Abstract
The effect of 0.5–1.5 mM salicylic acid (SA) on modulating reactive oxygen species metabolism and ascorbate–glutathione cycle in NaCl-stressed Nitraria tangutorum seedlings was investigated. The individual plant fresh weight (PFW) and plant dry weight (PDW) significantly increased under 100 mM NaCl while remained unchanged or decreased under 200–400 mM NaCl compared to the control. Superoxide anion (O·−2), hydrogen peroxide (H2O2), thiobarbituric acid reactive substances (TBARS), reduced ascorbate (AsA), dehydroascorbate (DHA), reduced glutathione (GSH) and oxidized glutathione (GSSG) increased whereas the ratios of AsA/DHA and GSH/GSSG decreased under varied NaCl treatments. Ascorbate peroxidase (APX) and glutathione reductase (GR) activities were enhanced while dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR) activities remained unvaried under 100–400 mM NaCl stresses. In addition, exogenous SA further increased PFW, PDW and root/shoot ratio. SA effectively diminished O·−2 accumulation. H2O2 and TBARS decreased under 0.5 and 1.0 mM SA treatments compared to those without SA. 0.5 mM of SA increased while 1.0 and 1.5 mM SA decreased APX activities. DHAR activities were elevated by 0.5 and 1.0 mM SA but not by 1.5 mM SA. MDHAR and GR activities kept constant or significantly increased at varying SA concentrations. Under SA treatments, AsA and GSH contents further increased, DHA and GSSG levels remained unaltered, while the decreases in AsA/DHA and GSH/GSSG ratios were inhibited. The above results demonstrated that the enhanced tolerance of N. tangutorum seedlings conferred by SA could be attributed mainly to the elevated GR and DHAR activities as well as the increased AsA/DHA and GSH/GSSG ratios.
Keywords: Ascorbate–glutathione cycle, Halophyte, Reactive oxygen species metabolism, Salicylic acid, Salinity
Introduction
Salinity is an important abiotic stress factor that threatens agricultural productivity around the world, particularly in arid and semi-arid regions. Soil salinity is an increasing problem and affects around 800 million hectares of land, which accounts for over 7% of the world’s total land area (Muchate et al. 2016). Under salinity conditions, the physiological and metabolic activities of plants are adversely influenced by osmotic stress, ionic cytotoxicity, nutritional imbalance, or a combination of these aspects (Ashraf 2004). Short-term osmotic stress caused by the salt outside the root zone leads to the inhibition of water uptake capacity, cell elongation, leaf development and root growth, the reduction of new leaves, the injury to the cells in transpiring leaves, and reduction in the growth rate (Hasanuzzaman et al. 2013; Muchate et al. 2016). Long-term ionic stress occurs due to the toxic effects of salt inside the plant and results in the premature death of leaves and the inhibition of photosynthesis, which reduce the supply of photosynthates in plants and ultimately influence the yield (Munns 2005; Hasanuzzaman et al. 2013; Muchate et al. 2016).
In addition, salinity stress results in the over-generation of reactive oxygen species (ROS) (Chinnusamy et al. 2005). ROS in plants mainly include free radicals such as superoxide anion (O·−2), hydroxyl radical (·OH), and non-radical molecules like hydrogen peroxide (H2O2), singlet oxygen (1O2), and so forth (Sharma et al. 2012). ROS at high concentrations disrupt the normal metabolism of membrane lipid, protein, and nucleic acid via oxidative damage (Muchate et al. 2016).
In order to scavenge or detoxify the over-generated ROS, plants have developed a complex oxidative defense system comprising of both enzymatic and non-enzymatic antioxidants (Noctor and Foyer 1998). Enzymatic antioxidants include superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), guaiacol peroxidase (GPOX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11), dehydroascorbate reductase (DHAR, EC 1.8.5.1), monodehydroascorbate reductase (MDHAR, EC 1.6.5.4), glutathione reductase (GR, EC 1.8.1.7), etc. The non-enzymatic antioxidants including reduced ascorbate (AsA), reduced glutathione (GSH), α-tocopherol and carotenoids, etc. along with the enzymes of ascorbate–glutathione (AsA–GSH) cycle, also significantly contribute to the scavenging of ROS (Muchate et al. 2016). In plants, the AsA–GSH cycle operates in the cytosol, mitochondria, plastids and peroxisomes (Jiménez et al. 1998; Meyer 2008). Since AsA and GSH are present in high concentrations in plant cells, it is assumed that the AsA–GSH cycle plays a key role for H2O2 detoxification.
Salicylic acid (SA) functions as a plant hormone that plays an important role in regulating a number of physiological and biochemical processes, and has diverse effects on plant tolerance to biotic and abiotic stresses (Syeed et al. 2011). Although SA of high concentrations may induce oxidative stress to plants, partially due to the accumulation of H2O2, and lead to cell death, the results published so far indicate that SA of low concentrations facilitate tolerance to most kinds of abiotic stresses (Horváth et al. 2007). SA improves acclimation to salt stress in plants by ion exclusion and/or compartmentalization, osmoregulation, reduction in membrane lipid peroxidation, synthesis of protein kinase, regulation of antioxidative system, etc. (Mikolajczyk et al. 2000; Aftab et al. 2011; Syeed et al. 2011; Idrees et al. 2012; Bastam et al. 2013; Liu et al. 2014a; Khalifa et al. 2016; Yadu et al. 2017). Furthermore, the effect of exogenous SA on the stress tolerance of plants depends on numerous factors such as the application concentration, the application mode, as well as the overall state of the plant i.e. developmental stage, oxidative balance of the cells, and acclimation by previous biotic or abiotic stresses (Horváth et al. 2007).
Nitraria tangutorum Bobr. is a wild shrub belonging to Nitraria genus in Nitrariaceae family. It is a typical halophyte and xerophyte, mainly grows in the clay covered with aeolian sand in the arid deserts and desert steppes (Li et al. 2013a, b). It plays an important role in saline-alkaline land greening and improvement as well as ecological environment restoration due to its superior tolerance to salinity and drought (Yang et al. 2010a, b). The growth, physiological responses, metabolic profile changes and signal regulation of proline metabolism of N. tangutorum under salt stress were extensively studied using callus, suspension cells and seedlings, respectively (Yang et al. 2010a, b, 2013b; Kang et al. 2015; Ni et al. 2015). Yang et al. (2012, 2014) also investigated the effects of exogenous H2O2 and sodium nitroprusside (NO donor) on ROS generation, activities of antioxidative enzymes and proline metabolism using N. tangutorum callus. Additionally, our previous study revealed that exogenous Ca2+ promoted the salt tolerance of N. tangutorum seedlings through enhancing Na+ compartmentalization and elevating ion pump activities of tonoplast and plasma membrane in N. tangutorum leaves (Liu et al. 2014b). We also studied the effect of exogenous SA on the changes of plant biomass, leaf relative water content, chlorophyll content, lipid peroxidation, osmotic adjustment solutes, and antioxidant enzymes in N. tangutorum seedlings subjected to NaCl stress (Liu et al. 2016). The existing literature indicated that SA enhanced the tolerance of plants toward various abiotic stresses primarily due to the enhanced antioxidative capacity. Hence, we deduce that exogenous SA may influence AsA–GSH metabolism in plants under salinity stress. However, the effects of exogenous SA on ROS metabolism and AsA–GSH cycle in N. tangutorum seedlings under salt stress are still unknown.
In the present study, therefore, the changes of plant growth-related parameters, ROS metabolism, lipid peroxidation level along with the enzymatic and non-enzymatic antioxidants in AsA–GSH cycle in N. tangutorum seedlings under NaCl stress combined with exogenous SA treatment were studied. The objectives were to evaluate the potential increase of tolerance in N. tangutorum seedlings by SA application and investigate the possible mechanism adopted by SA in modulating NaCl-induced oxidative stress, in terms of the response of AsA–GSH cycle.
Materials and methods
Plant materials and growth conditions
On March 15, 2015, N. tangutorum seeds were sowed in trays filled with pure river sand in the greenhouse of Northeast Agricultural University, Harbin, China. When the seedlings reached fifth-leaf stage, they were transplanted in plastic pots (10 cm × 10 cm) and grew for about 1 year. The culture substrate was a mixture of garden soil and vermiculite (1:1, v/v). On May 1, 2016, the uniform seedlings were selected and transplanted into plastic pots (15 cm × 15 cm) containing well-washed river sand, with three plants in each pot. The seedlings were irrigated with 1/2 Hoagland full nutrient solution and allowed to recover prior to further experimental treatments. During the growth period, the average air temperatures in the greenhouse were 22 °C in the daytime and 17 °C at night. The average daily photosynthetically active radiation (PAR) intensity was 400 mmol m−2 s−1.
NaCl and SA treatments
Salt stress was conducted with NaCl at four levels of 100, 200, 300 and 400 mM. SA (C7H6O3, Sigma-Aldrich, ≥ 99.0%) was applied at four concentrations of 0, 0.5, 1.0 and 1.5 mM. Control seedlings were watered by Hoagland full nutrient solution without NaCl and SA. A total of 17 treatments, each treatment with three replicates, were set and processed. Each treatment was initiated with Hoagland + 50 mM NaCl, and then the NaCl concentration was stepped up by 50 mM per day until the designed concentration was attained. Then, the seedlings of all treatments were continuously processed for 9 days, watered once each day. The watering amount was 2 times of sand water-holding capacity. On the second day after the treatments, the seedlings were sampled.
Measurement of growth-related parameters
After NaCl and SA treatments, the individual plant fresh weight (PFW) of three seedlings was respectively weighed for each treatment. Then, the seedling was divided into shoot and root, and dried to constant weight. Thus, the individual plant dry weight (PDW) for each treatment was obtained. The root/shoot (R/S) ratio = root dry weight/shoot dry weight.
Determination of O·−2 and H2O2 contents
The generation rate of O·−2 in the leaf of N. tangutorum seedling was measured according to the method of Wang et al. (2012) with modifications. The absorbance at 530 nm was measured by spectrophotometer and the generation rate of O·−2 was calculated based a standard curve of NaNO2. The protein content in the leaf was quantified according to the method of Bradford (1976) using bovine serum albumin (Sigma) as a standard. The O·−2 content was expressed as µmol min−1 g−1 protein.
H2O2 content was assayed according to the method of Liu et al. (2010). The absorbance was measured at 410 nm. The content of H2O2 was calculated with an H2O2 solution-derived standard curve and expressed as nmol g−1 FW.
Lipid peroxidation assay
Thiobarbituric acid reactive substances (TBARS) content was measured by using the method of Palma et al. (2009). The absorbance at 532 nm was determined and the value for non-specific absorption at 600 nm was subtracted. TBARS amount was calculated using the extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 FW.
Analyses on the activities of APX, DHAR, MDHAR and GR
APX activity was determined according to the method of Nakano and Asada (1981) with some modifications. The changes in the absorbance at 290 nm were recorded at 25 °C for 1 min after the addition of H2O2. One unit of APX activity was defined as the amount of enzyme that oxidizes 1 µmol of AsA per min at 20 °C and the APX activity was expressed as U g−1 FW.
Estimation of DHAR activity was conducted by the method of Hossain and Asada (1984) with modifications. DHAR activity was determined by monitoring the increase in absorbance at 265 nm due to AsA production. The nonenzymatic reduction of DHA by GSH was subtracted. One unit of DHAR activity was defined as the amount of enzyme that produces 1 nmol of AsA per min at 25 °C and the DHAR activity was expressed as U g−1 FW.
MDHAR activity was determined according to the method of Miyake and Asada (1992) with slight modification. The determination was based on adding ascorbate oxidase (Sigma) to produce MDHA. MDHAR activity was tested by following the decrease in absorbance at 340 nm due to NADH oxidation. One unit of MDHAR activity was defined as the amount of enzyme that oxidizes 1 nmol of NADH per min at 25 °C and the MDHAR activity was expressed as U g−1 FW.
GR activity was assayed by the method of Foyer and Halliwell (1976) with modifications. GR activity was measured by following the decrease in absorbance at 340 nm due to NADPH oxidation. One unit of GR activity was defined as the amount of enzyme that oxidizes 1 nmol of NADPH per min at 25 °C and the GR activity was expressed as U g−1 FW.
Analyses on the contents of AsA, total ascorbate, GSSG and total glutathione
Extraction of antioxidants was conducted according to method of Nagalakshmi and Prasad (2001). The extract was neutralized with triethanolamine for subsequent detections. The contents of AsA and total ascorbate were measured according to the method of Jiang and Zhang (2001) with some modifications. The content of total ascorbate was measured by monitoring the absorbance at 525 nm. AsA content was determined in the absence of DTT and N-ethylmaleimide. DHA content was calculated as the difference between total ascorbate and AsA.
The contents of GSSG and total glutathione were measured according to the method of Griffith (1980) with modifications. For the determination of GSSG, the changes of absorbance values of the reaction mixture were read at 412 nm for the initial 3 min. The content of total glutathione was detected in the absence of 2-vinylpyridine. GSH content was calculated as the difference between total glutathione and GSSG.
Statistical analysis
All of the presented growth and physiological data were mean values of a representative experiment (n = 3) and shown as the mean ± SD. The data were analyzed using SPSS software version 17.0 (SPSS Inc., Cary, NC, USA). Two-way analysis of variance (ANOVA) was used to examine the influences of NaCl and SA treatments as well as their interaction on the tested traits in N. tangutorum seedlings. The statistical differences were analyzed based on the least significant differences (LSD) test, at significance level of P < 0.05.
Results
Effect of NaCl and SA on plant growth
Two-way ANOVA result revealed that two main effects (NaCl and SA) and their interaction (N × S) had significant influences (P < 0.05) on all three plant growth parameters except N × S on PDW (Table 1). As can be seen from Fig. 1a, b, the individual PFW and PDW significantly increased under 100 mM NaCl treatment and remained unchanged at 200 mM NaCl as compared to the control. However, these two parameters decreased when subjected to 300 and 400 mM NaCl stresses. With the application of SA, the PFW and PDW under 100 and 200 mM NaCl treatments were further enhanced. Moreover, the negative effects of 300 and 400 mM NaCl on PFW and PDW were alleviated to different extent by SA treatments. Generally, SA of medium (1.0 mM) and high (1.5 mM) concentrations exhibited stronger promoting effects on PFW and PDW of seedlings under NaCl stress.
Table 1.
Analysis of variance (ANOVA) for growth and physiological traits of N. tangutorum seedlings under salinity stress and exogenous SA application
| Source of variance | P values | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFW | PDW | R/S | O·−2 | H2O2 | TBARS | APX | DHAR | MDHAR | GR | AsA | DHA | AsA/DHA | GSH | GSSG | GSH/GSSG | |
| N | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0431 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| S | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0013 | 0.2236 | < 0.0001 | 0.0003 | 0.3063 | 0.0153 | < 0.0001 | 0.0897 | 0.0161 |
| N × S | 0.0163 | 0.3430 | 0.0028 | 0.1490 | 0.0913 | < 0.0001 | 0.0011 | 0.0459 | 0.5244 | 0.0029 | 0.0508 | 0.7867 | 0.5390 | 0.0007 | 0.0009 | 0.9989 |
N NaCl treatments; S salicylic acid treatments; PFW plant fresh weight; PDW plant dry weight; R/S root/shoot ratio; O·−2 superoxide anion; H2O2 hydrogen peroxide; TBARS thiobarbituric acid reactive substances; APX ascorbate peroxidase; DHAR dehydroascorbate reductase; MDHAR monodehydroascorbate reductase; GR glutathione reductase; AsA reduced ascorbate; DHA dehydroascorbate; AsA/DHA reduced ascorbate/dehydroascorbate ratio; GSH reduced glutathione; GSSG oxidized glutathione; GSH/GSSG reduced glutathione/oxidized glutathione ratio
Fig. 1.
Individual plant fresh weight (PFW, a), plant dry weight (PDW, b) and root/shoot (R/S) ratio (c) of N. tangutorum seedlings under combined NaCl and SA treatments. The seedlings were untreated (control) and treated with different combinations of NaCl (100, 200, 300 and 400 mM) and SA (0, 0.5 1.0 and 1.5 mM) for 9 days, respectively. Different lowercase letters mean significant differences (P < 0.05) among different treatments by least significant differences (LSD) test
The R/S ratios gradually increased with the increases of NaCl concentration (100–300 mM), and then declined to some extent (Fig. 1c). However, the R/S ratio at 400 mM NaCl was also significantly higher than the control (P < 0.05). Exogenous application of SA at 0.5–1.5 mM further increased R/S ratios (P < 0.05) except for the combination of 100 mM NaCl + 0.5 mM SA.
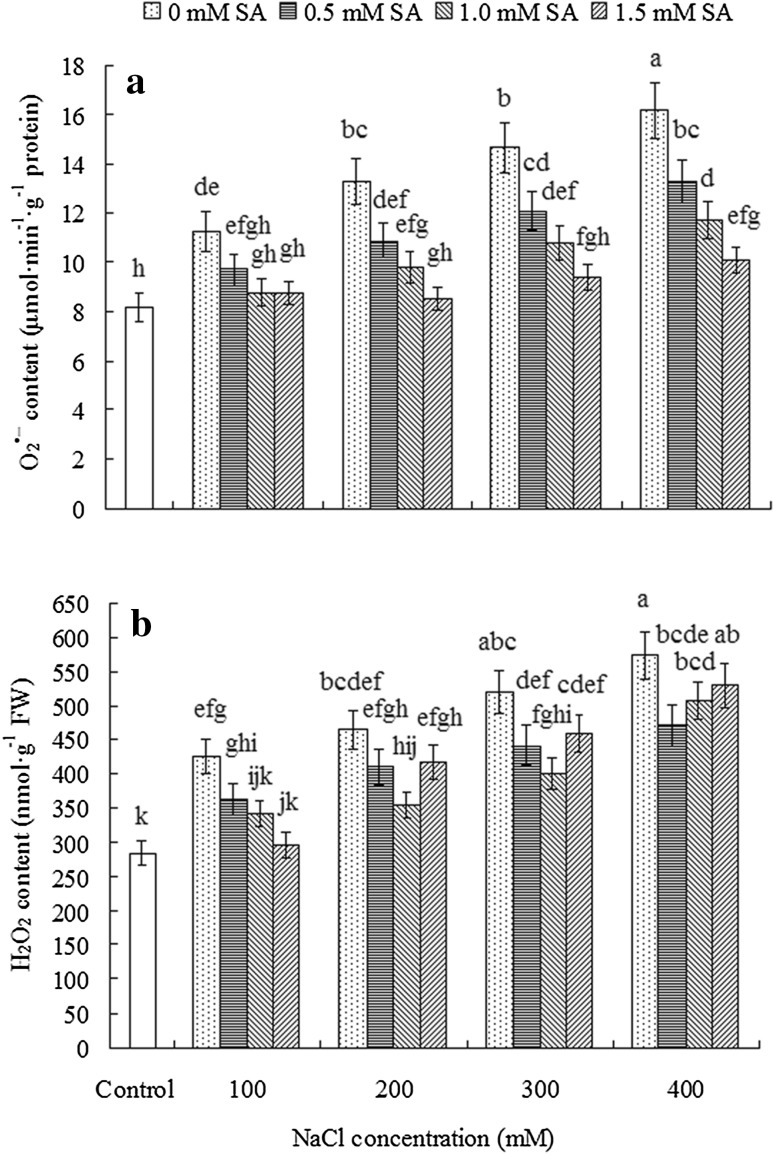
Effect of NaCl and SA on the contents of O·−2 and H2O2
NaCl and SA treatments but not their interaction exhibited significant effect (P < 0.05) on the contents of ROS investigated in the present study (Table 1). As was shown in Fig. 2, NaCl stress resulted in the significantly enhanced O·−2 and H2O2 levels (P < 0.05). The contents of O·−2 and H2O2 increased by 38–98 and 49–102% respectively, in seedlings exposed to 100–400 mM NaCl, as compared to the control. Exogenous application of SA markedly reduced the accumulation of O·−2 and H2O2. The mitigation effects of SA on O·−2 level increase were progressively related to the increases of SA concentration (Fig. 2a). Furthermore, 1.5 mM SA notably decreased the contents of O·−2 induced by 100–300 mM NaCl stresses to the level of the control. SA of low and medium concentrations (0.5 and 1.0 mM) exhibited relatively stronger inhibition effect on H2O2 accumulation (Fig. 2b). However, the effect of 1.5 mM SA on reducing H2O2 content was insignificant in 200–400 mM NaCl-stressed seedlings in comparison with those in the absence of SA.
Fig. 2.
Contents of superoxide anion radical (O·−2, a) and hydrogen peroxide (H2O2, b) in the leaf of N. tangutorum seedlings under combined NaCl and SA treatments. Different lowercase letters mean significant differences (P < 0.05) among different treatments by least significant differences (LSD) test
Effect of NaCl and SA on the content of TBARS
TBARS content was significantly (P < 0.05) affected by NaCl and SA treatments as well as their interaction (Table 1). Under NaCl stress, the content of TBARS proportionally increased with the increasing of NaCl concentration (Fig. 3). The TBARS values under 100–400 mM NaCl treatments were 156, 191, 238 and 283% of the control, respectively. With the application of SA, the increasing of TBARS level induced by NaCl stress was markedly inhibited. SA treatments of low and medium concentrations (0.5 and 1.0 mM) significantly lowered TBARS contents (P < 0.05) whereas the impact of high concentration (1.5 mM) SA was not so pronounced especially at low NaCl concentrations (100 and 200 mM).
Fig. 3.
Content of thiobarbituric acid reactive substances (TBARS) in the leaf of N. tangutorum seedlings under combined NaCl and SA treatments
Effect of NaCl and SA on the activities of APX, DHAR, MDHAR and GR
The activities of all four enzymes were significantly influenced (P < 0.05) by two main effects and their interaction except SA and N × S on MDHAR activity (Table 1). With the aggravation of NaCl stress extent, APX activities in the seedlings were dramatically elevated (P < 0.05). APX activities were further enhanced by 0.5 mM SA treatment (Fig. 4a). However, SA of medium and high concentrations (1.0 and 1.5 mM) inhibited APX activities, with APX activities declining to the levels of seedlings without SA treatments and even the control.
Fig. 4.
Contents of ascorbate peroxidase (APX, a), dehydroascorbate reductase (DHAR, b), monodehydroascorbate reductase (MDHAR, c) and glutathione reductase (GR, d) in the leaf of N. tangutorum seedlings under combined NaCl and SA treatments
Under NaCl stresses, the activities of DHAR and MDHAR kept constant except for the DHAR activity under 300 mM NaCl stress (Fig. 4b, c). When NaCl concentration was lower than 400 mM, 0.5 and 1.0 mM SA increased DHAR activities compared with the seedlings without SA treatments. Nevertheless, under 400 mM NaCl stress, SA of all the tested concentrations showed no obvious impact on DHAR activity. The effect of exogenous SA on MDHAR activity was insignificant except for the combinations of 300 mM NaCl + 0.5 mM SA, 300 mM NaCl + 1.0 mM SA and 400 mM NaCl + 1.0 mM SA (Fig. 4c).
Along with NaCl concentration increasing from 100 to 400 mM, GR activities exhibited an increasing trend (Fig. 4d). However, the differences in GR activity between 100 and 200 mM NaCl treatments as well as between 300 and 400 mM NaCl treatments were not significant. Exogenous application of SA at 0.5–1.5 mM significantly augmented GR activities of NaCl-stressed seedlings except for 400 mM, and the promoting effect of 1.0 mM SA was the strongest.
Effect of NaCl and SA on the contents of AsA, DHA, GSH, and GSSG as well as the ratios of AsA/DHA and GSH/GSSG
Table 1 revealed that two main effects demonstrated significant effects (P < 0.05) on ascorbate and glutathione pools except SA on the contents of DHA and GSSG. In addition, N × S significantly (P < 0.05) affected the contents of GSH and GSSG (Table 1). Notable changes in the ascorbate pool were observed in NaCl-stressed seedlings. The contents of AsA and DHA both showed increasing trends with the increases of NaCl concentration (Fig. 5a, b). However, the increments in DHA content were higher than those in AsA content, which lead to the decline in AsA/DHA ratio along with NaCl concentration (Fig. 5c). When NaCl concentration was lower than 400 mM, exogenous SA further enhanced AsA contents. However, the AsA levels remained unchanged under 400 mM NaCl stress. DHA contents kept constant with the application of SA. The above changing trends of AsA and DHA levels induced by exogenous SA resulted in the increases of AsA/DHA ratio. The greatest increments were observed when applying 1.0 mM SA.
Fig. 5.
Reduced ascorbate (AsA) content (a), dehydroascorbate (DHA) content (b), AsA/DHA ratio (c), reduced glutathione (GSH) content (d), oxidized glutathione (GSSG) content (e) and GSH/GSSG ratio (f) in the leaf of N. tangutorum seedlings under combined NaCl and SA treatments
As for the glutathione pool, the contents of GSH and GSSG in the leaf of N. tangutorum seedlings proportionally increased when exposed to 100–400 mM NaCl (Fig. 5d, e). Likewise, the increments in GSSG content were greater than those in GSH content in the presence of NaCl. Hence, the GSH/GSSG ratio appeared as a declining trend (Fig. 5f). Application of SA differently affected GSH and GSSG contents. The GSH contents were elevated (100–300 mM NaCl) or kept constant (400 mM NaCl) whereas the GSSG contents remained unchanged (100–300 mM NaCl) or declined (400 mM NaCl). Thus, the GSH/GSSG ratios increased to some extent with increasing NaCl concentration and some GSH/GSSG values were even recovered to the level of the control. The most pronounced effect was also found with 1.0 mM SA treatment.
Discussion
Salt stress is one of the major abiotic stresses limiting plant growth and productivity. In the present study, we observed that halophyte N. tangutorum exhibited good tolerance to low concentration salinity, as indicated by the equivalent or increased PFW and PDW under 100 and 200 mM NaCl treatments as compared to the control (Fig. 1a, b). The maintenance of growth and development for plants in salinity environments is partly associated with osmotic adjustment through the synthesis of osmotic adjustment solutes or in some halophytes, with the sequestration and accumulation of Na+ in the vacuole (Chinnusamy et al. 2005; Liu et al. 2014b). In the studies of Yang et al. (2010b) and Ni et al. (2015), it was also found that 50 and 100 mM NaCl was beneficial to the growth of N. tangutorum.
When NaCl concentration exceeded 200 mM, however, the PFW and PDW declined and the decrements in PFW was greater than those in PDW. These results indicated that under high concentration NaCl stress, the water absorption capacity of N. tangutorum roots was inhibited and hence the PFW decreased. High concentration of salt in soil involves both osmotic stress and ionic stress to plants. On one hand, the growth reduction is directly related to the osmotic stress of soil solution containing salt. The presence of high salt in the soil around the roots reduces the water absorption capacity of plant and results in the quick reduction in the growth rate (Hasanuzzaman et al. 2013). On the other hand, the toxic effect of excess salt inside the plant induces early leaf senescence, reduced photosynthesis rate and the decline in nutrient accumulation, which ultimately result in the inhibition of plant growth (Muchate et al. 2016).
Furthermore, the good tolerance of N. tangutorum seedlings to NaCl stress was also reflected by the significantly higher R/S ratios under different NaCl treatments as compared to the control (Fig. 1c). Similar results were found in N. tangutorum by Kang et al. (2015), who reported the increases in R/S ratio under salinity treatment in pot culture study. Generally, Na+ is not considered as a macronutrient and has not been shown to be essential for all higher plants (Subbarao et al. 1999). However, it has also been reported to have a stimulatory effect on the growth of some halophytes and xerophytes such as Atriplex halimus L. and Zygophyllum xanthoxylum (Martínez et al. 2005; Yue et al. 2012).
Our results found that exogenous SA stimulated the growth of N. tangutorum seedlings under NaCl stress and the promoting effect was more pronounced at medium and high SA concentrations (1.0 and 1.5 mM). Furthermore, in the presence of exogenous SA, NaCl-stressed N. tangutorum seedlings had even greater growth parameters than the control plants, as indicated by the PFW, PDW and R/S ratio in some combined treatments of NaCl and SA. These results are in accordance with some previous studies, which also reported the positive effect of SA on the growth parameters of Artemisia annua L., lemongrass, pistachio and lettuce, under salt stress (Aftab et al. 2011; Idrees et al. 2012; Bastam et al. 2013; Khalifa et al. 2016). The SA-mediated increases in the growth parameters of N. tangutorum seedlings might be as a result of the protective role of SA on membranes as well as the SA-induced antioxidant function and metabolic activity in seedlings (Gunes et al. 2005; Aftab et al. 2010).
In the present study, SA application significantly diminished O·−2 accumulation in the seedlings (Fig. 2a). The effect of exogenous SA on reducing the accumulation of O·−2 and on increasing SOD activity have been well documented in cotton and Pisum sativum L. (Liu et al. 2014a; Yadu et al. 2017). It is deduced that exogenous SA enhanced the scavenging of O·−2 via enhancement of SOD activity in N. tangutorum seedlings. The increases of SOD activity in the leaf of N. tangutorum seedlings subjected to combined treatments of NaCl and SA were observed in our previous study (Liu et al. 2016). Moreover, 0.5 and 1.0 mM SA significantly diminished H2O2 induced by NaCl stress. However, the effect of 1.5 mM SA was not pronounced, which may be attributed to the elevated H2O2 content induced by SA itself. Some references have demonstrated that physiologically relevant concentrations of SA can enhance H2O2 levels (Yang et al. 2013a). An increase in H2O2 concentration induced by SA is mediated via inhibition of CAT and APX activities through SA binding (Jayakannan et al. 2015b). In the present study, the APX activities were found to be significantly inhibited under 1.5 mM SA treatment (Fig. 4a). These results are in agreement with some other studies, which also found the increased H2O2 levels under SA treatments of relatively high concentrations (Aftab et al. 2011; Syeed et al. 2011).
NaCl stress remarkably induced the accumulation of TBARS, indicating the severe lipid peroxidation occurred in the leaves of N. tangutorum seedlings. The high levels of TBARS resulted from the reaction of overproduced O·−2 and H2O2 on membrane phospholipids. In addition, 0.5 and 1.0 mM SA decreased the degree of lipid peroxidation induced by NaCl stresses whereas 1.5 mM SA did not significantly reduced the oxidative damage in the seedlings (Fig. 3). The changes of TBARS level under combined treatments of NaCl and SA paralleled with the variations in H2O2 content. The protective effect of relatively low concentration SA against lipid peroxidation was also found in some other plants under salinity stress (Aftab et al. 2011; Syeed et al. 2011; Liu et al. 2014a; Khalifa et al. 2016; Yadu et al. 2017).
Despite the destructive effects, ROS are well described as second messengers and are involved in a variety of cellular processes including the tolerance of plants to environmental stresses (Sharma et al. 2012). Due to the multifunctional roles of ROS, it is vital to strictly control ROS level in cells to avoid any oxidative injury but not to eliminate them completely. In response to the elevated H2O2 levels induced by NaCl, the activities of APX increased whereas those of DHAR and MDHAR did not change significantly except for the DHAR activity under 300 mM NaCl stress (Fig. 4). It was also found salt stress significantly increased APX activities in rapeseed seedlings, the leaves of Vigna radiata (Linn.) Wilczek. and the roots of maize seedlings (Hasanuzzaman et al. 2014; Shan and Liu 2017; Hu and Shan 2018). In addition, 0.5 mM SA further up-regulated APX activity in N. tangutorum seedlings subjected to NaCl stress. However, 1.0 and 1.5 mM SA significantly decreased APX activities as compared to those without SA application. In the presence of H2O2, SA serves as one electron donor for CAT or APX to convert oxidized intermediate into an inactive and partially reduced state. As SA donates one electron to oxidized intermediate of CAT or APX, SA is converted into an oxidized form, SA* (Rao et al. 1997). This provides the evidence why 1.0 and 1.5 mM SA inhibited APX activity in N. tangutorum seedlings subjected to NaCl stress in the present study. Nazar et al. (2015) also reported that 0.5 mM SA notably increased APX activity in mustard under salt stress. Moreover, the promotion effect of low concentration SA (20 μM) on APX activity was also found in the leaves of Nymphaea tetragona Georgi under Cd stress (Gu et al. 2018). The DHAR activities were elevated by 0.5 and 1.0 mM SA but not by 1.5 mM SA under 100–300 mM NaCl stresses. Furthermore, the DHAR activities remained unaltered with SA treatments under 400 mM NaCl stress. The MDHAR activities kept constant under the combined treatments of NaCl and SA in comparison with those in the absence of SA.
Under NaCl stress, the contents of AsA and DHA increased as compared to the control (Fig. 5a, b). However, the levels of AsA and DHA increased differently, resulting in a shift of the AsA/DHA ratio towards an oxidative state (Fig. 5c). Generally, a high redox ratio of AsA/DHA is vital for the prevention of oxidative injury in plants. Our results demonstrated lower AsA/DHA ratios in NaCl-stressed N. tangutorum seedlings compared to control. It was attributed to the active participation of APX, which resulted in comparatively higher increments in levels of DHA than AsA. In other words, such a response of AsA/DHA ratio could also be related to the slower acceleration rate in the activities of DHAR and MDHAR, compared to APX. In Fig. 4b, c, slight increases in DHAR activity rather than MDHAR activity were observed in NaCl-stressed seedlings, which indicated that DHAR rather than MDHAR mainly contributed to the increases in AsA content. The decreases in AsA/DHA ratio were also observed in rice seedlings, watermelon seedlings, the leaves of tomato seedlings and the roots of maize seedlings under salt stress (Rahman et al. 2016; Li et al. 2017; Zhou et al. 2017; Hu and Shan 2018). Moreover, SA application increased or kept steady the activities DHAR and MDHAR whereas at high concentrations inhibited APX activities. Thus, the levels of AsA and DHA increased to different extent, and resulted in the increases of AsA/DHA ratio in NaCl + SA-treated seedlings compared to NaCl-treated seedlings. The increases of AsA/DHA ratio have also been shown to confer salt stress tolerance to Cicer arietinum L., watermelon, tomato and maize (Garg and Bhandari 2016; Li et al. 2017; Zhou et al. 2017; Hu and Shan 2018).
Under NaCl stress, the contents of GSH and GSSG were elevated compared with the control (Fig. 5d, e). The same changing trends of GSH and GSSG were also found in salt-stressed rapeseed and rice seedlings (Hasanuzzaman et al. 2014; Rahman et al. 2016). The balance between GSH and GSSG is a key element in maintaining cellular redox state. However, the declines in GSH/GSSG ratio were observed in NaCl-stressed N. tangutorum seedlings especially at higher NaCl concentrations (Fig. 5f), which was due to the lower regeneration rates of GSH as compared to those of GSSG. Although GSH/GSSG ratios declined with the increasing of NaCl concentration, GR activities increased significantly compared to the control (Fig. 4d). High GR activity maintains the glutathione pool in the reduced state, allowing GSH to be used by DHAR to reduce DHA to AsA. The above phenomenon indicated that GSH regeneration was not inhibited. Instead, the consumption of GSH was faster than its regeneration. Moreover, the deficiency of NADPH rather than limitation in GR activity could be one of the reasons associated with the decreases in GSH/GSSG ratio (Garg and Bhandari 2016). The declines in GSH/GSSG ratio were also found in rapeseed seedlings, Cicer arietinum L., rice seedlings, watermelon seedlings, the leaves of tomato seedling and the roots of maize seedlings under salinity stress (Hasanuzzaman et al. 2014; Garg and Bhandari 2016; Rahman et al. 2016; Li et al. 2017; Zhou et al. 2017; Hu and Shan 2018).
SA of varying concentrations kept or further increased GR activities in N. tangutorum seedlings in comparison with those without SA treatments. The enhanced GR activities favored the increased accumulation of GSH. As can be seen in Fig. 5d–f, SA induced comparatively higher accumulation of GSH than GSSG, increased GSH/GSSG ratio, and thus improved redox stability within cells. Moreover, the increased GSH provides reducing substrates for the reduction of DHA to AsA by DHAR, then the produced AsA is used by APX to directly detoxify H2O2. In other words, the higher ratios of the redox buffers i.e. AsA/DHA and GSH/GSSG observed in SA-treated seedlings demonstrated the promoting effect of SA on maintaining the vigor of AsA–GSH cycle in scavenging the over-generated ROS under NaCl stress. The effect of SA on increasing the ratios of AsA/DHA and GSH/GSSG was also demonstrated in rapeseed, mustard and wheat under salt stress (Hasanuzzaman et al. 2014; Nazar et al. 2015; Fardus et al. 2017), in mustard under short-term drought stress (Alam et al. 2013), in rice under Cu stress (Mostofa and Fujita 2013), and in the leavers of safflower under Zn stress (Namdjoyan et al. 2017).
Furthermore, the effects of SA on the expressions of key genes encoding AsA–GSH cycle enzymes including glutathione-S-transferase (GST1 and GST2), glutathione peroxidase (GPX1), phospholipid hydroperoxide GPX (GPX2), DHAR (DHAR), MDHAR (MDHAR), GR (GR), and glutathione synthetase (GSHS) in stressed plants were investigated (Kang et al. 2013; Li et al. 2013a, b). The SA-mediated differential regulation of the transcript levels of these genes was regarded as a key mechanism underlying the role of SA in salinity tolerance via AsA and GSH (Li et al. Li et al. 2013a, b). SA is known as a signal molecule in the induction of defense mechanisms in plants. To activate the defense response, SA needs to bind to specific receptors. The non-expresser of pathogenesis-related gene 1 (NPR1) protein is a master redox receptor for SA (Wu et al. 2012). SA can also bind with NPR3 and NPR4, which in turn trigger the yield of active monomeric NPR1 in the cytoplasm (Fu et al. 2012). The monomeric NPR1 enters the nucleus and functions as a transcriptional co-activator of defense genes (Attaran and He 2012). These defense genes control programmed cell death as well as the osmotic and oxidative stress tolerance, which are all important bases for salt tolerance (Jayakannan et al. 2015a).
Conclusions
Nitraria tangutorum seedlings exhibited good growth status when subjected to 100 and 200 mM NaCl stresses, as indicated by the unvaried or increased PFW, PDW and R/S ratio. NaCl stress induced increased accumulation of O·−2, H2O2 and TBARS in the leaf of N. tangutorum seedlings. N. tangutorum seedlings increased the contents of AsA, DHA, GSH and GSSG along with the activities of APX and GR, but failed to combat with the NaCl stress-induced oxidative damage. Exogenous application of SA favored the growth of N. tangutorum seedlings and alleviated the membrane lipid peroxidation induced by NaCl stress. Moreover, SA not only enhanced the activities of DHAR and GR, but also promoted the ratios of AsA/DHA and GSH/GSSG toward reduced states. Based on the above results, we conclude that exogenous SA improves the salinity tolerance of N. tangutorum seedlings partly through the regulation of AsA–GSH cycle. The data obtained in the present study suggest that SA influences the growth and physiology especially the ROS metabolism of N. tangutorum seedlings. SA, therefore, possessed the potential to be developed as effective plant growth promoters and as substitute of biofertilizers under field conditions. In the present study, the expression patterns of the key genes encoding AsA–GSH cycle enzymes in NaCl-stressed N. tangutorum seedlings were not measured, which should be conducted in subsequent study. In addition, systematic study using biochemical and genetic approaches to reveal the SA signal transduction pathway is a promising area of future research.
Acknowledgements
The authors gratefully acknowledge the financial supports by National Natural Science Foundation of China (31770437), Natural Science Foundation of Heilongjiang Province (C201427), China Postdoctoral Science Foundation (2014M551209) and Heilongjiang Postdoctoral Fund (LBH-Z14035).
References
- Aftab T, Khan MMA, Idrees M, Naeem M, Moinuddin Salicylic acid acts as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J Crop Sci Biotech. 2010;13:183–188. doi: 10.1007/s12892-010-0040-3. [DOI] [Google Scholar]
- Aftab T, Khan MMA, da Silva JAT, Idrees M, Naeem M, Moinuddin Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul. 2011;30:425–435. doi: 10.1007/s00344-011-9205-0. [DOI] [Google Scholar]
- Alam MM, Hasanuzzaman M, Nahar K, Fujita M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by upregulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2013;7:1053–1063. [Google Scholar]
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora. 2004;199:361–376. doi: 10.1078/0367-2530-00165. [DOI] [Google Scholar]
- Attaran E, He SY. The long-sought-after salicylic acid receptors. Mol Plant. 2012;5:971–973. doi: 10.1093/mp/sss086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastam N, Baninasab B, Ghobadi C. Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regul. 2013;69:275–284. doi: 10.1007/s10725-012-9770-7. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Jagendorf A, Zhu JK. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45:437–448. doi: 10.2135/cropsci2005.0437. [DOI] [Google Scholar]
- Fardus J, Matin MA, Hasanuzzaman M, Hossain MS, Nath SD, Hossain MA, Rohman MM, Hasanuzzaman M. Exogenous salicylic acid mediated physiological responses and improvement in yield by modulating antioxidant defense system of wheat under salinity. Not Sci Biol. 2017;9:219–232. doi: 10.15835/nsb929998. [DOI] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Bhandari P. Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate–glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma. 2016;253:1325–1345. doi: 10.1007/s00709-015-0892-4. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Gu CS, Yang YH, Shao YF, Wu KW, Liu ZL. The effects of exogenous salicylic acid on alleviating cadmium toxicity in Nymphaea tetragona Georgi. S Afr J Bot. 2018;114:267–271. doi: 10.1016/j.sajb.2017.11.012. [DOI] [Google Scholar]
- Gunes A, Inal A, Alpaslan M, Cicek N, Guneri E, Eraslan F, Guzelordu T. Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.) Arch Agron Soil Sci. 2005;51:687–695. doi: 10.1080/03650340500336075. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and responses of plants under salt stress. New York: Springer; 2013. pp. 25–87. [Google Scholar]
- Hasanuzzaman M, Alam MM, Nahar K, Mahmud JA, Ahamed KU, Fujita M. Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci. 2014;8:631–639. [Google Scholar]
- Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul. 2007;26:290–300. doi: 10.1007/s00344-007-9017-4. [DOI] [Google Scholar]
- Hossain MA, Asada K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 1984;25:85–92. [Google Scholar]
- Hu H, Shan C. Effect of cerium (Ce) on the redox states of ascorbate and glutathione through ascorbate–glutathione cycle in the roots of maize seedlings under salt stress. Cereal Res Commun. 2018;46:31–40. doi: 10.1556/0806.45.2017.057. [DOI] [Google Scholar]
- Idrees M, Naeem M, Khan MN, Aftab T, Khan MMA, Moinuddin Alleviation of salt stress in lemongrass by salicylic acid. Protoplasma. 2012;249:709–720. doi: 10.1007/s00709-011-0314-1. [DOI] [PubMed] [Google Scholar]
- Jayakannan M, Bose J, Babourina O, Shabala S, Massart A, Poschenrieder A, Rengel Z. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J Exp Bot. 2015;66:1865–1875. doi: 10.1093/jxb/eru528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. 2015;76:25–40. doi: 10.1007/s10725-015-0028-z. [DOI] [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Pastori G, del Río LA, Sevilla F. Role of the ascorbate–glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang GZ, Li GZ, Liu GQ, Xu W, Peng XQ, Wang CY, Zhu YJ, Huo TC. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate–glutathione cycle. Biol Plantarum. 2013;57:718–724. doi: 10.1007/s10535-013-0335-z. [DOI] [Google Scholar]
- Kang J, Zhao W, Zhao M, Zheng Y, Yang F. NaCl and Na2SiO3 coexistence strengthens growth of the succulent xerophyte Nitraria tangutorum under drought. Plant Growth Regul. 2015;77:223–232. doi: 10.1007/s10725-015-0055-9. [DOI] [Google Scholar]
- Khalifa GS, Abdelrassoul M, Hegazi AM, Elsherif MH. Attenuation of negative effects of saline stress in two lettuce cultivars by salicylic acid and glycine betaine. Gesunde Pflanzen. 2016;68:177–189. doi: 10.1007/s10343-016-0376-2. [DOI] [Google Scholar]
- Li G, Peng X, Wei L, Kang G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene. 2013;529:321–325. doi: 10.1016/j.gene.2013.07.093. [DOI] [PubMed] [Google Scholar]
- Li Q, Xu J, Li H, Wang S, Yan X, Xin Z, Jiang Z, Wang L, Jia Z. Effects of aspect on clonal reproduction and biomass allocation of layering modules of Nitraria tangutorum in nebkha dunes. PLoS ONE. 2013;8:e79927. doi: 10.1371/journal.pone.0079927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chang J, Chen H, Wang Z, Gu X, Wei C, Zhang Y, Ma J, Yang J, Zhang X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci. 2017;8:295. doi: 10.3389/fpls.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Guo Y, Bai J. Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J Plant Growth Regul. 2010;29:171–183. doi: 10.1007/s00344-009-9121-8. [DOI] [Google Scholar]
- Liu S, Dong Y, Xu L, Kong J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014;73:67–78. doi: 10.1007/s10725-013-9868-6. [DOI] [Google Scholar]
- Liu W, Yuan X, Zhang Y, Xuan Y, Yan Y. Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiol Plant. 2014;36:2183–2193. doi: 10.1007/s11738-014-1595-8. [DOI] [Google Scholar]
- Liu W, Zhang Y, Yuan X, Xuan Y, Gao Y, Yan Y. Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ J Plant Physiol. 2016;63:132–142. doi: 10.1134/S1021443716010118. [DOI] [Google Scholar]
- Martínez JP, Kinet JM, Bajji M, Lutts S. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J Exp Bot. 2005;56:2421–2431. doi: 10.1093/jxb/eri235. [DOI] [PubMed] [Google Scholar]
- Meyer AJ. The integration of glutathione homeostasis and redox signaling. J Plant Physiol. 2008;165:1390–1403. doi: 10.1016/j.jplph.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. doi: 10.1105/tpc.12.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radical in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Mostofa MG, Fujita M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology. 2013;22:959–973. doi: 10.1007/s10646-013-1073-x. [DOI] [PubMed] [Google Scholar]
- Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD. Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev. 2016;82:371–406. doi: 10.1007/s12229-016-9173-y. [DOI] [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. N Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi N, Prasad MNV. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001;160:291–299. doi: 10.1016/S0168-9452(00)00392-7. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Namdjoyan S, Kermanian H, Soorki AA, Tabatabaei SM, Elyasi N. Interactive effects of salicylic acid and nitric oxide in alleviating zinc toxicity of Safflower (Carthamus tinctorius L.) Ecotoxicology. 2017;26:752–761. doi: 10.1007/s10646-017-1806-3. [DOI] [PubMed] [Google Scholar]
- Nazar R, Umar S, Khan NA. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate–glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav. 2015;10:e1003751. doi: 10.1080/15592324.2014.1003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Yang X, Zhu J, Liu Z, Ni Y, Wu H, Zhang H, Liu T. Salinity-induced metabolic profile changes in Nitraria tangutorum Bobr. suspension cells. Plant Cell Tissue Organ Cult. 2015;122:239–248. doi: 10.1007/s11240-015-0744-0. [DOI] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Palma F, Lluch C, Iribarne C, García-Garrido JM, Tejera García NA. Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul. 2009;58:307–316. doi: 10.1007/s10725-009-9380-1. [DOI] [Google Scholar]
- Rahman A, Hossain MS, Mahmud JA, Nahar K, Hasanuzzaman M, Fujita M. Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants. 2016;22:291–306. doi: 10.1007/s12298-016-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Liu R. Exogenous hydrogen peroxide up-regulates the contents of ascorbate and glutathione in the leaves of Vigna radiata (Linn.) Wilczek. exposed to salt stress. Braz J Bot. 2017;40:583–589. doi: 10.1007/s40415-016-0354-z. [DOI] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:217037. [Google Scholar]
- Subbarao GV, Wheeler RM, Stutte GW, Levine LH. How far can sodium substitute for potassium in red beet? J Plant Nutr. 1999;22:1745–1761. doi: 10.1080/01904169909365751. [DOI] [PubMed] [Google Scholar]
- Syeed S, Nazar Anjum NA, Iqbal R, Masood A, Khan NA. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol Plant. 2011;33:877–886. doi: 10.1007/s11738-010-0614-7. [DOI] [Google Scholar]
- Wang C, He M, Li Y, Jiang C, Tian L, Wang Q. Comparative study on different detection methods of superoxide radicals in plant tissues. Environ Chem. 2012;31:726–730. [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012;1:639–647. doi: 10.1016/j.celrep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Yadu S, Dewangan TL, Chandrakar V, Keshavkant S. Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol Mol Biol Plants. 2017;23:43–58. doi: 10.1007/s12298-016-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi R, Wei X, Fan Q, An L. Effect of salinity on antioxidant enzymes in calli of the halophyte Nitraria tangutorum Bobr. Plant Cell Tissue Organ Cult. 2010;102:387–395. doi: 10.1007/s11240-010-9745-1. [DOI] [Google Scholar]
- Yang Y, Wei X, Shi R, Fan Q, An L. Salinity-induced physiological modification in the callus from halophyte Nitraria tangutorum Bobr. J Plant Growth Regul. 2010;29:465–476. doi: 10.1007/s00344-010-9158-8. [DOI] [Google Scholar]
- Yang YL, Zhang YY, Lu J, Zhang H, Liu Y, Jiang Y, Shi RX. Exogenous H2O2 increased catalase and peroxidase activities and proline content in Nitraria tangutorum callus. Biol Plantarum. 2012;56:330–336. doi: 10.1007/s10535-012-0094-2. [DOI] [Google Scholar]
- Yang W, Zhu C, Ma X, Li G, Gan L, Ng D, Xia K. Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung mean seedlings. PLoS ONE. 2013;8:e84580. doi: 10.1371/journal.pone.0084580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang F, Li X, Shi R, Lu J. Signal regulation of proline metabolism in callus of the halophyte Nitraria tangutorum Bobr. grown under salinity stress. Plant Cell Tissue Organ Cult. 2013;112:33–42. doi: 10.1007/s11240-012-0209-7. [DOI] [Google Scholar]
- Yang F, Ding F, Duan X, Zhang J, Li X, Yang Y. ROS generation and proline metabolism in calli of halophyte Nitraria tangutorum Bobr. to sodium nitroprusside treatment. Protoplasma. 2014;251:71–80. doi: 10.1007/s00709-013-0527-6. [DOI] [PubMed] [Google Scholar]
- Yue LJ, Li SX, Ma Q, Zhou XR, Wu GQ, Bao AK, Zhang JL, Wang SM. NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J Arid Environ. 2012;87:153–160. doi: 10.1016/j.jaridenv.2012.06.002. [DOI] [Google Scholar]
- Zhou Y, Wen Z, Zhang J, Chen X, Cui J, Xu W, Liu HY. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci Hortic. 2017;220:90–101. doi: 10.1016/j.scienta.2017.02.021. [DOI] [Google Scholar]