Abstract
The finding described in this study is the first report of leaf spot disease of cotton caused by Curvularia verruculosa surveyed in the state of Maharashtra (India). The isolated phytopathogenic fungal strain was identified using morphological characteristics and molecular identification of ITS gene sequence (MF784436) and D1D2 region of LSU gene (KY978073). The ability of fungal strain to secrete hydrolytic enzymes viz., pectinase, xylanase, protease, cellulase and lipase was detected. The secretion profile of hydrolytic enzymes by C. verruculosa was also examined in planta and in vitro. The secretion of cellulase, xylanase and protease was found to be inducible on cotton-stalk powder containing media; while secretion of pectinase and lipase was constitutive in glucose containing medium. The hydrolytic enzymes secretion during etiological progression of disease was detected on cotton leaves at regular interval of 24 h up to 10 days. A significant correlation (P < 0.05) was observed between hydrolytic enzymes secretion and disease severity index. The increased level of hydrolytic enzymes in infected plant sample indicates their role in disease progression. The newly documented fungal phytopathogen Curvularia verruculosa was deposited at National Fungal Culture Collection of India, Pune with accession number of NFCCI-4119.
Keywords: Curvularia verruculosa, Transgenic Bt cotton, Leaf spot disease, Phytopathogen
India is the second largest cotton producer in the world (22% of the global cotton production) with largest cultivation area (30% of global cotton area) among the major cotton growing countries (WWF-India report 2012).
The cotton crop is prone to several fungal, bacterial and viral diseases; among these infections the fungal infections are predominant on cotton and cause the major yield loss (Bell 1999). In India, the various fungal diseases reported on cotton includes fusarium wilt, verticillium wilt, grey mildew, alternaria leaf spot, myrothecium leaf spot, root rot and anthracnose (AICCIP 2005–06, 2013–14). The disease establishment by phytopathogenic fungi utilizes different mechanisms like synthesis and liberation of cell wall degrading hydrolytic enzymes, toxin production and synthesis of metabolites which interfere with the normal growth of host plant. The hydrolytic enzymes are foremost virulence factors of the fungal phytopathogen for disease establishment (Gour and Dube 1975; Have et al. 2002; Kikot et al. 2009; Bellincampi et al. 2014; Kubicek et al. 2014). The present study focused on isolation of fungal phytopathogen associated with the infected cotton plant leaves and detection of its enzymatic virulence factors.
In the cotton cropping seasons of 2013–14 and 2014–15 (June–September), the cotton plants in Maharashtra (India) were surveyed. The plants were observed with leaf spots that started as minute, irregular in shape (5–18 mm in diameter) with chlorotic area around the spots. However, the mature spots turned into necrotic lesion with a halo center inducing leaf chlorosis and premature senescence. The estimated disease incidence and disease severity index (DSI) were 36 and 49.38% respectively. The maximum disease incidence was recorded in the month of August. The disease prone climatic conditions in this month (temperature, 25–32 °C; humidity, 55–90%) could have supported the fungal infection.
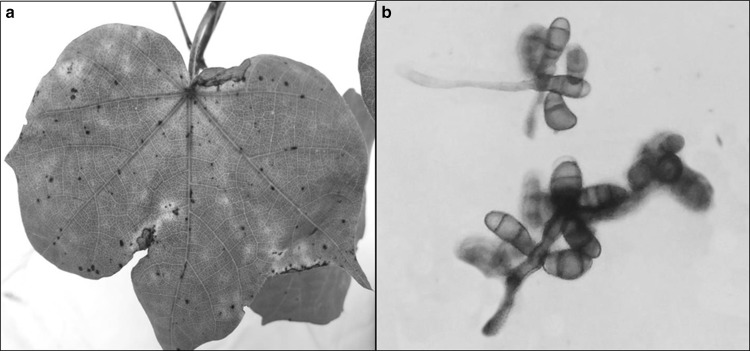
The infected leaves with prominent brown lesions surrounded with chlorotic region (Fig. 1a) were collected from different cotton growing fields in the surveyed area. The fungus was isolated from the section of diseased leaf on Czapek Dox Agar (CDA) media containing streptomycin (50 µg/ml). The morphological identification was carried out by the key described by Webster and Weber (2007) and Watanabe (2010). The fungus attained 90 mm wide colony on CDA in 6 days at 28 °C. The colony appeared as greenish gray on CDA, having pale colored fimbriate margin with grayish black reverse. The colony had circular, flat, filamentous appearance with slightly hairy, spreading and aerial mycelia with abundant sporulation. The hyphae were septate, branched, sub-hyline to brown in color. The conidiophore aroused singly, simple to rarely branched, macronematous, septate, sub-hyline to brown in color (Fig. 1b) on CDA. The conidia developed 17–38 µm long and 10–17 µm wide, with 2–4 cells broadly ellipsoidal, straight to slightly curved with one of the central cell being darker and larger, second and third cells appeared brown to pale brown while apical and basal cells were sub-hyaline, without hilum basally on slide culture assembly.
Fig. 1.
a Symptomatic cotton leaf, b conidiophore of C. verruculosa
The morphology of the fungus is similar with that described for genus Curvularia (Kusai et al. 2016). The molecular characterization of isolated fungal culture was carried out at National Fungal Culture Collection of India (NFCCI-ARI) Pune, India. The ITS gene was amplified with fungal universal primers ITS-4 and ITS-5. The D1D2 region of LSU-rDNA gene was amplified by using primers LROR and LR5. The sequencing PCR was set up with ABI-Big Dye terminator v 3.1 cycle sequencing kit. The BLAST analysis and sequence identity of ITS and D1D2 region by Clustal W identified the fungal isolate as Curvularia verruculosa. The sequences of ITS and D1D2 region of LSU-rDNA genes of C. verruculosa were deposited to NCBI with Genbank accession numbers MF784436 and KY978073 respectively. This newly isolated fungal phytopathogen—Curvularia verruculosa was deposited at National Fungal Culture Collection of India, Agharkar Research Institute (NFCCI-ARI) Pune, India with deposition accession number of NFCCI-4119.
The pathogenicity of isolated fungus was assayed by spraying the spore suspension (1 × 106 spores ml−1) on healthy plants at the four-leaf stage (18 days older plant). The control plants were treated with sterile distilled water. All the tested cotton plant leaves developed typical disease symptoms after 10–12 days of inoculation while control plants were symptomless. The vigor index and disease severity index (DSI) of control and test plants were measured as per Islam and Borthakur (2012) and Zhao et al. (2014) respectively. The DSI of test plants was 30 and a decrease of 29.75% in vigor index over control plant was also observed. The fungus was re-isolated from the leaf spots of treated cotton plants as an endorsement of Koch’s postulates.
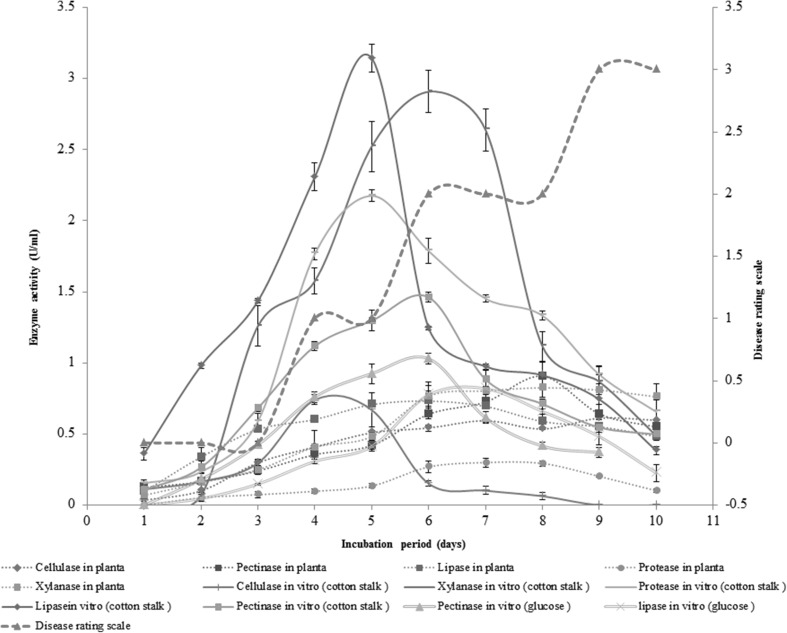
Hydrolytic enzymes are considered as most commonly produced than any other virulence factors by the fungal pathogens for disease establishment. The role of hydrolytic enzymes in penetration and break down of cuticular, cellulosic and pectic layers of plant cell wall is well documented (Have et al. 2002; Bellincampi et al. 2014; Kubicek et al. 2014). The detection of extracellular hydrolytic enzyme (viz. pectinase, xylanase, protease, cellulase and lipase) secretion by C. verruculosa was detected on solid media containing respective substrates. The zones of hydrolysis were produced around the fungal colony as a result of secretion of hydrolytic enzymes. The activities of hydrolytic enzymes were also determined in basal liquid media [g/l; NaNO3, 2; K2HPO4, 1; MgSO4, 0.5; KCl, 0.5; FeSO4, 0.01] supplemented with 1% cotton stalk powder and 1% glucose. The secretion of pectinase, xylanase, protease, cellulase and lipase in basal liquid medium (supplemented with 1% cotton stalk powder) was recorded as 1.46, 2.91, 2.18, 0.74 and 3.14 U/ml respectively (Fig. 2). The activity of pectinase and lipase in liquid medium supplemented with 1% glucose powder was 1.03 and 0.82 U/ml respectively, whereas the activity of xylanase, cellulase and protease was not detected in the glucose containing liquid medium (Fig. 2). These results indicated that the expression of cellulase, xylanase and protease was inducible while the expression of pectinase and lipase was constitutive in Curvularia verruculosa. According to the study of Kapat et al. (1998), the constitutive as well as induced type of enzyme secretion is important for disease development. The constitutive enzyme secretion during early phase of infection may help the pathogen for faster penetration.
Fig. 2.
In vitro secretion of hydrolytic enzymes by C. verruculosa in basal liquid medium containing cotton stalk powder/glucose as a carbon source and In planta secretion of hydrolytic enzymes by C. verruculosa during pathogenesis and its correlation with disease rating scale
The secretion of hydrolytic enzymes during pathogenesis was analyzed on cotton leaves after the regular interval of 24 h up to 10 days post inoculation (Fig. 2). The secretion of pectinase was found maximum (0.91 U/ml) among the studied enzymes. The secretion of pectinase was observed from 1st day of inoculation; the level of enzyme secretion was continuously increased and found maximum at 8th day of inoculation. The xylanase secretion in cotton leaf tissues was negligible at 1st day of inoculation but rose progressively and found maximum at 8th day. The considerable level of lipase was detected at 1st day of inoculation and a linear increase in activity was noticed till 6th day of inoculation, later on, a gradual decrease in activity was noticed. Cellulase secretion was found maximum at 6th day with a gradual increase in activity, right from 1st day of inoculation. The protease was least secreted enzyme among all the hydrolytic enzymes. The secretion of protease was observed from 2nd day of inoculation and found maximum at 7th day.
The hydrolytic enzyme secretion and disease development was correlated by analyzing the enzyme secretion and disease severity index (leaf area covered by disease) over 10 days of incubation. The enzyme secretion potential and DSI values were found to be elevated with increase in period of incubation (Fig. 2). The correlation between hydrolytic enzyme (pectinase, xylanase, protease, cellulase and lipase) production and disease rating scale was studied by Pearson Correlation Coefficient (P < 0.05). A significant correlation between hydrolytic enzymes (pectinase, xylanase, protease, cellulase and lipase) secretion and DSI was observed (P < 0.05). The presence of significant correlation between enzyme production and disease development indicated that, the hydrolytic enzymes act as an arsenal for penetration and colonization of phytopathogenic fungus within the host, which in turn develops disease. It was also observed that extracellular hydrolytic enzyme secretion pattern greatly differs during in planta and in vitro condition. This may be due to some inducing factors present in plant as well as some plant defense reactions and pH of cell sap.
In the present study, the phytopathogen associated with leaf spot disease of cotton was isolated and identified as Curvularia verruculosa. Although, C. verruculosa has been previously reported as a causative agent of leaf spot disease of Typha (Tandon and Bilgrami 1962), Sorghum, Triticum, Oryza (Sivanesan 1987) and Cynodon (Huang et al. 2005), the incidence of C. verruculosa infection to cotton plant has not been reported. Hence, to the best of our knowledge, this is the first report of C. verruculosa causing leaf spot disease of cotton. Moreover, this is the first approach to study in planta and in vitro secretion of hydrolytic enzymes by C. verruculosa on host plant.
Though the leaf spot disease caused by Curvularia sp. is concerned as minor disease, the outcomes of disease survey and pathogenicity test indicated that, C. verruculosa can cause serious outbreaks in the state of Maharashtra, which may lead to severe loss of cotton production under unpredictable global climatic vicissitudes. The findings of this study could be helpful for development of potential control measures and management of the newly isolated fungus in India. In addition to this, the studied enzymatic virulence factors may be appropriate targets for antifungal therapy.
Acknowledgements
The authors are thankful to Dr. S. K. Singh, National Fungal Culture Collection of India, Agharkar Research Institute (NFCCI-ARI) Pune, India for molecular identification and deposition of fungal strain.
References
- AICCIP Annual Report (2005–06) All India Coordinated Cotton Improvement Project, Coimbatore, Tamil Nadu
- AICCIP Annual Report (2013–14) All India Coordinated Cotton Improvement Project, Coimbatore, Tamil Nadu
- Bell AA. Diseases of cotton. In: Wayne Smith C, Tom Cothren J, editors. Cotton: origin, history, technology and production. New York: Wiley; 1999. pp. 553–559. [Google Scholar]
- Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall related susceptibility in plant–pathogen interactions. Front Plant Sci. 2014;5:1–8. doi: 10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gour HN, Dube HC (1975) Production of pectic enzymes by Curvularia lunata causing leaf spot of cotton. In: Proceedings of the Indian National Science Academy—Part B: Biological Science, vol 41, pp 480–485
- Have TA, Tenberge KB, Benen J, Tudzynski P, Visser J, Van Kan JAL. The contribution of cell wall degrading enzymes to pathogenesis of fungal plant pathogens. Mycota. 2002;11:341–358. [Google Scholar]
- Huang J, Zheng L, Hsiang T. First report of leaf spot caused by Curvularia verruculosa on Cynodon sp. in Hubei, China. Plant Pathol. 2005;54:253. doi: 10.1111/j.1365-3059.2005.01126.x. [DOI] [Google Scholar]
- Islam NF, Borthakur SK. Screening of mycota associated with Aijung rice seed and their effects on seed germination and seedling vigour. Plant Pathol Quar. 2012;2:75–85. doi: 10.5943/ppq/2/1/11. [DOI] [Google Scholar]
- Kapat A, Zimand G, Elad Y. Biosynthesis of pathogenicity hydrolytic enzymes by Botrytis cinerea during infection of bean leaves and in vitro. Mycol Res. 1998;102:1017–1024. doi: 10.1017/S0953756297006023. [DOI] [Google Scholar]
- Kikot GE, Hours RA, Alconada TM. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. J Basic Microbiol. 2009;49:231–241. doi: 10.1002/jobm.200800231. [DOI] [PubMed] [Google Scholar]
- Kubicek CP, Starr TL, Glass NL. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 2014;52:427–451. doi: 10.1146/annurev-phyto-102313-045831. [DOI] [PubMed] [Google Scholar]
- Kusai NA, Azmi MMZ, Zulkifly S, Yusof MT, Zainudin NAIM. Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rend Fis. 2016;27:205–214. doi: 10.1007/s12210-015-0458-6. [DOI] [Google Scholar]
- Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap. 1987;158:1–261. [Google Scholar]
- Tandon RN, Bilgrami KS. A new pathogenic species of genus Curvularia. Curr Sci. 1962;31:254. [Google Scholar]
- Watanabe T. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. Boca Raton: CRC Press; 2010. pp. 234–238. [Google Scholar]
- Webster J, Weber R. Introduction to fungi. New York: Cambridge University Press; 2007. pp. 472–475. [Google Scholar]
- WWF-India report (2012) Cotton market and sustainability in India. WWF-India-Yes Bank, p 7
- Zhao B, Yan J, Zhang S, Liu X, Gao Z. Phylogeny and pathogenicity of Fusarium spp. isolated from greenhouse melon soil in Liaoning Province. Saudi J Biol Sci. 2014;21:374–379. doi: 10.1016/j.sjbs.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]