Abstract
Stress increases risk for psychopathology, and diet may moderate the impact of stress on mental health. A “Western” diet has been linked to psychopathology in humans; animal studies also show that diet can influence negative valence behavior in the presence or absence of stress, but findings are inconsistent. Contradictions in existing studies may result from differences in macronutrient content of diets and presence of metabolic syndrome. The present study exposed mice to 10 days of high fat or high sucrose diet concurrent with social defeat stress exposure and examined negative valence behavior at acute (<five days) and long-term (>30 days) time points after stress/diet exposure. Predictably, stress increased negative valence behavior in the social interaction, open field, elevated zero maze, and tail suspension tests at the acute time point. While most stress-induced behaviors normalized after the 30-day recovery period, social avoidance was still highly significant for stress-exposed mice, supporting the hypothesis that avoidance of a trauma-related cue persists beyond non-specific anxiety-like behaviors. Supporting the hypothesis that an unhealthy diet contributes to psychopathology, non-stressed mice fed high fat or high sucrose diets spent less time exploring the center of the open field. This effect was no longer present after a 30-day recovery. Intriguingly, mice previously fed either high fat or high sucrose diets exhibited increased rearing behavior in the elevated zero maze 30 days post stress and diet exposure. This finding could be evidence that short-term diet administration can initiate a long-term increase in risk-assessment behavior.
Keywords: Social defeat stress, High fat diet, High sucrose diet, Negative valence
Highlights
-
•
Social stress increased negative valence in short-term behavioral tests.
-
•
Social avoidance persisted for stress exposed mice thirty days post stress exposure.
-
•
Unhealthy diet decreased exploration in the center of the open field.
-
•
Non-stress, control diet mice had the least anxiety-like behavior in open field.
-
•
Unhealthy diets increased rearing behavior 30 days post stress exposure.
1. Introduction
Exposure to chronic stress or a traumatic event increases the risk of developing psychiatric disorders, most notably major depressive disorder (MDD) and post-traumatic stress disorder (PTSD). Increasingly, researchers are also recognizing the importance of diet in mental health. A “Western” diet high in fat and sugar has been linked to psychopathology in humans (Jacka et al., 2010a, 2010b; Sinclair et al., 2016). A diet rich in processed meats, sugary foods, and high-fat dairy products is associated with an increased risk for depression (Akbaraly et al., 2009). Even after controlling for socioeconomic status and age, men who consumed a diet high in processed meats, fried food, and carbohydrates had a higher prevalence of symptoms of depression (Le Port et al., 2012). Longitudinal studies show that women who consume a diet high in sugar have a higher incidence of depression (Gangwisch et al., 2015). Experimentally, HSD administered to healthy weight and overweight humans resulted in higher depressive symptoms for both groups (Breymeyer et al., 2016).
The effects of diet on stress-sensitivity have also been explored in animal models, but results are inconsistent. Some studies support the hypothesis that an “unhealthy” but palatable diet decreases the impact of stress exposure. For example, when C57BL/6J mice were fed high fat diet (HFD, 45% kcal fat, Research Diets), the effects of a social defeat stress (SDS) x overcrowding procedure were minimized, measured by decreased depressive-like behavior in the forced swim test (FST) and decreased anxiety-like behavior in the light-dark box, compared to controls fed a low fat diet (LFD, 10% kcal fat, Research Diets) (Finger et al., 2011). Similarly, Wistar rats fed HFD (61% kcal fat) were protected from effects of SDS compared to rats fed a low fat, high carbohydrate diet (63% kcal carbohydrate), measured by normal locomotor activity and normalized body weight gain (Buwalda et al., 2001). Access to palatable diet with 45% kcal fat content also reduced stress-sensitivity in juvenile Sprague-Dawley rats exposed to seven days SDS (MacKay et al., 2017).
Other studies support the hypothesis that HFD exacerbates the impact of stress on behavioral and endocrine outcomes and have deleterious effects on behavior even without additional stressors. For example, in C57BL/6J mice, SDS plus 30 days on a HFD (42% kcal fat) led to greater social avoidance in the social interaction test compared non-stressed mice or SDS mice on control diet (4% kcal fat) (Chuang et al., 2010). C57BL/6J mice fed HFD (60.3% kcal fat, Harlan Laboratories) had increased anxiety-like behavior in the open field test (OF) and elevated zero maze (EZM), cognitive impairment in the Y maze, and increased depressive-like behavior in the FST compared to controls fed a standard diet (18% kcal fat, Harlan Laboratories) (Almeida-Suhett et al., 2017). In mice, six weeks of HFD (58% kcal fat) caused anhedonia in a sucrose-preference test and anxiety-like behavior in the elevated plus maze (EPM) compared to mice fed an ingredient-matched low fat control diet (11% kcal fat) (Sharma et al., 2013). Similarly, 12 weeks of HFD (58% kcal from fat, research diets) decreased time in the open arms of the EPM and time in the center of the OF, and increased immobility in the FST (Sharma and Fulton, 2013). Two months of HFD (45% kcal fat) in CD-1 mice increased negative valence behaviors in the OF and hole board test, but actually decreased immobility in the FST (Del Rosario et al., 2012).
While many diet x stress reports focus on HFD, high sucrose diets (HSD) may also increase risk for psychopathology in humans, and HSD has also been shown to modify the impact of stress in animal studies. Twelve weeks of HSD (74.2% carbohydrate, 5.8% fat, 20% protein, custom made of condensed milk, sugar, and Labina chow diet) in BALB/c mice, exacerbated the impact of 2-h restraint stress on anxiety-like behavior in the EPM, enhanced aversive memory in a fear conditioning task, and increased depression-like behavior in the tail suspension test (TST) (Santos et al., 2016). In absence of stress, Wistar Hannover rats fed HSD (25% sucrose) had increased anxiety-like behavior in the OF (Pinto et al., 2016). Other studies show that HSD decreases anxiety- and depressive-like behavior. Thirteen weeks on a custom-made “cafeteria” diet high in both fat and sugar decreased Swiss mice immobility in the FST and TST and increased time in the open arms of the EPM (Leffa et al., 2015).
The majority of animal studies employ long-term diet administration, which causes numerous downstream effects including metabolic syndrome, complicating the interpretation. Although less studied, short-term diet administration is also associated with neurological, physiological, and behavioral impacts. For example, impaired novel object recognition was observed after just one week of HFD (Gainey et al., 2016), and 10-days of HFD altered the cellular composition of the arcuate nucleus of the hypothalamus in mice (Balland and Cowley, 2017). Furthermore, previous research has suggested that the influence of HFD on anxiety-like behavior may vary by duration of diet exposure (Sweeney et al., 2017), providing additional rationale for evaluating the impact of a shorter term diet administration.
Very few studies have compared the influence of more than one diet on behavior, some animal models neglect to identify the source and macronutrient content of diets (e.g. (Baran et al., 2005)), others use a HFD that also has a high sucrose content (e.g. (Finger et al., 2011)), and still others employ a “control” LFD that is actually high in sucrose (e.g. (Balsevich et al., 2014)), highlighting the need for additional research on this topic. The present study employs a high fat/low sucrose diet (HFD), a high sucrose/low fat diet (HSD), and a control diet (chow). This study tests the hypothesis that unhealthy diets will influence sensitivity to social stress and allows us to determine if the effects differ for HSD and HFD. To test this hypothesis, mice are exposed to 10-days of SDS with concurrent exposure to one of the three diets. Tests of anxiety- and depressive-like behavior are conducted at acute and long-term time points following SDS and diet exposure.
2. Materials and methods
2.1. Animals and housing
All procedures and protocols were reviewed and approved by the Grand Valley State University Institutional Animal Care and Use Committee (IACUC). In accordance with IACUC, all efforts were made to minimize pain, suffering, and number of animals used.
Male C57BL/6N (C57, n = 78) mice were purchased from Charles River Laboratories (Portage, MI) at four weeks old and were seven weeks old (∼20g) at the start of testing. Male CD-1 retired breeder mice (CD-1 n = 29) were purchased from Harlan (Lansing, MI). Mice were housed in polycarbonate cages with wire tops. Mice were housed four to a cage prior to group assignment. At the start of the study, non-stress (NS) mice were housed two per cage-one on each side of a Plexiglas divider. During the 10-day SDS procedure, SDS mice were housed in a separate room in the home cage of a CD-1 mouse but separated with a Plexiglas divider. After the 10-day SDS procedure, SDS mice were moved back to the original housing room and were housed two per cage-one on each side of a Plexiglas divider. At this time, CD-1 mice were also housed in the same room, but on a different cage rack than the experimental mice.
Water and food were available ad libitum. Both housing rooms used in this study were temperature and humidity-controlled with a 12 h light-dark cycle (lights on at 21:00).
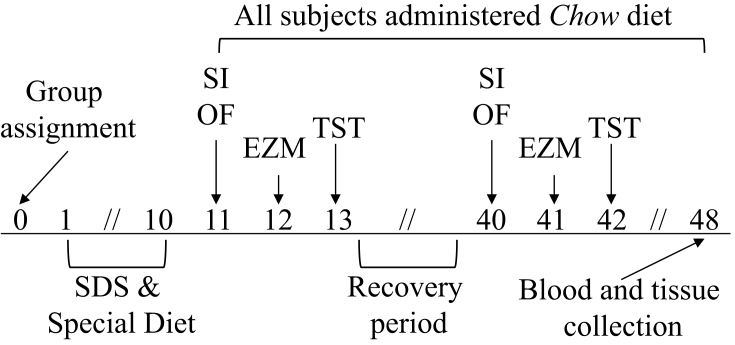
In a 3 × 2 design, mice were assigned to SDS or NS and one of three diets: Chow, HSD, or HFD (n = 11–14/group). Group assignments are balanced for baseline body weight and baseline behavior in the OF (See Supplement). Content and nutritional details of control Chow, HSD, and HFD are shown in Table 1. Experimental timeline is shown in Fig. 1.
Table 1.
Dietary content.
| Chow | HSD | HFD | ||
|---|---|---|---|---|
| Fat | %Kcal | 11.41 | 10.52 | 58.02 |
| Source | Cholesterol, linoleic acid, arachidonic acid, omega-3 fatty acids, porcineanimal fat | Soybean oil and hydrogenated coconut oil | Soybean oil and hydrogenated coconut oil | |
| Sucrose | %Kcal | 3.25 | 60.16 | 12.66 |
| Source | Cane molasses | Maltodextrin 10 and unspecified source | Maltodextrin 10 and unspecified source | |
| Protein | %Kcal | 24.13 | 16.44 | 16.44 |
| Source | Unspecified | Casein and DL-methionine | Casein and DL-methionine | |
| Minerals | Calcium, Phosphorus, Potassium, Magnesium, Sulfur, Sodium, Chloride, Fluorine, Iron, Zinc, Manganese, Copper, Cobalt, Iodine, Chromium, Selenium. | Calcium, Magnesium, Potassium Citrate, Potassium Sulfate, sodium chloride, Chromium Potassium Sulfate, Cupric Carbonate, Potassium Iodate, Iron, Manganous Carbonate, Sodium Selenite, and Zinc Carbonate. | ||
| Vitamins | Carotene; Vitamin K, A, D, and E; Thiamin; Riboflavin; Niacin; Pantothenic acid; Choline Chloride; Folic acid; Pyridoxine; Biotin; and Ascorbic acid. | Vitamin A, D3, E, B12, B6, B2, and B1; Menadione, Biotin, Folic acid, Niacin, and Pantothenic acid. | ||
| Product Information | 50017 Labdiet, St. Louis, MO | D123298 Research Diets Inc., New Bruswick, NJ | D123318 Research Diets Inc., New Bruswick, NJ | |
Fig. 1.
Experimental timeline.
2.2. Food intake and body weight
Mouse body weights and food weights were recorded daily during the ten days of SDS, during short term and long term behavioral testing. Any other time of the study, mice and food were weighed every other day.
2.3. Social defeat stress
SDS was conducted during the dark cycle under red light and was similar to that published in Nature Protocols (Golden et al., 2011). SDS procedure performed in a room separate from NS mice; SDS mice were also housed in this separate room for the 10 days/nights. Intruder C57 mice were placed into the home cage of a CD-1 mouse for a 5-min exposure. After the physical exposure, C57's and CD-1's were separated by a perforated Plexiglas divider for the remaining 24 h, allowing sensory but not physical contact. This procedure was repeated for ten days with a novel CD-1 aggressor each day. Because we observed an increase in violent behavior across the ten days, we employed a strategy to minimize physical injuries to the C57 mice similar to previous reports (e.g. (Goto et al., 2014)). Specifically, each defeat session was observed in real time and the session was paused or stopped to limit physical injuries. This meant that physical exposure was limited to 15–60 s intervals during the 5-min session with decreased intervals each day.
2.4. Behavioral tests
Behavioral tests were performed during the dark phase of the light cycle, beginning at least 1 h after lights off. Subjects were habituated to the testing room for at least 1 h before testing. Testing rooms had background white noise of approximately 60 dB. For OF and SI tests, videos were taken from above with a Fujifilm YV5x2-7R4B2 and sent to a connected Dell Inspiron 15 PC. For EZM, videos were taken from above with two Logitech HD Webcams C615 and sent to a connected Dell Inspiron 15 PC. For the TST, videos were taken with a Sony HDR-CX405 HD Handycam and later transferred to a Dell Inspiron 15 PC.
2.4.1. Open field test
The OF test was performed in a beige Multi Field OF Maze made of ABS plastic. Each of the four arenas are 1′ x 1′ x 1′3” (San Diego Instruments; San Diego, CA). Each arena was illuminated by floor lamps to approximately 80 lux. Mice were placed into the center of the arena. Total locomotion and percent time in the center were calculated for the 5-min test.
2.4.2. Social interaction test
Immediately following the OF, mice were tested for social avoidance. Mice were placed into the OF arena which now contained an empty wire-mesh cage placed against an inner wall (social target absent phase). After 5 min, mice were removed from the arena and placed into a temporary holding cage. A novel CD-1 aggressor mouse was then placed under an identical wire-mesh cage and the C57 was returned to the arena for an additional 5 min (social target present phase). Social interaction ratio was calculated as time in the interaction zone with the Social Target Present vs. time spent in the interaction zone with the Social Target Absent.
2.4.3. Elevated zero maze
Overhead lights illuminated the open areas of the maze to approximately 320 lux. Mice were placed into a closed area of a beige EZM made of ABS plastic and an outer wall circumference of 75.4″ and inner wall circumference of 62.8” (San Diego Instruments; San Diego, CA) and allowed to explore for 5 min. Total locomotion and percent time in the open area were calculated. Rearing behavior was manually keyed in real time by a research student blind to the treatment conditions. Due to the labor-intense nature of this endeavor, we were unable to score all mice. In the first EZM, rearing behavior was not recorded for half of the subjects (Day 12; n = 33 included). In the second EZM, rearing behavior was not recorded for 10 subjects (Day 41; n = 68 included).
2.4.4. Tail suspension test
The testing room was illuminated by overhead light to approximately 400 lux. Tails were taped to a horizontal beam of a ring stand elevated 50 cm from the surface. Latency to immobility and time immobile were scored during the 6-min trial by a research student blind to the treatment conditions. Percent time immobile was calculated. On day 42, we were unable to collect data from three mice because the tape came off the tails before the 6 min were complete (n = 75 included).
2.5. Data analysis
Behavior in the OF, SI, and EZM was tracked in real time using ANY-maze (Stoelting Co.; Chicago, IL). TST videos were scored manually using the “observer” mode program in ANY-maze. Statistical analysis was conducted using IBM SPSS Statistics V22.0 (Armonk, NY). Two-way analysis of variance (ANOVA) was performed for main effect of stress, main effect of diet, and stress × diet interaction. Post-hoc Tukey analyses were performed where appropriate for data with a significant F-statistic. Figures were created with GraphPad Prism (San Diego, California). Results are reported as significant at the 0.05 level.
3. Results
3.1. Social interaction
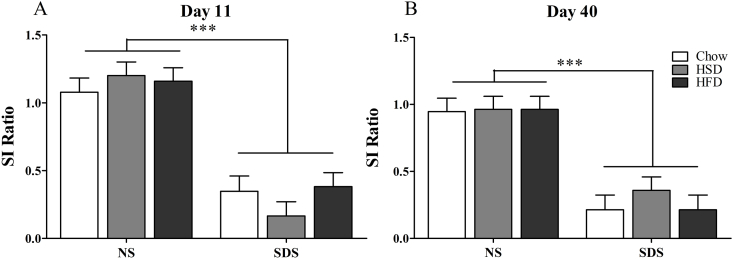
Mice exposed to SDS exhibited elevated social avoidance as measured by decreased social interaction ratio one day following SDS, on experimental day 11 (See Timeline, Fig. 1) (main effect of stress ANOVA F(1,72) = 99.60, p < 0.001, Fig. 2A). This effect persisted 30 days later, on experimental day 40 (F(1,72) = 62.62, p < 0.001, Fig. 2B). Data showed no effect of diet at either time point as a main effect or an interaction with stress.
Fig. 2.
Social interaction. SDS-exposed mice exhibit decreased social interaction compared to NS controls at both acute (A) and long-term (B) time points. There was no effect of diet. (n = 11–14 per group; *p < 0.05; **p < 0.01; ***p < 0.001).
3.2. Open field
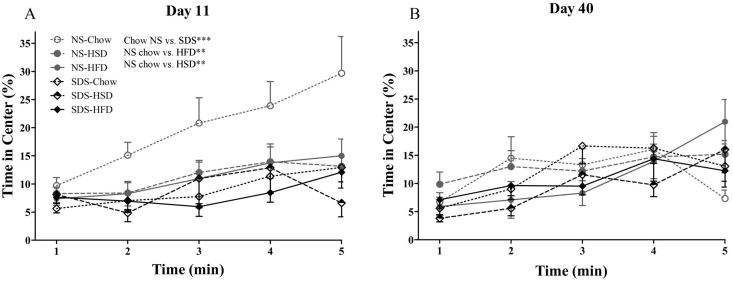
One day following SDS (experimental day 11) chow fed mice in the NS group spent the most time exploring the center of the OF while NS mice on HFD and HSD behaved similarly to stress-exposed mice (Fig. 3A). Examining percent time in OF over the 5 min trial; the difference between groups appears to grow and there was a significant interaction between stress and diet (between subject effects of repeated measures ANOVA: (F(2,72) = 3.747, p = 0.028, Fig. 3A). Post-hoc Bonferroni analysis of the interaction revealed that, within the NS group, chow-fed mice spent significantly more time in the center of the OF compared to mice fed HFD (p = 0.003) and HSD (p = 0.003). Similarly, within the chow-fed diet group, there was a significant difference between NS and SDS mice (p < 0.001). Within the SDS group, there were no significant effects of diet and within the other diet groups, there were no significant effects of stress. Thirty days later, this stress × diet interaction was no longer present (ANOVA F(2,72) = 0.313, p = 0.732, Fig. 3B).
Fig. 3.
Percent time in center of open field across 5-min test. NS mice on chow diet spend more time exploring the center of the OF than SDS-exposed mice (all diets) and NS mice on the HFD or HSD (A) but was no longer observed at day 40 (B; n = 11–14 per group; *p < 0.05; **p < 0.01; ***p < 0.001).
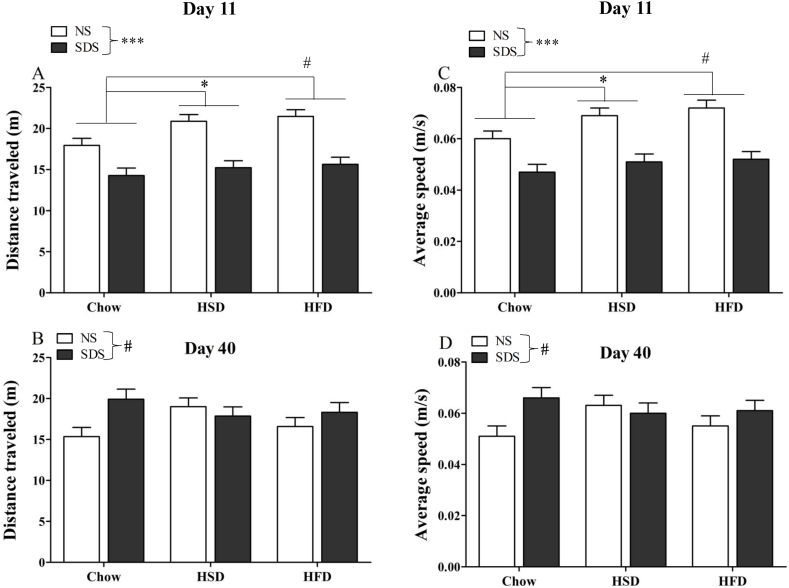
Total distance traveled and speed of locomotion were lower for SDS-exposed mice than NS controls in the 5-min OF test at day 11 (main effect of stress on total distance traveled: F(1,72) = 52.87, p < 0.001, Fig. 4A; main effect of stress on average speed of locomotion: F(1,72) = 52.66, p < 0.001, Fig. 4C). On day 40, the main effect of stress was reversed; previously stressed mice trended towards increased total distance traveled (F(1,72) = 3.49, p = 0.066, Fig. 4B) and increased average speed (F(1,72) = 3.50, p = 0.066, Fig. 4D) compared to NS controls.
Fig. 4.
Open field. At day 11, there was a main effect of stress on total distance traveled (A) and average speed of locomotion (C), with stress mice covering less distance and traveling slower than NS mice. At day 40, the main effect of stress was partially reversed; previously stressed mice trended toward increased total distance traveled (B) and average speed (D). At day 11, there was also a main effect of diet on total distance traveled (A) and average speed of locomotion (C) with chow-fed mice exhibiting decreased distance and speed relative to both HSD- and HFD-fed mice. There were no effects of diet on day 40 and no stress × diet interactions at either time point (n = 11–14 per group; #p = 0.066; *p < 0.05; **p < 0.01; ***p < 0.001).
There was also a main effect of diet on total distance traveled (F(2,72) = 4.46, p = 0.015, Fig. 4A) and average speed of locomotion (F(2,72) = 4.56, p = 0.014, Fig. 4C) in the OF at day 11. Post-hoc Tukey revealed that mice on the Chow diet travel less distance than mice on the HFD (p = 0.017) or HSD (p = 0.076, trend), and had a slower average speed than HFD (p = 0.015) and HSD (p = 0.082, trend). On day 40, there was no effect of diet on distance traveled or average speed either as a main effect or as an interaction with stress.
3.3. Elevated zero maze
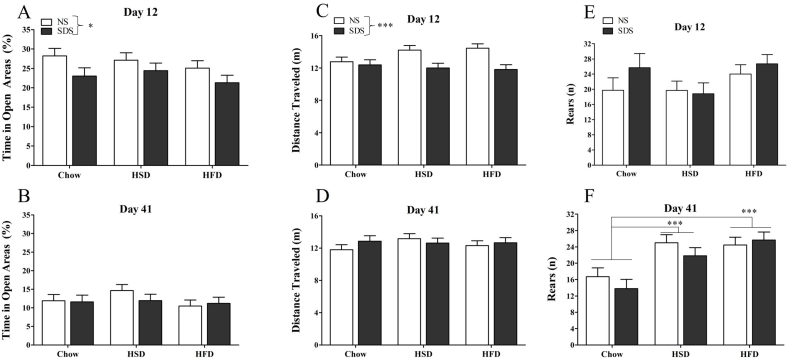
Stressed mice exhibited decreased percent time in open area of the EZM on experimental day 12 (main effect of stress: F(1,72) = 5.972, p = 0.017 Fig. 5A). This effect was not observed at day 41 (F(1,72) = 0.308, p = 0.581, Fig. 5B). There was no effect of diet for percent time in open areas at either time point either as a main effect or as an interaction with stress.
Fig. 5.
Elevated zero maze. Percent time in open areas was lower for SDS mice at day 12 (A; n = 11–14 per group), but not day 41 (B; n = 11–14 per group). Distance traveled in the EZM was lower for SDS-exposed mice at day 12 (C; n = 11–14 per group), but not 41 (D; n = 11–14 per group). Mice fed HFD and HSD reared more frequently than chow-fed mice at day 41(F; n = 9–12 per group) but not day 12 (E; n = 3–7 per group). There was no effect of diet on percent time open, or distance traveled, and no effect of stress on rearing behavior (*p < 0.05; **p < 0.01; ***p < 0.001).
Stressed mice traveled less distance in the EZM at day 12 (main effect of stress: F(1,72) = 13.169, p < 0.001, Fig. 5C) but not at day 41 (F(1,72) = 0.318, p = 0.575, Fig. 5D). There was no effect of diet on distance traveled in the EZM at either time point either as a main effect or as an interaction with stress.
There was a trend for diet to influence rearing at day 12 (main effect of diet: F(2,27) = 2.817, p = 0.077, Fig. 5E). At day 41, there was a significant main effect of diet (F(2,62) = 12.301, p < 0.001, Fig. 5F); Chow-fed mice reared less frequently compared to mice fed HSD (Post-hoc Tukey p < 0.001) and HFD (Post-hoc Tukey p < 0.001). Stress had no effect on the number of rears in the EZM at either time point either as a main effect or as an interaction with diet.
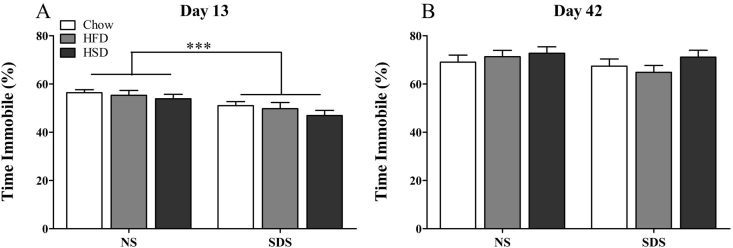
3.4. Tail suspension test
Compared to NS mice, stressed mice spent less time immobile in the 6-min TST at day 13 (F(1,72) = 13.133, p < 0.001, Fig. 6A). This effect was no longer present at day 42 (F(1,69) = 1.191, p = 0.279, Fig. 6B). Diet did not influence immobility at either time period either as a main effect or as an interaction with stress. Latency to immobility was not influenced by stress, diet, or their interaction at either time point (Supplemental Fig. 2).
Fig. 6.
Tail suspension test. SDS-exposed mice spent less time immobile than NS mice at day 13 (A; n = 11–14 per group), but not day 42 (B; n = 10–14 per group). There was no effect of diet on time immobile (***p < 0.001).
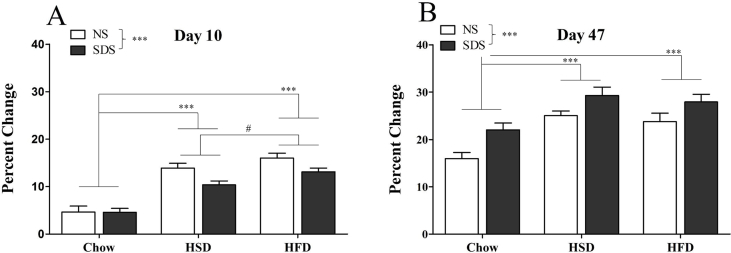
3.5. Body weight
Stressed mice gained weight more slowly than NS mice during the ten day diet and SDS exposure (day 10 main effect of stress: F(1,72) = 7.58, p < 0.001) but gained more weight than NS mice by the end of the study (day 47 main effect of stress: F(1,72) = 14.95, p < 0.001). Special-diet fed mice gained weight more rapidly during the 10-day exposure, and HFD-fed mice gained slightly more weight than HSD mice (day 10 main effect of diet: F(2,72) = 56.32, p < 0.001; Post-hoc Tukey Chow vs. HSD p < 0.001; Chow vs. HFD p < 0.001; HFD vs. HSD p = 0.032). This difference between chow and special diets was maintained even after returning to chow diet such that mice previously fed HFD and HSD still had a greater percent change than chow-fed diet at the final day of the study (day 47 main effect of diet: F(2,78) = 15.89, p < 0.001; Post-hoc Tukey chow vs. HFD p < 0.001; Chow vs. HSD p < 0.001). There was no difference in percent change body weight between mice previously fed HFD and HSD. There was no stress × diet interaction at either time point (Fig. 7).
Fig. 7.
Percent change body weight. Stressed mice gained weight more slowly than NS mice during the ten-day diet and SDS exposure (Day 10; A) but gained more weight than NS mice by the end of the study (Day 47; B). Mice fed HFD and HSD gained weight more rapidly than chow-fed mice during the 10-day exposure, and HFD mice trended towards greater weight gain than HSD mice (Day 10; A). Mice previously fed HFD and HSD also had a greater percent change in body weight than chow-fed diet at the final day of the study (Day 47; B). There was no difference in percent change body weight between mice previously fed HFD and HSD at day 47 and no stress × diet interaction at either time point (n = 11–14 per group; #p = 0.032; ***p < 0.001).
4. Discussion
The present study was designed to test the hypothesis that “unhealthy” HFD or HSD will influence behavioral sensitivity to social stress. We did identify an interaction between stress and diet on behavior in the OF test one day following SDS; NS mice on the Chow diet spent more time in the center of the OF arena than any other group while NS mice on the HSD or HFD spent no more time in the center of the OF arena than stress-exposed mice. In contrast to our original hypothesis, these data suggest that “unhealthy” diets may increase anxiety even in the absence of additional stress.
We observed several main effects of diet on behavioral outcomes. In the EZM, after the 30-day recovery period, mice previously exposed to HFD or HSD reared more frequently than chow-fed controls. The increase in rearing behavior may represent hyperactive and exploratory behavior (Crossland et al., 2017; Casarrubea et al., 2017) or increased risk-assessment (Arrant et al., 2013). These results support findings from previous studies showing that an “unhealthy” diet can influence behavior in the absence of other environmental or physiological stressors (e.g. (Almeida-Suhett et al., 2017; Sharma et al., 2013; Sharma and Fulton, 2013; Del Rosario et al., 2012)).
Main effects of stress observed in our study support previous data in this field. Shortly after stress-exposure, SDS mice almost entirely avoided social interaction, and displayed increased anxiety-like behavior as measured by decreased exploration in the OF and EZM tests. At this time point, SDS mice also displayed increased mobility in the TST compared to NS mice. While a decrease in mobility in the TST is often interpreted as increased “behavioral despair” (e.g. (Iniguez et al., 2016; Rygula et al., 2005)), our findings are consistent with other reports showing increased negative valence behaviors in approach/avoidance tasks but increased immobility in the FST (e.g. (Del Rosario et al., 2012)) and support the hypothesis that increased mobility represents “active coping” behavior (de Kloet and Molendijk, 2016), which is also promoted by elevations of the stress neuropeptide corticotropin releasing factor (CRF) (e.g. (Lu et al., 2008; Garcia-Lecumberri and Ambrosio, 2000)). After a 30-day recovery period, main effects of stress on behavior in the OF, EZM, and TST normalized while social avoidance in the SI test was still significant. These data support the hypothesis that avoidance of a trauma-related cue persists beyond non-specific avoidance behaviors and suggests that “fear generalization” did not occur in SDS-exposed mice in this study (Sauerhofer et al., 2012).
There was also a main effect of stress on percent change in body weight. SDS-exposed mice gained weight more slowly than NS mice during the ten-day stress exposure, but then over-compensated for this early loss, ultimately gaining more weight than NS mice by the final day of the study. This pattern has been observed before and was believed to be caused by a stress-induced increase in food intake (Goto et al., 2014). In the present study, we attempted to record food intake, however, many of the mice mixed small pieces of food in with bedding such that it was not possible to reliably measure the food weight.
In contrast to pilot data showing that 10-days of special diet was insufficient to alter body weight, special-diet fed mice in the present study gained weight more rapidly than chow-fed mice during the 10-day exposure. This main effect of diet on body weight was maintained even after returning to chow diet such that, at the final day of the study, mice previously fed HFD or HSD had a greater percent change in body weight than chow-fed mice.
Although differences in body weight can confound behavioral results by decreasing overall locomotor activity, the differences in body weight in the present study did not reach overweight or obesity. Furthermore, we observed an increase in rearing behavior in mice previously fed HFD or HSD relative to chow-fed mice, which suggests that increased anxiety-like behavior in the open field and elevated zero maze were not due a generalize deficit in locomotion secondary to differences in body weight.
One potential limitation in this study is that all mice were returned to chow diet one day following SDS, which meant that they were still on “special” diets during the acute OF and SI tests, but not acute EZM and TST. Therefore, the impact of diet on acute behavior in the EZM could be due to withdrawal of palatable diet as previously reported (Ulrich-Lai et al., 2015; Avena et al., 2009). However, because the impact of diet on rearing behavior in the EZM was significant 30 days after discontinuing the special diet, it is unlikely that withdrawal explains the effects of diet on acute EZM behavior.
Another limitation of this study is that the strain of mice (C57BL/6N) has elevated baseline anxiety-like behavior compared to the related C57BL/6J strain (See Supplemental Material). Therefore, it is possible that lack of effect of diet within the stress-exposed group, particularly in the open field test where an interaction between stress and diet was observed, is due to a ceiling effect.
Future studies should continue this line of research using both male and female subjects, diving deeper into the role of specific sources of macronutrients, and also exploring the impact of stress × diet interactions on endocrine and gene-expression changes that may contribute to the influence of diet on mental health.
Conflicts of interest
Authors declare no competing interests.
Funding
GVSU Catalyst.
GVSU McNair.
GVSU Psychology Department.
Role of funding source
Funding sources had no role in “the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.”
Acknowledgements
Thank you to Stephanie Phillips and Steven Northup-Smith for assistance in setting up equipment and preparing for the study. Thank you to Rachel Bomya for assisting in data analysis. Thank you to Kevin Mittner for assisting in lab set-up, data collection, and analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.05.005.
Contributor Information
Deseree M. Eudave, Email: desieudave@gmail.com.
McKenna N. BeLow, Email: Mckenna.below@gmail.com.
Elizabeth I. Flandreau, Email: flandree@gvsu.edu.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Akbaraly T.N., Brunner E.J., Ferrie J.E., Marmot M.G., Kivimaki M., Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry. 2009;195(5):408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Suhett C.P., Graham A., Chen Y., Deuster P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1beta expression in specific brain regions. Physiol. Behav. 2017;169:130–140. doi: 10.1016/j.physbeh.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Arrant A.E., Schramm-Sapyta N.L., Kuhn C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013;256:119–127. doi: 10.1016/j.bbr.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena N.M., Rada P., Hoebel B.G. Sugar and fat bingeing have notable differences in addictive-like behavior. J. Nutr. 2009;139(3):623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balland E., Cowley M.A. Short-term high-fat diet increases the presence of astrocytes in the hypothalamus of C57BL6 mice without altering leptin sensitivity. J. Neuroendocrinol. 2017;29(10) doi: 10.1111/jne.12504. [DOI] [PubMed] [Google Scholar]
- Balsevich G., Uribe A., Wagner K.V., Hartmann J., Santarelli S., Labermaier C., Schmidt M.V. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J. Endocrinol. 2014;222(1):15–26. doi: 10.1530/JOE-14-0129. [DOI] [PubMed] [Google Scholar]
- Baran S.E., Campbell A.M., Kleen J.K., Foltz C.H., Wright R.L., Diamond D.M., Conrad C.D. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16(1):39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breymeyer K.L., Lampe J.W., McGregor B.A., Neuhouser M.L. Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite. 2016;107:253–259. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B., Blom W.A., Koolhaas J.M., van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol. Behav. 2001;73(3):371–377. doi: 10.1016/s0031-9384(01)00493-0. [DOI] [PubMed] [Google Scholar]
- Casarrubea M., Faulisi F., Pensabene M., Mendola C., Dell'Utri R., Cardaci M., Santangelo A., Crescimanno G. Effects of the benzodiazepine inverse agonist FG7142 on the structure of anxiety-related behavior of male Wistar rats tested in hole board. Psychopharmacology (Berlin) 2017;234(3):381–391. doi: 10.1007/s00213-016-4474-8. [DOI] [PubMed] [Google Scholar]
- Chuang J.C., Krishnan V., Yu H.G., Mason B., Cui H., Wilkinson M.B., Zigman J.M., Elmquist J.K., Nestler E.J., Lutter M. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol. Psychiatr. 2010;67(11):1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland R.F., Balasa A., Ramakrishnan R., Mahadevan S.K., Fiorotto M.L., Van den Veyver I.B. Chronic maternal low-protein diet in mice affects anxiety, night-time energy expenditure and sleep patterns, but not circadian rhythm in male offspring. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170127. e0170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Molendijk M.L. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosario A., McDermott M.M., Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br. J. Nutr. 2012;108(7):1143–1149. doi: 10.1017/S0007114511006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger B.C., Dinan T.G., Cryan J.F. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Gainey S.J., Kwakwa K.A., Bray J.K., Pillote M.M., Tir V.L., Towers A.E., Freund G.G. Short-term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front. Behav. Neurosci. 2016;10:156. doi: 10.3389/fnbeh.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch J.E., Hale L., Garcia L., Malaspina D., Opler M.G., Payne M.E., Rossom R.C., Lane D. High glycemic index diet as a risk factor for depression: analyses from the Women's Health Initiative. Am. J. Clin. Nutr. 2015;102(2):454–463. doi: 10.3945/ajcn.114.103846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lecumberri C., Ambrosio E. Differential effect of low doses of intracerebroventricular corticotropin-releasing factor in forced swimming test. Pharmacol. Biochem. Behav. 2000;67(3):519–525. doi: 10.1016/s0091-3057(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Kubota Y., Tanaka Y., Iio W., Moriya N., Toyoda A. Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behav. Brain Res. 2014;270:339–348. doi: 10.1016/j.bbr.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Iniguez S.D., Aubry A., Riggs L.M., Alipio J.B., Zanca R.M., Flores-Ramirez F.J., Hernandez M.A., Nieto S.J., Musheyev D., Serrano P.A. Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 2016;5:54–64. doi: 10.1016/j.ynstr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka F.N., Kremer P.J., Leslie E.R., Berk M., Patton G.C., Toumbourou J.W., Williams J.W. Associations between diet quality and depressed mood in adolescents: results from the Australian Healthy Neighbourhoods Study. Aust. N. Z. J. Psychiatr. 2010;44(5):435–442. doi: 10.3109/00048670903571598. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Pasco J.A., Mykletun A., Williams L.J., Hodge A.M., O'Reilly S.L., Nicholson G.C., Kotowicz M.A., Berk M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatr. 2010;167(3):305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Le Port A., Gueguen A., Kesse-Guyot E., Melchior M., Lemogne C., Nabi H., Goldberg M., Zins M., Czernichow S. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051593. e51593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffa D.D., Valvassori S.S., Varela R.B., Lopes-Borges J., Daumann F., Longaretti L.M., Dajori A.L., Quevedo J., Andrade V.M. Effects of palatable cafeteria diet on cognitive and noncognitive behaviors and brain neurotrophins' levels in mice. Metab. Brain Dis. 2015;30(4):1073–1082. doi: 10.1007/s11011-015-9682-0. [DOI] [PubMed] [Google Scholar]
- Lu A., Steiner M.A., Whittle N., Vogl A.M., Walser S.M., Ableitner M., Refojo D., Ekker M., Rubenstein J.L., Stalla G.K., Singewald N., Holsboer F., Wotjak C.T., Wurst W., Deussing J.M. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol. Psychiatr. 2008;13(11):1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- MacKay J.C., Kent P., James J.S., Cayer C., Merali Z. Ability of palatable food consumption to buffer against the short- and long-term behavioral consequences of social defeat exposure during juvenility in rats. Physiol. Behav. 2017;177:113–121. doi: 10.1016/j.physbeh.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Pinto B.A., Melo T.M., Flister K.F., Franca L.M., Kajihara D., Tanaka L.Y., Laurindo F.R., Paes A.M. Early and sustained exposure to high-sucrose diet triggers hippocampal ER stress in young rats. Metab. Brain Dis. 2016;31(4):917–927. doi: 10.1007/s11011-016-9830-1. [DOI] [PubMed] [Google Scholar]
- Rygula R., Abumaria N., Flugge G., Fuchs E., Ruther E., Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162(1):127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Santos C.J., Ferreira A.V., Oliveira A.L., Oliveira M.C., Gomes J.S., Aguiar D.C. Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr. Neurosci. 2016:1–7. doi: 10.1080/1028415X.2016.1213529. [DOI] [PubMed] [Google Scholar]
- Sauerhofer E., Pamplona F.A., Bedenk B., Moll G.H., Dawirs R.R., von Horsten S., Wotjak C.T., Golub Y. Generalization of contextual fear depends on associative rather than non-associative memory components. Behav. Brain Res. 2012;233(2):483–493. doi: 10.1016/j.bbr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Sharma S., Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013;37(3):382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- Sharma S., Fernandes M.F., Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013;37(9):1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Millar L., Allender S., Snowdon W., Waqa G., Jacka F., Moodie M., Petersen S., Swinburn B. The cross-sectional association between diet quality and depressive symptomology amongst Fijian adolescents. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161709. e0161709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney P., O'Hara K., Xu Z., Yang Y. HFD-induced energy states-dependent bidirectional control of anxiety levels in mice. Int. J. Obes. 2017;41(8):1237–1245. doi: 10.1038/ijo.2017.112. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Fulton S., Wilson M., Petrovich G., Rinaman L. Stress exposure, food intake and emotional state. Stress. 2015;18(4):381–399. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.