biomed Opt. 2015;20(8):088001. doi: 10.1117/1.JBO.20.8.088001. [DOI] [PubMed] [Google Scholar]
- 60.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain. Neuropsychiatr Dis Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159–2175. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 63.DiMauro TM, Attawia M, Holy C, Lilienfeld S, Sutton JK, Ward M. Red light implant for treating Parkinson’s disease. Google Patents 2007 [Google Scholar]
- 64.Darlot F, Moro C, Massri N, Chabrol C, Johnstone DM, Reinhart F, Agay D, Torres N, Bekha D, Auboiroux V. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann Neurol. 2016;79(1):59–75. doi: 10.1002/ana.24542. [DOI] [PubMed] [Google Scholar]
- 65.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pitzschke A, Lovisa B, Seydoux O, Zellweger M, Pfleiderer M, Tardy Y, Wagnières G. Red and NIR light dosimetry in the human deep brain. Phys Med Biol. 2015;60(7):2921. doi: 10.1088/0031-9155/60/7/2921. [DOI] [PubMed] [Google Scholar]
- 67.Johnstone D, El Massri N, Moro C, Spana S, Wang X, Torres N, Chabrol C, De Jaeger X, Reinhart F, Purushothuman S. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism–An abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 68.Moro C, Massri NE, Torres N, Ratel D, De Jaeger X, Chabrol C, Perraut F, Bourgerette A, Berger M, Purushothuman S. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice: Laboratory investigation. J Neurosurg. 2014;120(3):670–683. doi: 10.3171/2013.9.JNS13423. [DOI] [PubMed] [Google Scholar]
- 69.Johnstone D, Coleman K, Moro C, Torres N, Eells J, Baker G, Ashkan K, Stone J, Benabid A, Mitrofanis J. The potential of light therapy in Parkinson’s disease. Chrono Physiol Ther. 2014;4:1–14. [Google Scholar]
- 70.Lapchak PA. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert Rev Med Devices. 2012;9(1):71–83. doi: 10.1586/erd.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed Laser Surg. 2017;35(8):432–441. doi: 10.1089/pho.2016.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burchman MA. Using photobiomodulation on a severe Parkinson’s patient to enable extractions, root canal treatment, and partial denture fabrication. J Laser Dent. 2011;19:297–300. [Google Scholar]
- 73.Zhao G, Guo K, Dan J. case analysis of Parkinson’s disease treated by endonasal low energy He-Ne laser. Acta Acad Med Qingdao Univ. 39:398. [Google Scholar]
- 74.Lim L. The Potential of intranasal light therapy for brain stimulation. Presented, NAALTA Conference; Palm Beach Gardens, Florida. 2013. [Google Scholar]
- 75.Sun L, Peräkylä J, Kovalainen A, Ogawa KH, Karhunen PJ, Hartikainen KM. Human brain reacts to transcranial extraocular light. PLoS ONE. 2016;11(2):e0149525. doi: 10.1371/journal.pone.0149525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timonen M, Nissilä J, Liettu A, Jokelainen J, Jurvelin H, Aunio A, Räsänen P, Takala T. Can transcranial brain-targeted bright light treatment via ear canals be effective in relieving symptoms in seasonal affective disorder?–A pilot study. Med Hypotheses. 2012;78(4):511–515. doi: 10.1016/j.mehy.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 77.Jurvelin H, Takala T, Nissilä J, Timonen M, Rüger M, Jokelainen J, Räsänen P. Transcranial bright light treatment via the ear canals in seasonal affective disorder: a randomized, double-blind dose-response study. BMC psychiatry. 2014;14(1):288. doi: 10.1186/s12888-014-0288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamblin MR. Shining light on the head: photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54(4):2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haeussinger FB, Heinzel S, Hahn T, Schecklmann M, Ehlis A-C, Fallgatter AJ. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PloS One. 2011;6(10):e26377. doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strangman GE, Zhang Q, Li Z. Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. Neuroimage. 2014;85:136–149. doi: 10.1016/j.neuroimage.2013.04.090. [DOI] [PubMed] [Google Scholar]
- 83.White D, Widdowson E, Woodard H, Dickerson J. The composition of body tissues. (II) Fetus to young adult. Br J Radiol. 1991;64(758):149–159. doi: 10.1259/0007-1285-64-758-149. [DOI] [PubMed] [Google Scholar]
- 84.Firbank M, Hiraoka M, Essenpreis M, Delpy D. Measurement of the optical properties of the skull in the wavelength range 650–950 nm. Phys Med Biol. 1993;38(4):503. doi: 10.1088/0031-9155/38/4/002. [DOI] [PubMed] [Google Scholar]
- 85.Salehpour F, Rasta SH, Mohaddes G, Sadigh-Eteghad S, Salarirad S. Therapeutic effects of 10-HzPulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg Med. 2016;48(7):695–705. doi: 10.1002/lsm.22542. [DOI] [PubMed] [Google Scholar]
- 86.Lapchak PA, Boitano PD, Butte PV, Fisher DJ, Hölscher T, Ley EJ, Nuño M, Voie AH, Rajput PS. Transcranial near-infrared laser transmission (NILT) profiles (800 nm): systematic comparison in four common research species. PloS One. 2015;10(6):e0127580. doi: 10.1371/journal.pone.0127580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One. 2012;7(10):e47460. doi: 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lapchak PA, Boitano PD. Transcranial near-infrared laser therapy for stroke: how to recover from futility in the NEST-3 clinical trial. Acta Neurochir Suppl. 2016;121:7–12. doi: 10.1007/978-3-319-18497-5_2. [DOI] [PubMed] [Google Scholar]
- 89.Aulakh K, Zakaib S, Willmore WG, Winnie NY. SPIE BiOS. Vol. 9706. International Society for Optics and Photonics; 2016. Transcranial light-tissue interaction analysis; pp. 97061–97065. [Google Scholar]
- 90.Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40(4):1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 91.Yaroslavsky A, Schulze P, Yaroslavsky I, Schober R, Ulrich F, Schwarzmaier H. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol. 2002;47(12):2059. doi: 10.1088/0031-9155/47/12/305. [DOI] [PubMed] [Google Scholar]
- 92.Hart NS, Fitzgerald M. A New Perspective on Delivery of Red-Near-Infrared Light Therapy for Disorders of the Brain. Discov Med. 2016;22(120):147–156. [PubMed] [Google Scholar]
- 93.Karu TI. Cellular mechanism of low power laser therapy: new questions. Lasers Med Dent. 2003;3:79–100. [Google Scholar]
- 94.Hode T, Duncan D, Kirkpatrick S, Jenkins P, Hode L. SPIE BiOS Biomedical Optics. Vol. 7165. International Society for Optics and Photonics; 2009. The importance of coherence in phototherapy; p. 716507. [Google Scholar]
- 95.Litscher D, Litscher G. Laser therapy and dementia: A database analysis and future aspects on LED-based systems. Int J Photoenergy. 2014 doi: 10.1155/2014/268354. [DOI]
- 96.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, Ando T, Xu T, Huang Y-Y, Hamblin MR. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One. 2013;8(1):e53454. doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg Med. 2012;44(3):218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34(6):1021. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- 100.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B, Biol. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 102.Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5(6):a011304. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 104.Wong-Riley MT, Bai X, Buchmann E, Whelan HT. Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport. 2001;12(14):3033–3037. doi: 10.1097/00001756-200110080-00011. [DOI] [PubMed] [Google Scholar]
- 105.Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci. 2008;28(50):13511–13521. doi: 10.1523/JNEUROSCI.3457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ying R, Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone-and MPP+-induced neurotoxicity. Brain Res. 2008;1243:167–173. doi: 10.1016/j.brainres.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sommer AP, Bieschke J, Friedrich RP, Zhu D, Wanker EE, Fecht HJ, Mereles D, Hunstein W. 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: basis for treatment of alzheimer’s disease? Photomed Laser Surg. 2012;30(1):54–60. doi: 10.1089/pho.2011.3073. [DOI] [PubMed] [Google Scholar]
- 108.Trimmer PA, Schwartz KM, Borland MK, De Taboada L, Streeter J, Oron U. Reduced axonal transport in Parkinson’s disease cybrid neurites is restored by light therapy. Mol Neurodegener. 2009;4(1):26. doi: 10.1186/1750-1326-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infrared laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett. 2002;323(3):207–210. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 110.Lapchak PA, Boitano PD. A novel method to promote behavioral improvement and enhance mitochondrial function following an embolic stroke. Brain Res. 2016;1646:125–131. doi: 10.1016/j.brainres.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 111.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 112.Dong T, Zhang Q, Hamblin MR, Wu MX. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab. 2015;35(9):1435–1444. doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007;25(3):180–182. doi: 10.1089/pho.2007.2064. [DOI] [PubMed] [Google Scholar]
- 114.Ferraresi C, Kaippert B, Avci P, Huang YY, Sousa MV, Bagnato VS, Parizotto NA, Hamblin MR. Low-level Laser (Light) Therapy Increases Mitochondrial Membrane Potential and ATP Synthesis in C2C12 Myotubes with a Peak Response at 3–6 h. Photochem Photobiol. 2015;91(2):411–416. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mintzopoulos D, Gillis TE, Tedford CE, Kaufman MJ. Effects of Near-Infrared Light on Cerebral Bioenergetics Measured with Phosphorus Magnetic Resonance Spectroscopy. Photomed Laser Surg. 2017;35(8):395–400. doi: 10.1089/pho.2016.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodell AB, O’Keefe G, Rowe CC, Villemagne VL, Gjedde A. Cerebral blood flow and Aβ-amyloid estimates by WARM analysis of [11C] PiB uptake distinguish among and between neurodegenerative disorders and aging. Front Aging Neurosci. 2016;8:321. doi: 10.3389/fnagi.2016.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borghammer P, Cumming P, Østergaard K, Gjedde A, Rodell A, Bailey CJ, Vafaee MS. Cerebral oxygen metabolism in patients with early Parkinson’s disease. J Neurol Sci. 2012;313(1):123–128. doi: 10.1016/j.jns.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 118.Nagafusa Y, Okamoto N, Sakamoto K, Yamashita F, Kawaguchi A, Higuchi T, Matsuda H. Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. J Affect Disord. 2012;140(3):296–299. doi: 10.1016/j.jad.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 119.Sadigh-Eteghad S, Mahmoudi J, Babri S, Talebi M. Effect of alpha-7 nicotinic acetylcholine receptor activation on beta-amyloid induced recognition memory impairment. Possible role of neurovascular function. Acta Cir Bras. 2015;30(11):736–742. doi: 10.1590/S0102-865020150110000003. [DOI] [PubMed] [Google Scholar]
- 120.Litscher G, Min L, Passegger CA, Litscher D, Li M, Wang M, Ghaffari-Tabrizi-Wizsy N, Stelzer I, Feigl G, Gaischek I. Transcranial Yellow, Red, and Infrared Laser and LED Stimulation: Changes of Vascular Parameters in a Chick Embryo Model. Integr Med Int. 2015;2(1–2):80–89. [Google Scholar]
- 121.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010;42(6):566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 122.Lee HI, Lee S-W, Kim SY, Kim NG, Park K-J, Choi BT, Shin Y-I, Shin HK. Pretreatment with light-emitting diode therapy reduces ischemic brain injury in mice through endothelial nitric oxide synthase-dependent mechanisms. Biochem Biophys Res Commun. 2017;486(4):945–950. doi: 10.1016/j.bbrc.2017.03.131. [DOI] [PubMed] [Google Scholar]
- 123.Salgado AS, Zângaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. J Lasers Med Sci. 2015;30(1):339–346. doi: 10.1007/s10103-014-1669-2. [DOI] [PubMed] [Google Scholar]
- 124.Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 125.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013 doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed]
- 126.Rodriguez-Rodriguez A, Jose Egea-Guerrero J, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21(10):1201–1211. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 127.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Chen AC-H, Huang Y-Y, Arany PR, Hamblin MR. SPIE BiOS: Biomedical Optics. International Society for Optics and Photonics; 2009. Role of reactive oxygen species in low level light therapy; pp. 716502–716511. [Google Scholar]
- 130.Chen AC, Arany PR, Huang Y-Y, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PloS one. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tafur J, Mills PJ. Low-intensity light therapy: exploring the role of redox mechanisms. Photomedicine and laser surgery. 2008;26(4):323–328. doi: 10.1089/pho.2007.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pal G, Dutta A, Mitra K, Grace MS, Amat A, Romanczyk TB, Wu X, Chakrabarti K, Anders J, Gorman E. Effect of low intensity laser interaction with human skin fibroblast cells using fiber-optic nano-probes. Journal of Photochemistry and Photobiology B: Biology. 2007;86(3):252–261. doi: 10.1016/j.jphotobiol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 133.Wang F, Xing D, Chen T-S. Fourth International Conference on Photonics and Imaging in Biology and Medicine. Vol. 6047. International Society for Optics and Photonics; 2006. High fluence laser irradiation induces reactive oxygen species generation in human lung adenocarcinoma cells; pp. 60473–60476. [Google Scholar]
- 134.Yang X, Askarova S, Sheng W, Chen J, Sun AY, Sun GY, Yao G, Lee J-M. Low energy laser light (632.8 nm) suppresses amyloid-β peptide-induced oxidative and inflammatory responses in astrocytes. Neuroscience. 2010;171(3):859–868. doi: 10.1016/j.neuroscience.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone-and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153(4):963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu Z, Li Z, Liu N, Jizhang Y, McCarthy TJ, Tedford CE, Lo EH, Wang X. Near infrared radiation protects against oxygen-glucose deprivation-induced neurotoxicity by down-regulating neuronal nitric oxide synthase (nNOS) activity in vitro. Metab Brain Dis. 2015;30(3):829–837. doi: 10.1007/s11011-015-9663-3. [DOI] [PubMed] [Google Scholar]
- 137.Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med. 2002;31(4):283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 138.Sutalangka C, Wattanathorn J, Muchimapura S, Thukham-mee W, Wannanon P, Tong-un T. Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J Acupunct Meridian Stud. 2013;6(5):247–251. doi: 10.1016/j.jams.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 139.Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M. Astrocytic and microglial nicotinic acetylcholine receptors: An overlooked issue in Alzheimer’s disease. J Neural Transm. 2016;123(12):1359–1367. doi: 10.1007/s00702-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 140.Lim W, Kim J, Kim S, Karna S, Won J, Jeon SM, Kim SY, Choi Y, Choi H, Kim O. Modulation of Lipopolysaccharide-Induced NF-κB Signaling Pathway by 635 nm Irradiation via Heat Shock Protein 27 in Human Gingival Fibroblast Cells. Photochem Photobiol. 2013;89(1):199–207. doi: 10.1111/j.1751-1097.2012.01225.x. [DOI] [PubMed] [Google Scholar]
- 141.Meredith GE, Sonsalla PK, Chesselet M-F. Animal models of Parkinson’s disease progression. Acta Neuropathol. 2008;115(4):385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 143.Chen AC-H, Huang Y-Y, Sharma SK, Hamblin MR. Effects of 810-nm laser on murine bone-marrow-derived dendritic cells. Photomed Laser Surg. 2011;29(6):383–389. doi: 10.1089/pho.2010.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choi D-H, Lim JH, Lee K-H, Kim MY, Kim HY, Shin CY, Han S-H, Lee J. Effect of 710-nm visible light irradiation on neuroprotection and immune function after stroke. Neuroimmunomodulation. 2012;19(5):267–276. doi: 10.1159/000335547. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Q, Zhou C, Hamblin MR, Wu MX. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J Cereb Blood Flow Metab. 2014;34(8):1391–1401. doi: 10.1038/jcbfm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee HI, Lee SW, Kim NG, Park KJ, Choi BT, Shin YI, Shin HK. Low-level light emitting diode (LED) therapy suppresses inflammasome-mediated brain damage in experimental ischemic stroke. J Biophotonics. 2017;10(11):1502–1513. doi: 10.1002/jbio.201600244. [DOI] [PubMed] [Google Scholar]
- 148.Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S. Intranasal Cerebrolysin Attenuates Learning and Memory Impairments in D-galactose-Induced Senescence in Mice. Exp Gerontol. 2017;87:16–22. doi: 10.1016/j.exger.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 149.Obulesu M, Lakshmi MJ. Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res. 2014;39(12):2301–2312. doi: 10.1007/s11064-014-1454-4. [DOI] [PubMed] [Google Scholar]
- 150.Da Costa CA, Checler F. Apoptosis in Parkinson’s disease: is p53 the missing link between genetic and sporadic Parkinsonism? Cell Signal. 2011;23(6):963–968. doi: 10.1016/j.cellsig.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 151.Desagher S, Martinou J-C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 152.Gronbeck KR, Rodrigues CM, Mahmoudi J, Bershad EM, Ling G, Bachour SP, Divani AA. Application of Tauroursodeoxycholic Acid for Treatment of Neurological and Non-neurological Diseases: Is There a Potential for Treating Traumatic Brain Injury? Neurocrit Care. 2016;25(1):153–166. doi: 10.1007/s12028-015-0225-7. [DOI] [PubMed] [Google Scholar]
- 153.Majdi A, Mahmoudi J, Sadigh-Eteghad S, Golzari SE, Sabermarouf B, Reyhani-Rad S. Permissive role of cytosolic pH acidification in neurodegeneration: A closer look at its causes and consequences. J Neurosci Res. 2016;94(10):879–887. doi: 10.1002/jnr.23757. [DOI] [PubMed] [Google Scholar]
- 154.Qian Y-f, Wang H, Yao W-b, Gao X-d. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Cell Biol Int. 2008;32(2):304–311. doi: 10.1016/j.cellbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 155.Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115(7):1461–1469. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- 156.Duan R, Zhu L, Liu TCY, Li Y, Liu J, Jiao J, Xu X, Yao L, Liu S. Light emitting diode irradiation protect against the amyloid beta 25–35 induced apoptosis of PC12 cell in vitro. Lasers Surg Med. 2003;33(3):199–203. doi: 10.1002/lsm.10216. [DOI] [PubMed] [Google Scholar]
- 157.Wong-Riley M, Whelan H, Dhokalia A, Das R, Hammamieh R, Liang H, Eells J, Jett M. cDNA microarray analysis of the visual cortex exposed to light-emitting diode treatment in monocularly enucleated rats. Soc Neurosci Abstr. 131:20. [Google Scholar]
- 158.Ghanbari A, Ghareghani M, Zibara K, Delaviz H, Ebadi E, Jahantab M. Light-Emitting Diode (LED) therapy improves occipital cortex damage by decreasing apoptosis and increasing BDNF-expressing cells in methanol-induced toxicity in rats. Biomed Pharmacother. 2017;89:1320. doi: 10.1016/j.biopha.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 159.Gavish L, Asher Y, Becker Y, Kleinman Y. Low level laser irradiation stimulates mitochondrial membrane potential and disperses subnuclear promyelocytic leukemia protein. Lasers Surg Med. 2004;35(5):369–376. doi: 10.1002/lsm.20108. [DOI] [PubMed] [Google Scholar]
- 160.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72(1):12–19. [PubMed] [Google Scholar]
- 161.Weinreb O, Bar-Am O, Amit T, Chillag-Talmor O, Youdim MB. Neuroprotection via pro-survival protein kinase C isoforms associated with Bcl-2 family members. FASEB J. 2004;18(12):1471–1473. doi: 10.1096/fj.04-1916fje. [DOI] [PubMed] [Google Scholar]
- 162.Zhang L, Xing D, Zhu D, Chen Q. Low-power laser irradiation inhibiting Aβ25–35-induced PC12 cell apoptosis via PKC activation. Cell Physiol Biochem. 2008;22(1–4):215–222. doi: 10.1159/000149799. [DOI] [PubMed] [Google Scholar]
- 163.Zhang L, Zhang Y, Xing D. LPLI inhibits apoptosis upstream of Bax translocation via a GSK-3β-inactivation mechanism. J Cell Physiol. 2010;224(1):218–228. doi: 10.1002/jcp.22123. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H, Wu S, Xing D. Inhibition of Aβ 25–35-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012;24(1):224–232. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 165.Liang J, Liu L, Xing D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radic Biol Med. 2012;53(7):1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 166.Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt. 2014;19(10):108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Telerman A, Lapter S, Sharabi A, Zinger H, Mozes E. Induction of hippocampal neurogenesis by a tolerogenic peptide that ameliorates lupus manifestations. J Neuroimmunol. 2011;232(1):151–157. doi: 10.1016/j.jneuroim.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J Neurosci. 2013;33(33):13505–13517. doi: 10.1523/JNEUROSCI.0918-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.El Massri N, Lemgruber AP, Rowe IJ, Moro C, Torres N, Reinhart F, Chabrol C, Benabid A-L, Mitrofanis J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp Brain Res. 2017;235(6):1861–1874. doi: 10.1007/s00221-017-4937-0. [DOI] [PubMed] [Google Scholar]
- 170.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 171.Yun Y-C, Jang D, Yoon S-B, Kim D, Choi D-H, Kwon O, Lee Y-M, Youn D. Laser Acupuncture Exerts Neuroprotective Effects via Regulation of Creb, Bdnf, Bcl-2, and Bax Gene Expressions in the Hippocampus. Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/7181637. [DOI] [PMC free article] [PubMed]
- 172.Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- 173.Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31(9):1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naeser MA, Martin PI, Ho MD, Krengel MH, Bogdanova Y, Knight JA, Yee MK, Zafonte R, Frazier J, Hamblin MR. Transcranial, Red/Near-Infrared Light-Emitting Diode Therapy to Improve Cognition in Chronic Traumatic Brain Injury. Photomed Laser Surg. 2016;34(12):610–626. doi: 10.1089/pho.2015.4037. [DOI] [PubMed] [Google Scholar]
- 175.Sporns O. Structure and function of complex brain networks. Dialogues in Clinical Neuroscience. 2013;15(3):247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park H-J, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 177.Xiao H, Yang Y, Xi J-h, Chen Z-q. Structural and functional connectivity in traumatic brain injury. Neural Regeneration Research. 2015;10(12):2062–2071. doi: 10.4103/1673-5374.172328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kringelbach ML, Green AL, Aziz TZ. Balancing the brain: resting state networks and deep brain stimulation. Front Integr Neurosci. 2011;5:8. doi: 10.3389/fnint.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de la Plata CDM, Garces J, Kojori ES, Grinnan J, Krishnan K, Pidikiti R, Spence J, Devous MD, Moore C, McColl R. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol. 2011;68(1):74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012;59(1):511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naeser M, Ho M, Martin P, Treglia E, Krengel M, Hamblin M, Baker E. Improved language after scalp application of red/near-infrared light-emitting diodes: pilot study supporting a new, noninvasive treatment for chronic aphasia. Procedia Soc Behav Sci. 2012;61:138–139. [Google Scholar]
- 182.Johnstone DM, Mitrofanis J, Stone J. Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light. Neural Regen Res. 2015;10(3):349. doi: 10.4103/1673-5374.153673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Petrie SR, Hamblin MR, Ionescu DF, Cusin C, Yeung A, Cassano P. Photobiomodulation in Patients with Low Back Pain: A Case Control Series for the Effect on Depression. Qual Prim Care. 2016;24(1):33–38. [Google Scholar]
- 184.Zalewska-Kaszubska J, Obzejta D. Use of low-energy laser as adjunct treatment of alcohol addiction. Lasers Med Sci. 2004;19(2):100–104. doi: 10.1007/s10103-004-0307-9. [DOI] [PubMed] [Google Scholar]
- 185.Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36(3):171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 186.Muili KA, Gopalakrishnan S, Meyer SL, Eells JT, Lyons J-A. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS One. 2012;7(1):e30655. doi: 10.1371/journal.pone.0030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sommer AP, Trelles MA. Laser Surg. Mary Ann Liebert, Inc; 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA: 2014. Photomed. [Google Scholar]
- 188.Tuby H, Maltz L, Oron U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers Surg Med. 2011;43(5):401–409. doi: 10.1002/lsm.21063. [DOI] [PubMed] [Google Scholar]
- 189.Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 190.Oron A, Oron U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: a minireview. Photomed Laser Surg. 2016;34(12):627–630. doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- 191.Farfara D, Tuby H, Trudler D, Doron-Mandel E, Maltz L, Vassar RJ, Frenkel D, Oron U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2015;55(2):430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 192.Romeo S, Vitale F, Viaggi C, di Marco S, Aloisi G, Fasciani I, Pardini C, Pietrantoni I, Di Paolo M, Riccitelli S. Fluorescent light induces neurodegeneration in the rodent nigrostriatal system but near infrared LED light does not. Brain Res. 2017;1662:87–101. doi: 10.1016/j.brainres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 193.Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR. Low-level laser therapy for fat layer reduction: A comprehensive review. Lasers Surg Med. 2013;45(6):349–357. doi: 10.1002/lsm.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paolillo FR, Borghi-Silva A, Arena R, Parizotto NA, Kurachi C, Bagnato VS. Effects of phototherapy plus physical training on metabolic profile and quality of life in postmenopausal women. J Cosmet Laser Ther. 2017;19(6):364–372. doi: 10.1080/14764172.2017.1326610. [DOI] [PubMed] [Google Scholar]
- 195.Berman MH, Halper JP, Nichols TW. Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition. J Neurol Neurosci. 2017;8(1):176. doi: 10.21767/2171-6625.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maksimovich IV. Dementia and Cognitive Impairment Reduction after Laser Transcatheter Treatment of Alzheimer’s Disease. World J Neurosci. 2015;5(3):189. [Google Scholar]
- 197.Maloney R, Shanks S, Maloney J. The application of low-level laser therapy for the symptomatic care of late stage Parkinson’s disease: a non-controlled, non-randomized study. Lasers Surg Med. 2010:61–61. [Google Scholar]
- 198.Hesse S, Werner C, Byhahn M. Transcranial Low-Level Laser Therapy May Improve Alertness and Awareness in Traumatic Brain Injured Subjects with Severe Disorders of Consciousness: a Case Series. Int Arch Med. 2015;6:1. [Google Scholar]
- 199.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C. Infrared laser therapy for ischemic stroke: A new treatment strategy. Stroke. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 200.Hacke W, Schellinger PD, Albers GW, Bornstein NM, Dahlof BL, Fulton R, Kasner SE, Shuaib A, Richieri SP, Dilly SG. Transcranial laser therapy in acute stroke treatment. Stroke. 2014;45(11):3187–3193. doi: 10.1161/STROKEAHA.114.005795. [DOI] [PubMed] [Google Scholar]
- 201.Ab Boonswang N, Chicchi M, Lukachek A, Curtiss D. A new treatment protocol using photobiomodulation and muscle/bone/joint recovery techniques having a dramatic effect on a stroke patient’s recovery: a new weapon for clinicians. BMJ Case Rep. 2012 doi: 10.1136/bcr.08.2011.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimul. 2016;9(5):780–787. doi: 10.1016/j.brs.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cassano P, Cusin C, Mischoulon D, Hamblin MR, De Taboada L, Pisoni A, Chang T, Yeung A, Ionescu DF, Petrie SR. Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatr J. 2015 doi: 10.1155/2015/352979. [DOI] [PMC free article] [PubMed]
- 204.Duch W, Oentaryo RJ, Pasquier M. Cognitive Architectures: Where do we go from here? AGI. 2008;171:122–136. [Google Scholar]
- 205.Newson RS, Kemps EB, Luszcz MA. Cognitive mechanisms underlying decrements in mental synthesis in older adults. Aging Neuropsychol Cogn. 2003;10(1):28–43. [Google Scholar]
- 206.Vargas E, Barrett DW, Saucedo CL, Huang L-D, Abraham JA, Tanaka H, Haley AP, Gonzalez-Lima F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci. 2017;32(5):1153–1162. doi: 10.1007/s10103-017-2221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol. 2014;88(4):584–593. doi: 10.1016/j.bcp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 208.Jack C. Treating cognitive impairment with transcranial low level laser therapy. J Photochem Photobiol B, Biol. 2017;168:149–155. doi: 10.1016/j.jphotobiol.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 209.Moghadam HS, Nazari MA, Jahan A, Mahmoudi J, Salimi MM. Beneficial Effects of Transcranial Light Emitting Diode (LED) Therapy on Attentional Performance: An Experimental Design. Iran Red Crescent Med J. 2017 (In Press) [Google Scholar]
- 210.Blanco NJ, Saucedo CL, Gonzalez-Lima F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem. 2017;139:69–75. doi: 10.1016/j.nlm.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 211.Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg. 2011;29(12):785–787. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 212.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B, Biol. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 213.Choi D-H, Lee K-H, Kim J-H, Kim MY, Lim JH, Lee J. Effect of 710nm visible light irradiation on neurite outgrowth in primary rat cortical neurons following ischemic insult. Biochem Biophys Res Commun. 2012;422(2):274–279. doi: 10.1016/j.bbrc.2012.04.147. [DOI] [PubMed] [Google Scholar]
- 214.Vos M, Lovisa B, Geens A, Morais VA, Wagnières G, Van Den Bergh H, Ginggen A, De Strooper B, Tardy Y, Verstreken P. Near-infrared 808 nm light boosts complex IV-dependent respiration and rescues a Parkinson-related pink1 model. PLoS One. 2013;8(11):e78562. doi: 10.1371/journal.pone.0078562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fukuzaki Y, Shin H, Kawai HD, Yamanoha B, Kogure S. 532 nm Low-Power Laser Irradiation Facilitates the Migration of GABAergic Neural Stem/Progenitor Cells in Mouse Neocortex. PloS One. 2015;10(4):e0123833. doi: 10.1371/journal.pone.0123833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fukuzaki Y, Sugawara H, Yamanoha B, Kogure S. 532 nm low-power laser irradiation recovers γ-secretase inhibitor-mediated cell growth suppression and promotes cell proliferation via Akt signaling. PloS One. 2013;8(8):e70737. doi: 10.1371/journal.pone.0070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Y, Huang Y-Y, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Montell C. The history of TRP channels, a commentary and reflection. Pflug Arch Eur J Phy. 2011;461(5):499–506. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- 219.Litscher D, Litscher G. Laser therapy and stroke: quantification of methodological requirements in consideration of yellow laser. Int J Photoenergy. 2013 doi: 10.1155/2013/575798. [DOI]
- 220.Konstantinovi LM, Jeli MB, Jeremi A, Stevanovi VB, Milanovi SD, Filipovi SR. Transcranial application of near-infrared low-level laser can modulate cortical excitability. Lasers Surg Med. 2013;45(10):648–653. doi: 10.1002/lsm.22190. [DOI] [PubMed] [Google Scholar]
- 221.Grover F, Jr, Weston J, Weston M. Acute Effects of Near Infrared Light Therapy on Brain State in Healthy Subjects as Quantified by qEEG Measures. Photomed Laser Surg. 2017;35(3):136–141. doi: 10.1089/pho.2015.4036. [DOI] [PubMed] [Google Scholar]
- 222.Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3(3):031404. doi: 10.1117/1.NPh.3.3.031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Qu C, Cao W, Fan Y, Lin Y. Retinal Degenerative Diseases. Springer; 2010. Near-infrared light protect the photoreceptor from light-induced damage in rats; pp. 365–374. [DOI] [PubMed] [Google Scholar]
- 224.Chu-Tan JA, Rutar M, Saxena K, Wu Y, Howitt L, Valter K, Provis J, Natoli R. Efficacy of 670 nm light therapy to protect against photoreceptor cell death is dependent on the severity of damage. Int J Photoenergy. 2016 doi: 10.1155/2016/2734139. [DOI]
- 225.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540(7632):230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meyer DM, Chen Y, Zivin JA. Dose-finding study of phototherapy on stroke outcome in a rabbit model of ischemic stroke. Neurosci Lett. 2016;630:254–258. doi: 10.1016/j.neulet.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 227.Wu X, Alberico SL, Moges H, De Taboada L, Tedford CE, Anders JJ. Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg Med. 2012;44(3):227–232. doi: 10.1002/lsm.22004. [DOI] [PubMed] [Google Scholar]
- 228.Lapchak P, Salgado K, Chao C, Zivin J. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 229.Hamblin MR, de Sousa MVP, Agrawal T. Handbook of Low-Level Laser Therapy. Pan Stanford Publishing Pte. Ltd; 2016. [Google Scholar]
- 230.Hashmi JT, Huang YY, Sharma SK, Kurup DB, De Taboada L, Carroll JD, Hamblin MR. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42(6):450–466. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mantsch HH. The evolution of biomedical vibrational spectroscopy: A personal perspective. Biomed Spectrosc Imaging. 2015;4(4):315–329. [Google Scholar]
- 232.Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol. 2004;556(2):337–345. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Barolet D, Boucher A, Bjerring P. In vivo human dermal collagen production following LED-based therapy: The importance of treatment parameters. Lasers Surg Med. 2005;36:76. [Google Scholar]
- 234.Castano AP, Dai T, Yaroslavsky I, Cohen R, Apruzzese WA, Smotrich MH, Hamblin MR. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg Med. 2007;39(6):543–550. doi: 10.1002/lsm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Giuliani A, Lorenzini L, Gallamini M, Massella A, Giardino L, Calzà L. Low infra red laser light irradiation on cultured neural cells: effects on mitochondria and cell viability after oxidative stress. BMC Complement Altern Med. 2009;9(1):8. doi: 10.1186/1472-6882-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Purushothuman S, Johnstone DM, Nandasena C, van Eersel J, Ittner LM, Mitrofanis J, Stone J. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci Lett. 2015;591:155–159. doi: 10.1016/j.neulet.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 237.da Luz Eltchechem C, Salgado ASI, Zângaro RA, da Silva Pereira MC, Kerppers II, da Silva LA, Parreira RB. Transcranial LED therapy on amyloid-β toxin 25–35 in the hippocampal region of rats. Lasers Med Sci. 2017;32(4):749–756. doi: 10.1007/s10103-017-2156-3. [DOI] [PubMed] [Google Scholar]
- 238.Shaw VE, Spana S, Ashkan K, Benabid AL, Stone J, Baker GE, Mitrofanis J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J Comp Neurol. 2010;518(1):25–40. doi: 10.1002/cne.22207. [DOI] [PubMed] [Google Scholar]
- 239.Peoples C, Spana S, Ashkan K, Benabid A-L, Stone J, Baker GE, Mitrofanis J. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(5):469–476. doi: 10.1016/j.parkreldis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 240.Shaw VE, Peoples C, Spana S, Ashkan K, Benabid A-L, Stone J, Baker GE, Mitrofanis J. Patterns of cell activity in the subthalamic region associated with the neuroprotective action of near-infrared light treatment in MPTP-treated mice. Park Dis. 2012 doi: 10.1155/2012/296875. [DOI] [PMC free article] [PubMed]
- 241.Moro C, Torres N, El Massri N, Ratel D, Johnstone DM, Stone J, Mitrofanis J, Benabid A-L. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: evidence from two mouse strains. BMC Neurosci. 2013;14(1):40. doi: 10.1186/1471-2202-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Reinhart F, El Massri N, Darlot F, Torres N, Johnstone DM, Chabrol C, Costecalde T, Stone J, Mitrofanis J, Benabid A-L. 810nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neurosci Res. 2015;92:86–90. doi: 10.1016/j.neures.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 243.El Massri N, Moro C, Torres N, Darlot F, Agay D, Chabrol C, Johnstone DM, Stone J, Benabid A-L, Mitrofanis J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp Brain Res. 2016;234(11):3225–3232. doi: 10.1007/s00221-016-4720-7. [DOI] [PubMed] [Google Scholar]
- 244.Moro C, El Massri N, Darlot F, Torres N, Chabrol C, Agay D, Auboiroux V, Johnstone DM, Stone J, Mitrofanis J. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016;1648:19–26. doi: 10.1016/j.brainres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 245.Reinhart F, Massri NE, Chabrol C, Cretallaz C, Johnstone DM, Torres N, Darlot F, Costecalde T, Stone J, Mitrofanis J. Intracranial application of near-infrared light in a hemi-parkinsonian rat model: the impact on behavior and cell survival. J Neurosurg. 2016;124(6):1829–1841. doi: 10.3171/2015.5.JNS15735. [DOI] [PubMed] [Google Scholar]
- 246.Reinhart F, El Massri N, Johnstone DM, Stone J, Mitrofanis J, Benabid A-L, Moro C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp Brain Res. 2016;234(7):1787–1794. doi: 10.1007/s00221-016-4578-8. [DOI] [PubMed] [Google Scholar]
- 247.Reinhart F, El Massri N, Torres N, Chabrol C, Molet J, Johnstone DM, Stone J, Benabid A-L, Mitrofanis J, Moro C. The behavioural and neuroprotective outcomes when 670nm and 810nm near infrared light are applied together in MPTP-treated mice. Neurosci Res. 2016;117:42–47. doi: 10.1016/j.neures.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 248.DeTaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med. 2006;38(1):70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 249.Lapchak PA, Han M-K, Salgado KF, Streeter J, Zivin JA. Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke. 2008;39(11):3073–3078. doi: 10.1161/STROKEAHA.108.516393. [DOI] [PubMed] [Google Scholar]
- 250.Huisa BN, Chen Y, Meyer BC, Tafreshi GM, Zivin JA. Incremental treatments with laser therapy augments good behavioral outcome in the rabbit small clot embolic stroke model. Lasers Med Sci. 2013;28(4):1085–1089. doi: 10.1007/s10103-012-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Oron A, Oron U, Streeter J, Taboada LD, Alexandrovich A, Trembovler V, Shohami E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 252.Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J Neurotrauma. 2012;29(2):408–417. doi: 10.1089/neu.2010.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mohammed HS. Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med Sci. 2016;31(8):1651–1656. doi: 10.1007/s10103-016-2033-5. [DOI] [PubMed] [Google Scholar]
- 254.Wu J-H, Chang W-D, Hsieh C-W, Jiang J-A, Fang W, Shan Y-C, Chang Y-C. Effect of low-level laser stimulation on EEG. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/951272. [DOI] [PMC free article] [PubMed]
- 255.Chaieb L, Antal A, Masurat F, Paulus W. Neuroplastic effects of transcranial near-infrared stimulation (tNIRS) on the motor cortex. Front Behav Neurosci. 2015;9:147. doi: 10.3389/fnbeh.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Werner C, Byhahn M, Hesse S. Non-invasive brain stimulation to promote alertness and awareness in chronic patients with disorders of consciousness: Low-level, near-infrared laser stimulation vs. focused shock wave therapy. Restor Neurol Neurosci. 2016;34(4):561–56925. doi: 10.3233/RNN-150624. [DOI] [PubMed] [Google Scholar]
- 257.Xuan W, Huang L, Hamblin MR. Repeated transcranial low-level laser therapy for traumatic brain injury in mice: biphasic dose response and long-term treatment outcome. J Biophotonics. 2016;9:1263–1272. doi: 10.1002/jbio.201500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, DJM Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Science translational medicine. 2014;6:238ra269. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Buscone S, Mardaryev AN, Raafs B, Bikker JW, Sticht C, Gretz N, Farjo N, Uzunbajakava NE, Botchkareva NV. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg Med. 2017 Sep;49(7):705–718. doi: 10.1002/lsm.22673. [DOI] [PubMed] [Google Scholar]