Abstract
Although basal medium optimization is a key factor in the success of tissue culture, its mineral composition is frequently disregarded when optimizing in vitro propagation protocols. A previous work on Eucalyptus dunnii micropropagation suggests that excessive callus formation and leaf chlorosis are related to specific nutritional conditions of the basal media. Recently, a novel basal medium based on the mineral nutrient analysis of E. dunnii young stump shoots was developed and successfully tested in plant regeneration and micropropagation of E. dunnii, avoiding all these issues. Considering this basal medium as an ideal growth condition, a mild deprivation of each macro and micronutrient and of the total organic fraction was imposed to E. dunnii in vitro cultures for 30 d. As a result, K, Mg, Mn, Cl, Zn, Mo, Ni or Co deprivation quantitatively affected growth and development of axillary shoots. Moreover, leaf chlorosis and the development of organogenic callus under Fe deficiency, and leaf drop along with shoot tip necrosis under N deficiency were observed. These symptoms suggest that nutrient content in E. dunnii tissues needs to be above 420.3 mg kg−1 for Fe and 27.7 g kg−1 for N to avoid the symptoms of leaf chlorosis and shoot tip necrosis. Additionally, the main role of Mn in quantitative responses and the antagonism between ions, especially for Mg/K and Mg/Zn, were denoted by the multivariate analysis. Overall, these results make a relevant contribution to the optimization of in vitro propagation of E. dunnii and other hard-to-propagate related species.
Keywords: Mineral nutrition, Deficiency symptoms, Micropropagation, Eucalypts, Chlorosis
Introduction
Eucalyptus dunnii has gained importance for its tolerance to cold stress among cultivated subtropical eucalypts (Arnold et al. 2004; Smith and Henson 2007). However, the deployment of improved genetic material of E. dunnii is restrained by poor seed availability and low rooting rates of cuttings (Graça 1987). Undoubtedly, mass clonal propagation of elite genotypes through micropropagation can help in resolving these problems by producing large quantities of healthy, high quality plants. To achieve this, a better understanding of E. dunnii mineral nutrition is essential, since main issues of in vitro propagation of this species are related to basal medium composition (Oberschelp and Gonçalves 2016).
It is well known that suboptimal culture media cause physiological disorders or even the death of the cultures (Nas and Read 2004). Furthermore, tissues and cells response to growth regulators is affected because of these nutritional imbalances (Ramage and Williams 2002; Kothari et al. 2004) and can induce characteristic symptoms, which can be used for diagnosis (Dell et al. 2001). Accordingly, chlorosis and necrosis of tissues and their patterns of development are the most important criteria for visual diagnosis of mineral nutrient deficiencies (Römheld 2012).
A novel basal medium, which attempts to mimic the mineral nutrient concentration of E. dunnii young stump shoots, has been successfully used for micropropagation, organogenesis and plant regeneration of this species (Oberschelp et al. 2015; Oberschelp and Gonçalves 2016). Although this medium avoided typical issues involved in E. dunnii in vitro propagation, it was not possible to identify which nutrients were the most relevant.
The present study aims to extend our previous work to provide an integrated view of the nutrients in EDMm basal media (Oberschelp and Gonçalves 2016) and its effects on growth and development. By accounting for the effects of mild deprivation of mineral and organic nutrients on morpho-physiological responses and on N, P, K, Ca, Mg, S, Cu, Fe, Mn and Zn tissue content, we characterized growth and development reduction, characteristic N and Fe deficiency symptoms and mineral nutrient concentrations associated with each growth condition in E. dunnii in vitro multiplication.
Materials and methods
Plant material. Four E. dunnii clones (ED006, ED015, ED020, and ED023) selected for high productivity were obtained from the Instituto Nacional de Tecnología Agropecuaria (INTA) Tree Breeding Program. In vitro cultures were established from epicormic shoots of stem sections, as described in Oberschelp and Gonçalves (2016).
The axillary buds developed into shoots were transferred to EDMm basal medium (Oberschelp and Gonçalves 2016), supplemented with 0.01 mg L−1 naphthaleneacetic acid (NAA), 0.2 mg L−1 6-benzylaminopurine (BAP), 5 g L−1 agar–agar cat. no. A7921 (Sigma-Aldrich®, St. Louis, MO, USA) and 20 g L−1 sucrose into 250-ml glass jars (40 ml aliquots), and subcultured eight times every 30 d, until the beginning of the experiments. Explants taken from these cultures were standardized to four shoot clusters without callus and oxidation symptoms for the experiment (Fig. 1a, b).
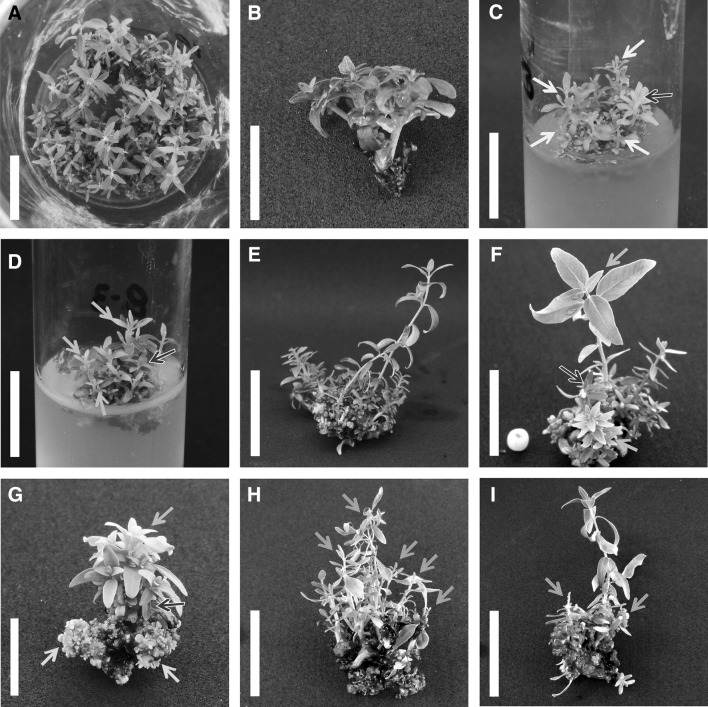
Fig. 1.
a Explant source from ED_020 clone in EDMm multiplication medium. b Detail of the standard explant from ED_014 clone. c Axillary bud cluster of ED_020 clone at 10 d of culture with new growing buds. d Axillary bud cluster of ED_020 clone at 10 d of culture under Fe deprivation showing chlorosis in new buds whereas older buds and leaves remain green. e Axillary bud cluster of ED_020 clone at 30 d of culture in full EDMm medium. f Axillary bud cluster of ED_023 clone at 30 d of culture under Fe deprivation. g Axillary bud cluster of ED_021 clone at 30 d of culture under Fe deprivation. h Axillary bud cluster of ED_020 clone at 30 d of culture under N deprivation. i Axillary bud cluster of ED_023 clone at 30 d of culture under N deprivation. Yellow arrows = healthy new buds; red arrows = chlorotic new buds; black arrows = old shoots from the original explant; blue arrows = shoot tip necrosis; green arrows = organogenic callus. Bar = 2 cm in (a) (b) (c), (d), (e), (f), (h) and (i); 1 cm in (b) and (g)
Culture media preparation and growth conditions were the same as those described in Oberschelp and Gonçalves (2016).
Experimental conditions. EDMm basal media (Oberschelp et al. 2015; Oberschelp and Gonçalves 2016) was modified for each deprivation treatment according to Table 1 and supplemented with 0.01 mg L−1 NAA, 0.1 mg L−1 BAP, 5 g L−1 agar–agar cat. no. A7921 (Sigma-Aldrich®, St. Louis, MO, USA) and 20 g L−1 sucrose. Culture media preparation and growth conditions were the same as those described in Oberschelp and Gonçalves (2016). Cultures were performed in 50-ml glass test tubes containing 10-ml of media.
Table 1.
Original and modified concentration, nutrient availability (NA) and components modified by each mild nutritional deprivation treatment imposed on the EDMm basal medium
| Nutritional deprivation treatment | Original concentration (mmol L−1)a | Modified concentration (mmol L−1) | NA (%) | Components modified from the EDMm basal medium |
|---|---|---|---|---|
| Full | – | – | 100 | None—full EDMm basal mediuma |
| –N | Total = 26.9 | 17.41 | 65 | |
| NO3− = 20.7 | 15.97 | 77 | NH4NO3 = 0 mg L−1 | |
| NH4+ = 6.2 | 1.44 | 23 | ||
| –P | 1.84 | 0.37 | 20 | KH2PO4 = 50 mg L−1 + K2SO4 = 130 mg L−1 |
| –K | 7.18 | 1.84 | 26 | KNO3 = 0 mg L−1 + NH4NO3 = 595 mg L−1 |
| –Ca | 4.11 | 0.85 | 21 | Ca(NO3)2.4H2O = 200 mg L−1 + NH4NO3 = 640 mg L−1 |
| –Mg | 1.21 | 0.25 | 21 | Mg(NO3)2.6H2O = 65 mg L−1 + NH4NO3 = 460 mg L−1 |
| –S | 0.89 | 0.17 | 19 | (NH4)2SO4 = 0 mg L−1 + NH4NO3 = 440 mg L−1 |
| –B | 0.05 | 0.00 | 0 | H3BO3 = 0 mg L−1 |
| –Cu | 0.04 | 0.00 | 0 | CuSO4.5H2O = 0 mg L−1 |
| –Fe | 0.26 | 0.05 | 19 | o,o-EDDHA/Fe = 2.8 mg L−1 (56 mg L−1 de Basafer® Plus) |
| –Mn | 0.12 | 0.00 | 0 | MnSO4.H2O = 0 mg L−1 |
| –Zn | 0.014 | 0.00 | 0 | ZnSO4.7H2O = 0 mg L−1 |
| –Co | 0.001 | 0.00 | 0 | CoCl2.6H2O = 0 mg L−1 |
| –Mo | 0.0006 | 0.00 | 0 | Na2MoO4.2H2O = 0 mg L−1 |
| –Ni | 0.0001 | 0.00 | 0 | Cl2Ni.6H2O = 0 mg L−1 |
| –NaCl | Cl = 0.21 | Cl = 0.027 | 13 | NaCl = 0 mg L−1 |
| Na = 0.21 | Na = 0.012 | 6 | ||
| –ORG | Standarda | 0.00 | 0 | All the organics were omitted |
aAccording to (Oberschelp and Gonçalves. 2016)
Plant tissue mineral analyses. After 30 d of culture, four samples of complete clusters (20 g fresh weight per sample) were collected for each deprivation treatment (nutrient deprivation x clone), rinsed three times with distilled water to remove residues of culture media, dried to constant weight at 65 ± 1 °C, milled with a porcelain mortar and pestle, sieved (841-μm mesh), and stored in plastic Eppendorf tubes. The chemical analyses of N, P, K, Ca, Mg, S, Cu, Fe, Mn and Zn were performed at the Laboratory of Plant Tissue, Department of Soil Science - ESALQ/USP, following the methodology proposed by Sarruge and Haag (1974). Organics, B, Cl, Na, Co, Mo and Ni were not analyzed due to sample size and technical limitations.
Experimental design and data analyses. A completely randomized design (CRD) under a 20 × 4 factorial arrangement (nutrient deprivation x clone) with five replicates was used. The experimental unit was a 50-ml glass test tube containing one explant (Fig. 1b). Data were collected after 30 d of culture; the following parameters were recorded: number of buds per cluster (NB); number of elongated buds (shoots > 20 mm in length) per cluster (NEB); multiplication rate (MR), calculated based on Halloran and Adelberg (2011); and growth rate (GR), calculated based on Oberschelp and Gonçalves (2016). Biomass weight gain (BWG) was calculated using the formula:
where FBW = explant final biomass weight at 30 d of culture, IBW = initial biomass weight (10 explants sample average),
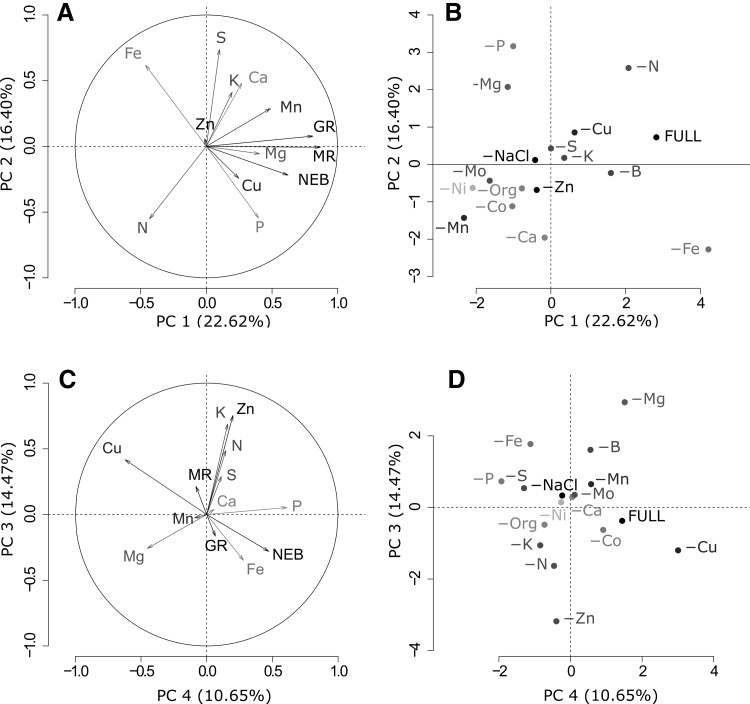
Before analysis, outliers, homogeneity of variance, and normality of errors were checked. Box-Cox transformations (Box and Cox 1964) were applied if variables did not meet the assumptions for parametric analysis after variance analysis and Tukey’s HSD test. Furthermore, the data obtained from chemical analysis and measurements of MR, GR, and NEB were subjected to a principal component analysis (PCA) (Jolliffe 2002) and were represented in biplot graphs (Gabriel and Odoroff 1990). In biplots, each variable is displayed as a vector in a correlation circle (radius = 1), which indicates the combined strength of its relationships with the two principal components (vector length) and whether these relationships are positive or negative (vector direction). The angle between two vectors indicates the degree of correlation between two variables. A 90° angle indicates that variables are uncorrelated; zero or 180º indicates complete positive or negative correlation. Treatments are displayed in an additional biplot.
For statistical analysis of tissue mineral nutrient concentration, the mean effect of deprivation of each nutrient was considered for variance analysis and Tukey’s HSD test.
All the analyses were performed using R 3.0.2 program (R Core Team 2013) with ‘FactoMineR’, ‘laercio’ and ‘MASS’ packages.
Results and Discussion
Four components of the PCA explained 64% of the variance from the 12 independent variables assessed, in which significant correlations between nutrient deprivation levels and morpho-physiological responses were observed (Table 2).
Table 2.
Principal components (PC), cumulative proportional variance (CPV) and significant correlations of the original independent variables with each component for four E. dunnii clones cultured in EDMm basal medium under 30 d in vitro nutritional deprivation
| PC | CPV (%) | Significant independent variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Cu | Fe | Mn | Zn | MR | GR | NEB | ||
| PC1 | 22.6 | − 0.43º | − 0.46º | 0.49* | 0.87** | 0.81** | 0.62** | |||||||
| PC2 | 39.0 | − 0.55* | − 0.54* | 0.41º | 0.48* | 0.74** | 0.62** | |||||||
| PC3 | 53.5 | 0.49* | 0.69** | 0.41º | 0.75** | |||||||||
| PC4 | 64.1 | 0.61** | − 0.45º | − 0.62** | 0.47º | |||||||||
º P ≤ 0.1; * P ≤ 0.05 and ** P ≤ 0.01
Biplot from the PCA analyses shows that Mn deficiency reduces growth and development of cultures (Fig. 2a, b). Coincidently, our previous work suggested that Mn plays a major role in in vitro propagation of E. dunnii, given its high concentration in EDMm media and physiological relevance (Oberschelp and Gonçalves 2016). Moreover, high Cu concentrations had negative effects on shoot elongation (Fig. 2c, d). This finding suggests that Cu concentration should be reduced in EDMm medium to optimize this response.
Fig. 2.
Biplot graphs of the PCA for mineral nutrient content, GR, MR and NEB for four E. dunnii clones grown under nutrient deprivation. a Biplot for vectors of the original variables and b clustering of the observations for each nutrient deprivation treatment when considering PC1 and PC2. c Biplot for vectors of the original variables and d observations clustering for each nutrient deprivation treatment when considering PC3 and PC4. In (a) and (c), the correlation circle describes the correlation between each vector (original variable) and principal components. Vector length indicates the strength of the relationship and the angle between two vectors shows the degree of correlation (adjacent = highly correlated, orthogonal = uncorrelated, and opposite = negatively correlated)
Characteristic deficiency symptoms in E. dunnii cultures were observed only under Fe and N mild deprivation (Fig. 1d, f, g, h, i). Fe deficiency generates internerval chlorosis in young leaves in a wide variety of crops (Bergmann 1992; Broadley et al. 2012); these symptoms were observed in vitro in Corylus avellana (Al Kai et al. 1984), Passiflora edulis (Monteiro et al. 2000), Rosa hibrida (Van Der Salm et al. 1994), E. globulus microcuttings (Schwambach et al. 2005), Juglans major 209 × J. regia (Licea-Moreno et al. 2015) and E. dunnii cultures (Oberschelp and Gonçalves 2016). Moreover, organogenic callus formation was observed under Fe deprivation (Fig. 1g). Similarly, Reed et al. (2013) found that callus formation decreases with increased Fe concentrations in in vitro cultures of pear.
Although differences were not significant and did not quantitatively affect growth and development, the lowest N content in tissues was observed in the N deprivation treatment (Table 3, 4). N mild deprivation induced oxidation (browning of tissues), shoot tip necrosis (STN), leaf senescence and leaf abscission (Fig. 1h, i); these symptoms are generally related to nutrient-starved shoots in the culture medium. STN is commonly observed under suboptimal culture conditions and can be related to several factors (Bairu et al. 2009). In pear rootstocks, excess of NO3− and NH4+ is pointed by Jamshidi et al. (2016) as one of the main factors responsible for STN. However, in E. dunnii, it can be attributed to N shortage, since it was observed only under this nutrient deprivation treatment.
Table 3.
Mean and standard deviation of NB, NEB, BWG, MR and GR of E. dunnii in vitro shoots grown in each nutritional deprivation treatment in EDMm basal media after 30 d of culture
| Nutritional deprivation | NB | NEB | BWG (mg explant−1) | MR | GR |
|---|---|---|---|---|---|
| FULL | 15.10 ± 6.41ab | 0.60 ± 0.75a | 85.57 ± 31.70a | 3.78 ± 1.60ab | 10.42 ± 2.07a |
| –N | 11.95 ± 4.58bcde | 0.55 ± 0.83a | 72.25 ± 29.58a | 2.99 ± 1.15bcde | 8.84 ± 2.09abc |
| –P | 11.60 ± 5.47bcde | 0.20 ± 0.41a | 69.82 ± 28.74a | 2.90 ± 1.37bcde | 8.44 ± 1.86abcd |
| –K | 10.55 ± 4.06cde | 0.35 ± 0.49a | 74.92 ± 36.59a | 2.64 ± 1.01cde | 8.79 ± 2.74abcd |
| –Ca | 11.30 ± 2.98bcde | 0.45 ± 0.69a | 75.01 ± 34.44a | 2.83 ± 0.74bcde | 8.98 ± 2.70abc |
| –Mg | 9.68 ± 3.87cde | 0.32 ± 0.48a | 66.48 ± 36.42a | 2.42 ± 0.97cde | 7.65 ± 2.80abcd |
| –S | 10.65 ± 3.59bcde | 0.30 ± 0.57a | 65.49 ± 30.84a | 2.66 ± 0.90bcde | 7.79 ± 2.10abcd |
| –B | 12.45 ± 5.12bcd | 0.55 ± 0.89a | 72.59 ± 36.48a | 3.11 ± 1.28bcd | 8.58 ± 3.00abcd |
| –Cu | 12.05 ± 4.96bcde | 0.55 ± 0.69a | 75.89 ± 38.40a | 3.01 ± 1.24bcde | 8.85 ± 2.95abcd |
| –Fe | 17.65 ± 7.15a | 0.55 ± 0.76a | 81.59 ± 40.32a | 4.41 ± 1.79a | 9.58 ± 3.18ab |
| –Mn | 8.60 ± 3.78cde | 0.30 ± 0.47a | 63.09 ± 28.74a | 2.15 ± 0.94cde | 7.62 ± 2.61abcd |
| –Zn | 8.45 ± 2.84de | 0.50 ± 0.69a | 70.92 ± 29.85a | 2.11 ± 0.71de | 8.59 ± 2.08abcd |
| –Co | 8.20 ± 3.12de | 0.60 ± 0.68a | 59.63 ± 28.11a | 2.05 ± 0.78de | 7.12 ± 2.10bcd |
| –Mo | 7.70 ± 3.18e | 0.40 ± 0.60a | 54.90 ± 29.56a | 1.93 ± 0.80e | 6.55 ± 2.72cd |
| –Ni | 8.10 ± 3.42de | 0.35 ± 0.49a | 52.33 ± 22.82a | 2.03 ± 0.85de | 6.37 ± 1.80cd |
| –NaCl | 11.25 ± 4.29bcde | 0.40 ± 0.50a | 62.55 ± 34.00a | 2.81 ± 1.07bcde | 7.26 ± 2.58bcd |
| –ORG | 12.95 ± 5.05bc | 0.20 ± 0.41a | 67.85 ± 32.05a | 3.24 ± 1.26bc | 8.10 ± 2.92abcd |
Different lowercase letters for each columns indicate differences according to Tukey’s HSD test (P = 0.05)
Table 4.
Mean and standard deviation of mineral nutrient content of E. dunnii in vitro shoots grown in each nutritional deprivation treatment after 30 d of culture
| Nutritional deprivation | N | P | K | Ca | Mg | S | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |
| Full | 35.5 ± 7.0a | 5.7 ± 1.0a | 19.6 ± 1.2abc | 7.1 ± 0.3a | 1.4 ± 0.2ab | 1.7 ± 0.2ab | 104.3 ± 4.5b | 1173.7 ± 264.3a | 607.8 ± 35.0a | 181.2 ± 4.6ab |
| –N | 27.7 ± 4.9a | 3.7 ± 0.4ab | 21.0 ± 2.7abc | 8.3 ± 0.5a | 1.8 ± 0.1ab | 1.6 ± 0.1ab | 88.3 ± 6.0b | 1075.0 ± 27.6a | 651.8 ± 26.5a | 164.0 ± 4.2b |
| –P | 35.5 ± 6.5a | 1.6 ± 0.1b | 21.8 ± 1.6abc | 6.1 ± 1.0a | 1.5 ± 0.1ab | 2.0 ± 0.1a | 99.8 ± 3.9b | 1101.8 ± 19.4a | 509.0 ± 54.4a | 177.3 ± 3.9ab |
| –K | 38.8 ± 6.4a | 4.8 ± 0.7a | 14.3 ± 2.2c | 7.3 ± 0.7a | 2.1 ± 0.2a | 1.6 ± 0.3ab | 104.3 ± 6.3b | 1140.0 ± 39.7a | 600.3 ± 40.2a | 181.3 ± 16.3ab |
| –Ca | 40.8 ± 11.7a | 4.3 ± 0.2a | 18.6 ± 3.9bc | 2.0 ± 0.3b | 1.3 ± 0.3bc | 1.3 ± 0.2bc | 92.8 ± 13.4b | 857.5 ± 51.7a | 613.7 ± 85.5a | 189.8 ± 4.7ab |
| –Mg | 40.4 ± 5.2a | 4.5 ± 0.8a | 26.8 ± 1.1a | 8.0 ± 0.0a | 0.8 ± 0.1c | 1.8 ± 0.3ab | 102.8 ± 6.7b | 1148.5 ± 19.8a | 630.3 ± 44.9a | 217.3 ± 17.3a |
| –S | 36.5 ± 6.6a | 3.8 ± 0.0a | 22.6 ± 2.7abc | 7.4 ± 0.5a | 1.8 ± 0.2ab | 1.2 ± 0.0c | 111.8 ± 28.6ab | 985.5 ± 70.7a | 616.3 ± 44.9a | 188.5 ± 1.4ab |
| –B | 41.8 ± 10.0a | 5.2 ± 0.6a | 24.9 ± 0.5ab | 7.2 ± 0.1a | 1.8 ± 0.1ab | 1.6 ± 0.1ab | 106.8 ± 1.1ab | 896.8 ± 102.9a | 583.3 ± 7.4a | 190.5 ± 6.4ab |
| –Cu | 35.2 ± 6.9a | 5.2 ± 0.1a | 20.4 ± 1.9abc | 6.9 ± 0.6a | 1.4 ± 0.3abc | 1.5 ± 0.3abc | 14.2 ± 1.4c | 1067.2 ± 211.3a | 531.5 ± 34.9a | 188.3 ± 4.7ab |
| –Fe | 37.4 ± 10.6a | 5.5 ± 0.4a | 21.7 ± 2.5abc | 7.2 ± 0.9a | 1.7 ± 0.3ab | 1.3 ± 0.1bc | 142.0 ± 9.6a | 420.3 ± 148.9b | 587.7 ± 48.7a | 191.8 ± 9.9ab |
| –Mn | 39.8 ± 2.5a | 5.0 ± 1.4a | 18.4 ± 3.3bc | 6.1 ± 0.7a | 1.3 ± 0.3bc | 1.5 ± 0.3abc | 94.2 ± 12.8b | 945.3 ± 145.0a | 51.6 ± 2.0b | 197.0 ± 25.1ab |
| –Zn | 36.6 ± 2.1a | 4.3 ± 0.2a | 17.3 ± 1.9bc | 6.4 ± 1.0a | 1.4 ± 0.3abc | 1.2 ± 0.2bc | 95.7 ± 6.0b | 1049.2 ± 199.8a | 522.3 ± 43.6a | 48.0 ± 7.4c |
| –Co | 40.1 ± 0.3a | 4.6 ± 1.0a | 17.6 ± 3.2bc | 6.5 ± 0.7a | 1.4 ± 0.1bc | 1.2 ± 0.0bc | 95.5 ± 4.2b | 1066.5 ± 92.0a | 543.3 ± 7.4a | 179.5 ± 12.0ab |
| –Mo | 40.4 ± 0.9a | 4.7 ± 0.4a | 19.5 ± 3.8abc | 6.7 ± 1.2a | 1.6 ± 0.0ab | 1.4 ± 0.1bc | 101.8 ± 2.5b | 1075.5 ± 35.4a | 522.8 ± 14.5a | 190.3 ± 16.6ab |
| –Ni | 40.4 ± 0.0a | 4.1 ± 0.4a | 19.1 ± 0.0abc | 6.4 ± 0.4a | 1.5 ± 0.0ab | 1.3 ± 0.1bc | 92.0 ± 0.7b | 1020.3 ± 64.0a | 511.8 ± 31.5a | 182.8 ± 0.4ab |
| –NaCl | 39.0 ± 4.4a | 4.1 ± 0.1a | 21.4 ± 2.2abc | 7.0 ± 0.4a | 1.7 ± 0.1ab | 1.4 ± 0.1bc | 89.8 ± 0.4b | 1005.8 ± 35.0a | 544.8 ± 7.4a | 180.3 ± 11.0ab |
| –Org | 36.8 ± 5.8a | 4.1 ± 0.6a | 16.1 ± 2.8c | 6.4 ± 1.3a | 1.2 ± 0.3bc | 1.0 ± 0.1c | 88.8 ± 10.4b | 1006.7 ± 387.2a | 549.8 ± 131.1a | 182.8 ± 34.9ab |
Different lowercase letters for each column indicate differences according to Tukey’s HSD test (P = 0.05)
The nutrient concentrations found in this work, both for N and Fe should be used as a baseline when monitoring media and tissues in E. dunnii in vitro propagation. The symptoms observed suggest that nutrient content in tissues needs to be above 420.3 mg kg−1 for Fe and 27.7 g kg−1 for N to avoid them (Table 4). Similarly, 17.4 mmol L−1 (243.6 mg L−1) of N and 0.05 mmol L−1 (2.8 mg L−1) of Fe in basal media should be considered critical for 30-d subcultures (Table 1). Additionally, lower Fe concentrations need to be studied in order to improve organogenic callus induction in E. dunnii.
Visual symptoms are noticeable under strong deficiencies, when growth has already been affected (Römheld 2012). If the deficiency of a nutrient is not strong enough and symptoms are not present, growth inhibition will be barely detected (Bergmann 1992). Nevertheless, in this study Fe deficiency produced symptoms without a negative effect on growth and development, a response that can be attributed to the role of Fe in photosynthesis and the mixotrophic conditions of in vitro culture. Additionally, Fe deprivation induced Cu accumulation in tissues; this effect is probably related to a rise in the Cu/Zn-superoxide dismutase, as already observed in other Fe-deficient plants (Iturbe-Ormaetxe et al. 1995; Sharma et al. 2004; Tewari et al. 2005) and/or due to cation competition effect (Broadley 2012).
Among the deprivation treatments, only -K, -Mn and -Zn induced a significant reduction of growth and development along with a lower nutrient content, according to the variance analysis and Tukey HSD test (Table 3, 4). Although Cl, Na, Co, Mo and Ni content was not analyzed in tissues due to technical limitations, their omission from EDMm medium formulation also caused negative effects on growth and development (Table 3).
NaCl is a standard component of EDMm medium, and its omission negatively affected cultures growth, which was indicated by the vectors and clustering in biplots (Fig. 2a, b) and by the lower MR (Table 3). Even though low concentrations of Na are favorable for plants due to its capacity to partially substitute K (Subbarao et al. 2003; Raposo Almeida et al. 2010; Kronzucker et al. 2013), the observed responses can be hardly attributed to this nutrient considering the high amount of K in EDMm media when NaCl was omitted (Table 1). Furthermore, beneficial role of Cl in many crop cultures was demonstrated (Marschner 1995; Chen et al. 2010). Lee and de Fossard (1977) also found growth reduction in Fragaria x ananassa under Cl deprivation in the culture media. However, references in the literature are mostly related to excess and negative effects of these nutrients in tissue culture, probably due to the concentrations tested, which is generally over 20 mmol L−1 (Morabito et al. 1994; Chen et alet al. 1998; Kennedy and De Filippis 1999; Shibli et al. 2003).
Co relevance in tissue culture as an ethylene synthesis inhibitor has been demonstrated (Roustan et al. 1989; Taiz and Zeiger 2006). In this study, explants were grown in non-ventilated vessels for 30 d, a system with high risk of ethylene accumulation (Santana-Buzzy et al. 2006). Therefore, Co might have prevented ethylene effects; this assumption is supported by the growth and development reduction observed under Co deprivation in basal medium (Table 3).
In NO3−-fed plants, there is a positive relationship between Mo supply, nitrate reductase activity in leaves and yield (Hawkesford et al. 2012). Although most plant tissues and organs uptake more N and grow faster in media with both N forms, NH4+ and NO3− (George and De Klerk 2008), the preference for one or another ionic form is a species-dependent characteristic (Edwards and Horton 1982). Considering that EDMm has both sources of N and that Mo deprivation induced lower NB, GR and MR, it can be inferred that E. dunnii cultures uptake and assimilate NO3− preferentially, as reported for other eucalypts species under ex vitro and in vitro conditions (Schwambach et al. 2005; Warren 2009; Máximo et al. 2015).
Ni omission also affected growth and development (Table 3). Its relevance in E. dunnii nutrition may be related to the need for stored N, mainly when explants are under N depletion in culture media since Ni plays a key role in recycling nitrogen during senescence or during seasonal changes (Winter et al. 2015).
As many other culture media, the organic fraction of EDMm medium has Cystein (27% of S), which is the first amino acid synthetized by plants in S metabolism (Marschner 1995). S deprivation and organic fraction omission from EDMm medium failed to significantly affect morpho-physiological responses (Table 2); yet, both produce a significant S reduction in tissues (Table 3), suggesting that E. dunnii cultures takes up and assimilates both forms. Conversely, a rise in S concentration was observed in P deprivation treatment, mainly because of the addition of K2SO4 (Table 1).
All nutrients that were chemically analyzed in tissues showed a reduction of the mean tissue concentration when reduced or omitted from the EDMm medium (Table 4), showing that deprivation was effectively induced. However, deficiency or excess does not always produce noticeable symptoms, initial effects are disturbance in metabolic process, accumulation of intermediate products and the delay or stop of the energetic metabolism (Bergmann 1992). Nutrient storage and/or accumulation by explants coming from favorable growth conditions and/or nutrients incompletely deprived in medium and/or insufficient time under deprivation should be considered to explain why many nutrients that affect growth and development did not induce characteristic symptoms of deficiency in this work.
Biplots from PCA suggest interactions among nutrients. The iron-phosphate precipitation is a common event in basal media and hydroponic nutrient solutions, which can be worsened by increasing either P and/or Fe free concentration (Dalton et al. 1983; George and De Klerk 2008). According to the biplots (Fig. 2a, b), this antagonism was present in EDM medium, as denoted by the direction and length of their vectors.
Antagonism between Mg and K was identified in both deprivation treatments (Fig. 2c, d). Highly hydrated Mg2+ ions have low binding strength and are therefore easily displaced by K+ in the exchange sites of cell walls (Marschner 1995); this is a well-known effect in Mg deficiencies caused by K excess (Hawkesford et al. 2012). Mg also interacts negatively with Zn because of their similar ionic radius (Boardman and McGuire 1990), as denoted by the vectors and observations in biplots (Fig. 2c, d). Given the high relevance of Zn and K in auxin-mediated cell elongation (Christian et al. 2006; George and De Klerk 2008) and their synergistic effect in EDMm medium (Fig. 2c, d), rising Mg concentrations should be monitored so that they do not suppress Zn and/or K uptake.
Deprivation of B and Cu did not affect growth or development of cultures; however, this does not necessarily mean that these nutrients are not essential. Nutrient storage of explants was probably enough to supply these nutrients throughout the 30 d of culture and symptoms of deprivation would have been appeared in successive subcultures.
The combination of PCA analysis and variance analysis allowed us to identify associations among nutrients and between nutrients and morpho-physiological variables that variance analysis alone could not. Additionally, this holistic statistical perspective recognized synergism and antagonism between nutrients that are well known when working with ex vitro nutrient solutions but poorly recognized in in vitro cultures. Considering the reduction of growth and development caused by almost all these nutrients, these interactions should be taken in account when adjusting nutrient concentration in culture media, avoiding adjusting them individually.
In summary, accurate management of N, K, Mg, Fe, Mn, Zn, Co, Mo and Ni concentrations is essential to achieve optimal growth and development of E. dunnii cultures. Although Mg/K and Mg/Zn antagonisms did not affect growth and development; care must be taken to avoid side effects when increasing nutrient concentrations beyond the ranges tested in this work. Moreover, 420.3 mg kg−1 of Fe and 27.7 g kg−1 of N in tissues caused the characteristic visual symptoms, affecting quality of shoots and leaves. Timely identification of these symptoms could help to correct culture medium rapidly and therefore to avoid growth and development decline of in vitro cultures.
Acknowledgements
The authors thank the Instituto Nacional de Tecnología Agropecuaria (INTA) and the Escola Superior de Agricultura Luiz de Queiroz, University of São Paulo (ESALQ/USP) for the support.
Author contributions
Author 1 (corresponding author): Design of the work, data collection, data analysis and interpretation, drafting the article and critical revision of the article.
Author 2: Conception of the work, support, data interpretation and critical revision of the article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Al Kai H, Salesses G, Mouras A. Multiplication in vitro du noisetier (Corylus avellana L.) Agronomie. 1984;4:399–402. doi: 10.1051/agro:19840412. [DOI] [Google Scholar]
- Arnold RJ, Luo J, Clarke B (2004) Trials of cold-tolerant eucalypt species in cooler regions of south central China. ACIAR Technical Reports 57, Canberra
- Bairu MW, Stirk WA, Staden JA. Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tiss Org Cult. 2009;98:239–248. doi: 10.1007/s11240-009-9560-8. [DOI] [Google Scholar]
- Bergmann W. Nutritional disorders of plants: development, visual and analytical diagnosis. Jena: Gustav Fischer; 1992. [Google Scholar]
- Boardman R, McGuire DO. The role of zinc in forestry. I. Zinc in forest environments, ecosystems and tree nutrition. For Ecol Manag. 1990;37:167–205. doi: 10.1016/0378-1127(90)90054-F. [DOI] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B Stat Methodol. 1964;26:211–234. [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. Function of nutrients: micronutrients. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. San Diego: Academic Press; 2012. pp. 191–248. [Google Scholar]
- Chen W, Zl He, Xe Yang, Mishra S, Pj Stoffella. Chlorine nutrition of higher plants: progress and perspectives. J Plant Nutr. 2010;33:943–952. doi: 10.1080/01904160903242417. [DOI] [Google Scholar]
- Chen DM, Keiper FJ, Filippis LF. Physiological changes accompanying the induction of salt tolerance in Eucalyptus microcorys shoots in tissue culture. J Plant Physiol. 1998;152:555–563. doi: 10.1016/S0176-1617(98)80277-0. [DOI] [Google Scholar]
- Christian M, Steffens B, Schenck D, Burmester S, Böttger M, Lüthen H. How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol (Stuttg) 2006;8:346–352. doi: 10.1055/s-2006-923965. [DOI] [PubMed] [Google Scholar]
- Dalton CC, Iqbal K, Turner DA. Iron phosphate precipitation in Murashige and Skoog media. Physiol Plant. 1983;57:472–476. doi: 10.1111/j.1399-3054.1983.tb02771.x. [DOI] [Google Scholar]
- Dell B, Malajczuk N, Xu D, Grove TS. Nutrient disorders in plantation eucalypts. 2. Canberra: ACIAR; 2001. [Google Scholar]
- Gabriel KR, Odoroff CL. Biplots in biomedical research. Stat Med. 1990;9:469–485. doi: 10.1002/sim.4780090502. [DOI] [PubMed] [Google Scholar]
- George EF, De Klerk G-J. The components of plant tissue culture media I: macro- and micro-nutrients. In: George EF, Hall MA, De Klerk G-J, editors. Plant propagation by tissue culture. 3. Dordrecht: Springer; 2008. [Google Scholar]
- Graça MEC. Avaliação do florescimento e do potencial de produção de sementes de Eucalyptus dunnii Maiden no Brasil. Boletim de Pesquisa Florestal. 1987;14:2–12. [Google Scholar]
- Halloran SM, Adelberg J. A macronutrient optimization platform for micropropagation and acclimatization: using turmeric (Curcuma longa L.) as a model plant. In Vitro Cell Dev Biol Plant. 2011;47:257–273. doi: 10.1007/s11627-011-9364-5. [DOI] [Google Scholar]
- Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P. Functions of macronutrients. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. San Diego: Academic Press; 2012. pp. 135–189. [Google Scholar]
- Iturbe-Ormaetxe I, Moran JF, Arrese-Igor C, Gogorcena Y, Klucas RV, Becana M. Activated oxygen and antioxidant defenses in iron-deficient pea plants. Plant, Cell Environ. 1995;18:421–429. doi: 10.1111/j.1365-3040.1995.tb00376.x. [DOI] [Google Scholar]
- Jolliffe IT. Principal component analysis. 2. Berlin, Heidelberg: Springer; 2002. [Google Scholar]
- Jamshidi S, Yadollahi A, Ahmadi H, Arab MM, Eftekhari M. Predicting In vitro culture medium macro-nutrients composition for pear rootstocks using regression analysis and neural network models. Front Plant Sci. 2016;7:274. doi: 10.3389/fpls.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BF, Filippis FD. Physiology and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and salt sensitive Grevillea arenaria. J Plant Physiol. 1999;155:746–754. doi: 10.1016/S0176-1617(99)80092-3. [DOI] [Google Scholar]
- Kothari SL, Agarwal K, Kumar S. Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet—Eleusine coracana (L.) Gaertn. In Vitro Cell Dev Biol Plant. 2004;40:515–519. doi: 10.1079/IVP2004564. [DOI] [Google Scholar]
- Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT. Sodium as nutrient and toxicant. Plant Soil. 2013;369:1–23. doi: 10.1007/s11104-013-1801-2. [DOI] [Google Scholar]
- Lee ECM, de Fossard RA. Some factors affecting multiple bud formation of strawberry (Fragaria X ananassa Duch.) Acta Hort. 1977;78:187–195. doi: 10.17660/ActaHortic.1977.78.24. [DOI] [Google Scholar]
- Licea-Moreno RJ, Contreras A, Morales AV, Urban I, Daquinta M, Gomez L. Improved walnut mass micropropagation through the combined use of phloroglucinol and FeEDDHA. Plant Cell Tiss Organ Cult. 2015;123:143–154. doi: 10.1007/s11240-015-0822-3. [DOI] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2. San Diego: Academic Press; 1995. [Google Scholar]
- Máximo WPF, Santos PAA, Mendonca EG, Santos BR, Paiva L. Nitrate (NO3−) and ammonium (NH4+) ratios for propagation of Eucalyptus hybrid in two different in vitro cultivation systems. Aust J Crop Sci. 2015;9:1242–1248. [Google Scholar]
- Monteiro ACBA, Higashi EN, Gonçalves AN, Rodriguez APM. A novel approach for the definition of the inorganic medium components for micropropagation of yellow passionfruit (Passiflora edulis Sims. F. flaviacarpa Deg.) In Vitro Cell Dev Biol Plant. 2000;36:527–531. doi: 10.1007/s11627-000-0094-3. [DOI] [Google Scholar]
- Morabito D, Mills D, Prat D, Dizengremel P. Response of clones of Eucalyptus microtheca to NaCl in vitro. Tree Physiol. 1994;14:201–210. doi: 10.1093/treephys/14.2.201. [DOI] [PubMed] [Google Scholar]
- Nas MN, Read PE. A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic. 2004;101:189–200. doi: 10.1016/j.scienta.2003.10.004. [DOI] [Google Scholar]
- Oberschelp GPJ, Gonçalves AN, Calderan Meneghetti E, Mendes Graner E, Almeida M. Eucalyptus dunnii Maiden plant regeneration via shoot organogenesis on a new basal medium based on the mineral composition of young stump shoots. In Vitro Cell Dev Biol Plant. 2015;51:626–636. doi: 10.1007/s11627-015-9715-8. [DOI] [Google Scholar]
- Oberschelp GPJ, Gonçalves AN. Assessing the effects of basal media on the in vitro propagation and nutritional status of Eucalyptus dunnii Maiden. In Vitro Cell Dev Biol Plant. 2016;52:28–37. doi: 10.1007/s11627-015-9740-7. [DOI] [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Cited 05 may 2013
- Ramage CM, Williams RR. Mineral nutrition and plant morphogenesis. In Vitro Cell Dev Biol Plant. 2002;38:116–124. doi: 10.1079/IVP2001269. [DOI] [Google Scholar]
- Raposo Almeida JC, Laclau JP, De Moraes Gonçalves JL, Ranger J, Saint-André L. A positive growth response to NaCl applications in Eucalyptus plantations established on K- deficient soils. For Ecol Manag. 2010;259:1786–1795. doi: 10.1016/j.foreco.2009.08.032. [DOI] [Google Scholar]
- Reed BM, Wada S, DeNoma J, Niedz RP. Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol Plant. 2013;49:343–355. doi: 10.1007/s11627-013-9504-1. [DOI] [Google Scholar]
- Römheld V. Diagnosis of deficiency and toxicity of nutrients. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. San Diego: Academic Press; 2012. pp. 299–312. [Google Scholar]
- Roustan JP, Latche A, Fallot J. Stimulation of Daucus carota somatic embryogenesis by inhibitors of ethylene synthesis: cobalt and nickel. Plant Cell Rep. 1989;8:182–185. doi: 10.1007/BF00716836. [DOI] [PubMed] [Google Scholar]
- Santana-Buzzy N, Canto-Flick A, Iglesias-Andreu LG, Montalvo-Peniche MC, Lopez-Puc G, Barahona-Perez F. Improvement of in vitro culturing of Habanero pepper by inhibition of ethylene effects. HortScience. 2006;41:405–409. [Google Scholar]
- Sarruge JR, Haag HP. Análises químicas em plantas. Piracicaba: ESALQ/USP; 1974. [Google Scholar]
- Schwambach J, Fadanelli C, Fett-Neto AG. Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus. Tree Physiol. 2005;25:487–494. doi: 10.1093/treephys/25.4.487. [DOI] [PubMed] [Google Scholar]
- Sharma PN, Kumar P, Tewari RK. Early signs of oxidative stress in wheat plants subjected to zinc deficiency. J Plant Nutr. 2004;27:449–461. doi: 10.1081/PLN-120028873. [DOI] [Google Scholar]
- Shibli RA, Shatnawi MA, Swaidat IQ. Growth, osmotic adjustment, and nutrient acquisition of bitter almond under induced sodium chloride salinity in vitro. Commun Soil Sci Plan. 2003;34:1969–1979. doi: 10.1081/CSS-120023231. [DOI] [Google Scholar]
- Smith HJ, Henson M. Achievements in forest tree genetic improvement in Australia and New Zealand 3: Eucalyptus dunnii Maiden. Aust For. 2007 [Google Scholar]
- Subbarao GV, Ito O, Berry WL, Wheeler RM. Sodium: a functional plant nutrient. Crit Rev Plant Sci. 2003;22:391–416. [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 4. Massachusetts: Sinauer Associates; 2006. [Google Scholar]
- Tewari RK, Kumar P, Sharma PN. Signs of oxidative stress in the chlorotic leaves of iron starved plants. Plant Sci. 2005;169:1037–1045. doi: 10.1016/j.plantsci.2005.06.006. [DOI] [Google Scholar]
- Van Der Salm TPM, Van Der Toorn CJG, Hanisch Ten Cate CH, Dubois LAM, De Vries DP, Dons HJM. Importance of the iron chelate formula for micropropagation of Rosa hybrid L. ‘Moneyway’. Plant Cell Tiss Organ Cult. 1994;37:73–77. doi: 10.1007/BF00048120. [DOI] [Google Scholar]
- Warren CR. Uptake of inorganic and amino acid nitrogen from soil by Eucalyptus regnans and Eucalyptus pauciflora seedlings. Tree Physiol. 2009;29:401–409. doi: 10.1093/treephys/tpn037. [DOI] [PubMed] [Google Scholar]
- Winter G, Todd CD, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534. doi: 10.3389/fpls.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]