Abstract
This study comprehensively addresses the phenotype, function and whole transcriptome of primary human and rodent Schwann cells (SCs) and highlights key species-specific features beyond the expected donor variability that account for the differential ability of human SCs to proliferate, differentiate and interact with axons in vitro. Contrary to rat SCs, human SCs were insensitive to mitogenic factors other than neuregulin and presented phenotypic variants at various stages of differentiation, along with a mixture of proliferating and senescent cells, under optimal growth-promoting conditions. The human SC responses to cAMP-induced differentiation featured morphological changes and cell cycle exit without a concomitant increase in myelin-related proteins or lipids. Human SCs efficiently extended processes along those of other SCs (human or rat) but failed to do so when placed in co-culture with sensory neurons under conditions supportive of myelination. Indeed, axon contact-dependent human SC aligment, proliferation and differentiation was not observed and could not be overcome by growth factor supplementation. Strikingly, RNA-seq data revealed that ~44 of the transcriptome contained differentially expressed genes in human and rat SCs. A bioinformatics signal transduction approach further highlighted that representative SC-specific transcripts encoding myelin-related and axon growth-promoting genes were significantly affected and that a deficient expression of key transducers of cAMP and adhesion signaling explained the fairly limited potential of human SCs to differentiate and respond to axonal cues. These results confirmed the significance of combining traditional bioassays and high resolution genomics methods to characterize human SCs and identify genes predictive of cell function and/or therapeutic value.
Keywords: Differentiation, proliferation, glia-neuron interactions, transcriptome, cAMP signaling, adhesion signaling
INTRODUCTION
Primary human Schwann cells (SCs) are useful in vitro models of normal and abnormal SC function relevant to a variety of conditions affecting peripheral nerves. The use of primary human SCs in transplantation also remains a potential therapeutic intervention for the treatment of spinal cord and peripheral nerve injuries in humans [1,2]. SCs provide a unique advantage for autologous grafting given the fact that a relatively small biopsy from a patient’s own nerve can provide the mother cells from which the purified, expanded populations are derived. Propagation of human SCs can be achieved via methods similar to those used in the culturing of cells from experimental animals. Provision of neuregulin, an agonist of the receptor tyrosine kinase ErbB/HER, and forskolin, a potent inducer of cyclic adenosine monophosphate (cAMP), are crucial for SC expansion. In fact, the discovery of neuregulin as the primary human SC mitogen, and forskolin as synergistic enhancer of neuregulin-dependent proliferation [3,4] made it possible to greatly improve the harvest and amplification potential of the adult nerve-derived populations over several passages [reviewed in [5]].
Despite the availability of standardized protocols rendering high numbers of purified SCs, studies that use cultured human SCs are relatively scarce. This is due not only to limitations in the availability of human tissues but also to the poor performance of cultured human SCs in standard functional assays that were originally designed for achieving differentiation of rodent cells [6]. Whereas both human and rodent SCs adapt well to in vitro culture and sequential rounds of expansion using chemical mitogens [4,7,8], only rodent SCs were shown to support neuronal health and faithfully accomplish axon ensheathment and myelin formation in vitro [9,10]. Independent lines of evidence support the idea that the SC product derived from human nerves has special characteristics [11] and only partially resembles the one obtained from experimental animals. In fact, the cellular and molecular basis for the myelination defects of primary human SCs has been elusive for over 25 years. Thus, to address this long-standing issue, we conducted a side-by-side comparison of the phenotypic and functional characteristics of human and rat SCs by means of SC-specific bioassays for proliferation, differentiation and SC-neuron interactions along with whole transcriptome profiling through next generation whole transcriptome sequencing or RNA-seq.
We found that human SCs propagated in vitro using standard methods shared common basic features with rat SCs, such as the expression of selected markers deemed specific to the SC lineage, ability to align with one another forming typical bundles of cells and responsiveness to the mitogenic action of neuregulin and forskolin. Nevertheless, we found important differences in the human SCs concerning their phenotypic heterogeneity, partial reactivation of myelin gene expression and impaired association with sensory axons. As opposed to rat SCs, human SCs were migratory and had a tendency to form SC aggregates on the axons, which altogether precluded an effective alignment, proliferation and differentiation in response to axonal growth factors. These differences were highlighted at the level of the transcriptome as a whole and in specific categories of transcrips encoding myelination-associated genes, neurotrophins, adhesion molecules and other identifiers of the SC phenotype. A bioinformatics pathway analysis revealed that modules of signal transduction that are known to underlie mechanisms of differentiation and adhesion in SCs were deficiently expressed in human SCs. Though important variability was observed in human SC cultures obtained from various organ donors and types of nerves, our results support the idea that intrinsic species-specific differences rather than donor variability accounted for the unexpected functional features of human SCs in vitro.
MATERIALS AND METHODS
Chemicals and reagents
The cell permeable cAMP derivative 8-(4-Chlorophenylthio) adenosine-3′, 5′-cyclic monophosphate (CPT-cAMP, cat.#C010) was obtained from Biolog (US distributor, Axxora LLC, San Diego, CA). Recombinant purified heregulin-β1177-244 (cat.#100-03), a soluble peptide consisting of the EGF homology domain of β1-heregulin, was acquired from Peprotech (Rocky Hill, NJ). Recombinant human PDGF-BB (cat.# 220-BB), IGF-1 (cat.# 291-G1), FGF-basic (cat# 233-FB), TGF-β1 (cat.# 240-B) and nerve growth factor (cat#.556-NG) were acquired from R&D Systems. Defined fetal bovine serum (FBS) was acquired from HyClone (Logan, UT). Forskolin (cat.# F68861), cholera toxin (cat.# 8052), pertussis toxin (cat.# 0847), poly-L-lysine (PLL, cat.# P2636) and mouse laminin (cat.# L2020) were acquired from Sigma (St. Louis, MO). The β-Galactosidase staining kit (cat.#9860) was purchased from Cell Signaling Tech (Beverly, MA). The Click-iT EdU (5-ethynyl-2′-deoxyuridine) Alexa-Fluor594 Imaging Kit (cat.# C10339) was obtained from Life Technologies. [3H]-thymidine (6.7 Ci/mmol) and Solvable™ were acquired from Perkin-Elmer (Boston, MA).
Antibodies and hybridoma cell lines
Commercially available antibodies were acquired from the following sources. From DAKO (Carpinteria, CA): S100 (cat.# Z0311) and glial fibrillar acidic protein (GFAP, cat.# Z0334). From Santa Cruz Biotech (Dallas, TX): fibronectin (cat.# sc-8422); pan-cadherin (cat.# sc-10733); β1-Integrin (cat.# sc-53711); horse radish peroxidase (HRP)-conjugated anti-mouse (cat.# sc-2055) and anti-rabbit (cat.# sc-2301). From Cell Signaling Tech. (Beverly, MA): phosphorylated (Ser217/221)-MEK (cat.#9121); phosphorylated (Ser-473)-Akt (cat.# 4051); β-actin (cat.# 8457); and FAK (cat.# 13009). From Chemicon (Temecula, CA): myelin-associated glycoprotein (cat.# MAB1567); chicken neurofilament (cat.# ab5539). From BD Transduction Labs: paxillin (cat.# 610051); and N-cadherin (cat.# 610920). Other antibodies were as follows: neurofilament 70 kDa, clone DA2 (EMD-Millipore, cat.# MAB1615); and vinculin (Sigma, cat.# V9264). Polyclonal (rabbit) Krox-20 and periaxin sera were kindly provided by Drs. Dies Meijer and Peter Brophy, respectively. The hybridoma cell lines Thy 1.1/TIB-103 (rodent-specific Thy1.1), 192-IgG (rodent-specific p75NGFR) and HB-8737 (human/primate-specific p75NGFR) were procured from the American Type Culture Collection (ATCC, Manassas, VA). The hybridoma cell line HL1099 (neurofilament medium chain) was provided by Dr. G. Shaw. The polyclonal antibodies recognizing L1- and N-CAM and the hybridoma cell lines producing O1 and O4 monoclonal antibodies were provided by Dr. M. Schachner. Conditioned medium from the above referred hybridoma cell cultures was collected and used as source of monoclonal antibodies, as described previously [1,12]. The reactivity and cell type specificity of the antibodies was confirmed experimentally using appropriate controls of human and rat SCs, myelin, fibroblasts and neurons via immunostaining and/or Western blot. In the case of p75NGFR, the species-specific monoclonal antibodies mentioned above were used exclusively in human and rat SC cultures, respectively. The binding affinity of the antibodies in human and rat samples was not determined experimentally. Additional technical information on the products and procedures used will be made available upon request.
Primary cultures of adult nerve-derived human Schwann cells
Human peripheral nerves from normal cadaveric de-identified donors were made available by the Life Alliance Organ Recovery Agency of the University of Miami Miller School of Medicine, as described previously [13]. The donors (ages 10 to 66) had negative serology for blood borne viruses and no reported history of peripheral neuropathy or cancer. The nerves were harvested in iced-cold Belzer’s solution and processed for cell isolation within 4–24 h after death/aortic clampling. Protocols for the use of human and animal tissues/cells were reviewed and approved by the respective Institutional Review Boards of the University of Miami. Of note, experimentation with de-identified human cells constituted non-human subjects research. Experiments used human SCs derived from the nerve roots comprising the cauda equine unless otherwise noted. Sural, intercostal and sciatic nerves were used in selected experiments to confirm the results in cultures derived from different types of nerves. Cells were used irrespective of the gender and age of the donor. We have not found obvious differences in the quality of the cultures derived from males/females or young/aged donors despite the observation that cells from younger donors tend to expand at higher rates [14]. Human SC cultures displaying an abnormal pattern of growth, floating debris or excessive fibroblast contamination (>10%) were excluded from the analysis.
The protocol used to obtain and expand human SCs was reported previously [7] with minor modifications [15]. Briefly, the nerve fascicles were pulled out of their surrounding connective tissue and cut into small fragments (approximately 3–5 cm in length) prior to incubation for 8–10 days at 37°C in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% heat-inactivated FBS, 2 μM forskolin, 10 nM β1-heregulin (herein referred to as neuregulin), 1% penicillin/streptomycin and 25 μg/ml gentamicin. To facilitate the enzymatic dissociation, isolate viable cells and remove myelin debris the nerve fragments were allowed to predegenerate in this complete media, which was was used throughout plating and expansion, unless otherwise noted [7]. The pre-degenerated nerve fragments were dissociated by incubation in DMEM containing 10% FBS, 0.5 mg/ml collagenase type I (cat.#4196, Worthington, Lakewood, NJ) and 2.5 mg/ml dispase II (cat.#165-869, Roche, Indianapolis, IN), without antibiotics, overnight at 37 °C. The resulting cell suspension was plated onto mouse laminin-coated 10-cm tissue culture dishes in DMEM supplemented as described above with FBS, neuregulin, forskolin and antibiotics. Of note, the concentrations of these supplements were maintained throughout unless otherwise noted. The cells were allowed to propagate in number up until reaching confluence and subjected to serial passaging at a ratio of 1:10 in each round of subculture for cryopreservation or experimentation, as described below.
Purification and characterization of human Schwann cells and fibroblasts
Human SCs were routinely purified at an early passage (prior to cryopreservation) via immunopanning using human-specific p75NGFR monoclonal antibodies [15]. Immunopanning plates were prepared by incubating 10-cm polystyrene plates (non-tissue culture treated) with 1:100 dilution of goat anti-mouse IgG,-A,-M (cat.#55486, ICN/Cappel) in 0.05 M Tris buffer (pH: 9.5) overnight at 4 °C followed by incubation for 2 h (at 4 °C) with undiluted culture supernatant of HB-8737 cells produced in house. Cells were collected by trypsinization and resuspended in 10 mL of cold Leibovitz’s (L15) medium for plating onto the immunopanning plates at 4 °C on a flat surface. Non-adherent cells were removed by performing a gentle wash with cold L15 medium within 30 min of incubation or as determined by phase contrast microscopy observation. The adherent cells (SCs) were detached by gentle scraping in L15 medium, collected by centrifugation, resuspended and plated onto laminin-coated dishes for propagation. The purity of the human SC cultures was confirmed to be >90% based on immunostaining with S100, p75NGFR (human-specific) and fibronectin antibodies.
Fibroblast cultures were established from the cell populations that had the capacity to promptly adhere to non-coated plastic dishes and proliferate in response to serum factors, as described previously [16]. Fibroblasts were expanded up to passage-2 in DMEM containing 10% FBS and antibiotics (without added mitogenic factors) prior to cryopreservation. Fibroblasts were discriminated from SCs based on their S100 negative, p75NGFR negative, fibronectin positive phenotype. These cultures were >95% fibroblasts as judged by co-immunostaining with fibronectin and S100 antibodies.
Primary cultures of adult nerve-derived rat Schwann cells
SCs were prepared from the sciatic nerves of three months-old male Fisher rats by a modification of a previously reported method, as described recently in [17]. The sciatic nerves of 4 animals were processed together to obtain a large enough population of SCs for cryopreservation at early passage. The sciatic nerve tissue was allowed to pre-degenerate in vitro for 8–10 days by cultivating small tissue explants (0.5 cm in length) in non-coated cell culture dishes. Explants were maintained in DMEM containing 10% FBS, 1% penicillin/streptomycin and 25 μg/ml gentamicin until enzymatic dissociation with a mixture of dispase and collagenase, as described for human SCs. The resulting cell suspensions were plated on PLL-coated dishes in DMEM-10% FBS (with antibiotics) for 1 day and contaminating fibroblasts were removed by a complement reaction using Thy 1.1 conditioned medium produced in house. Cells were expanded as described above for human SCs. The purified cultures consisted of >98% SCs based on immunostaining with S100, p75NGFR (rat-specific) and Thy1.1 antibodies. Adult SCs used in these studies were tested for their ability to differentiate in response to cAMP and form myelin upon co-culture with DRG neurons [18]. All cultures were routinely inspected for purity, sterility and normal pattern of growth prior to use in experimentation.
Cryopreservation, thawing and plating of cell stocks
All experiments performed in these studies used purified, expanded SCs and fibroblasts derived from cryopreserved stocks. Cryopreservation was carried out by resuspending single cell suspensions collected by trypsinization in medium consisting of 90% FBS/10% DMSO, following standard practice. Cryopreservation does not alter the viability (usually >90% after thawing) or other biological properties of the cells [1]. Cultures were cryopreserved at passages 1–2, thawed and expanded to be used in experimentation at passages 2–3. Sub-culturing of human SCs over passage-3 was avoided to prevent enrichment in senescent cells. Human SC cultures did not experience notorious changes in morphology, expression of markers or proliferation controls up to passage-3. Of note, human and rat SC stocks were thawed, plated, propagated in vitro under identical culture conditions. All cell-based assays were carried out in multi-well dishes coated sequentially with PLL and mouse laminin. Experimentation and analysis of human and rat SCs was carried out in parallel samples.
Cultures of purified embryonic neurons from the dorsal root ganglia
Purified embryonic rat DRG neurons (dissociated, day 15) were established essentially as described previously in [17]. Briefly, the DRG bodies were dissected out from the embryos and enzymatically dissociated with 0.25% trypsin (37°C, 45 min). Dissociated ganglia were resuspended in neurobasal medium containing B27 supplement (Invitrogen, Carlsbad, CA), 10 ng/ml nerve growth factor, 1 mM L-glutamine (Life Technologies), 1% FBS and gentamycin. Cells were placed in the center of a well of a laminin-coated 24-well dish (cat.# 354659, BD Biosciences) in the form of 30–50 μl droplets containing 5,000–10,000 cells each. Cells were allowed to attach to the substrate for at least 2 h before addition of fresh culture medium (450 μl/well). Cultures were purified of non-neuronal cells by a single cycle of treatment with the anti-mitotic agent 5-fluoro-2′deoxyuridine (10 μM), which was provided one day after cell plating and removed after 3 days. This method allows the neuronal bodies to localize at high density in the center of the culture well while the axonal outgrowth extends radially throughout the remaining available surface. For use in co-culture experiments, the DRG neurons were maintained in culture for at least 12–15 days to ensure that neurites have extended to the edge of the well. Individual neuronal cultures were scrutinized to be free off cellular debris and contaminating non-neuronal cells prior to plating the glial cells.
Proliferation assays and assessment of cell expansion
Sub-confluent cultures of SCs growing on PLL-laminin-coated 24-well plates (80,000 cells/well) were deprived of mitogens for 2 d in DMEM-10% FBS and then for 1 d in HEPES-buffered DMEM containing 1% FBS and gentamycin. Whereas the removal of the mitogenic stimulus allows SCs to return to quiescence, the addition of a non-mitogenic concentration of FBS (1%) prevents apoptotic cell loss and maintains the cells attached to the laminin substrate. The use of 1% FBS also helps to rule out any possible contribution of fibroblast proliferation to incorporated tritium counts. Cells were exposed to medium containing [3H]-thymidine (0.25 μCi/ml) under the conditions described in the figure legends. Incorporated tritium into the cells was determined by liquid scintillation counting typically 48–72 h post-stimulation. To achieve a maximal response in the incorporation of tritium counts, a condition in which neuregulin (10 nM) was provided in combination with forskolin (2 μM) and FBS (10%) was included as positive control [13].
The incorporation of the thymidine analog EdU into nuclear DNA by fluorescence microscopy was also used as means to determine S-phase entry in individual cells and corroborate the results obtained by [3H]-thymidine incorporation assays. Briefly, mitogen and serum-starved SCs were exposed to medium containing EdU labeling reagent (0.1μM) under the conditions described in the figure legends. The cultures were fixed with 4% paraformaldehyde and −20°C methanol and blocked with 5% normal goat serum (NGS) prior to detecting EdU incorporation by using the Click-iT EdU labeling kit according to the manufacturer’s instructions. Nuclei was counterstained with Hoechst. [3H]-thymidine incorporation assays were preferred to provide a quantitative comparison among treatments in experiments consisting of multiple variables (assayed in triplicate samples) based on cost, sensitivity and reproducibility.
To determine the temporal course of cell expansion, cells were detached from their dishes and plated onto PLL-laminin-coated 6-well plates at a density of 100,000 cells/well in medium containing 10% FBS, mitogens and gentamycin. Cells were allowed to propagate under standard culture conditions prior to trypsinization and counting. A Bio-Rad TC20™ automated cell counter (Bio-Rad, Hercules, CA) was used to determine the number of live/dead cells at each time point using cells in suspension, as described in [1].
Senescence-associated β-galactosidase activity
The expression of pH-dependent β-galactosidase activity was determined by means of a cytochemical assay using the chromogenic substrate 5-bromo-4-chloro-3-indoyl β-D-galactopyranoside, which yields an insoluble blue product when cleaved by β-galactosidase. Enzymatic activity at pH 6, a known characteristic of senescent cells, was determined in fixed cells using the Senescence β-Galactosidase Staining Kit according to the manufacturer’s instructions. Cultures were stained with DAPI prior to imaging by fluorescence and bright field microscopy. Senescent cells were identified as those containing a blue intracellular precipitate.
Differentiation assays
Isolated SCs were differentiated by prolonged treatment with the cell permeable analog of cAMP, CPT-cAMP, as reported previously [19]. For differentiation, cells were plated at a density that renders a sub-confluent culture (80,000 cells/well) based on the observation that SCs (rat) would not induce the expression of key markers of myelination in over-confluent cultures [19]. To maintain low density, maximize the effect of the inductive stimulus and simplify the interpretation of results, assays were carried out by adding CPT-cAMP (250 μM) in DMEM medium containing 1% FBS (and gentamycin), as this low serum medium supports the induction of myelin gene expression (differentiation) but not the proliferation of cultured (rat) SCs [19,20]. In addition, these conditions ensure that the responses to the differentiating action of CPT-cAMP are not altered by agents present in the extracellular medium and/or the induction of proliferation during the course of treatment, which may in turn alter cell density and cell-cell interactions. Exceptions were experiments such as EdU incorporation assays, which were carried out in medium containing 10% FBS, neuregulin, forskolin and gentamycin (in the absence and presence of CPT-cAMP). These conditions were required to support high levels of SC proliferation and O4 expression in the control group, as both of these variables are rapidly reduced if cells are deprived of mitogenic factors and serum. We have previously shown that prolonged treatment with high doses of CPT-cAMP is required to allow SCs to achieve a differentiated, post-mitotic phenotype exhibiting high levels of expression of myelination-associated genes. Most importantly, the presence of FBS and other growth factors in the culture medium does not preclude CPT-cAMP-induced myelin gene expression [19].
Cultures were analyzed for the expression of general SC-specific markers (S100), mature/myelinating SC markers (Krox-20, O4, O1, periaxin, and MAG), immature SC markers (p75NGFR) and changes in cell morphology by means of immunofluorescence microscopy over a time period of 3–4 days (ratSCs) and 7 days (human SCs). Cultures maintained from the outset in the absence of CPT-cAMP served as controls for undifferentiated, immature cells expressing low or negligible levels of myelin-associated proteins and lipids. Some cultures were analyzed by means of EdU incorporation assays, as CPT-cAMP treatment induces cell cycle exit and this can be detected as a reduction in the incorporation of the EdU label. To simplify the interpretation of results, the detection of cell proliferation (EdU) and expression of phenotypic markers was done in parallel cultures.
Assessment of axon contact-induced SC proliferation and differentiation in SC-neuron cultures
Assays for axon contact-induced SC proliferation are based on the ability of SCs to readily undergo mitosis upon establishing cell-to-cell contact with neurites from sensory neurons in the absence of exogenously added soluble growth factors [21]. Axon contact-induced proliferation was determined by seeding a single cell suspension of purified SCs (human or rat) onto a bed of purified dissociated DRG neurons in the presence of EdU labeling reagent. For this, mitogen and serum-starved SCs were trypsin-dissociated and immediately seeded (usually 80,000 cells) in a drop-by-drop manner onto the neuronal cultures. Co-cultures were established and maintained in Neurobasal medium (supplemented as described previously) for its potential to support neuronal health but not SC proliferation. Proliferating cells were detected by means of EdU incorporation assays in medium containing EdU labeling reagent, as described above. To prevent possible detrimental effects of the treatments on the physical interaction between SCs and axons, which is a crucial prefatory step for proliferation, SCs were allowed to attach to and extend processes along the axonal surface for 4 h prior to the addition of growth factors or EdU. It is known that SCs (rat) attach to and begin extending processes along the axonal surface right after co-culture initiation [22]. Human SCs co-cultured with neurons were revealed by immunostaining with S100, p75NGFR (human-specific), and O4 antibodies, as described below. SC-neuron cultures were also routinely stained with neurofilament antibodies (neuronal/axonal marker) and DAPI (nuclear marker), which served as reference controls for the extension of the axonal web and the location of the cells, respectively. Only healthy (non-senescent) proliferative human SC cultures were used for the co-culture experiments. The viability of human SCs in co-culture was not compromised at any time as assessed routinely via co-staining with propidium iodide and Hoechst [1]. Transmission electron microscopy (TEM) analysis of human SC-DRG neuron cultures grown on laminin-coated coverslips was done as described previously [18].
Assessment of cell adhesion and alignment in co-cultures of human and rat SCs
Co-cultures were established to assess cell-cell interactions, adhesion and alignment between individual rat and human SCs. To easily visualize and discriminate the species of origin by live cell fluorescence microscopy, rat and human SCs were transduced with lentiviral vectors encoding for the green and red fluorescent proteins EGFP and mCherry, respectively. Cells at passage-2 were infected overnight at a multiplicity of infection of 10 in medium containing mitogens. The efficiency of EGFP and mCherry expression was >90% at 3 days post-transduction. For co-culturing of human and rat SCs, single cell suspensions of EGFP-human and mCherry-rat SCs were combined at different ratios and plated onto PLL-laminin-coated 24-well dishes in complete medium. The cells were allowed to settle down onto the substrate and imaged by fluorescence microscopy 24–72 h after plating. Importantly, the expression of fluorescent proteins did not change the dynamics of SC differentiation and SC-axon interactions typical of non-infected cells [20].
Fluorescence microscopy and immunostaining of live and fixed cells
Detection of the cell surface antigens O1 (galactocerebroside), O4 (sulfatide) and p75NGFR was done by live cell labeling (20 min, RT) using hybridoma culture supernatant produced in house. Alternatively, cultures were fixed with 4% paraformaldehyde (10 min) and −20°C methanol (5 min), blocked in 5% NGS in PBS and incubated overnight with the appropriate dilution of the primary antibody. Commercially available antibodies were used at 1:200 and hybridoma culture supernatants were used without dilution. Alexa-conjugated (594, 488 or 647) antibodies (1:300) were used as secondary antibodies. Cells were mounted with Vectashield containing DAPI (Vector Labs, Burlingame, CA) and analyzed by fluorescence microscopy using an inverted Olympus IX70 inverted microscope. Microscopic analysis of large surface areas was performed via high throughput automated fluorescence microscopy using a Thermo Scientific Cellomics ArrayScan VTI High Content System Reader, version 6.6.2.0.
For quantitative analysis based on image data, pictures from random fields were taken at low magnification and the proportion of cells that labeled positive for the indicated markers was determined in reference to the total number of cells (DAPI staining). Cells were classified as positive or negative for the expression of Krox-20 (nuclear staining), O4/O1 (membrane staining), and EdU (nuclear staining) in comparison to non-treated controls and without regard to the staining intensity shown by individual cells. For high throughput microscopy, 10x magnification images of S100 (546), O4 (488), neurofilament (647) and DAPI (UV) were taken as serial images (ranging from 80–150) starting from the center toward the periphery of the well (24-well format).
Western blots
Total lysates from human SCs were prepared and denatured as described previously [23]. Equal protein samples (usually 1–5 μg protein/lane) were subjected to polyacrylamide gel electrophoresis under denaturing conditions and then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA) by a liquid transfer system (BioRad). Membranes were blocked with ECL blocking agent (GE Healthcare) in Tris-Buffer saline containing 0.05% Tween-20 (TBS-T) and incubated overnight with a 1:500–1:2000 dilution of each primary antibody or undiluted hybridoma culture supernatant. The membranes were washed with TBS-T prior to incubation with HRP-conjugated secondary antibodies. Immunoreactive protein bands were detected by enhanced chemiluminescence using ECL Advanced or Plus (GE Healthcare) based on signal intensity. Western blot films (Hyperfilm ECL) were scanned at a resolution of 400 dpi and quantified using Image Studio Lite Software (version 5.2). The expression of β-actin served as a control for the equal loading of protein samples.
Statistical analysis of data from cell-based assays
Statistical significance of quantitative data derived from cell-based assays was determined relative to each control group by means of the Student t-Test or one-way analysis of variance (ANOVA) using Sigma Plot 12.0 software. For multiple comparisons, Bonferroni post-test was used. Experimental data presented in the graphs was expressed as the mean ± standard deviation (SD) of a representative experiment out of at least 3 experiments performed independently using cultures of human SCs from different donors and a typical rat SC population for comparison, unless otherwise noted in the figure legends. Triplicate samples from the control and each experimental condition were used for quantification in [3H]-thymidine incorporation assays and fluorescence microscopy imaging. Statistical significance was accepted for p < 0.05. Values of significant correlations are indicated in the figures according to their degree of significance. Non-significant correlations were also indicated in some meaningful comparisons.
RNA isolation and sequencing by RNA-seq
Representative highly proliferative (non-senescent) human and rat SC cultures obtained at passage-3 were selected for RNA-seq analysis. Human SCs were derived from the cauda equine of an 18 year-old male donor whose tissue was processed within 2 h of harvesting. Cultures used were confirmed to be highly pure (>99% for rat SCs and >97% for human SCs) to minimize contamination with non-SC RNA. β-galactosidase activity assays were done prior to plating the cells for RNA-seq to confirm the absence of senescent cells. Human and rat SCs were plated at a density of 2×106 cells/well in PLL-laminin-coated 6-well dishes(in triplicate) in medium containing 10% FBS, neuregulin, forskolin and gentamycin. Cells were collected by mild trypsinization overnight after plating (to minimize cell density-dependent effects) and snap-frozen as pellets in liquid nitrogen. RNA was extracted from the cell pellets using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Briefly, cells were lysed and homogenized in a lysis buffer and DNAse was added to the samples to allow for extraction of RNA without contaminating DNA. The samples were passed through a column followed by two washes and a final elution step. RNA was analyzed for quality and quantity on the Agilent Bioanalyzer (Agilent, Santa Clara, CA) and 560 ng of total RNA was used for sequencing. Total RNA was prepared for sequencing on the Illumina platform using the ScriptSeq Complete Gold Kit (human, mouse, rat; Epicentre, Madison, WI) according to manufacturer’s instructions using the low input protocol. Total RNA was ribodepleted and remaining RNA was converted to cDNA using random hexamer primers. Prepared cDNA was then ligated with adapters for Illumina sequencing and submitted to twelve cycles of PCR. Samples were pooled three samples per lane on a high output flow cell (Illumina, San Diego, CA) using the HiSeq SBS Kit v3 (200 cycles) on the cBot (Illumina, San Diego, CA) and sequenced on the HiSeq 2000 (Illumina, San Diego, CA).
Bioinformatics and statistical analysis of RNA-seq data
Raw sequencing reads were aligned to the reference genome (Homo sapiens or Rattus norvegicus, http://www.ensembl.org/index.html) using STAR [24] and then quantified using HTSeq [25]. A list of homologous genes across species was taken from MGI Vertebrate Homology (ftp://ftp.informatics.jax.org/pub/reports/HOM_AllOrganism.rpt). Statistically significant differential expression for each gene was assessed using three software packages: edgeR[26], DESeq [25], and BaySeq [27]. Sequence reads matching microbial genes were not found. Heat-maps were generated using read counts per million (generated using edgeR) and the commercially available program heatmap.2 (http://www.insider.org/packages/cran/gplots/docs/heatmap.2). Only genes flagged as differentially expressed by all three software packages were included in further analysis. Pathway enrichment analysis was performed using MetaCore™ (http://www.genego.com, GeneGo, St Joseph, MI), [28]. Additional technical and scientific information on specific genes was obtained from GeneCards (http://www.genecards.org/). Annotated names were used for the identification of gene transcripts in the figures. Only the most relevant isoforms/variants in each gene group were selected for presentation. Selection was based on their relative levels of expression as well as their known relevance to SC function based on existing literature.
RESULTS
Human SCs were highly responsive to the mitogenic action of neuregulin: synergistic effect of cAMP-stimulating agents and specificity of proliferative responses
Neuregulin is a potent mitogenic factor for cultured SCs. However, stimulation with neuregulin alone is insufficient to sustain long-term expansion of human and rat SC populations in the absence of forskolin or other agents that increase cAMP [29,4]. To address the responses of human SCs to the mitogenic action of neuregulin, serum and other growth factors, we stimulated purified primary human SCs with mitogens alone and in combination of cAMP-stimulating agents, and subsequently measured S-phase entry by means of [3H]-thymidine and EdU incorporation assays. Throughout these studies, cultures of rat SCs treated under identical conditions were used for reference and comparative purposes, as their responses in proliferation and differentiation have been characterized more extensively [30,18].
Results indicated that the provision of nanomolar doses of a neuregulin peptide that is known to bind to and activate ErbB/HER receptors [31] was sufficient to induce a significant but modest increase in S-phase entry, as assessed by the incorporation of [3H]-thymidine (Fig. 1a,b) and EdU into nuclear DNA (Fig. 1c). As expected, neuregulin-dependent DNA synthesis was greatly enhanced by co-administration of diverse cAMP-stimulating agents, including forskolin (Fig. 1b,c), cholera toxin (Fig. 1b), and pertussis toxin (Fig. 1b). Human SCs did not increase the incorporation of [3H]-thymidine in response to provision of cAMP-stimulating agents alone (Fig. 1b) but modestly proliferated in response to serum factors (Figs. 1d and f). Similar to rat SCs, human SCs synergistically increased their proliferation upon combined neuregulin and forskolin treatment (fig. 1b, d–e). However, only rodent SCs exhibited a synergistic mitogenic response when forskolin was administrated together with FBS (Figs.1d–f), platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), and basic fibroblast growth factor-2 (FGF-2), (Figs.1g–h). Our assays did not provide an indication that human or rodent SCs would proliferate in response to transforming growth factor-β (TGF-β), which is another potential SC mitogen [32], when provided alone or together with forskolin (Figs. 1g,h). It was noteworthy to observe that human SCs would remain quiescent unless stimulated with recombinant neuregulin (Fig. 1g). No other purified growth factor was shown to be effective at eliciting human SC proliferation.
Figure 1.
Regulation of cell proliferation in adult human and rat SCs. (a–c). Synergistic action of cAMP-stimulating agents on neuregulin-induced S-phase entry in human SCs. The effect of increasing doses of neuregulin provided alone and in combination with forskolin, cholera toxin (200 ng/ml) and pertussis toxin (200 ng/ml) was determined by means of [3H]-thymidine (a,b) and EdU (c) incorporation assays (see Methods). Neuregulin was used at 10 nM and forskolin at 2 μM unless otherwise noted. (d–f). Effect of serum (FBS, 10%) and forskolin on DNA synthesis in human and rat SCs. (g–i). Responses of human and rat SCs to various growth factors provided alone or together with forskolin. The growth factors were used as follows: PDGF-BB (20 ng/ml), IGF-1 (50 ng/ml), FGF-2 (20 ng/ml); TGF-β (20 ng/ml). The time course of growth factor-dependent activation of MEK and Akt in human and rat SCs was obtained via Western blot detection of Akt and MEK phosphorylation on key activating residues (Ser-473 and Ser-217/221, respectively). Note that of all of the growth factors tested, neuregulin was the only one that rendered high levels of MEK and Akt phosphorylation that were sustained over time. In this and all subsequent graphs, bar heights are means of triplicate determinations; error bars represent SD. Results are from one representative experiment out of at least 3 independent experiments performed using human SC populations known to efficiently proliferate in response to neuregulin. Experiments were carried out using cells from an 18 year-old (yr) male (cauda equine) unless specified in the figure legends. Panel b, 52 yr male (passage-1). Panels d,f, 37 yr female (passage-2). Statistical significance is indicated as follows, * p < .05, * * p < .01 and *** p < .001 (ANOVA). Experimental treatments showing non-significant differences (ns) with respect to the control (no growth factors added; clear bars) are also indicated.
To more conclusively address the immediate responses of human and rat SCs to the growth factors mentioned above, the time course of activation/phosphorylation of MEK and Akt, which are downstream kinase effectors of ligand-activated receptor tyrosine kinases, was determined by means of Western blot assays (Fig. 1i). Results indicated that neuregulin was the only factor able to induce a strong and sustained phosphorylation of MEK and Akt on key activating residues, which is a requirement for cell cycle re-entry in SCs [13]. Though human SCs moderately responded to PDGF and IGF-1 by increasing MEK and/or Akt phosphorylation, the activation was transient and of a much lesser magnitude than the one evoked by neuregulin, and thus likely not sufficient to elicit a proliferative response [13].
It has been reported that expansion of human SCs over multiple passages can be achieved without overwhelming fibroblast contamination in medium supplemented with neuregulin and cAMP-inducing agents [4]. However, mitogen-expanded human SCs often contain a variable proportion (usually <10%) of fibroblasts that proliferate in response to serum factors (Supplementary Fig. 1a,b). As opposed to SCs, fibroblasts were insensitive to the mitogenic action of neuregulin either when provided alone or in combination with forskolin. In fact, forskolin reduced the rate of fibroblast proliferation in response to FBS (Supplementary Fig. 1b), which confirms the SC specificity of incorporated tritium counts [16].
Collectively, the evidence provided above confirmed that human and rat SCs shared a high sensitivity to neuregulin and a synergistic mitogenic response to cAMP-stimulating agents. However, some species-specific differences clearly emerged as rodent but not human SC proliferation could be elicited by a broader panel of growth factors including PDGF and IGF-1.
Human SC cultures consisted of mixed populations of proliferating cells, senescent cells and cells at different stages of differentiation: donor variability and heterogeneity of O4 expression
The assignation of phenotype of an expanded SC population is mostly based on immuno-detection of markers such as S100 (a calcium binding protein with specificity to the SC lineage in peripheral nerves), p75NGFR (the low affinity neurotrophin receptor typical of neural crest-derived cells), and GFAP (an intermediate filament protein with exclusive SC localization in peripheral nerves), [33,34]. Immunostaining of human SC cultures with antibodies against S100, p75NGFR and GFAP confirmed the SC phenotype while revealing the elongated roughly bipolar morphology of the cells along with their ability to align with one another forming parallel bundles (Fig. 2a,b and Supplementary Fig. 2). Peripheral nerve fibroblasts were clearly different from SCs on the basis of their expanded morphology, strong expression of fibronectin and lack of S100 (Supplementary Fig. 1a).
Figure 2.
Immuno-detection of S100, GFAP and O4 in human SCs: donor variability and heterogeneity of O4 expression. (a) Panoramic view of a representative proliferative human SC culture (cauda equine) stained with antibodies against S100 (right panel, red) and O4 (left panel, green). Human SCs were plated at 80,000 cells/well and stimulated for 3 days in SC medium consisting of DMEM supplemented with neuregulin, forskolin and 10% FBS. This formulation is referred to as ‘mitogens’ throughout the figure legends. The culture shown on the right panel was allowed to incorporate EdU for the whole time period of the experiment. EdU staining (proliferating cells, red) was carried out after live cell labeling with O4 antibodies (green). Observe the pattern of bundling and alignment typical of cultured SCs on a bi-dimensional substrate along with the homogeneous levels of S100 staining. This is in contrast to the pattern of O4 immuno-detection which identifies only a minor proportion of the cells. Of note, proliferating (EdU positive) SCs could be identified as O4 positive or negative cells (inset). (b) Higher magnification images of a representative human SC culture (cauda equine) co-immunostained with GFAP and O4 antibodies. (c,d) A representative image (c) and a quantification (d) of O4 expression in selected SC cultures from cauda equine, sural, intercostal and sciatic nerves. Data in the bar graph was obtained from cultures collected at passage-2 that were plated in triplicate samples and analyzed at the same time by means of O4 immunolabeling. A representative experiment out of 2 independent ones rendering similar results is shown. ***, p <.001 (t-Test). The proportion of O4 positive SCs was variable but typically <15% in cultures established from human nerves, regardless of the type of nerve or other donor-specific features (d). Strikingly, some cultures were devoid of O4 positive cells. In panel (d), donor’s age and gender was registered as follows (from left to right): 50 yr/female, 43 yr/female, 10 yr/female, 66 yr/male, 51 yr/male, 29 yr/female, 20 yr/male, 53 yr/male. Arrowheads in (b) indicate GFAP positive, O4 negative human SCs. Nuclei were labeled with DAPI (blue channel) in all fluorescence microscopy images unless otherwise noted.
A distinctive feature of human SCs was their subjacent phenotypic heterogeneity which could not be revealed by immuno-detection of general SC markes (Fig. 2a,b). This heterogeneity was particularly denoted by the expression of plasma membrane lipid antigens reactive to O4 antibodies consisting of sulfated analogues of galactosylceramide [35]. Despite some variability was observed in the percentage of O4 positive cells when comparing human SC populations obtained from different donors and nerve sources, the proportion of O4 positive cells was consistently low (typically <15%) under conditions that supported strong and homogeneous O4 expression in rat SC cultures (Fig. 2b–d). Indeed, some human SC cultures were devoid of O4 positive cells regardless of the nerve of origin and the rounds of subculture (Fig. 2d, and data not shown).
Human SCs expanded in number at lower rates than rat SCs (Fig. 3a). Indeed, EdU incorporation and β-galactosidase activity assays revealed that most human SC cultures consisted of mixed populations of proliferating and senescent cells (Fig. 3b–d). The proportion of proliferating cells was consistently lower in human than rat SCs subjected to identical culture conditions (Fig. 3b–c). Whereas human SCs generally contained <60% EdU positive cells and various proportions of β-galactosidase positive cells, those of the rat contained >90% EdU positive cells and undetectable levels of senescence-dependent β-galactosidase activity (Fig. 3b,d). Substantial variability among independent preparations of human donor cells was observed (Figs.3c–d), aligned with the expected variability in the growth rates [14]. Human SC cultures that exhibited a low proliferation index usually contained a higher proportion of senescent cells (Fig. 3c,d). Nevertheless, a comparative analysis of independently obtained preparations did not show a clear relationship between O4 expression and EdU incorporation (or β-galactosidase activity) as a function to the type of nerve, the passage number (up to passage-3) or donor-specific features such as age or gender. Proliferating cells could display an O4 positive or negative phenotype (Fig. 2a, right panel). β-galactosidase positive and negative cells were essentially indistinguishable on the basis of cell morphology and levels of S100 or p75NGFR (Fig. 3b,d and data not shown).
Figure 3.
Heterogeneity of human SC cultures as manifested by the proportion of proliferating and senescent cells under optimal conditions for cell growth. (a) Growth curves of representative populations of human and rat SCs plated and cultured in mitogens medium. (b) Human and rat SCs stained for EdU (red nuclei) and senescence-dependent β-galactosidase activity (β-gal, black intracellular staining). Mitogen-starved human and rat SCs were incubated with mitogens for 3 days in the absence (β-gal) or presence of EdU labeling reagent. Note the heterogeneity of EdU labeling and the presence of β-galactosidase positive cells in human but not rat SC cultures. EdU incorporation in the control condition was negligible denoting the growth factor dependency of proliferation in rat and human SCs (not shown, see Fig. 1c,f). (c–d) Variability in the proportion of proliferative (c, EdU positive) and senescent cells (d, β-gal positive) in SC cultures established from different nerve sources and donors. In panels c,d, donor information matches the description provided in Fig. 2 (legend). In panel a, cells from a 51 yr male (passage-3) were used. Sibling cultures were processed for EdU incorporation and β-gal activity, as identified by the numbers (1–6) assigned to each culture (c,d) of human (H) and rat (R) SCs. Note the relatively low percentage (typically <60%) of proliferating cells in human SC cultures with respect to those of the rat. Human SC cultures that exhibited a low proliferation index (c) were usually enriched in β-gal positive cells; yet, these cultures maintained high viability and a normal pattern of growth, as shown in panels c,d. ***, p <.001 (t-Test).
Altogether, we conclude that immunochemical detection of phenotypic markers deemed specific to the SC lineage revealed the identity and purity but not the intrinsic phenotypic heterogeneity of the human SC preparations. Distinctive subpopulations of human SCs could be established as determined by O4 expression, β-galactosidase activity and proliferative capacity. All rat SC cultures analyzed so far were homogeneous with regards to the abovementioned characteristics.
Human SCs did not undergo extensive differentiation in response to cAMP: morphological changes, cell growth arrest and deficits in the reactivation of myelin gene expression
Induction of myelin gene expression in isolated rat SCs can be successfully achieved via long-term treatment with high doses of cAMP derivatives in the absence or presence of serum, neuregulin or other growth factors [19,20]. However, standard differentiation protocols for rodent cells rendered suboptimal results when applied to cultures of human SCs. Figs. 4 and 5 illustrate the most relevant changes observed in human and rat SCs subjected to treatment with CPT-cAMP, a phosphodiesterase (PDE)-resistant cell permeable cAMP derivative that effectively drives myelin gene expression of isolated rat SCs in vitro [19,18]. cAMP-treated human SCs exhibited the expected changes in cell size and shape, including significant enlargement and flattening of the cells along with a reticulated cytoplasm (Fig. 4a, see S100 and p75NGFR immuno-detection). Similar to rat SCs, cAMP-treated human SCs acquired high levels of nuclear Krox-20 expression (Fig. 4b,c), which is a cAMP-dependent transcription factor linked to the onset of myelination [36]. In addition to this, and as evidenced by the lack of EdU labeling, cAMP-treated human SCs acquired a growth arrested (non-proliferative) state even under conditions supportive of maximal proliferation (i.e. medium containing 10% FBS and chemical mitogens), (Fig. 5a,b).
Figure 4.
Differentiation of human and rat SCs induced by prolonged treatment with CPT-cAMP. SCs were plated at low density and treated with CPT-cAMP (250 μM) under conditions non-supportive of proliferation, as described in Methods. Cultures were analyzed by immunofluorescence microscopy using the antibodies indicated in the figure 7 days (human SCs) and 4 days (rat SCs) after CPT-cAMP administration. As opposed to rat SCs, cAMP-treated human SCs exhibited morphological changes (see S100 and p75NFGR staining in panel a, and O4 staining in panel b) and high levels of Krox-20 expression (b–c). Nonetheless, they only moderately elevated O4 (c) without enhancing O1 (a,c) or reducing p75NGFR expression (a). The heterogeneity of O4 expression in human SC cultures was not revealed on the basis of S100 or p75NGFR expression even after CPT-cAMP treatment. *p<.05, **p<.01 and ***p<.001 (t-Test). Arrowheads in panel b indicate O4 negative, Krox-20 positive human SCs.
Figure 5.
Induction of growth arrest and O4 expression in response to prolonged treatment with CPT-cAMP in human and rat SCs. SCs were plated in mitogens medium (control), treated with CPT-cAMP (250 μM) for 7 days (human SCs) and 4 days (rat SCs), respectively, and analyzed for EdU incorporation (a,b) and O4 expression (c,d) via fluorescence microscopy. Similar to rat SCs, cAMP-treated human SCs acquired a growth arrested (non-proliferative) state (a,b, EdU incorporation). Contrary to rat SCs, human SCs only moderately elevated O4 (c,d) despite prolonged incubation with CPT-cAMP. In (d), representative cultures of human SCs (cauda equine) from three different donors were analyzed in parallel to rat SC cultures to reveal their levels of O4 expression under the conditions described above. Donor information was registered as follows (from left to right): 53 yr, male; 18 yr, male; 33 yr, male. Representative results derived from 2 independent rounds of experimentation are shown. *p<.05, **p<.01 and ***p<.001 (t-Test). Arrowheads in panel c indicate O4 negative human SCs.
Despite these changes, human SCs failed to reduce the expression of p75NGFR (Fig. 4a), an early event that highlights the transition into a myelinating phenotype [37]. Most strikingly, cAMP-treated human SCs did not increase the expression of myelin-specific markers such as O1 (galactocerebroside, Figs. 4a,c), periaxin (Prx, Fig. 4a), and MAG (not shown). Assessment of O4 expression in various human SC populations exposed to CPT-cAMP treatment confirmed a modest increase in the proportion of O4 positive cells in the absence (Fig. 4b,c) or presence of mitogenic factors and serum (Fig. 5c,d). By contrast, >95% of cAMP-treated rat SCs displayed a strong O4 positive phenotype regardless of the presence of serum and mitogenic factors (Figs. 4, b,c and 5c,d), [38,19]. It is worth mentioning that efficient upregulation of O4 (or other myelinating SC markers) was not observed despite using human SCs from various donors at low or high passage (Fig. 5d), plating the cells at different densities, including growth factors or extending the time of culture (not shown).
To conclude, human SCs only partially undergo cAMP-induced differentiation under conditions that are optimal for myelin gene expression in rat cells. Strikingly, human SCs changed their morphology, exited the cell cycle and induced Krox-20 expression without re-activating the expression of key myelinating SC markers or reducing p75NGFR expression.
Human SCs did not establish a stable physical interaction with axons: preference for SC-SC association and lack of axon contact-dependent alignment, proliferation and differentiation
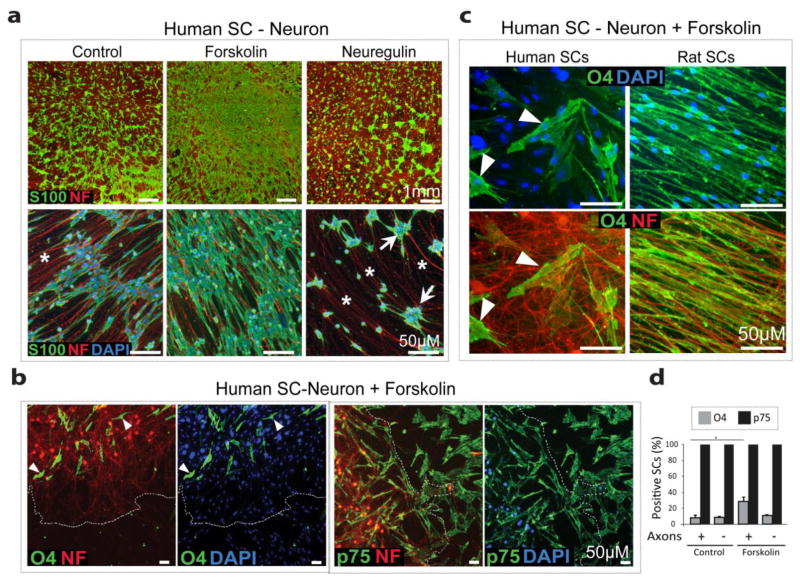
Cultures of SCs and DRG neurons have been extensively used to investigate the cellular and molecular basis of SC proliferation, differentiation and myelin synthesis. All of these events are known to be dependent on the ability of SCs to establish extensive physical contact with the axonal membrane, which is required for direct (juxtacrine) axon-to-SC signaling [39,40]. Thus, to test the ability of SCs to physically interact, proliferate and differentiate in response to axon contact, we established SC-DRG neuron cultures by seeding a single cell suspension of human SCs onto purified dissociated DRG neurons. Rat SCs were processed identically and used as reference controls due to their well-known ability to proliferate and differentiate upon axon contact under our experimental conditions [30,18]. Results indicated that human SCs were impaired to readily adhere to, extend processes along the axons and acquire the bipolar, elongated morphology characteristic of axon-associated SCs (Fig. 6) regardless of the donor, passage number or nerve source (not shown).
Figure 6.
Failure of human SCs to establish a stable physical association with sensory axons. (a–b) Differential adhesion properties of human and rat SCs placed in co-culture with DRG neurons. SCs were seeded as a single cell suspension onto cultures of purified, dissociated DRG neurons in medium non-supportive of SC proliferation, as described in Methods. Cells were fixed 2 days after co-culture initiation (a) or imaged live at the times indicated (b). In a, cultures were stained with antibodies against S100 (green) and neurofilament (NF, red, axonal marker) to reveal the SC cytoplasm and the axonal outgrowth, respectively. Images from phase contrast (a, upper panels and b) and immuno-fluorescence microscopy (a, lower panels) from representative areas are provided. As expected, regional variability was noticeable. Whereas rat SCs readily extended processes along the axons and acquired the typical bipolar shape, human SCs formed numerous cell clumps (ab, arrowheads) that increased in size and number over time and ultimately gave rise to tightly packed spheroid structures (b, arrows). Note that extensive axonal areas (NF) were left without ensheathment by SC processes in human but not rat SC-neuron cultures (a,b, asterisks). Formation of SC spheroids contributed to the eventual loss of the cultures due to substratum detachment, which most often progressed from the periphery to the center of the culture well (b). (c) Effective adhesion and alignment of human and rat SCs. Single cell suspensions of lentivirally infected human SCs (GFP, green) and rat SCs (mCherry, red) were placed in co-culture at the proportions indicated in the figure in mitogens medium. Low and high magnification pictures of the live cells were taken 3 days after co-culture initiation to show the pattern of alignment (left panels) and the elongated morphology of individual cells (right panels), respectively. Observe that human and rat SCs (white arrowheads) effectively intermingled with one another to produce a stable configuration that was maintained over time.
Human SCs extensively migrated along the neurite outgrowth and formed numerous cellular aggregates (Fig. 6a–b, arrowheads) that acquired a roughly spherical shape with time (Fig. 6b, arrows). The migratory behavior of human SCs readily led to axon bundling, as many axons were left deprived of SC processes (Fig. 6a,b, asterisks). Prolonged co-culture inevitably led to widespread SC clumping and substratum detachment, which usually started in peripheral areas and progressed rapidly towards to the center of well (Fig. 6b). Spontaneous reversal of this phenomenon or clump disaggregation was not observed in any case. Indeed, substratum detachment was the most important determinant for the eventual loss of human SC-neuron cultures.
To further test whether cell clumping was an attribute of human SC adhesion to rodent cells in general, human and rat SCs were made distinguishable from one another by overexpression of GFP and mCherry, respectively, which was achieved through lentiviral infection prior to co-culture initiation (Fig. 6c). Labeling with fluorescent proteins allowed us to not only properly identify each cell type but also analyze their capacity for SC-SC adhesion and alignment by live cell fluorescence microscopy. Contrary to the above mentioned phenomenon of axon segregation, effective integration, alignment and pattern formation between human and rat SCs was observed within 24 hours and maintained over several days without signs of instability or clump formation (Fig. 6c). Consistent with the lack of effective adhesion to axons, axon contact-dependent human SC proliferation and differentiation did not occur regardless of the characteristics of the human SC population used to initiate the co-culture. This is shown by the representative images of EdU incorporation assays using human SCs from different donors (Fig. 7a). Contrary to rat SCs, human SCs did not upregulate or maintain O4 expression when placed in co-culture with DRG neurons (Fig. 7b). The failure of human SCs to proliferate and/or increase O4 expression was an expected consequence (and a clear indication) of impaired axon-to-SC signaling via membrane contact, as axons are potent inducers of mitogenesis and the O4 phenotype in SCs in vitro and during development [38].
Figure 7.
Failure of human SCs to undergo proliferation and increase O4 expression in response to axonal growth factors. (a) Human SCs from 3 different donors (Human-1 to 3) and rat SCs were seeded as a single cell suspension onto cultures of DRG neurons in medium non-supportive of SC proliferation containing EdU labeling reagent (Methods). Cells were fixed 2 days after co-culture initiation and processed for EdU detection (EdU, red nuclei). Corresponding images of areas containing equivalent neuronal density are provided, as visualized by phase contrast (righ panels) and fluorescence microscopy (left panels). Note that rat but not human SCs proliferated extensively. EdU incorporation in human SC-DRG neuron cultures was negligible (<0.5%) regardless of the source of human SCs used. Cells from over 10 donors were tested in independent rounds of experimentation with identical results. Image data from 3 representative cultures are shown, as follows: Human-1 (29 yr, female), Human-2 (10 yr, female), and Human-3 (66 yr, male). (b) Human and rat SCs were analyzed for O4 expression at 1 and 4 days after being plated onto DRG neurons under the conditions described in (a). The relatively high levels of O4 expression present in the initial human SC populations was lost within 4 days of co-culture initiation (right panels). Human SC migration, clumping and detachment prevented extended co-culture. In panel (b), cells from a 29 yr, female were used. Arrows highlight representative neuronal bodies; arrowheads, areas of aggregation of SC nuclei; asterisks, axonal areas devoid of SC processes.
Growth factor supplementation was not sufficient to promote human SC association or differentiation in response to axonal cues
Additional experiments revealed that the formation of SC aggregates on the axons, along with the lack of proliferation was independent of the initial levels of expression of O4 in the human SC cultures, the type of substrate used to establish the neuronal cultures, or donor-specific features (see examples provided in Fig. 7a). Cell aggregation occurred over a time period that ranged from a few hours to a few days after co-culture initiation as determined primarily by the following factors: (1) the initial plating density of the human SCs, as higher cell densities led to faster clumping and detachment (not shown); and (2) the addition of supplements to the culture medium (Fig. 8a). In particular, provision of cAMP-stimulating agents such as forskolin (Fig. 8) or CPT-cAMP (not shown) prior to or at the time of human SC plating onto the neurons delayed, but did not prevent, SC aggregation. Provision of soluble neuregulin, on the contrary, exacerbated human SC migration and consequently, accelerated cell clustering and detachment of the cultures (Fig. 8a, right panels). Provision of L-ascorbic acid (vitamin C), which is a critical factor for collagen synthesis and basal lamina assembly by SCs [41], had no apparent effect either when provided alone or together with serum and/or cAMP-stimulating agents. Indeed, an organized basal lamina or deposition of extracellular matrix (ECM) components were absent in ascorbate-treated human SC-neuron cultures (not shown), consistent with available data [9]. Axon-related human SCs did not efficiently enhance the expression of O4, O1 or MAG in spite of the presence of cAMP-inducing agents (Fig. 8b–d, shown only for O4). The rarely seen O4 positive human SCs did not properly align along the axons or acquired a bipolar shape (Fig. 8c, left panels). This is in contrast to axon-related rat SCs that extended processes along the axons and maintained high levels of O4 expression either in the absence or presence of forskolin (Fig. 7b and 8c, right panels). Despite a subset of human SC cultures exhibited some apparent alignment along the axons (Fig. 7b, left panels), this conformation was transient (Fig. 7b, right panels). Human SCs usually migrate rapidly on the axons and accumulate in clumps of a wide range of shapes and sizes (Supplementary Fig. 3).
Figure 8.
Effect of forskolin and neuregulin on human SC-neuron cultures. Co-cultures were left untreated (control) or treated with neuregulin (10 nM) or forskolin (2 μM) for 3 days, as indicated. Cultures were fixed, stained as described in the figure, and visualized by fluorescence microscopy at low (a, upper panels, and b) and high magnification (a, lower panels, and c). Forskolin reduced SC migration and delayed cell aggregation (a) but poorly increased O4 expression in axon-related human SCs (c–d). Contrastingly, neuregulin caused rapid migration of SCs into dense clusters (arrows, a), thus leaving most axons devoid of SC processes. These axonal areas are denoted by the asterisks. In (b), SCs were stained with p75NGFR antibodies (human-specific) to reveal the location of the human SCs both within and outside the neurite extension. Dotted lines in (b) were traced to demark the limit of the neurite outgrowth. A quantification of the proportion of O4 positive human SCs with respect to the total number of SCs (p75NGFR positive SCs) in axon-containing and axon-free areas is depicted in panel (d). No O1 expression was detected under these conditions despite using cells from a variety of donors. Notice that rat SCs display high and homogeneous levels of O4 expression and an elongated morphology under identical culture conditions (c).
To summarize, the inability of human SCs to properly adhere to, proliferate and differentiate into an O4 positive phenotype in response to axon contact provided a strong rationale for the myelination defects reported previously [9]. Given that human SCs effectively aligned and maintained their processes along those of rat SCs, we conclude that heterophilic interaction with axons rather than homophilic interaction with SCs (human or rat) was the primary defective attribute in the human SC cultures.
RNA-seq profiling revealed only partial overlap between the human and rat transcriptomes: differential expression of S100 isoforms, myelin-related genes and other SC-specific transcripts
Human and rodent SCs shared a common basic core of SC-specific attributes but diverged significantly in function. To investigate the molecular basis for the distinct behavior of human SCs, we used the latest transcriptome sequencing technology, RNA-seq, and bioinformatics analysis as a means to describe and compare the molecular signatures of the rat and human SCs (Fig. 9). Two representative populations of adult nerve-derived, highly purified rat and human SC cultures growing in the presence of mitogenic factors were processed for RNA isolation and sequencing. As identified by the algorithms of all three statistical programs (BaySeq, edgeR, and DESeq), there were 7,245 coding genes and 39 non-coding RNAs (including long intergenic non-coding RNAs) that were differentially expressed in rat versus human SCs out of a total of 16,483 genes that were included in the analysis (Fig. 9a). Of the differentially expressed transcripts, 3,471 were upregulated and 3,813 were down-regulated in rat SCs compared to human SCs. As illustrated by the general graphical representation (Fig. 9b, heat-map), changes were extensive across many discrete genes. To our surprise, the percentage of differentially expressed RNAs in the human and rat transcriptomes was estimated to be as high as 44.19 %.
Figure 9.
RNA-seq analysis of human and rat SCs. (a) Venn diagram representation of statistical analysis of RNA-seq data showing the number of genes found to be differentially expressed by three different statistical analysis programs. (b) Heat-map showing relative representation of differentially expressed genes clustered according to expression pattern across samples (3 per group). Relative expression is shown in z-scores per gene where red represents high expression and blue represents low expression. (c–h) Representation of total (bar graphs) and differentially expressed genes (heat-maps) in assigned categories. Individual sequence reads for each gene were aligned, counted and normalized to the total number of reads in each sample.
It was also surprising to find that nearly all gene families known to represent the SC phenotype contained a proportion of differentially expressed genes. One example was the wide repertoire of S100 isoforms with multiple variants of the A-type being expressed prevalently in rat SCs and the B-type (used as SC marker, see Fig. 2) in human SCs (Fig. 9c). Several myelin-specific genes were predominantly expressed in rat SCs (Fig. 9e), in line with the immunodetection profiles of myelin-related proteins and lipids shown in Figs. 4, 5 and 8. Examples include transcripts encoding myelin-associated proteins such as myelin protein-zero or MPZ (Fig. 9e, left panel) and enzymes involved in myelin lipid metabolism, such as ATP-citrate lyase or ACLY (Fig. 9e, right panel), which is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA required for the generation of elongated fatty acids in myelin sphingolipids. The relationship between the levels of expression of mRNA for Krox-20 (Egr2) and c-Jun (JUN, an inhibitor of myelination) was inverted in human and rat SCs. Whereas rat SCs expressed higher basal levels of Krox-20 mRNA, human SCs expressed higher basal of c-Jun (Fig. 9f). Human SCs were superior than rat SCs in regards to the expression of neurotrophin receptors such as p75NGFR, and neurotrophic factors, such as NGF, GDNF and BDNF (Fig. 9g), that are crucial for SC-mediated nerve repair and promotion of axon growth after injury [42]. As expected based on the common neuregulin-dependent control of SC proliferation (Fig. 1), the transcripts encoding ErbB2 and ErbB3 were well represented in the human transcriptome with negligible representation of ErbB4 and EGFR (Fig. 9d).
Pathway analysis of human SC RNA-seq data highlighted critical deficiencies in signal transduction pathways controlling differentiation, adhesion and cytoskeletal remodeling
To better understand the cause for the differentiation defects of human SCs, and narrow down the scope of the present study, a focus was placed on gene transcripts responsible for axon contact-dependent signal transduction in SCs. By using pathway enrichment bioinformatics tools we found that the following two ubiquitous pathways had many components that were downregulated (or deficiently expressed) in human SCs with respect to those of the rat: (1) The adenylyl cyclase (AC)/cAMP signaling pathway (Supplementary Fig. 4); and (2) The ECM- and adhesion-dependent control of cytoskeletal organization and dynamics (Fig. 10).
Figure 10.
Pathway analysis of adhesion signaling. (a) Schematic diagram of the adhesion-dependent control of cytoskeletal organization and dynamics. The diagram highlights some of the molecular intermediates that were found to be deficiently expressed in human SCs (grey elements). The gene families/isoforms were highlighted by MetaCore™ pathway enrichment analysis, and subsequently identified by individual searches in the transcriptome database. This selection was based on relative levels of expression and current literature of SC biology. (b–i) Quantitative changes in gene expression of selected transcripts/transcript isoforms. Molecules involved in cell-cell and cell-ECM interactions: cadherins (b), CAMs (c), and integrin receptors (d). Intracellular transducers of adhesion signaling: FAK, talin, paxillin, vinculin, cofilin and LIMK (e). Structural cytoskeletal proteins: actin (f), tubulin (g), neurofilament low (NEFL) and medium (NEFM) (h), GFAP (h), vimentin and plectin (i). Genes of interest were indicated with their statistical significance, as follows: (**), differentially expressed genes identified by all statistical programs (BDE); (*), differentially expressed genes identified by two statistical programs (DE).
Within the cAMP pathway we observed that human SCs exhibited a deficient expression of transcripts encoding for various AC isoforms (Supplementary Fig. 4c), A-Kinase anchoring proteins, AKAPs (Supplementary Fig. 4d), and transducers of cAMP signaling such as the exchange protein-activated by cAMP (EPAC, Rap-GEF, Supplementary Fig. 4h) and its direct downstream effector, the small G protein Rap1 (Supplementary Fig. 4i). This expression profile, along with the high levels of cAMP-specific PDEs such as PDE4-B and PDE4-D in human SCs (Supplementary Fig. 4e), suggest that both cAMP biosynthesis and transduction through key effectors required for SC differentiation [30] may be compromised in human SCs. Despite these differences, the following components of the cAMP pathway exhibited high levels of expression in both groups: (1) the adhesion receptor Gpr126 (Supplementary Fig. 4b), which is the only known GPCR that signals through cAMP to control myelination [43]; (2) the catalytic and regulatory subunits of the cAMP-dependent protein kinase (PKA) (Supplementary Fig. 4f); and (3) the cAMP-responsive transcription factor CREB (Supplementary Fig. 4g), which are critical mediators of cAMP-dependent SC proliferation [16,30,44].
Multiple deficiencies in cytoskeletal remodeling networks were observed in human SCs, including the expression of cell surface molecules involved in binding to other cells and/or the ECM such as members of the cell adhesion molecule (CAM) family (Fig. 10c) Yet, human SCs displayed high levels of expression of cadherins such as cadherin-19 (CDH19, Fig. 10b), which are involved in homophilic cell-cell binding [45,22] and are highly expressed in SC precursors [46]. Human SCs exhibited high levels of expression of the mRNA encoding for integrin receptor family members such as β1- and α6-integrin (Fig. 10d), which are well-characterized components of the laminin receptor in SCs [47]. However, key transducers of integrin-mediated signal transduction to the cytoskeleton were deficiently expressed in human SCs, as shown for the transcripts of focal adhesion kinase (FAK), talin, paxillin, vinculin and cofilin (Fig. 10e). These molecules, which form part of an effective signal transduction module linking the cell surface to the cytoskeleton, are responsible for SC motility and myelin membrane wrapping [48]. A lower representation of transcripts encoding key cytoskeletal elements was identified in human SCs. Examples include the expression of β-tubulin (TUBB, Fig. 10g) and intermediate filaments, such as GFAP, the low (NEFL) and medium (NEFM) chains of neurofilament (Fig. 10h), and vimentin (VIM, Fig. 10j). Plectin, an intermediate filament-associated scaffolding protein involved in compartmentalization and stabilization of the myelin sheath [49] was also significantly reduced in human SCs (Fig. 10). The ubiquitous markers β-actin (ACTB, Fig. 10f) and α-tubulin (TUBA, Fig. 10g) are shown as controls for equal mRNA expression in both types of cells.
Differential expression of adhesion molecules, transducers of adhesion signaling and cytoskeletal elements may underly the preference of human SCs to interact with other SCs rather than axons
One of the most striking features of human SCs revealed by cell-based assays was their affinity for other SCs as opposed to axons. The property of homophilic binding was manifestated not only by the clustering of human SCs but also the exclusion of the axons. This phenomenon was clearly observable in human SC-neuron cultures visualized by TEM, which revealed many overlapping SC processes and extensive areas of membrane-to-membrane apposition between adjacent SCs (Fig. 11a, left panel). Not only axons were left without ensheathment but they were segregated and confined into discrete pockets (Fig. 11a, right panel) under a thick layer of SC processes stacking on top of each other. This behavior of human SCs in co-culture provides a basis for the formation of loosely attached SC aggregates as well as the detachment of the axons from the laminin substrate. TEM analysis also revealed the human SCs to be characterized by an elaborated cytoplasm that contained multiple intracellular vesicles, phagosomes and enlarged lysosomes (Fig. 11a), consistent with the recently reported phagocytic activity of human SCs [11]. Importantly, aggregated human SCs displayed no signs of impaired viability.
Figure 11.
(a) Ultrastructural evidence of SC aggregation and axonal exclusion in human SC-DRG neuron cultures. TEM imaging of a representative culture 3 days after co-culture initiation. The low magnification panel on the left shows multiple layers of SC processes (SC) and exclusion of the axonal outgrowth (Ax, arrows) towards the outer surface of the culture. Axons were grouped together within small pocket areas (inset, a′) under a thick layer of SC processes loosely attached to the laminin substrate (bottom left area in the image). Extensive areas of direct SC-SC membrane apposition were found whereas SC adhesion to axons was poor. Notice that the SC cytoplasm featured many intracellular phagosome-like structures and enlarged lysosomes (L). SC heath was maintained, as revealed by the integrity of the cytoplasm and nucleus (N). (b) Western blot profiles of selected proteins involved in adhesion signaling in human and rat SCs.
Given that our traditional and transcriptomics approaches both inferred adhesion signaling to underlie the unique responses of human SCs to axon contact, we used Western blot to assess the levels of expression of some key molecules highlighted by RNA-seq to potentially mediate adhesion signaling in human SCs. For this, human SC samples from 2 different donors were processed along with an equal protein sample from rat SCs growing under identical conditions and the intensity of the immunoreactive bands obtained for any given marker was compared qualitatively (Fig. 11b) and quantitatively via densitometric analysis (Supplementary Fig. 5). Western blot was used to validate the results because the protein expression profiles of signal transduction molecules provide a stronger link to cell function than simple mRNA levels. As expected, human SCs exhibited exhacerbated immunoreactivity for cadherin-related proteins (including N-cadherin) when compared to rat samples, along with higher levels of β1-integrin. On the contrary, rat SCs were enriched in immunoreactive bands for antibodies against CAMs (including N-CAM and L1-CAM) and intermediate filament molecules such as GFAP and neurofilament. Noticeably, the low and medium chains of neurofilament were nearly undetected in human SCs, essentially as highlighted by RNA-seq (Fig. 10h). This may explain the human SC inability to organize and/or maintain elongated processes along the axonal axis. Variable but still reasonably lower levels of FAK, paxillin and vinculin were seen in samples of human SCs with respect to those of the rat, which may be linked to reduced transduction of adhesion-dependent signals from the membrane to the cytoskeleton in human SCs.
Collectively, these studies revealed the power and usefulness of RNA-seq analysis in combination with cell-based assays to understand human SC function in vitro and identify signal transduction modules likely to underlie the atypical response of human SCs to axon-contact and adhesion signaling.
DISCUSSION
As summarized in Table 1, the in vitro assessment of proliferation, differentiation and SC-axon interactions revealed that human SCs only partially mimic those derived from rats. These species-specific features illustrate the unique nature of human SCs and may explain the reasons for their limited differentiation potential in vitro.
Table 1.
Comparison of human and rat SC cultures based on their performance in standard functional assays of proliferation, differentiation (with CPT-cAMP) and SC-axon interactions.
| Functional Assay | Attribute | Human | Rat |
|---|---|---|---|
| Proliferation | Responsiveness to soluble neuregulin and serum factors | Yes | Yes |
| Synergistic action of cAMP on neuregulin-induced proliferation | Yes | Yes | |
| Responsiveness to PDGF, insulin, IGF-1 and FGF | No | Yes | |
| Senescence-associated growth arrest | Yes | No | |
| Extended passage or immortalization in vitro | No | Yes | |
|
| |||
| Differentiation | Heterogeneity of O4 expression | Yes | No |
| Morphological differentiation and cell cycle exit | Yes | Yes | |
| Enhancement of Krox-20 expression | Yes | Yes | |
| Enhancement of O1, periaxin and MAG expression | No | Yes | |
| Reduction of p75NGFR expression | No | Yes | |
|
| |||
| SC-Axon Interactions | Attachment and process extension along DRG neurites | No | Yes |
| Migration and clump formation on the axons | Yes | No | |
| Axon contact-induced proliferation | No | Yes | |
| Axon contact-induced O4 expression | No | Yes | |
| Axon ensheathment, basal lamina formation and myelination | No | Yes | |
Differentiation and adhesion deficits in cultured human SCs
Previous attempts to establish in vitro systems consisting of myelinating human SCs rendered suboptimal [10] or negative results [9], as human SCs do not maintain neuronal health or axon ensheathment in cultures of rat or human neurons. Whereas human DRG axons are successfully ensheathed and myelinated by rat SCs, they do not support human SC growth and basal lamina formation [9], indicating that intrinsic properties of the human SCs rather than a species mismatch between SCs (human) and neurons (rat) underlie the deficits in myelination. Despite human SCs effectively elongated processes along those of other SCs (human or rat), they exhibited an impaired early response to axon contact, as extensive process elongation, axon-contact dependent proliferation or differentiation did not occur. Substantial SC migration and formation of sphere-like structures restricted the long-term maintenance of human SC-neuron cultures regardless of the origin of the human SCs or the use of human DRG neurons as substrate (not shown). Not only were human SCs defective in associating with axons but they also consistently failed to efficiently acquire O4 expression in response to cAMP stimulation and/or co-culture with DRG neurons, which are two inducers of the O4 phenotype in rodent SCs [38,33]. Importantly, O4 positive human SCs did not proliferate or maintain O4 expression when plated on an axonal substrate, possibly due to the lack of proper adhesion to axons. These observations, along with the incomplete activation of myelin gene expression in response to cAMP, suggest human SCs to be fairly undifferentiated. Extensive migration and SC-SC association are features typical of immature SCs [50]. It has been reported that human SCs display a proteomics and transcriptomics signature consistent with the repair SC phenotype on the basis of their high levels of c-Jun expression, active myelin degradation and incorporation of cell-extrinsic components via phagocytosis [11]. Bioassays that can faithfully model myelination are not available for primary nerve-derived human SCs. Human SC aggregation could be prevented temporarily via provision of cAMP-stimulating agents. Yet, other factors were required, as cAMP elevation was not sufficient to concurrently foster proliferation and/or differentiation of axon-related human SCs. We have interpreted the clumping behavior as sign of lack of adhesion of human SCs to the axons, as provision of non-adherent conditions is sufficient to create SC spheroids that offer resemblance to the ones reported herein [51]. Restoring the expression of heterophillic adhesion molecules on the SC surface may be crucial, as data is consistent with a SC deficiency to express adhesion molecules/receptors for axon recognition rather than an axonal deficiency to express adhesion molecules/receptors for SCs.
Transplantation studies have supported the differentiation potential of cultured human SCs in models of peripheral nerve injury [52,53] and the fact that still-to-be identified factors are necessary for human SC myelination to occur in vitro. Whereas an extensive body of literature has shown that rat SCs myelinate regenerated axons in the injured spinal cord, to the best of our knowledge, human and rat SCs have not been compared side-by-side in transplantation studies. A recent study that introduced xenografts of human SCs into the injured (contused) rat spinal cord showed long-term transplant survival, greater volumes of preserved white matter, axon ingrowth in the lesion site and myelination [14]. Nevertheless, the peripheral myelin within the transplants was most likely provided by endogenous SCs, as no evidence of human myelin was found on the basis of human-specific myelin protein-zero immunodetection [14].
Human SC phenotyping for basic and clinical use: the impact of heterogeneity and donor variability
Obtaining human SCs from peripheral nerves is still the preferred method for clinical applications [5]. One advantage of using adult nerves for human SC isolation is that sufficient numbers of SCs can be produced using a modest nerve segment. The other advantage is that SCs can be purified and greatly expanded in number in a relatively short time. Nonetheless, the underlying heterogeneity and variability of individual human SC batches cannot be overlooked when appreciating the responses of these cells in vitro and in vivo after transplantation [14]. For the purpose of clinical cell therapy, the need to obtain an independent preparation of cells for each individual recipient imposes a critical challenge for cell characterization and potency assay development. Human SCs display subsets of proliferating, senescent and O4 negative cells in various proportions under conditions that allow rat SCs to homogeneously express O4 and extensively proliferate without undergoing senescence. Coincidently, human SCs have a limited lifespan and stop proliferating once reaching a certain number of passages [54,55], while rodent SCs immortalize without losing their characteristics of normal primary cells [56].
Many factors can account for these differences as well as the variability observed in the human populations. SCs from peripheral nerves are intrinsically a heterogeneous group of glial cells in terms of lineage specification, as both myelinating and non-myelinating phenotypes may be represented in different proportions according to the type of nerve. Cell culture methods may favor the growth of specific sub-types and/or introduce a variety of phenotypic or functional alterations, such assenescence-dependent cell cycle arrest, that can affect the properties of the cells. As a result, the final populations may diverge quite significantly from the cells of origin. Each human nerve is unique. Factors such as age, gender, ethnicity, medical condition, and lifestyle choices of the donor cannot be ruled out when appreciating the complexity of the starting material. Facing multiple sources of known and unknown variability is a common aspect when investigating the properties of human cells and tissues in general. Special challenges are specific to the use of SCs, as the availability of technologies to target and identify SCs in a rigorous manner still lag behind those available for other cell types.
Despite their heterogeneity, the attributes of human SCs summarized in Table 1 were invariable and independent of donor-specific features. The fact that cells from a wide range of donors displayed notorious deficits in axon binding and myelin gene reactivation indicate that these features were general rather than specific of a given human SC population. Representative cultures of rat SCs competent to myelinate axons were used as reference controls for the effectiveness of treatment and validation of outcome measures in all experiments. Accumulated research using rat SCs have confirmed their responsiveness to differentiating cues irrespective of developmental stage [20], gender, strain and method used for isolation [1].
Novel approaches for cell identification
Work with cultured SCs has been interpreted under the assumption that SCs derived from different nerve sources, species and/or developmental stages should display similar properties in vitro, including proliferation controls and potency for lineage specification [57]. Another long-standing assumption is that in vitro expansion of SCs in the absence of neurons maintains the original characteristics of the cells [29]. Novel data indicates that SCs are a more extensive family of closely related phenotypes than initially assumed. In fact, some features of the SCs in vivo may persist in vitro, as SC cultures may vary significantly in their ability to support regeneration as a function of the type and location of the nerve tissue used to derive the SC populations [58].
The advent of gene microarrays and whole transcriptome sequencing has recently enabled a broader and unambiguous description of a cell’s RNA expression profile. Our RNA-seq comparison of the human and rat SC transcriptomes allowed us to identify species-specific differences relevant to human SC characterization that would not have been resolved otherwise by traditional methods. The information extracted from the RNA-seq data not only supported and complemented that of cell-based assays but also predicted the involvement of candidate molecules within the cAMP and adhesion signaling pathways to potentially mediate human SC-axon interactions and differentiation. Despite a subset of genes could be confirmed at the protein level via immunological methods, whether any given gene product underlies a functional feature in the human SCs remains to be determined by an independent and systematic cell signaling approach. Indeed, additional RNA-seq studies are needed to confirm our findings and address the influence of factors such as age and gender on donor variability.
Conclusions and perspectives
A vast literature on cultured SCs dating from the 1950s has paved the way to the conception of our modern ideas on SC development and myelination. However, much work is still needed to validate these concepts in human models. We conclude from these studies that traditional methods for cell characterization are not sufficient to reveal the diversity and special properties of human SCs, as these only partially mimic those derived from experimental animals. Our side-by-side comparison of human and rat SCs highlighted species-specific differences not previously appreciated that hinder a direct comparison of human and animal cells along with the possibility to test the potency of human SCs using bioassays set up for rodent cells. From the practical perspective, our data supports the feasibility and significance of using high resolution genomic methods such as RNA-seq to more thoroughly characterize the SC phenotype and establish identity-function correlates predictive of therapeutic value. A better understanding of the human SCs as well as the development of human specific-tests is needed for the evaluation of their bioactivity. In view of our still limited understanding of the diversity and special properties of SCs derived from human donors, the concepts and methodologies presented here may prove useful in furthering our understanding and potential applications of endogenous peripheral nerve human SCs and those derived from from stem cell sources via in vitro differentiation.
Supplementary Material
Supplementary Figure 1: Characterization of contaminating fibroblasts in human SC cultures. (a) Discrimination of SCs and fibroblasts by means of cell type-specific immunolabeling with S100 (red) and fibronectin (green) antibodies. (b) Fibroblast proliferation in response to serum growth factors.
Supplementary Figure 2: Representative image of a confluent human SC culture co-immunostained with antibodies against S100 (red) and p75NGFR (green).
Supplementary Figure 3: Human SCs plated onto purified cultures of DRG neurons. Human SC aggregates (spheroids) can adopt a wide range of shape and sizes within the culture area, as illustrated in the images selected from a typical culture 3 days after co-culture initiation. SCs and axons were identified by S100 (green) and neurofilament (NF) immunostaining (red), as described in Figure 8a.
Supplementary Figure 4: Pathway analysis of cAMP signaling. (a) Schematic diagram highlighting the molecular intermediates found to be deficiently expressed in human SCs with respect to those of the rat (grey objects). The gene families/isoforms were highlighted by MetaCoreTM pathway enrichment analysis, and subsequently identified by individual searches in the transcriptome database. Selection was based on relative levels of expression and current literature of SC biology. (b–i). Quantitative changes in gene expression of selected transcripts encoding GPCRs (b), ACs (c), AKAPs (d), PDEs (e), PKA (f), CREB (g), EPAC (h) and Rap1 (i). Whereas elements within the PKA-CREB signaling seemed to be well represented in human SCs, a deficiency in EPAC-Rap1 was apparent. This profile is consistent with the synergistic responses to cAMP in proliferation rather than differentiation in human SCs. Genes of interest were indicated with their statistical significance: **, differentially expressed genes identified by all statistical programs (BDE); *, differentially expressed genes identified by two statistical programs (DE). Brackets indicate transcripts not expressed in the human transcriptome.
Supplementary Figure 5: Western blot profiles of selected proteins involved in adhesion signaling in human and rat SCs. The data in the bar graphs corresponds to the densitometric analysis of Western blot bands presented in Figure 11b.
Acknowledgments
We thank the technical assistance provided by Ketty Bacallao, Michael McGrath, Talia Robinson, Kristine Ravelo and Natalia Andersen with cell culture and Derek Van Booven with RNA-seq. We acknowledge the human SC manufacturing team of The Miami Project to Cure Paralysis for evaluating the purity and viability of cell stocks and Yan Shi for assisting in automated image analysis. We greatly appreciate the contribution of Patrick Wood for communicating unpublished data from many years of research and facilitating access to cryopreserved stocks of human SCs, hybridoma cell lines and antibodies. We thank the important contribution made by Mary Bunge and Margaret Bates on TEM studies. This work was supported by the National Institutes of Health Grants 1R21NS084326 (to P.V.M.), 1R01NS089525 (to G.W.), The Craig H. Neilsen Foundation (339576 to P.V.M.), The Miami Project to Cure Paralysis and The Buoniconti Fund. The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Andersen ND, Srinivas S, Pinero G, Monje PV. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci Rep. 2016;6:31781. doi: 10.1038/srep31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KD, Guest JD, Dietrich WD, Bunge MB, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerstrom-Noga E, Wood P, Levi AD. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci U S A. 1995;92(5):1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi AD, Bunge RP, Lofgren JA, Meima L, Hefti F, Nikolics K, Sliwkowski MX. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15(2):1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge M, Monje PV, Khan A, Wood PM. From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Handbook of Clinical Neurology (Spinal Cord Injury) 2017 doi: 10.1016/bs.pbr.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci. 1989;9(2):625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17(4):327–338. doi: 10.1002/(SICI)1098-1136(199608)17:4<327::AID-GLIA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Levi AD. Characterization of the technique involved in isolating Schwann cells from adult human peripheral nerve. J Neurosci Methods. 1996;68(1):21–26. doi: 10.1016/0165-0270(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Morrissey TK, Bunge RP, Kleitman N. Human Schwann cells in vitro. I. Failure to differentiate and support neuronal health under co-culture conditions that promote full function of rodent cells. J Neurobiol. 1995;28(2):171–189. doi: 10.1002/neu.480280205. [DOI] [PubMed] [Google Scholar]
- 10.Morrissey TK, Kleitman N, Bunge RP. Human Schwann cells in vitro. II. Myelination of sensory axons following extensive purification and heregulin-induced expansion. J Neurobiol. 1995;28(2):190–201. doi: 10.1002/neu.480280206. [DOI] [PubMed] [Google Scholar]
- 11.Weiss T, Taschner-Mandl S, Bileck A, Slany A, Kromp F, Rifatbegovic F, Frech C, Windhager R, Kitzinger H, Tzou CH, Ambros PF, Gerner C, Ambros IM. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia. 2016;64(12):2133–2153. doi: 10.1002/glia.23045. [DOI] [PubMed] [Google Scholar]
- 12.Ravelo K, Andersen N, Monje P. Magnetic-activated cell sorting for the fast and efficient separation of human and rodent Schwann cells from mixed cell populations. Methods in Molecular Biology Schwann cells: methods and protocols. 2017 doi: 10.1007/978-1-4939-7649-2_6. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Monje PV, Bartlett Bunge M, Wood PM. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia. 2006;53(6):649–659. doi: 10.1002/glia.20330. [DOI] [PubMed] [Google Scholar]
- 14.Bastidas J, Athauda G, De La Cruz G, Chan WM, Golshani R, Berrocal Y, Henao M, Lalwani A, Mannoji C, Assi M, Otero PA, Khan A, Marcillo AE, Norenberg M, ADL, Wood PM, Guest JD, Dietrich WD, Bartlett Bunge M, Pearse DD. Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia. 2017;65(8):1278–1301. doi: 10.1002/glia.23161. [DOI] [PubMed] [Google Scholar]
- 15.Fregien NL, White LA, Bunge MB, Wood PM. Forskolin increases neuregulin receptors in human Schwann cells without increasing receptor mRNA. Glia. 2005;49(1):24–35. doi: 10.1002/glia.20091. [DOI] [PubMed] [Google Scholar]
- 16.Monje PV, Athauda G, Wood PM. Protein kinase A-mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. J Biol Chem. 2008;283(49):34087–34100. doi: 10.1074/jbc.M802318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinero G, Berg R, Andersen ND, Setton-Avruj P, Monje PV. Lithium Reversibly Inhibits Schwann Cell Proliferation and Differentiation Without Inducing Myelin Loss. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacallao K, Monje PV. Requirement of cAMP signaling for schwann cell differentiation restricts the onset of myelination. PLoS One. 2015;10(2):e0116948. doi: 10.1371/journal.pone.0116948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monje PV, Rendon S, Athauda G, Bates M, Wood PM, Bunge MB. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57(9):947–961. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monje PV, Soto J, Bacallao K, Wood PM. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem. 2010;285(40):31024–31036. doi: 10.1074/jbc.M110.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- 22.Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J Neurosci. 2002;22(10):4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto J, Monje PV. Axon contact-driven Schwann cell dedifferentiation. Glia. 2017;65(6):864–882. doi: 10.1002/glia.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anders S, Pyl PT, Huber W. HTSeq - A Python framework to work with high-throughput sequencing data. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardcastle TJ, Kelly KA. baySeq: empirical Bayesian methods for identifying differential expression in sequence count data. BMC Bioinformatics. 2010;11:422. doi: 10.1186/1471-2105-11-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolsky Y, Kirillov E, Zuev R, Rakhmatulin E, Nikolskaya T. Functional analysis of OMICs data and small molecule compounds in an integrated “knowledge-based” platform. Methods Mol Biol. 2009;563:177–196. doi: 10.1007/978-1-60761-175-2_10. [DOI] [PubMed] [Google Scholar]
- 29.Porter S, Clark MB, Glaser L, Bunge RP. Schwann cells stimulated to proliferate in the absence of neurons retain full functional capability. J Neurosci. 1986;6(10):3070–3078. doi: 10.1523/JNEUROSCI.06-10-03070.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacallao K, Monje PV. Opposing roles of PKA and EPAC in the cAMP-dependent regulation of schwann cell proliferation and differentiation. PLoS One. 2013;8(12):e82354. doi: 10.1371/journal.pone.0082354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbacci EG, Guarino BC, Stroh JG, Singleton DH, Rosnack KJ, Moyer JD, Andrews GC. The structural basis for the specificity of epidermal growth factor and heregulin binding. J Biol Chem. 1995;270(16):9585–9589. doi: 10.1074/jbc.270.16.9585. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AJ, Davis JB, Stroobant P, Land H. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol. 1989;109(6 Pt 2):3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 34.Bianchini D, De Martini I, Cadoni A, Zicca A, Tabaton M, Schenone A, Anfosso S, Akkad Wattar AS, Zaccheo D, Mancardi GL. GFAP expression of human Schwann cells in tissue culture. Brain Res. 1992;570(1–2):209–217. doi: 10.1016/0006-8993(92)90583-u. [DOI] [PubMed] [Google Scholar]
- 35.Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24(4):548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- 36.Zorick TS, Lemke G. Schwann cell differentiation. Curr Opin Cell Biol. 1996;8(6):870–876. doi: 10.1016/s0955-0674(96)80090-1. [DOI] [PubMed] [Google Scholar]
- 37.Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112(3):457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirsky R, Dubois C, Morgan L, Jessen KR. 04 and A007-sulfatide antibodies bind to embryonic Schwann cells prior to the appearance of galactocerebroside; regulation of the antigen by axon-Schwann cell signals and cyclic AMP. Development. 1990;109(1):105–116. doi: 10.1242/dev.109.1.105. [DOI] [PubMed] [Google Scholar]
- 39.Wood PM, Bunge RP. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]
- 40.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 41.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105(2):1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunge MB, Wood PM. Realizing the maximun potential of Schwann cells to promote recovery from spinal cord injury. Handbook of Clinical Neurology. 2012;109 doi: 10.1016/B978-0-444-52137-8.00032-2. (Spinal Cord Injury) [DOI] [PubMed] [Google Scholar]
- 43.Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33(46):17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MM, Badache A, DeVries GH. Phosphorylation of CREB in axon-induced Schwann cell proliferation. J Neurosci Res. 1999;55(6):702–712. doi: 10.1002/(SICI)1097-4547(19990315)55:6<702::AID-JNR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Luo J, Redies C. Cadherin-19 expression is restricted to myelin-forming cells in the chicken embryo. Neuroscience. 2010;165(1):168–178. doi: 10.1016/j.neuroscience.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 46.Wanner IB, Guerra NK, Mahoney J, Kumar A, Wood PM, Mirsky R, Jessen KR. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006;54(5):439–459. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- 47.Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156(1):199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, Bates ML, Bunge MB, Lambert S, Fernandez-Valle C. The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci. 2012;32(15):5284–5297. doi: 10.1523/JNEUROSCI.6207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walko G, Wogenstein KL, Winter L, Fischer I, Feltri ML, Wiche G. Stabilization of the dystroglycan complex in Cajal bands of myelinating Schwann cells through plectin-mediated anchorage to vimentin filaments. Glia. 2013;61(8):1274–1287. doi: 10.1002/glia.22514. [DOI] [PubMed] [Google Scholar]
- 50.Wanner IB, Mahoney J, Jessen KR, Wood PM, Bates M, Bunge MB. Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia. 2006;54(5):424–438. doi: 10.1002/glia.20389. [DOI] [PubMed] [Google Scholar]
- 51.Vadivelu RK, Ooi CH, Yao RQ, Tello Velasquez J, Pastrana E, Diaz-Nido J, Lim F, Ekberg JA, Nguyen NT, St John JA. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci Rep. 2015;5:15083. doi: 10.1038/srep15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14(3 Pt 1):1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stratton JA, Kumar R, Sinha S, Shah P, Stykel M, Shapira Y, Midha R, Biernaskie J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. eNeuro. 2017;4(3) doi: 10.1523/ENEURO.0307-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emery E, Li X, Brunschwig JP, Olson L, Levi AD. Assessment of the malignant potential of mitogen stimulated human Schwann cells. J Peripher Nerv Syst. 1999;4(2):107–116. [PubMed] [Google Scholar]
- 55.Casella GT, Wieser R, Bunge RP, Margitich IS, Katz J, Olson L, Wood PM. Density dependent regulation of human Schwann cell proliferation. Glia. 2000;30(2):165–177. doi: 10.1002/(sici)1098-1136(200004)30:2<165::aid-glia6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Mathon NF, Malcolm DS, Harrisingh MC, Cheng L, Lloyd AC. Lack of replicative senescence in normal rodent glia. Science. 2001;291(5505):872–875. doi: 10.1126/science.1056782. [DOI] [PubMed] [Google Scholar]
- 57.Wewetzer K, Radtke C, Kocsis J, Baumgartner W. Species-specific control of cellular proliferation and the impact of large animal models for the use of olfactory ensheathing cells and Schwann cells in spinal cord repair. Exp Neurol. 2011;229(1):80–87. doi: 10.1016/j.expneurol.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 58.Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Experimental Neurology. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Characterization of contaminating fibroblasts in human SC cultures. (a) Discrimination of SCs and fibroblasts by means of cell type-specific immunolabeling with S100 (red) and fibronectin (green) antibodies. (b) Fibroblast proliferation in response to serum growth factors.
Supplementary Figure 2: Representative image of a confluent human SC culture co-immunostained with antibodies against S100 (red) and p75NGFR (green).
Supplementary Figure 3: Human SCs plated onto purified cultures of DRG neurons. Human SC aggregates (spheroids) can adopt a wide range of shape and sizes within the culture area, as illustrated in the images selected from a typical culture 3 days after co-culture initiation. SCs and axons were identified by S100 (green) and neurofilament (NF) immunostaining (red), as described in Figure 8a.
Supplementary Figure 4: Pathway analysis of cAMP signaling. (a) Schematic diagram highlighting the molecular intermediates found to be deficiently expressed in human SCs with respect to those of the rat (grey objects). The gene families/isoforms were highlighted by MetaCoreTM pathway enrichment analysis, and subsequently identified by individual searches in the transcriptome database. Selection was based on relative levels of expression and current literature of SC biology. (b–i). Quantitative changes in gene expression of selected transcripts encoding GPCRs (b), ACs (c), AKAPs (d), PDEs (e), PKA (f), CREB (g), EPAC (h) and Rap1 (i). Whereas elements within the PKA-CREB signaling seemed to be well represented in human SCs, a deficiency in EPAC-Rap1 was apparent. This profile is consistent with the synergistic responses to cAMP in proliferation rather than differentiation in human SCs. Genes of interest were indicated with their statistical significance: **, differentially expressed genes identified by all statistical programs (BDE); *, differentially expressed genes identified by two statistical programs (DE). Brackets indicate transcripts not expressed in the human transcriptome.
Supplementary Figure 5: Western blot profiles of selected proteins involved in adhesion signaling in human and rat SCs. The data in the bar graphs corresponds to the densitometric analysis of Western blot bands presented in Figure 11b.