Abstract
Rice husks (RHs) as an agro-waste generated from rice production, while its application is limited. This study was designed to introduce a full utilization of rice husks, which extracted the phytochemical at first and then produced cellulose nanocrystals (CNCs) as the use of the residue. Furthermore, the phytochemicals extracted from rice husk was identified and its biological activity, including antioxidant activity, cellular antioxidant activity (CAA) and antiproliferative activity, had been detected as well. Results showed the bound fraction of rice husk had higher antioxidant than common fruit and grain. Free fraction of rice husk deserved to have further analysis in antiproliferative activity due to its low cytotoxicity. The CNCs produced by residue was using delignification process and acid hydrolysis treatments. The chemical composition of the residue obtained after phytochemical extraction was determined. CNCs morphological investigation was performed using an optical microscope and atomic force microscopy (AFM). Our strategy is to achieve a comprehensive utilization of rice husks with both economy and environment benefits.
Introduction
The utilization of waste material and byproduct plays a vital part in solving economic issues at present. Rice husk is the byproduct of rice milling and the major agro-waste from rice production. World production of rice annually was 741 million tons, and approximately 148 million tons of husks were generated, corresponding to 20% of grain weight1. However, due to their tough structure and huge bulk, rice husk has limited applications and was treated by burying underground or open field burning. Thus, it is of great benefit to both the economy and environment to effectively convert rice husk into valuable products.
Recently researches regarding obtaining higher values from RHs have been investigated, such as deriving bioactive phytochemicals due to its protection against oxidative damage2,3. The strong link between antioxidant activity and phytochemical especially phenolics had been demonstrated by numerous reports, which indicate that phenolic compounds could be the dominant factor of antioxidant capacity4. Furthermore, phenolic compounds were found to inhibit the growth of tumor cell lines from human cancers5. Several literatures had been reported the in vitro antioxidant activity and phenolic compounds of rice husk2,6. However, none of these researches reported the cellular antioxidant activity of rice husk. Moreover, the antiproliferative activity of phytochemical extracted from rice husk was limited.
In addition, agricultural solid residues with high cellulose content have been proposed to use in cellulose and nanocellulose production7,8. Studies have shown that rice husk contains about 35–40% cellulose, 15–20% hemicellulose, and 20–25% lignin9. Hence, another way to take advantage of rice husk includes the utilization of the cellulose section. Johar et al.10 had successfully produced cellulose fibers and nanocrystals from rice husk using an acid hydrolysis treatment. CNCs has attracted a great deal of attention for its wide application, including reinforced nanocomposites, optically transparent functional materials11,12. Moreover, their use in films, membranes, catalyst support materials, and functionalized drug carriers has being explored recently13. In the study of Wijaya et al.14, CNC was extracted from passionfruit peels and used as tetracycline antibiotic drug carrier. Therefore, the use of rice husk as the primary source for producing cellulose nanocrystals is promising.
Thus, the objective of this study was to introduce a comprehensive approach to utilize rice husk by deriving phytochemicals primarily and gaining CNCs from residue. Moreover, phenolic compounds, antioxidant and antiproliferative activities were evaluated and morphological investigation of the CNCs was conducted. Through this method, phytochemical extracted from rice husk can be a potential ingredient in antioxidants or anticancer products. And the CNC made from the residue can be applied to other areas that generate economic value such as reinforcement materials and drug carriers.
Results and Discussion
Phenolic and Flavonoid Contents of Rice Husk
The contents of phenolic and flavonoid of rice husk were presented in Table 1. The bound fraction was 91.95 and 76.30% contribution to the total phenolics and flavonoids, respectively, which demonstrated that most of the phytochemicals were existed in bound fraction both in phenolic and flavonoid groups. Consistent with our result, Wanyo et al.2 claimed that bound phenolic contents of rice husk extracted by acetone accounted for 73.3 and 80.0% contributes to total phenolic and flavonoid contents, respectively. In addition, the content of phenolics had little difference compared to the content of flavonoids in free fraction. The total phenolic and flavonoid contents of rice husk were 14.90 ± 0.7 mg GAE/g and 3.08 ± 0.17 mg CE/g, which were consistent with results reported by Seungcheol et al.15. It is known that corn has a higher total phenolic content in whole grain and its total phenolic content was about 2.65 mg GAE/g16, which accounted for about 20% of that in rice husk. The same phenomenon was observed between some kinds of common fruit17, vegetable18 and rice husk, which strongly indicated the potential biological activity in rice husk extracts.
Table 1.
The content and phenolic profile of free, bound and total phenolics and flavonoids of rice husk.
| free | bound | total | |
|---|---|---|---|
| total phenolics (mg GAE/g) | 1.20 ± 0.06 (8.05%)a | 13.70 ± 0.67 (91.95%) | 14.90 ± 0.70 |
| total flavonoids (mg CE/g) | 0.73 ± 0.07 (23.70%) | 2.35 ± 0.12 (76.30%) | 3.08 ± 0.17 |
| p-hydroxybenzoic acid (mg/100 g) | 1.39 ± 0.06 | 11.16 ± 0.94 | 12.55 ± 0.93 |
| caffeic acid (mg/100 g) | 0.38 ± 0.02 | 3.30 ± 0.13 | 3.68 ± 0.14 |
| p-coumaric acid (mg/100 g) | 1.01 ± 0.02 | 264.4 ± 2.4 | 265.4 ± 2.4 |
| ferulic acid (mg/100 g) | 1.03 ± 0.07 | 32.61 ± 1.02 | 33.64 ± 1.01 |
Data are expressed as mean ± standard deviation of triplicate samples.
aValues in parentheses indicate percentage contribution of this fraction to the corresponding total fraction.
Phytochemical Profiles of Rice Husk
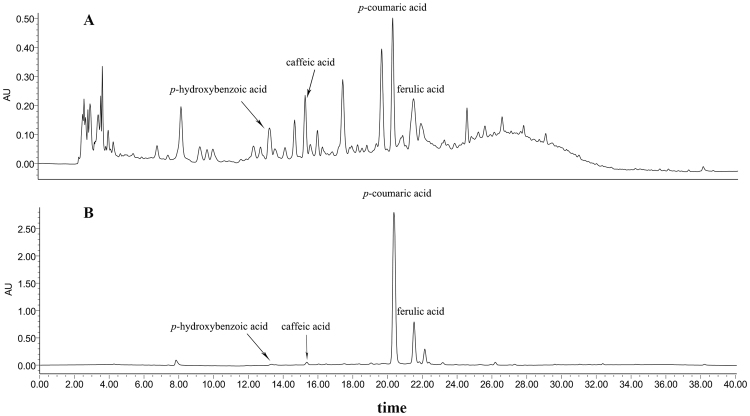
The HPLC chromatogram of free and bound phenolic acids of rice husk was shown in Fig. 1. Four phenolic acids, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid and ferulic acid, were identified and quantified and their contents were summarized in Table 1. As shown in Fig. 1A, a wide variety of the phytochemical components was observed in free fraction of rice husk, while there was little difference in their content. However, an opposite tendency was displayed in bound fraction in Fig. 1B. P-coumaric acid and ferulic acid were the major phenolic acids in bound fraction and the contents were 265.4 ± 2.4 and 33.64 ± 1.01 mg/100 g, respectively. This data was significantly higher than those in brown rice, millet, and other cereals19–21. Previous reported showed the antiperoxidative potential of p-coumaric acid against adjuvant-induced arthritis in rats22. Janicke et al. also reported ferulic acid and p-coumaric acid could produce antiproliferative effects by acting on the cell cycle of Caco-2 cells23. Our data demonstrated the high content of p-coumaric acid and ferulic acid in rice husk extract, therefore, the antioxidant and antiproliferative activities of phytochemical extracted from rice husk were evaluated below.
Figure 1.
HPLC chromatograms of free (A) and bound (B) phenolic compounds in rice husk. Flow rate: 1.0 mL/min; Detection is at 280 nm.
In vitro and Cellular Antioxidant Activity of Rice Husk
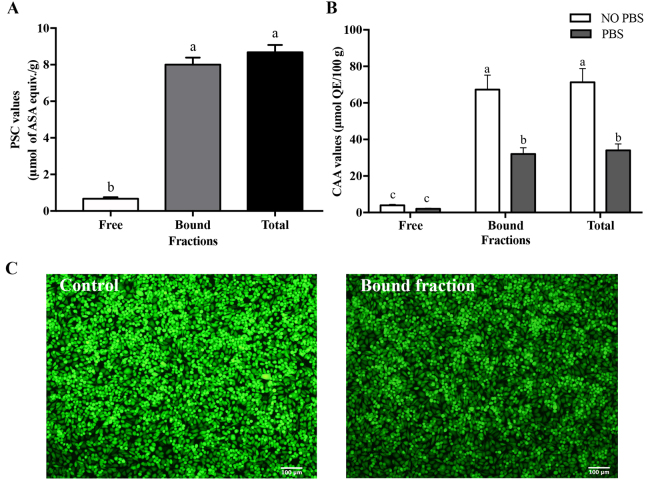
PSC and CAA assays were used here to quantify the in vitro antioxidant and cellular antioxidant activity of rice husk extract, respectively. On the whole, the PSC value (Fig. 2A) of the bound fraction had a dominant effect in samples, accounting for approximately 90% contribution to the total PSC values, which was in accordance with the content of phenolic. The high consistence between in vitro antioxidant activity and total phenolic contents were similar to previous study reported by Zhang et al.24 Furthermore, the total PSC value of rice husk extracts was 8.68 ± 0.40 μmol ASA equiv./g, which was about 3.98 times higher than that of bound fraction of whole rice25, respectively. Our results indicated that the antioxidant of rice husk extracts, especially bound fraction, had higher antioxidant activity.
Figure 2.
Antioxidant activity of the free, bound and total fraction of rice husk. (A) Antioxidant activity of rice husk expressed as PSC values (mean ± SD, n = 3). Bars with no letters in common are significantly different (p < 0.05); (B) Cellular antioxidant activity of rice husk expressed as CAA values (mean ± SD, n = 3). Bars with no letters in common are significantly different (p < 0.05); (C) The fluorescence picture of control and bound fraction of rice husk taken by fluorescence microscopy (Olympus IX83 Inverted Microscope).
The CAA assay is a more biologically relevant method because it simulates some of the cellular processes including cell uptake, metabolism, and distribution to predict the antioxidant behavior in biological systems26. PBS wash protocol and no PBS wash protocol were used to detect the degree of uptake and membrane association of samples. The CAA values (Fig. 2B) for bound fractions were 67.27 ± 7.94 and 32.00 ± 3.43 μmol QE/100 g in no PBS wash and PBS wash protocols, respectively, which were significantly higher than corresponding free fractions (3.99 ± 0.45 and 2.00 ± 0.36 μmol QE/100 g, respectively). The total CAA value of rice husk extracts was approximately 1.48 and 3.70 times higher than that of cranberry and red grape in no PBS protocol. Also, approximately 2.27 and 3.09 times higher than above fruit had been found in PBS protocol26. The fluorescence picture (Fig. 2C) taken by fluorescence microscopy (Olympus IX83 Inverted Microscope) could observe the antioxidant activity of bound fraction more directly. Bound fraction of rice husk could prevent oxidation of DCFH and reduced the formation of the fluorescent DCF, in that produce a darker field of view than control. The high cellular antioxidant activity of rice husk extracts demonstrated the potentials of rice husk, especially the bound fraction of rice husk, as natural sources of antioxidants with health benefits. Butsat and Siriamornpun also reported that rice husk can be regarded as valuable sources of bioactive components due to its high antioxidant properties3.
Antiproliferative Activity of Rice Husk
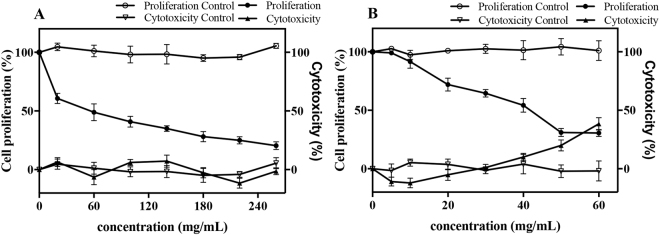
The antiproliferative activities and cytotoxicity were evaluated by HepG2 cells and shown in Fig. 3. On the whole, the inhibitions of HepG2 cells proliferation by the free (Fig. 3A) and bound (Fig. 3B) fractions were shown in a dose-dependent manner. At the concentration of 260 mg/mL, free fraction of rice husk showed no cytotoxicity and its cell proliferation was inhibited by 80%. At the concentration of 40 mg/ml, bound fraction of rice husk did not exhibited cytotoxicity, but inhibited cell proliferation by 54% specificly. Final values of antiproliferative activity and cytotoxicity were presented as the median inhibition dose (IC50) and the median cytotoxicity dose (CC50). The IC50 value of free fraction was 46.90 ± 2.79 mg/mL, while bound fraction had a higher antiproliferative activity at IC50 37.58 ± 4.40 mg/mL and at CC50 67.86 ± 1.62 mg/mL. It was observed that concentration at IC50 of free fraction showed no cytotoxicity, which indicated that the inhibitory effect of free fraction was not due to cytotoxicity but to the antiproliferative effect. Previous researches indicated p-coumaric can inhibit MCF-7 cells proliferation with an EC50 value at 1856.90 ± 70.25 μM27, and ferulic acid at 10 μM concentration can inhibit 21.09% growth of HepG2 cells28. Results indicated that the antiproliferative activitiy of the free fraction of rice husk deserved to have further study due to its low cytotoxicity. The antiproliferative activity of free and bound fraction revealed less consistence to phenolic content and antioxidant activity, which was in accord with the report of Nzaramba et al.29, who found no significant correlations between antiproliferative activity and antioxidant activity, phenolics in both HT-29 and LNCaP prostate cancer cell lines. This may be because of different effects, such as additive, synergistic and/or antagonistic effects among component presented in rice husk extracts. Our result demonstrated that the antiproliferative activities could not be explained only by their phenolic compound contents; Besides, the interaction among components presented in extracts should be considered30.
Figure 3.
Antiproliferative activity and cytotoxicity of free (A) and bound (B) fraction of rice husk against HepG2 human liver cancer cells (means ± SD, n = 3).
Chemical composition and morphological investigation of the CNCs
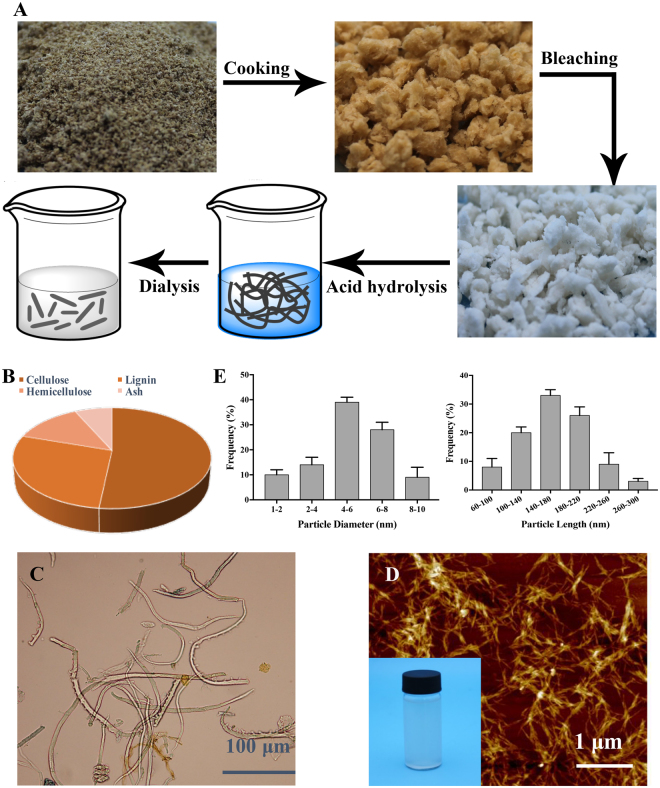
The chemical composition of rice husk was given in Supplementary file. The content of cellulose, hemicellulose, lignin, and ash were detected after phytochemical extracted from rice husk, as shown in Fig. 4B. The cellulose content of residue was above 50%, which suggested the promising production of CNCs. The procedure of CNCs production from residue was observed in Fig. 4A and details of cooking, bleaching, acid hydrolysis and dialysis were given in Materials and methods. Moreover, an optical microscope was used to observe the width and length of fibers obtained after bleaching treatment (Fig. 4C). Results showed the widths of bleached fiber were 5–15 μm and the corresponding lengths were 0.1–1 mm. The CNCs produced from residues was verified by AFM image and presented in Fig. 4D. The reason of agglomerated aspect of CNCs observed in the figure may be due to their high specific area and the strong hydrogen bonds between crystallites31. Furthermore, more than a hundred particles were chosen randomly and the diameter and length of these were measured, which was shown in Fig. 4E. A broad polydispersity, with a length ranging from 60 to 300 nm and a diameter between 1 and 10 nm, was observed. Diameter at 4–8 nm and length at 100–220 nm accounted for approximately 70% of the total. The application of CNCs obtained from rice husk was broad. Ooi et al. produced CNC-gelatin hydrogels to act as drug carriers, which could be used in controlled drug delivery systems32. Tang et al. also found the potential of polyrhodanine coated cellulose nanocrystals to be antimicrobial agent33.
Figure 4.
Chemical composition and morphological investigation of the CNCs. (A) Preparation of CNCs from the residue of rice husk; (B) Chemical composition of the residue; (C) Micrographs of the fibers after bleached; (D) Height mode AFM images of CNCs and picture of the real CNCs; (E) The frequency of particles diameter (nm) and length (nm) of CNCs.
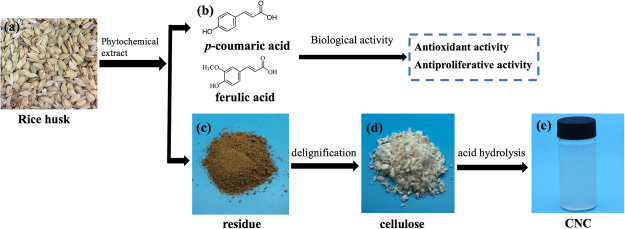
In conclusion, this work presented a fine example of the full application of rice husk, which is expected to promote effective utilization of ago-waste in the near future (Fig. 5). Our results reveal that the potentials of phenolic compounds extracted from rice husk as natural sources of antioxidants in bound and free fractions. Cellulose nanocrystals were successfully extracted from the residue after phytochemical extraction using an acid hydrolysis treatment, which was confirmed by chemical composition determination, morphological investigation. Rice husk as a wasted product can be used in the manufacture of nutritional supplements, the antimicrobial agent, food-packaging and other possible applications.
Figure 5.
A flowchart of rice husk full utilization. (a) the photo of rice husk; (b) the main phenolic compounds in rice husk; (c) the picture of residue after phytochemical extraction; (d) the cellulose gained after delignification; (e) the CNC prepared by acid hydrolysis from cellulose of rice husk.
Materials and Methods
Materials and Reagents
Rice husk was provided by the Rice Research Institute of Guangdong Academy of Agricultural Science, China. Folin-Ciocalteu reagent, fluorescein disodium salt, 2,2′-azobis (2-amidinopropane) dihydrochloride (ABAP), 2′,7′-Dichlorofluorescin diacetate (DCFH-DA), (+)-catechin, gallic acid, and chromatographic grade of p-coumaric acid, caffeic acid, p–coumaric acid, ferulic acid were purchased from Sigma-Aldrich Ltd. (St. Louis, MO, USA). Chromatographic grade of acetonitrile used for HPLC analysis was obtained from Anpel Ltd. (Shanghai, China). Human liver cancer cell line HepG2 (ATCC HB-8065) were purchased from ATCC company (Manassas, VA, USA). WME medium, fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS), Trypsin-EDTA solution and other cell culture reagents were purchased from Gibco Life Technologies Co. (Grand Island, NY, USA).
Extraction of Free and Bound Phenolic Compounds
Phenolics were extracted according to previously reported with a slight modification34. Briefly, the free phenolics in rice husk were extracted by chilled 80% acetone and then reconstituted using 70% methanol. The bound phenolics were digested and neutralized primarily, and then extracted five times with ethyl acetate. The ethyl acetate fractions reconstituted using 70% methanol. Both extractions and the remaining residue were stored at −40 °C for further use.
Determination of Phenolics and Flavonoids
The phenolic content was measured by Folin-Ciocalteu method as reported previously35 and gallic acid was used as the standard. The flavonoids were determined using the sodium borohydride/chloranil assay (SBC)36, using catechin as the standard. Data is expressed as milligram gallic acid equivalent per gram (mg GAE/g) and milligram catechin equivalent per gram (mg CE/g) of rice husk in triplicate, respectively.
Analysis of Phytochemical Composition
The identification of phytochemical was performed according to the method reported previously37 with slight modifications. The samples were analyzed by an HPLC system (Waters Co., USA), which was equipped with a 4.6 × 250 mm, 5 μm Sunfire C18 reversed phase column (Waters, USA). The mobile phase consisted of purified water with 0.1% trifluoroacetic acid (solvent A) and acetonitrile (solvent B) at a flow rate of 1.0 mL/ min using gradient elution as follows: 0–5 min 10% B, 5–20 min 25% B, 20–25 min 35% B, 25–40 min 90% B, 40–50 min 10% B, 50–60 min 10% B at 280 nm. Phenolic compounds in rice husk were identified by comparing the retention time and detected using an external standard method. Measured values were expressed as milligrams per 100 g of rice husk (mg/100 g).
Quantification of in vitro Antioxidant Activity
The in vitro antioxidant activity was evaluated by the peroxyl radical scavenging capacity (PSC) assays as described previously25. Ascorbic acid (ASA) was used as the standard. In brief, diluted samples and standard solutions were mixed with equivalent volume DCFH–DA and followed by ABAP. The reaction was performed at 485 nm excitation and 538 nm emission by the multi-mode microplate reader (Molecular Devices, Orleans Drive Sunnyvale, CA, USA). Results were calculated as micromoles of ascorbic acid equivalent per gram (μmol ASA equiv./g) and the data were reported as mean ± SD (n = 3).
Measurement of Cellular Antioxidant Activity
Human liver cancer cells HepG2 were cultured in WME medium supplemented with 2 mM L-glutamine, 10 mM Hepes, 5% fetal bovine serum, 5 μg/mL insulin, 50 units/mL penicillin, 0.05 μg/mL hydrocortisone, 50 μg/mL streptomycin and 100 μg/mL gentamycin at 37 °C in a humidified atmosphere of 5% CO2.
The CAA assay was conducted as described previously26. Quercetin was used as the standard. In brief, HepG2 cells were incubated at a density of 6.0 × 104 cells/well for 24 h and followed by medium containing samples which were under the cytotoxicity dose plus 25 μM DCFH-DA. ABAP was added after incubated for 1 h and the fluorescence intensity was monitored at 485 nm excitation and 535 nm emission every 5 min for 1 h (Molecular Devices). The CAA value was presented as micromoles of quercetin equivalent per 100 g of rice husk (μmol QE/100 g).
Cytotoxicity and Antiproliferative Activity Assays
The antiproliferative activity was measured in HepG2 cells by using a methylene blue assay that has been reported previously38. Briefly, cells were seeded at a concentration of 2.5 × 104 cells/well. For cytotoxicity test, cells were plated at the concentration of 4 × 104 cells/well. After incubated, a series concentration of diluted extracts in growth medium was added into each well and then stained for viable number counting. Absorbance was measured at 570 nm on Multi-Mode Microplate Reader (Molecular Devices). Each sample was measured at least three times. The cytotoxicity and antiproliferative activity of phytochemicals were assessed by half maximal cytotoxicity concentration (CC50) and median inhibition dose (IC50), respectively, which were expressed as mg/ml (mean ± SD, n = 3).
Chemical Composition of The Residue
The chemical composition of the residue was determined according to the Technical Association of Pulp and Paper Industry (TAPPI). T203 OS-7439 was used to measure the content of cellulose and hemicellulose and the lignin content was obtained according to T222 OS-8340. The ash content was determined after pyrolysis of the dry sample in a furnace at 525 °C for 6 h, following the standard procedure of T211 om-0241.
Preparation of Cellulose Nanocrystals from Residue
The preparation of cellulose nanocrystals from residue was conducted according to previously reported10 with slight modifications, which including delignification process and acid hydrolysis treatment. The residue was treated with the alkali solution (4% NaOH, w/v) and cooked at 165 °C for 90 min. Then the solid was washed with distilled water several times. Then, hydrogen peroxide (3%, v/v) and distilled water were added to ensure 12% concentration of pulp, followed by bleaching at 80 °C for 1 h. The mixture was filtered using excess distilled water. The acid hydrolysis treatment was conducted at 45 °C using concentrate sulphuric acid for 120 min under continuous stirring. The hydrolyzed material was washed by centrifugation at 10,000 rpm for 10 min. This step was repeated several times. And then the suspension was dialyzed using distilled water for several days until constant pH in the range of 5–6 was reached.
Optical Microscope and Atomic Force Microscopy
A drop of the cellulose fiber suspension after bleaching was placed on a glass slide for imaging with an optical microscope (OLYMPUS BX51). A drop of CNC suspension was placed on a piece of freshly cleaved mica, dried and then analyzed with Atomic Force Microscope (AFM, Nanoscope IIIa Multimode 8, Bruker)42. Lengths and diameters were obtained using the section analysis tool of the NanoScope Analysis software (Bruker, version 1.40) from height mode AFM image. More than a hundred CNCs were randomly measured in order to determine the average length and diameter.
Statistical Analysis
The data, expressed as the mean ± SD, included at least three replicates per sample. ANOVA and Tukey’s test were performed using Statistical Package for the Social Sciences (SPSS, version 21.0). Graphical representations were performed using Sigma plot version 12.3 (SPSS, Chicago, IL, USA). P-values < 0.05 were regarded as significant.
Electronic supplementary material
Acknowledgements
This work was supported by the Natural Science Foundation of Guangdong Province of China (2016A030312001) and Science and Technology Planning Project of Guangdong Province-China (2016B020233004).
Author Contributions
R.H.L., X.G., Z.F. and Y.G. conceived and designed the experiments; Y.G. and Y.L. performed the experiments; R.H.L., X.G., T.L., R.Z., L.Y. and M.Z. contributed chemicals and materials; Y.G., Y.L., X.G. and R.H.L. interpreted the data and wrote the draft manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27635-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinbo Guo, Email: xbg720@gmail.com.
Rui Hai Liu, Email: RL23@cornell.edu.
References
- 1.Oliveira JPd, et al. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chemistry. 2017;221:153–160. doi: 10.1016/j.foodchem.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 2.Wanyo P, Kaewseejan N, Meeso N, Siriamornpun S. Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products. Applied Biological Chemistry. 2016;59:1–12. doi: 10.1007/s13765-016-0173-8. [DOI] [Google Scholar]
- 3.Butsat S, Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chemistry. 2010;119:606–613. doi: 10.1016/j.foodchem.2009.07.001. [DOI] [Google Scholar]
- 4.Liu RH. Whole grain phytochemicals and health. Journal of Cereal Science. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- 5.Chandrasekara A, Shahidi F. Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. Journal of Functional Foods. 2011;3:159–170. doi: 10.1016/j.jff.2011.03.008. [DOI] [Google Scholar]
- 6.Lourith N, Kanlayavattanakul M. Appraisal of Thai glutinous rice husk for health promotion products. Journal of Cereal Science. 2013;57:343–347. doi: 10.1016/j.jcs.2012.12.005. [DOI] [Google Scholar]
- 7.Neto F, et al. Extraction and characterization of cellulose nanocrystals from;agro-industrial residue - Soy hulls. Industrial Crops & Products. 2013;42:480–488. doi: 10.1016/j.indcrop.2012.06.041. [DOI] [Google Scholar]
- 8.Satyanarayana KG, Arizaga GGC, Wypych F. Biodegradable composites based on lignocellulosic fibers—An overview. Progress in Polymer Science. 2009;34:982–1021. doi: 10.1016/j.progpolymsci.2008.12.002. [DOI] [Google Scholar]
- 9.Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Industrial & Engineering Chemistry Research. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- 10.Johar N, Ahmad I, Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Industrial Crops and Products. 2012;37:93–99. doi: 10.1016/j.indcrop.2011.12.016. [DOI] [Google Scholar]
- 11.Svagan AJ, Samir MASA, Berglund LA. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Advanced Materials. 2008;20:1263–1269. doi: 10.1002/adma.200701215. [DOI] [Google Scholar]
- 12.Nogi M. Optically Transparent Nanofiber Paper. Advanced Materials. 2009;21:1595–1598. doi: 10.1002/adma.200803174. [DOI] [Google Scholar]
- 13.Wang N, Ding E, Cheng R. Surface modification of cellulose nanocrystals. Nanoscale. 2014;6:7764–7779. doi: 10.1039/C4NR01756K. [DOI] [PubMed] [Google Scholar]
- 14.Wijaya CJ, et al. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydrate Polymers. 2017;175:370. doi: 10.1016/j.carbpol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, et al. Effect of Far-Infrared Radiation on the Antioxidant Activity of Rice Hulls. Journal of Agricultural & Food Chemistry. 2003;51:4400–4403. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- 16.Adom KK, Liu RH. Antioxidant activity of grains. Journal of Agricultural & Food Chemistry. 2002;50:6182. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Chu Y, Xianzhong Wu A, Hai Liu R. Antioxidant and Antiproliferative Activities of Common Fruits. Journal of Agricultural & Food Chemistry. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 18.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. Journal of Agricultural & Food Chemistry. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 19.Pedreschi R, Cisneros-Zevallos L. Phenolic profiles of Andean purple corn (Zea mays L.) Food Chemistry. 2007;100:956–963. doi: 10.1016/j.foodchem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Gong ES, et al. Phytochemical profiles and antioxidant activity of brown rice varieties. Food Chemistry. 2017;227:432–443. doi: 10.1016/j.foodchem.2017.01.093. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekara A, Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. Journal of Agricultural & Food Chemistry. 2010;58:6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 22.Pragasam SJ, Murunikara V, Sabina EP, Rasool M. Antiperoxidative potential of p-coumaric acid, a common dietary phenol, in adjuvant-induced arthritis in rats. Chinese Journal of Integrative Medicine. 2012;10:932–938. doi: 10.3736/jcim20120815. [DOI] [PubMed] [Google Scholar]
- 23.B J, et al. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutrition and Cancer. 2011;63:611–622. doi: 10.1080/01635581.2011.538486. [DOI] [PubMed] [Google Scholar]
- 24.Zhang MW, Zhang RF, Zhang FX, Liu RH. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. Journal of Agricultural and Food Chemistry. 2010;58:7580–7587. doi: 10.1021/jf1007665. [DOI] [PubMed] [Google Scholar]
- 25.Adom KK, Liu RH. Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. Journal of Agricultural & Food Chemistry. 2005;53:6572–6580. doi: 10.1021/jf048318o. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural & Food Chemistry. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 27.Zheng R, et al. Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Industrial Crops & Products. 2017;107:360–369. doi: 10.1016/j.indcrop.2017.05.050. [DOI] [Google Scholar]
- 28.Kumar N, et al. Ferulic acid amide derivatives as anticancer and antioxidant agents: synthesis, thermal, biological and computational studies. Medicinal Chemistry Research. 2016;25:1175–1192. doi: 10.1007/s00044-016-1562-6. [DOI] [Google Scholar]
- 29.Nzaramba MN, Reddivari L, Bamberg JB, Miller JC. Antiproliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. J Agric Food Chem. 2009;57:8308–8315. doi: 10.1021/jf901567k. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Liu RH. Synergistic Effect of Apple Extracts and Quercetin 3-β-d-Glucoside Combination on Antiproliferative Activity in MCF-7 Human Breast Cancer Cells in Vitro. Journal of Agricultural & Food Chemistry. 2009;57:8581–8586. doi: 10.1021/jf8039796. [DOI] [PubMed] [Google Scholar]
- 31.Normand ML, Moriana R, Ek M. Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydrate Polymers. 2014;111:979–987. doi: 10.1016/j.carbpol.2014.04.092. [DOI] [PubMed] [Google Scholar]
- 32.Ooi SY, Ahmad I, Amin MCIM. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Industrial Crops and Products. 2016;93:227–234. doi: 10.1016/j.indcrop.2015.11.082. [DOI] [Google Scholar]
- 33.Tang J, et al. Polyrhodanine coated cellulose nanocrystals - a sustainable antimicrobial agent. Acs Sustainable Chemistry & Engineering. 2015;3:1801. doi: 10.1021/acssuschemeng.5b00380. [DOI] [Google Scholar]
- 34.De lPC, Saldivar SO, Liu RH. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. Journal of Agricultural & Food Chemistry. 2007;55:4177–4183. doi: 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, et al. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.) Food Chemistry. 2017;217:773–781. doi: 10.1016/j.foodchem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 36.He X, Liu D, Liu RH. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. Journal of Agricultural & Food Chemistry. 2008;56:9337–9344. doi: 10.1021/jf070954+. [DOI] [PubMed] [Google Scholar]
- 37.Guo R, Guo X, Li T, Fu X, Liu RH. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chemistry. 2017;221:997–1003. doi: 10.1016/j.foodchem.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 38.Yoon H, Liu RH. Effect of 2alpha-hydroxyursolic acid on NF-kappaB activation induced by TNF-alpha in human breast cancer MCF-7 cells. Journal of Agricultural & Food Chemistry. 2008;56:8412–8417. doi: 10.1021/jf8012844. [DOI] [PubMed] [Google Scholar]
- 39.TAPPI T203 OS-74. Alpha-, beta- and gamma-cellulose in pulp. TAPPI Press, Atlanta, GA.
- 40.TAPPI T222 OS-83. Acid-insoluble lignin in wood and pulp. TAPPI Press, Atlanta, GA.
- 41.TAPPI T211 om-02. Ash in wood, pulp, paper and paperboard: combustion at 525°C. TAPPI Press, Atlanta, GA.
- 42.Ming S, et al. Effective dispersion of aqueous clay suspension using carboxylated nanofibrillated cellulose as dispersant. Rsc Advances. 2016;6:37330–37336. doi: 10.1039/C6RA03935A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.