Abstract
In order to evaluate the effect of inoculation and co-cultivation media elements on transformation frequency in Petunia hybrida, modified MS media with different elements were tested on Alvan and Large Flower Alvan (LF Alvan), two local cultivars. Leaf explants of both cultivars were inoculated with Agrobacterium tumefaciens strain LBA4404 (pBI121) containing neomycin phosphotransferase (nptII) and an intron-containing β-glucuronidase (gus) genes. When medium lacking KH2PO4, NH4NO3, KNO3, and CaCl2 was used as inoculation and co-cultivation medium, a higher frequency of transformation for Alvan (22%) and LF Alvan (16%) was obtained. Kanamycin resistant plantlets were stained blue by GUS assay. Furthermore, polymerase chain reaction (PCR) analysis revealed the presence of both gus and nptII genes in all putative transformants. Finally, southern blot hybridization confirmed insertion of 1–4 copies of gus gene in transgenic plants.
Keywords: Macro elements, Co-cultivation, Agrobacterium tumefaciens, Transformation, Petunia hybrida
Introduction
Petunia (Petunia hybrida), a dicotyledonous plant in family of Solanaceae, is one of the most popular ornamental plants which is valuable due to its beautiful and fragrant flowers. Petunia is used to extend novel varieties in bedding plant market (Gerats and Vandenbussche 2005). Moreover, it has become an important model plant because of its fast growth habit, short life cycle, clear genetic background, small genome and simple tissue operation method (Drummond et al. 2009). This species also attracted experimental interests in several studies such as biochemical pathway and flavonoid synthesis, floral development, transposon, self-incompatibility, male sterility and retroelement activity (Gerats and Vandenbussche 2005).
A. tumefaciens is widely used in plant transformation system. There are several reports for Agrobacterium-mediated transformation of Petunia cultivars (Fraley et al. 1984; Van der et al. 1999; Lutke 2006; Wylie et al. 2003; Li et al. 2013). The improvement of transformation efficiency in Petunia can facilitate genetic modification studies (Thirukumaran et al. 2009; Azadi et al. 2016). Host recognition by Agrobacterium is comprised of two independent processes including virulence gene activation and attachment to the host cell (McCullen and Binns 2006; Subramoni et al. 2014). Many factors including inoculation and co-cultivation media composition have a significant effect on Agrobacterium mediated transformation efficiency (Azadi et al. 2016). Removing some elements such as NH4NO3 (Hoshi et al. 2004), CaCl2 (Montoro et al. 2000) or all inorganic elements (Dupre et al. 2000) and also KH2PO4, NH4NO3, CaCl2 and KNO3 (Azadi et al. 2010a; Sharafi et al. 2014) from inoculation and co-cultivation media, significantly enhanced the transformation frequency in plant studied species. Ions have an important role in bacterial attachment to plants (Romantschuk 1992). CaCl2 might mediate bacteria or plant cell responses which impede the process of gene transfer (Montoro et al. 2000). Also the Ca+2 could hamper bacterial virulence genes to be expressed (Flego et al. 1997). The mechanism of the ion inhibitory effect on T-DNA transfer into plant cells remains unknown. In many plant species when half-strength salts MS medium was used as co-cultivation medium (Phelep et al. 1991; Tzfira et al. 1996; Machado et al. 1997; Zhang et al. 2003) the highest percentage of Agrobacterium-mediated gene transformation was obtained. One-tenth-strength MS medium has also led to the high percentage of transformation in wheat (Cheng et al. 1997; Ke et al. 2002).
In this study, the effect of eliminating macro and micro elements, vitamins and iron from inoculation and co-cultivation media on efficiency of gus gene transformation in Petunia was investigated.
Materials and methods
Plant material and bacterial strain
Seeds of two local cultivars of P. hybrida ‘Alvan’ and ‘Large Flower Alvan (LF Alvan)’ were sterilized by 70% ethanol for 30 s and sodium hypochlorite solution for 10 min, including a few drops of Tween 20. They were rinsed three times with sterile water and dried on sterile filter paper. Seeds were cultivated in MS medium containing 30 g/l sucrose and solidified with 7 g/l agar [Duchefa, Haarlem, The Netherlands]. The pH was adjusted to 5.8 with 1 N NaOH prior to autoclaving (121 °C for 20 min). Cultures were incubated at 25 ± 2 °C with 16/8 h photoperiod at 40 μmol m−2 s−1. Five weeks after germination, young leaves were cut into 6–8 mm segments, and used as explants for Agrobacterium inoculation. A. tumefaciens strain LBA4404 harbouring the plasmid pBI121 was used for inoculation. T-DNA of this plasmid consists of neomycin phosphotransferase gene (nptII) controlled by nos promoter, and the GUS reporter gene, ß-glucuronidase (uidA), controlled by 35S cauliflower mosaic virus promoter (CaMV35S) (Fig. 1). There is trfA out of T-DNA region under the control of a prokaryotic promoter to select transgenic bacteria (Durland et al. 1990; Chen et al. 2003).
Fig. 1.
Map of T-DNA region of plasmid pBI121. LB and RB left and right borders sequence of a T-DNA. nos-P, nopaline synthase gene promoter. nptII, neomycin phosphotransferase gene. nos-T, nopaline synthase gene terminator. 35S-P, CaMV 35S promoter. GUS, β-glucuronidase reporter gene (Chen et al. 2003; Valizadeh Kaji et al. 2013)
Kanamycin selection
Explants cultured on MS medium containing different concentrations of filter-sterilized kanamycin (0, 25, 50, 75, 100 and 150 mg/l) and kept at 25 ± 2 °C temperature and 16/8 h photoperiod under cool-white fluorescent light at 40 μmol m−2 s−1. Survival rate was calculated after 4 weeks (Table 1).
Table 1.
Effect of different concentration of kanamycin on plant sensitivity of Petunia hybrida
| Kanamycin concentration (mg/l) | No. of leaf explants | No. of shoot-forming explants | Survival rates (%)a |
|---|---|---|---|
| 0 | 30 | 30 ± 0.0a | 100 ± 0.0a |
| 25 | 30 | 24 ± 0.8b | 80.00 ± 1.5b |
| 50 | 30 | 19 ± 1.0c | 63.33 ± 2.5c |
| 75 | 30 | 14 ± 0.9c,d | 46.66 ± 1.0d |
| 100 | 30 | 9 ± 0.4e | 30.00 ± 0.9e |
| 150 | 30 | 0.0 ± 0.0f | 0.00 ± 0.0f |
aThe values represent the mean ± standard error of three replicates
Different letters are showing considerable differences at P ≤ 0.05
Agrobacterium-mediated transformation
A. tumefaciens was grown overnight in a liquid LB medium with 50 mg/l kanamycin and 50 mg/l rifampicin on a shaker incubator with 120–130 rpm at 28 °C. The bacterial suspension was centrifuged for 10 min (4000 g), then re-suspended in inoculation medium and diluted to OD600 = 0.8. Inoculation and co-cultivation media were considered as four treatments including: (a) full MS medium, (b) MS without KH2PO4, NH4NO3, KNO3, (c) MS without KH2PO4, NH4NO3, KNO3, CaCl2 and (d) only distilled water, sucrose and agar (no MS elements and vitamins) (Table 2). Leaf explants dried out on sterilized filter paper after inoculation with bacterial suspension and then co-cultivated on 7 g/l agar-solidified MS treatments including 30 g/l sucrose, and 10 mM MES with pH 5.6 for 3 days under dark condition. In order to eliminate Agrobacterium, explants were washed with liquid MS medium containing 500 mg/l cefotaxime [Sigma-Aldrich, Steinheim, Germany] and then transferred to selection medium containing 30 g/l sucrose, 7 g/l agar, 200 mg/l cefotaxime, 100 mg/l Kanamycin [Sigma–Aldrich, Steinheim, Germany] and 2 mg/l TDZ [Sigma–Aldrich, Steinheim, Germany]. Regenerated shoots on selection medium (2–3 cm) were rooted on half-strength MS medium supplemented with 30 g l−1 sucrose, 0.1 mg/l NAA [Duchefa, Haarlem, The Netherlands], 7 g/l agar and 50 mg/l kanamycin. The rooted plantlets were rinsed under running tap water to remove agar and transferred to the plastic pots containing a ratio of sterile vermiculite and perlite (1:1) mixture. They successfully acclimatized in a transparent plastic box in a growth chamber condition 16/8 h (light/dark) photoperiod, under fluorescent illuminations (40 μmol m−2 s−1 and 22 °C ± 1 °C) for 2 weeks.
Table 2.
Effect of different inoculation and co-cultivation media on Agrobacterium-mediated transformation of Petunia hybrida
| Inoculation and co-cultivation media | Kanamycin-resistant plantlet (%) | No. of PCR- positive plantletd | No. of GUS-positive plantlete | Transformation efficiency (%)f | ||||
|---|---|---|---|---|---|---|---|---|
| Alb | LF Alc | Al | LF Al | Al | LF Al | Al | LF Al | |
| Medium 1: MS full strength | 5.00 ± 1.00d | 4.00 ± 1.60c | 2/5 | 1/4 | 0/5 | 0/4 | 0.00 ± 0.00d | 0.00 ± 0.00c |
| Medium 2: MS—(KH2PO4, NH4NO3, KNO3)a | 12.00 ± 1.90c | 9.00 ± 1.30b,c | 9/12 | 6/9 | 7/12 | 4/9 | 7.00 ± 1.80c | 4.00 ± 1.50b |
| Medium 3: MS—(KH2PO4, NH4NO3, KNO3, CaCl2) | 30.00 ± 2.00a | 25.00 ± 1.90a | 28/30 | 21/25 | 22/30 | 16/25 | 22.00 ± 2.30a | 16.00 ± 2.50a |
| Medium 4: No MS elements and vitamins only distilled water, sucrose and agar | 20.00 ± 1.95b | 13.00 ± 1.60b | 16/20 | 10/13 | 13/20 | 8/13 | 13.00 ± 2.00b | 8.00 ± 1.60b |
In each treatment, 100 leaf explants from each cultivar were inoculated and the experiment was repeated five times. The data were collected as a mean of five replications ± standard error
Various letters shows significant differences at P ≤ 0.05 (n = 5)
aThe elements inside the bracket were removed from MS medium
bAlvan cultivar
cLF Alvan cultivar
dThe number of putative transgenic plantlets with positive PCR responses/The number of putative transgenic plantlets (kanamycin resistance)
eThe number of GUS-positive plantlets was obtained from the number of kanamycin resistant plantlets
fGUS-positive plantlets were used for investigation of percentage of transformation efficiency from 100 explants
DNA isolation and polymerase chain reaction analysis
Leaves of control and treated plants were used for DNA extraction following cetyltrimethylammonium bromide (CTAB) method according to Doyle and Doyle (1987) protocol. Gel electrophoresis and spectrophotometer were used for qualitative and quantitative evaluation of DNA, respectively. For polymerase chain reaction (PCR), specific primers of nptII, gus and trfA genes were used. The PCR amplifications were performed in 25 µl reaction mixture containing 1 µl of DNA template, 1 µl of each primer and 12.5 µl of master mix [i-Taq from iNtRON Biotechnology]. The primer sequences used for PCR reaction were as follows: 5 -GGTGGTCAGTCCCTTATGTTACG-3 and 5-CCGGCATAGTTAAAGAAATCATG-3for the 512 bp segment of the gus gene, 5-TCCGGCCGCTTGGGTGGAGAGGCT-3 and 5-TGGCGAACAGTTCGGCTGGCGCGA-3 for the 485 bp segment of the nptII gene, 5-ATCATTGACCCAGGCGTGTT-3 and 5-AATCGGACGTTTGACCGGAA-3 for the 1122 bp segment of the trfA gene. The PCR analysis was performed according to the conditions including: 30 cycles of 94 °C for 1 min, 58 °C for gus and trfA genes and 61 °C for nptII for 45 s, and 72 °C for 1 min, 72 °C for 10 min. After amplification, 7 µl of PCR products was loaded on 1% agarose gel at 80 V for 1 h by electrophoresis and finally visible bands were detected by UV light.
Histochemical gus assay
GUS assay was done using 5-bromo-4-chloro-3-indolyl β-D-glucuronidase (X-Gluc) solution [Wako Pure Chemical Industries Ltd, Osaka, Japan] (Jefferson et al. 1987). The leaves of putative transgenic plantlets from each treatment were incubated overnight in 50 mM sodium phosphate buffer (pH 7.0) with 1 mM X-Gluc at 37 °C under dark condition. Following incubation, chlorophyll was removed by 70% ethanol.
Southern blot hybridization
Southern blot analysis was carried out with 10–20 µg of genomic DNA extracted from leaves of PCR- and gus-positive plants and non-transformed control P. hybrida which were digested with BamHI with a single cut within the T-DNA (Fig. 1). The products were electrophoresed on 1% (W/V) agarose gel and subsequently were transferred to positively charged nylon membrane (Boehringer Mannheim GmbH, Mannheim, Germany). The gus probe (521 bp) related to the gus gene was prepared from plasmid pBI121 by labelling digoxigenin (DIG) using the PCR DIG probe synthesis kit (Roche Diagnostics, Mannheim, Germany). Pre-hybridization and hybridization were conducted using a high-SDS hybridization buffer containing 50% deionized formamide, 5 × SSC, 50 mM sodium phosphate (pH 7.0), 2% blocking solution, 0.1% N-lauroylsarcosine, and 7% SDS. Washing and detection steps were performed using instruction manual of the DIG labeling and detection system (Roche Diagnostics, Mannheim, Germany). Exposing of nylon membrane to the detection film (Lumi-Film Chemiluminescent Detection Film; Roche Diagnostics, Mannheim, Germany) led to detection of hybridization signals.
Statistical analysis
The experiments were based on a completely randomized design (CRD). The kanamycin selection experiment was done in three replications with 10 explants per replication and the transformation experiment was conducted in five replications with 20 explants per replication. Percentage data were subjected to arcsine (√x) transformation before statistical analysis. Analysis of data was conducted using ANOVA and Duncan’s multiple range test at P ≤ 0.05, via SAS 9.1 software, was applied to compare the means.
Results
Kanamycin selection
Kanamycin is widely used for the screening of transgenics with the ability to destroy the cell protein function and bind to the 30S ribosomal protein which is present in the organelles such as chloroplast. The most commonly used gene in the selection of transgenic plants is nptII gene, making plants resistant to kanamycin as glycoside antibiotic through the phosphorylation of the hydroxyl groups (Pandian et al. 2006). Kanamycin sensitivity of Petunia was examined during 4 weeks. Survival rate was significantly decreased with the increasing of kanamycin concentration and the lowest percentage of viability was obtained in the selection media with 100 and 150 mg/l kanamycin (Table 1). However, the appropriate concentration of 100 mg/l was considered for the selection of transgenic plants as the lowest concentration of the Kanamycin able to inhibit regeneration of the untransformed shoots, is preferred. The result of this assessment was similar for both cultivars.
Frequency of putative transgenic shoots
The effect of four inoculation and co-cultivation media were evaluated on production of kanamycin-resistant shoots of Petunia cultivars (Table 2). After two months shoots were induced on all explants (Fig. 3a, b) and successfully rooted within 1–2 months in rooting medium (Fig. 3c). In Alvan cultivar, the minimum rate of putative transgenic shoots (5%) was obtained in full strength MS medium (Medium 1). The frequency of putative transformed shoots was increased when medium 2 (MS medium lacking KH2PO4, NH4NO3, and KNO3) was used. In medium 3, in which CaCl2 was additionally removed, the highest frequency of putative transgenic shoots (30%) was observed. Also elimination of other elements such as micro and macro elements, vitamins, Na2EDTA, and FeSO4 (Medium 4) significantly increased the frequency of putative transgenic shoots compared to media 1 and 2 (Table 2).
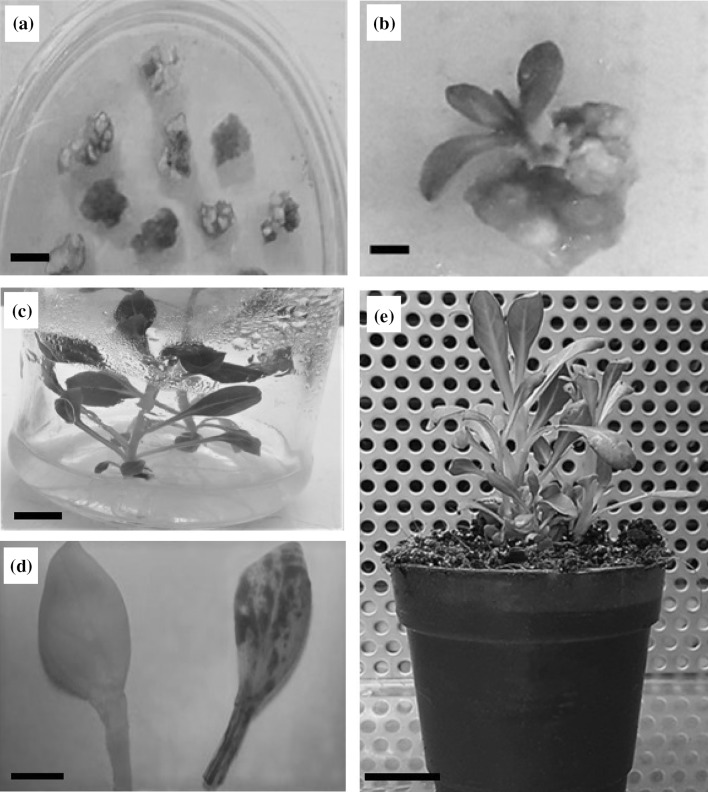
Fig. 3.
Transgenic plant regeneration of P. hybrida through Agrobacterium-mediated transformation. a Leaf explants showed resistance to kanamycin after 6 weeks on the selection medium (bar: 1 mm); b Regeneration of transgenic plant from selection medium, 2 months after co-cultivation (bar: 1 mm); c Rooted shoots on rooting medium (bar: 1 cm); d Histochemical GUS assay of leaf of transgenic plant (right) and non-transformed plant (left) (bar: 2 mm); e Two transgenic plantlets were acclimatized in phytotron (bar: 2.5 cm)
In LF Alvan cultivar the lowest kanamycin-resistant shoots (4%) was observed on medium 1 and no significant increase on frequency of putative transgenic shoots was observed in medium 2. The highest frequency of kanamycin-resistance shoots (25%) was obtained when KH2PO4, NH4NO3, KNO3 and CaCl2 were eliminated from inoculation and co-cultivation media (Medium 3). Compared with medium 1, the frequency of putative transgenics was significantly higher in medium 4 and there was no significant difference between media 2 and 4.
PCR analysis
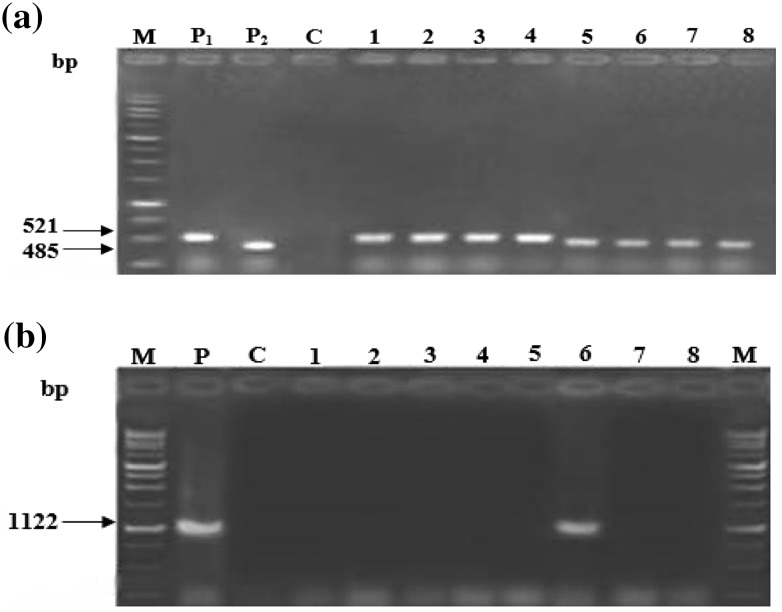
The presence of both nptII and gus inserts was confirmed by PCR amplification of DNA sequences (Fig. 2a). In both cultivars the lowest and the highest rate of transformation was observed in medium 1 and 3 respectively (Table 2). The absence of Agrobacterium impurity was studied by the absence of the 1122 bp band corresponding to the trfA gene and only one sample (sample 6) showed bacterial contamination (Fig. 2b).
Fig. 2.
PCR analysis of putative transgenic plants of P. Hybrida cv. Alvan regenerated on different media. a PCR analysis of putative transformed plants for detection of gus and nptII genes. Lane M, molecular size marker (1 Kb ladder Fermentase). Lane P1, positive control of gus gene (Plasmid pBI121 vector). Lane P2, positive control of nptII gene. Lane C, negative control (non-transformed plant). Lanes 1–4, amplification of 521 bp fragment of gus gene in putative transgenic plants regenerated on media one, two, three and four respectively. Lanes 5–8, amplification of nptII gene in putative transgenic plants regenerated on media one, two, three and four respectively. b PCR analysis of P. hybrida cv. Alvan on medium three for absence of trfA gene. Lane M, molecular size marker (1 Kb ladder Fermentase). Lane P, positive control of trfA gene (Plasmid pBI121 vector). Lane C, negative control (non-transformed plant). Lanes 1–5 and 7–8, putative transgenic plant with no bacterial contamination. Lane 6, the band with 1122 bp length shows bacterial contamination
GUS assay
Leaf samples of both putative transgenic and non-transgenic P. hybrida plantlets were tested for gus activity by histochemical gus assay and the leaves of kanamycin-resistant plantlets were stained blue (Fig. 3d). As shown in Table 2, no gus expression was observed in full-strength MS medium. None of putative transgenic plantlets for Alvan (0/5) and for LF Alvan (0/4) were gus positive in medium 1. The highest number of gus-positive plantlets for Alvan (22/30) and LF Alvan (16/25) were obtained on modified MS medium without KH2PO4, NH4NO3, KNO3, and CaCl2 (medium 3).
Transformation efficiency
For both cultivars no transformants were obtained on medium one. When KH2PO4, NH4NO3, and KNO3 were removed from MS medium, the frequency of transformation was significantly increased. The highest frequency of transformation for Alvan (22%) and LF Alvan (16%) was achieved on medium 3. In Alvan cultivar, a higher rate of transformation was occurred on medium 4, compared with media 1 and 2. In LF Alvan no significant difference was detected in the transformation efficiency between media 2 and 4. However, a significant increase was observed compared with control medium (Table 2).
Southern blot of transgenic plants
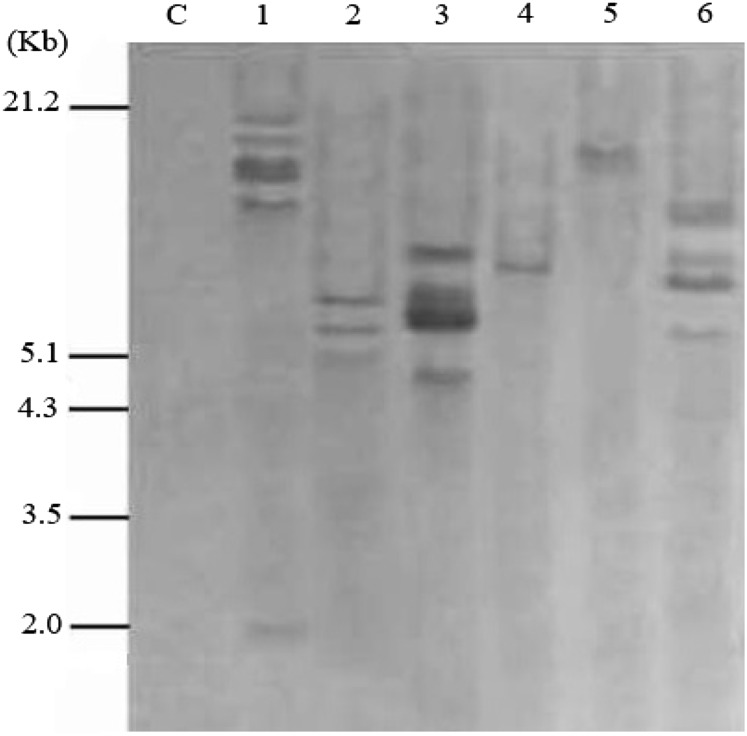
Six independent transformed lines, which showed strong gus activity and amplified PCR bands, were selected for southern blot hybridization by the uidA gene as a probe (Fig. 1). Two of six transformed plants (lines 4 and 5) had a single copy of gus integration, however four plants (Lines 1, 2, 3 and 6) showed multiple copies of gus integrations (Fig. 4). The non-transformed plant showed no hybridization signal. Well-developed transgenic plantlets were transferred to the plastic pots containing 1:1 ratio of vermiculite and perlite, and acclimatized under growth chamber condition (Fig. 3e).
Fig. 4.
Southern blot analysis of transgenic plants of P. Hybrida regenerated on medium three. DNA samples were digested with BamH1 and hybridized with a digoxigenin-labeled gus gene probe. Lane C, non-transformed plants. Lanes 1–6, transgenic plants (Lanes 1, 4 are FC Alvan cultivar and 2, 3, 5 and 6 are Alvan cultivar). Molecular size markers are indicated on the left
Discussion
In this study the effect of different types of modified MS media as inoculation and co-cultivation media was examined on Agrobacterium-mediated transformation of P. hybrida. When macro elements including KNO3, KH2PO4, CaCl2 and NH4NO3 were removed from inoculation and co-cultivation media, transformation efficiency was increased significantly. This result could accurately be observed in Agrobacterium-mediated transformation system in Lilium (Azadi et al. 2011) and hairy root induction of Papaver bracteatum (Sharafi et al. 2013). The transformation efficiency was achieved at maximum rate in Lilium (Azadi et al. 2010a) and Dracocephalum kotschyi (Sharafi et al. 2014) when macro elements such as NH4NO3, KNO3, CaCl2 and KH2PO4 were removed from the inoculation and co-cultivation media. Previous studies showed that biofilm formation of A.tumefaciens increased when inorganic phosphorous in co-cultivation medium was limited (Danhorn et al. 2004). Hoshi et al. (2004) observed a higher gus gene transformation in Lilium Acapulco’ cultivar when NH4NO3 was removed from co-cultivation medium. Likewise, proliferation of Agrobacterium was high in free-nitrogen medium. It has also been reported that elimination of CaCl2 from co-cultivation medium has rapidly improved the transformation efficiency in Hevea brasiliensis (Montoro et al. 2000). The shortage of PO4 could enhance VirG expression activity and create a proper positive signal to induce the infection of plants (Winans 1990; Subramoni et al. 2014).
Removing other elements such as MgSO4, micro elements, iron and vitamins from inoculation and co-cultivation media significantly increased transformation frequency compared with media 1 and 2 but reduced the percentage of transformation compared with medium 3 in both P. hybrida cultivars. Dupre et al. (2000) obtained the highest transformation efficiency in Ginkgo biloba when mineral elements-free co-cultivation medium was used.
This study revealed a possible role of macro elements (NH4NO3, KNO3, CaCl2 and KH2PO4) in reduction of the A. tumefaciens-mediated transformation in P. hybrida. Co-cultivation media, 3 and 4, were provided the highest bacterial proliferation around the explants compared with full strength MS medium. This experiment confirmed that macro elements partially prevent A. tumefaciens growth and proliferation during inoculation and co-cultivation conditions (Azadi et al. 2010b). This is important as removing of such macro elements from inoculation or co-cultivation media, may facilitate the transformation of recalcitrant species.
In conclusion, we have developed an effective system to significantly increase the agrobacterium-mediated transformation efficiency of P. hybrida by elimination of macro elements such as KNO3, NH4NO3, CaCl2 and KH2PO4 from inoculation and co-cultivation media.
Acknowledegments
This work was supported by Bu-ali Sina University, Hamedan, Iran, and Novin Giti Gene Biotech. Co. Biotechnology Incubator Center of National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
Abbreviations
- LF Alvan
Large Flower Alvan
- GUS
β-glucuronidase
- CTAB
Cetyltrimethylammonium bromide
- nptII
Neomycin phosphotransferase
- CaMV
Cauliflower mosaic virus
Contributor Information
Aso Nobakht Vakili, Email: a.nobakhtvakili@gmail.com.
Hedayat Bagheri, Email: bagheri.hedayat@gmail.com.
Pejman Azadi, Email: bagheri.hedayat@gmail.com.
References
- Azadi P, Chin DP, Kuroda K, Khan RS, Mii M. a) Macro elements in inoculation and co-cultivation medium strongly affect the efficiency of Agrobacterium-mediated transformation in Lilium. Plant Cell Tissue Organ Culture. 2010;101:201–209. doi: 10.1007/s11240-010-9677-9. [DOI] [Google Scholar]
- Azadi P, Otang NV, Chin DP, Nakamura I, Fujisawa M, Harada H, Misawa N, Mii M. b) Metabolic engineering of Lilium × formolongi using multiple genes of the carotenoid biosynthesis pathway. Plant Biotechnol Rep. 2010;4:269–280. doi: 10.1007/s11816-010-0147-y. [DOI] [Google Scholar]
- Azadi P, Otang NV, Supaporn H, Khan RS, Chin DP, Nakamura I, Mii M. Increased resistance to cucumber mosaic virus (CMV) in Lilium transformed with a defective CMV replicase gene. Biotech Lett. 2011;33:1249–1255. doi: 10.1007/s10529-011-0550-7. [DOI] [PubMed] [Google Scholar]
- Azadi P, Bagheri H, Nalousi AM, Nazari F, Chandler SF. Current status biotechnology advances in genetic engineering of ornamental plants. Biotechnol Adv. 2016;34(6):1073–1090. doi: 10.1016/j.biotechadv.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Chen P, Wang ChK, Soong ShCh. Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insersion from transgenic plants. Molecular Breeding. Inst Bioagric Sci. 2003;11:287–293. [Google Scholar]
- Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol. 1997;115:971–980. doi: 10.1104/pp.115.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C. Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR–PhoB regulatory system. J Bacteriol. 2004;186:4492–4501. doi: 10.1128/JB.186.14.4492-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation from small amount of fress tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Drummond RS, Martinezsanchez NM, Janssen BJ, Templeton KR, Simons JL, Quinn BD, Karunairetnam S, Snowden KC. Petunia hybrida carotenoid clieavage dioxygenase7 is involved in the production of negative and positive branching signals in Petunia. Plant Physiol. 2009;151:1867–1877. doi: 10.1104/pp.109.146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre´ P, Lacoux J, Neutelings G, Mattar-Laurain D, Fliniaux MA, David A, Jacquin-Dubreuil A. Genetic transformation of Ginkgo biloba by Agrobacterium tumefaciens. Physiol Plant. 2000;108:413–419. doi: 10.1034/j.1399-3054.2000.t01-1-100411.x. [DOI] [Google Scholar]
- Durland RH, Toukdarian A, Fang F, Helinski DR. Mutations in the trfA replication gene of the broad-host-range plasmid RK2 result in elevated plasmid copy numbers. J Bacteriol. 1990;172(7):3859–3867. doi: 10.1128/jb.172.7.3859-3867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flego D, Pirhonen M, Saarilahti H, Palva TK, Palva ET. Control of virulence gene expression by plant calcium in the phytopathogen Erwinia carotovora. Mol Microbiol. 1997;25:831–838. doi: 10.1111/j.1365-2958.1997.mmi501.x. [DOI] [PubMed] [Google Scholar]
- Fraley RT, Horsch RB, Mtzke MD, Chilton W, Sanders P. In vitro transformation of Petunia cells by an improved method of co-cultivation with A.tumefaciens strains. Plant Mol Biol. 1984;3:371–378. doi: 10.1007/BF00033384. [DOI] [PubMed] [Google Scholar]
- Grates T, Vandenbussche M. A model system for comparative research: petunia. Trends Plant Sci. 2005;10:251–256. doi: 10.1016/j.tplants.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kondo M, Mori S, Adachi Y, Nakano M, Kobayashi H. Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Rep. 2004;22:359–364. doi: 10.1007/s00299-003-0700-z. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke XY, McCormac AC, Harvey A, Lonsdale D, Chen DF, Elliott MC. Manipulation of discriminatory T-DNA delivery by Agrobacterium into cells of immature embryos of barley and wheat. Euphytica. 2002;126(3):333–343. doi: 10.1023/A:1019960309149. [DOI] [Google Scholar]
- Li F, Li Ch, Li M, Yu M, Fand Ch, Wang Sh. Microspores and Agrobacterium-mediated transient expression of β-glucuronidase (GUS) reporter gene. Int J Agric Biol. 2013;15:1098–1104. [Google Scholar]
- Lutke WK. Petunia (Petunia hybrida) Methods Mol Biol. 2006;344:339–349. doi: 10.1385/1-59745-131-2:339. [DOI] [PubMed] [Google Scholar]
- Machado LDOR, De Andrade GM, Barrueto Cid LP, Penchel RM, Brasileiro ACM. Agrobacterium strain specificity and shooty tumour formation in eucalypt (Eucalyptus grandis × E. urophylla) Plant Cell Rep. 1997;16:299–303. doi: 10.1007/BF01088285. [DOI] [PubMed] [Google Scholar]
- McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- Montoro P, Teinseree N, Rattana W, Kongsawadworakul P, Michaux-Ferriere N. Effect of exogenous calcium on Agrobacterium tumefaciens-mediated gene transfer in Hevea brasiliensis (rubber tree) friable calli. Plant Cell Rep. 2000;19:851–855. doi: 10.1007/s002990000208. [DOI] [PubMed] [Google Scholar]
- Pandian A, Hurlstone C, Liu Q, Singh S, Salisbury P, Green A. Agrobactrium-Mediated transformation protocol to overcome necrosis in elite Australian Brassica Juncea Lines. Plant Mol Bio Rep. 2006;24:103a–i. doi: 10.1007/BF02914050. [DOI] [Google Scholar]
- Phelep M, Petit A, Martin L, Duhoux E, Tempe J. Transformation and regeneration of a nitrogen-fixing tree, Allocasuarina verticillata Lam. Biotechnology. 1991;9:461–466. [Google Scholar]
- Romantschuk M. Attachment of plant pathogenic bacteria to plant surfaces. Annu Rev Phytopathol. 1992;30:225–243. doi: 10.1146/annurev.py.30.090192.001301. [DOI] [PubMed] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Razavi K, Ntui VO. A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum. Plant Cell Tissue Organ Culture. 2013;113:1–9. doi: 10.1007/s11240-012-0246-2. [DOI] [Google Scholar]
- Sharafi A, Sohi HH, Azadi P, Sharafi AA. Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants. 2014;20(2):257–262. doi: 10.1007/s12298-013-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramoni S, Nathoo N, Klimov E, Yuan Z. Agrobacterium tumefaciens response to plant-derived signaling molecules. Front Plant Sci. 2014;5:322–333. doi: 10.3389/fpls.2014.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirukumaran G, Ntuni VO, Khan RS, Mii M. Thidiazuron: an efficient plant growth regulator for enhancing Agrobacterium-mediated transformation in Petunia hybrida. Plant Cell Tissue Organ Culture. 2009;99:109–115. doi: 10.1007/s11240-009-9581-3. [DOI] [Google Scholar]
- Tzfira T, Yarnitzky O, Vainstein A, Altman A. Agrobacterium rhizogenes-mediated DNA transfer in Pinus halepensis. Plant Cell Rep. 1996;16:26–31. doi: 10.1007/BF01275443. [DOI] [PubMed] [Google Scholar]
- Valizadeh Kaji B, Ershadi A, Tohidfar M. Agrobacterium-Mediated Transformation of Pomegranate (Punica granatum L.) ‘Yousef Khani’ Using the gus Reporter Gene. Int J Hortic Sci Technol. 2013;1:31–41. [Google Scholar]
- Van der Meer IM. Agrobacterium-mediated transformation of Petunia leaf disks. Plant Cell Culture Protoc. 1999;111:327–334. doi: 10.1385/1-59259-583-9:327. [DOI] [PubMed] [Google Scholar]
- Winans SC. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SJ, Tjokrokusumo D, MCCOMB JA. Transformation of Petunia hybrida by the Agrobacterium suspension drop method. Analysis Vol: Mol Methods Plant; 2003. p. 23. [Google Scholar]
- Zhang W, Subbarao S, Addae P, Shen A, Armstrong C, Peschke V, Gilbertson L. Cre/lox-mediated marker gene excision in transgenic maize (Zea mays L.) plants. Theor Appl Genet. 2003;107(7):1157–1168. doi: 10.1007/s00122-003-1368-z. [DOI] [PubMed] [Google Scholar]