Abstract
Background
The American Thyroid Association (ATA) recommended the establishment of population specific reference ranges for thyroid hormones during pregnancy. Initial studies conducted in the United Arab Emirates (UAE) in 2003 and 2004 on pregnant women published a considerably higher upper limit for thyroid stimulating hormone (TSH) than that proposed by ATA. The UAE was classified as a country with mild iodine deficiency at the time of this initial study. After the implementation of aggressive strategies to address iodine deficiency over the last decade, the UAE was recently declared as iodine sufficient. The current study re-evaluates the reference intervals for thyroid hormones for pregnant women in the UAE after the declaration of iodine sufficiency status.
Methods
TSH and free thyroxin (FT4) from 414 UAE national pregnant females were analyzed to determine trimester specific reference ranges.
Results
The upper limits of the TSH reference ranges were found to be significantly lower than previously reported, but still higher than those recommended by ATA in 2011.
FT4 reference ranges were found to be slightly lower than previously reported.
Conclusion
TSH trimester specific reference ranges in UAE national pregnant women are higher than those recommended by ATA in 2011 but in keeping with the latest guidelines published in 2017. This should be considered while interpreting thyroid function tests in this population. Further studies including urinary iodine measurement, body mass index and larger numbers per partition in this population are recommended.
Keywords: Reference intervals, Gestation, Thyroid stimulating hormone, Pregnancy, Free thyroxin
1. Introduction
It is well established that complex hormonal and metabolic changes are observed in pregnant women. These changes include increased iodine requirement due to increased renal clearance; increased degradation of thyroid hormones thyroxin (T4) and triiodothyronine (T3) by placental type 3 deiodinases; secondary binding protein changes with an increase in serum thyroxin binding globulin (TBG) concentrations and a decrease in serum albumin; and stimulation of the Thyrotropin (TSH) receptor by human chorionic gonadotropin (HCG) [1]. These changes are responsible for altered thyroid function tests observed during pregnancy [2]. Consequently, major professional organizations such as the American Thyroid Association (ATA), the Endocrine Society and the European Endocrine Society recommend the use of trimester-specific and assay specific reference ranges for maternal serum TSH and FT4 [3], [4], [5].
The only published study of trimester specific reference ranges in UAE pregnant women was conducted in 2003–2004 [6]. At that time UAE was considered a country with mild iodine deficiency [7], [8], [9] and this was reflected in the upper limit of TSH observed [6]. Subsequently, the UAE Ministry of Health in collaboration with the Iodine Deficiency Federation implemented various prevention strategies that led to the declaration that the UAE is free of iodine deficiency by the International Council for Control of Iodine Deficiency Disorders in 2011 [10].
In the light of this development and the recent recommendations we carried out a retrospective study among healthy pregnant UAE women at different stages of pregnancy to establish gestational specific reference ranges in an iodine sufficient status to guide diagnosis and screening for thyroid disorders in this region.
2. Materials and methods
The study was conducted at Corniche Hospital, in Abu Dhabi during 2016 and 2017 using surplus material from samples submitted to Corniche Hospital laboratory for routine clinical investigations. The study was approved by the Ethics Review Committee of Corniche hospital (ethics approval reference CH140716020).
Only healthy pregnant women were included in the study. Patients with a history of thyroid disorders, chronic diseases, twin pregnancies or altered renal function were excluded
Sera from 452 pregnant women attending the hospital antenatal clinic, who fulfilled the above criteria were analyzed. Samples were analyzed for TSH, FT4 and thyroid peroxidase (TPO) antibodies. Samples found to be TPO antibodies positive were excluded from the statistical analysis.
Samples were collected in Becton Dickenson yellow top serum separator tubes (Catalog number 367957). Samples were allowed to clot for 30 min prior to centrifugation. Separated sera were frozen within two hours of collection and stored at − 20 °C until the date of the analysis, which was within one month of collection.
1st Trimester was defined as 4–12 weeks gestation, 2nd trimester was defined as 13–28 weeks gestation and 3rd trimester was defined as> 28 weeks gestation. Gestational ages were determined using the calculations based on the last menstrual period (LMP), or early obstetric scan if available.
A total of 147 women were in the first trimester, 160 were in the second trimester, and 145 were in the third trimester.
Serum TSH, FT4 and TPO antibodies were measured on the Roche e601 immunoassay analyzer using the electrochemiluminescence immunoassay “ECLIA” sandwich principle for TSH and the electrochemiluminescence immunoassay “ECLIA” competition principle for FT4 and TPO antibodies respectively. Results were determined via a calibration curve, which is instrument specifically generated by 2-point calibration, and a master curve provided via the reagent barcode. The analytical measuring range for TSH was 0.005 – 100 mIU/L and that of FT4 was 0.3 – 100 pmol/L [11]. A value of less than 34 kIU/L for TPO antibodies was considered negative as per the manufacturer quoted reference range [11]. Serum samples for TSH, FT4 and TPO antibodies are stable for one month when stored at − 20 °C [11].
The average imprecision (CV%) for 2 levels of internal quality control (QC) material used for each parameter were as follows: FT4 = 4.18% (3.34%&5.01% for normal and abnormal levels respectively) and TSH = 4.07% (5.64%&2.5% for normal and abnormal levels respectively).
3. Statistical analysis
Reference intervals were calculated for TSH and FT4 for each trimester using the EP Evaluator statistical software, which applies the non-parametric statistical method published by the Clinical Laboratory Standard Institute (CLSI) [12], [13]. This nonparametric method makes no assumption about the shape of the population distribution. The central 95%, which is an estimate of the normal range, was calculated for each analyte across the various gestational ages. The 90% confidence intervals and the confidence ratios were also calculated for each value. A confidence ratio value of 0.10 or less is desirable and values greater than 0.30 were flagged in the statistical report. Sub-analysis was also performed within the 1st trimester group for women who are 4–6 weeks pregnant and those who are 7–12 weeks. Comparisons between results for the various gestational groups were carried out using Analyse-it statistical program [14]. Polynomial regression analysis was performed between previously published reference ranges and the current ones to determine the effect of iodine sufficiency in this study as compared to previous values for TSH and FT4, p values of less than 0.05 were considered significant.
4. Results
A total of 452 patients were tested. Thirty three patients were positive for TPO antibodies and were excluded from further analysis. Additionally, one patient from the third trimester group was excluded due since the quantity of frozen serum was inadequate for analysis. The remaining 418 were included in the statistical analysis. On examination of the data 4 subjects were considered outliers as they exceeded 3 interquartile ranges and were further excluded from the reference ranges calculations. The central 95% intervals, 90% confidence intervals and the confidence ratios for the TSH and FT4 in each trimester are listed in Table 1. Histograms and comparison plots for the study population are displayed in Fig. 1, Fig. 2, Fig. 3.
Table 1.
Central 95% reference intervals, 90% confidence intervals and the confidence ratios for TSH&FT4 in various gestational groups.
| Test (Unit of measure) | 1st Trimester (n = 136) | 2nd Trimester (n = 146) | 3rd Trimester (n = 132) |
|---|---|---|---|
| FT4 (pmol/L) Reference Interval | 11.73 – 20.39 | 9.25 – 17.22 | 8.71 – 15.26 |
| − 90% CI for lower limit | 11.27 – 12.35 | 8.76 – 10.0 | 7.89 – 9.52 |
| − 90% CI for upper Limit | 19.12 – 22.16 | 16.06 – 18.27 | 14.49 – 15.54 |
| -Confidence ratio | 0.24 | 0.25 | 0.20 |
| TSH (mIU/L) Reference Interval | 0.094 – 3.33 | 0.052 – 4.56 | 0.44 – 4.75 |
| − 90% CI for lower limit | 0.012 – 0.13 | 0.005 – 0.54 | 0.32 – 0.62 |
| − 90% CI for upper Limit | 2.60 – 3.71 | 3.94 – 5.46 | 4.16 – 5.63 |
| -Confidence ratio | 0.19 | 0.20 | 0.21 |
Fig. 1.
Histograms for FT4 and TSH in the 1st Trimester (upper), 2nd Trimester (middle), 3rd Trimester (lower) panel.
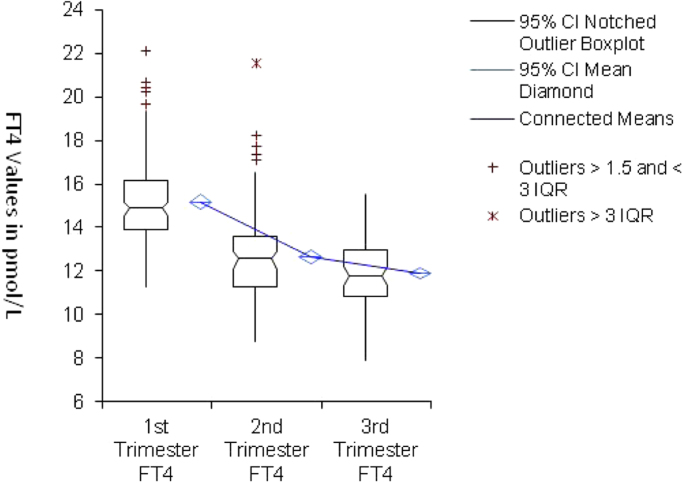
Fig. 2.
Comparison between FT4 mean, percentile and standard deviation plot at different trimesters. CI = Confidence interval, IQR = interquartile range.
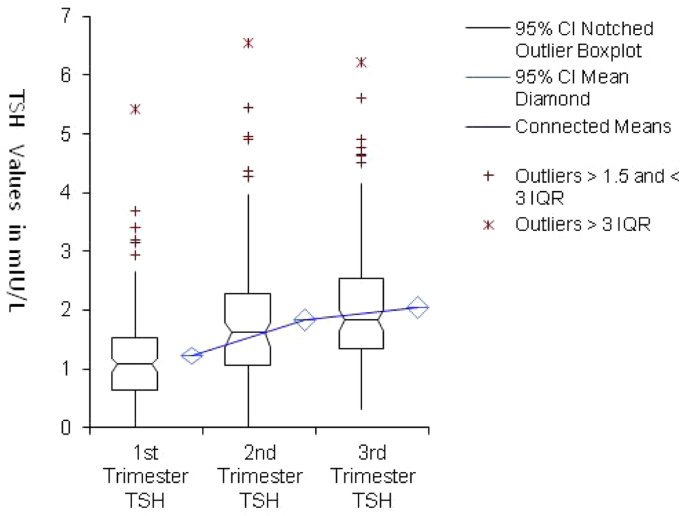
Fig. 3.
Comparison between TSH mean, percentile and standard deviation plot at different trimesters. CI = Confidence interval, IQR = interquartile range.
The upper limit for TSH at each trimester was significantly lower than previously reported (p .007) and it was still higher than the ATA 2011 recommendations.
There was no difference in the sub analysis between the 4–6 weeks and the 7–12 week groups this could be due to the fact that only 8 subjects were below 7 weeks and the remaining 128 subjects were all in the 7–12 weeks range.
5. Discussion
Altered maternal thyroid functions during pregnancy are associated with various maternal and child complications such as miscarriage, placenta abruption, preeclampsia and preterm delivery [15], [16]. Similarly, physiological alterations in the homeostatic control of thyroid hormones cause preterm delivery and impaired neurodevelopment of the child [17], [18], [19], [20], [21], [22], [23].
Differences in free thyroid hormones due to different analytical methods have been reported in pregnancy. There are also considerable population dependent differences in FT4 and TSH reference intervals using the same analytical method [24], [25]. Accordingly, current guidelines advocate the use of population based, and assay specific trimester ranges for thyroid function tests. When population specific ranges are unavailable, an upper TSH limit of 2.5 for the first trimester and 3.0 mIU/L for the second and the third trimesters were initially proposed by the ATA in 2011 [3]. However, subsequent studies have demonstrated that there is significant geographic and ethnic diversity in TSH levels in pregnancy and case doubt on the validity of the upper limit of 2.5 mIU/ [26], [27], [28], [29], [30], [31], [32], [33], [34]. For example, a study of 4800 pregnant 4Chinese women established a population specific first trimester upper limit of 4.87 mIU/L for serum TSH [26]. Interestingly, of 118 Chinese pregnant women who had TSH> 2.5 mIU/L in the first trimester, only 30% and 20.3% of them had TSH> 3.0mIU/L at 20th and 30th week of gestation. Similarly, first trimester TSH ranges were reported respectively in India (0.44–5.78 mIU/L) Spain (0.12–5.76 mIU/L) and Korea (0.01–4.10 mIU/L) [27], [28], [29], [30], [31], [32]. In light of these observations, the ATA published revised guidelines in 2017, recommending a new upper limit of normal of 4.0 mIU/L for TSH in the first trimester and a new treatment protocol for hypothyroidism [35]. The guidelines also recommended the use of assay specific, population specific and trimester specific reference ranges in pregnant women [35].
In the current study, TSH upper limit was 3.3 mIU/L, which is somewhat higher than initial ATA value of 2.5 mIU/L, but consistent with the revised ATA upper limit of 4.0 mIU/L. As expected, TSH values were lowest in the 1st trimester group and increased in the second and 3rd trimesters respectively, while FT4 levels showed the opposite trend (Fig. 2, Fig. 3).
Typically the lower end of TSH range is lower in the 1st trimester than the low end in the 2nd trimester. This was not true in our population were the low end for TSH in the 2nd trimester was actually lower than that of the first (0.05 versus 0.09 respectively). It is worth noting that our study population did not include patients with non- thyroidal illness.
We compared our results to those reported in different populations using the Roche method [1] and found significant variations especially from the USA data (Table 2) where the upper limit of TSH in the first trimester was only 1.06 mIU/L, versus 3.3 mIU/L for the UAE population.
Table 2.
Method and trimester specific TSH and FT4 medians and reference ranges using Roche Cobas e601/E-170, e411/ Elecsys methods.
| FT4 1st Trimester Median (Reference Interval) (n = ) | FT4 2nd Trimester Median (Reference Interval) (n = ) | FT4 3rd Trimester Median (Reference Interval) (n = ) | TSH 1st Trimester Median (Reference Interval) (n = ) | TSH 2nd Trimester Median (Reference Interval) (n = ) | TSH 3rd Trimester Median (Reference Interval) (n = ) | |
|---|---|---|---|---|---|---|
| UAE 2016 | 14.89 (11.73 – 20.39) (n = 136) | 12.57 (9.25 – 17.22) (n = 146) | 11.77 (8.71 – 15.26) (n = 132) | 1.1 (0.094 – 3.33) (n = 136) | 1.62 (0.052 – 4.56) (n = 146) | 1.84 (0.44 – 4.75) (n = 132) |
| India 2008 | 14.46 (12.00 – 19.45) (n = 107) | 13.4 (9.48 – 19.58) (n = 137) | 13.28 (11.3 – 17.7) (n = 87) | 2.1 (0.60 – 5.00) (n = 107) | 2.4 (0.40 – 5.78) (n = 137) | 2.1 (0.74 – 5.70) (n = 87) |
| Canada 2008 | 15.0 (11.0 – 19.0) (n = 224) | 13.5 (9.7 – 17.5) (n = 240) | 11.7 (8.1 – 15.3) (n = 211) | N/A | N/A | N/A |
| USA 2007 | N/A | N/A | N/A | 0.91 (0.28 – 1.06) (n = 71) | 1.03 (0.57 – 1.28) (n = 83) | 1.32 (0.69 – 2.87) (n = 62) |
| China 2013 | 15.84 (12.35 – 20.71) (n = 1024) | N/A | N/A | 1.66 (0.14 – 4.87) (n = 1024) | N/A | N/A |
| UK 2007 | 14.6 (10.7 – 19.4 (n = 1089) | N/A | N/A | 1.08 (0.14 – 3.19) (n = 1089) (n = 0893.19) | N/A | N/A |
Three data points for TSH were considered outliers as they exceeded three inter quartile ranges and were excluded; one point in the first trimester (5.43 mIU/L), one point in the 2nd trimester (6.56 mIU/L) and one point for the 3rd trimester (6.23 mIU/L). Also, one data point was considered an outlier for FT4 in the 2nd trimester (21.58 pmol/L) and was excluded. Examination of the medical records of these 4 subjects did not reveal any obvious thyroidal illness or other clinical findings that could explain these values.
Factors influencing thyroid function tests that were not addressed in our study include iodine status, body mass index (BMI) and inter-individual variation. We assumed that the study population is iodine sufficient based on the latest declaration by the UAE Ministry of Health and International Council for Control of Iodine Deficiency Disorders in 2011 [10]. Männistö et al. detected differences in the upper limits (95th percentile) of TSH among women with BMI> 30 kg/m2 and< 20 kg/m2 [36], [37]; upper limits were higher for TSH (3.50 mU/L and 2.86 mU/L respectively) and lower for FT4 (11.6 and 12.3 pmol/L respectively).
It is recommended to include a minimum of 400 individual measurements per partition due to the high inter-individual variability and skewness for TSH and FT4 [37], therefore a multicenter collaborative study on the national level may be required in the future to ensure the inclusion of adequate numbers. Our study did not address the issue of multi-ethnicity due to the fluctuating flux of migrating populations and rather focused on the local population constituting only one specific ethnic group. It would have been interesting to study age matched non-pregnant healthy control subjects alongside the study population for comparison; instead we verified the non-pregnant Roche quoted reference range on 20 healthy volunteers. The results of our study concur with the recent publications calling for an updated trimester and population specific TSH values.
6. Conclusion
TSH trimester specific reference ranges in UAE pregnant women are higher than those recommended by ATA in 2011 but in keeping with the latest guidelines published in 2017. This should be considered while interpreting thyroid function tests in this population. Further studies including urinary iodine measurement, BMI and larger numbers per partition in this population are recommended.
Acknowledgements
We like to acknowledger Roche Diagnostics for supplying the reagent kits free of charge to complete this study.
Conflict of interest
We like to confirm that the authors of the above manuscript didn’t receive any financial support for the manuscript presented and have no conflict of interests to disclose.
Contributor Information
Aly Bernard Khalil, Email: khalilab@icldc.ae.
Bashir Taha Salih, Email: BashirS@seha.ae.
Onismos Chinengo, Email: OnismosC@seha.ae.
Ma Remy D. Bardies, Email: BardiesR@seha.ae.
Andrew Turner, Email: TurnerA2@clevelandclinicabudhabi.ae.
Laila O. Abdel Wareth, Email: Warethl@clvelandclinicabudhabi.ae.
References
- 1.Lazarus J. Thyroid regulation and dysfunction in the pregnant patient. [Updated 2016 Jul 21] In: De Groot L.J., Chrousos G., Dungan K., editors. Endotext. MDText.com, Inc.; South Dartmouth (MA): 2000. 〈https://www.ncbi.nlm.nih.gov/books/NBK279059/〉 (Available from) [PubMed] [Google Scholar]
- 2.Soldin O.P. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Ther. Drug Monit. 2006;28(1):8–11. doi: 10.1097/01.ftd.0000194498.32398.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stagnaro-Green A., Abalovich M., Alexander E., Azizi F., Mestman J., Negro R., Nixon A., Pearce E.N., Soldin O.P., Sullivan S., Wiersinga W. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groot Leslie De, Abalovich Marcos, Alexander Erik K., Amino Nobuyuki, Barbour Linda, Cobin Rhoda H., Eastman Creswell J., Lazarus John H., Luton Dominique, Mandel Susan J., Mestman Jorge, Rovet Joanne, Sullivan Scott. Management of thyroid dysfunction during pregnancy and postpartum: anan endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus J., Brown R.S., Daumerie C., Hubalewska-Dydejczyk A., Negro R., Vaidya B. European Thyroid Association Guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid J. 2014. 2014;3(2):76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhatt G.S., Jayasundaram R., Wareth L.A., Nagelkerke N., Jayasundaram K., Darwish E.A., Lewis A. Thyrotrophin and free thyroxine trimester – specific reference intervals in a mixed ethinic pregnant population in the United Arab Emirates. Clin. Chim. Acta. 2006;370(1–2):147–151. doi: 10.1016/j.cca.2006.02.008. (Epub 2006 Mar 6) [DOI] [PubMed] [Google Scholar]
- 7.De Benoist B., Andersson M., Egli I., Takkouche B., Allen H. WHO Global Database on Iodine Deficiency. World Health Organization; Geneva: 2004. Iodine status worldwide. [PMC free article] [PubMed] [Google Scholar]
- 8.Azizi F., Malik M., Bebars E., Delshad H., Bakir A. Thyroid volumes in schoolchildren of the Emirates. J. Endocrinol. Investig. 2003;26(1):56–60. doi: 10.1007/BF03345123. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hosani H., Osman H., Abdel Wareth L., Saade D., Salah M. Prevalence of iodine deficiency disorders in the United Arab Emirates measured by raised TSH levels. East Mediterr. Health J. 2003;9(1–2):123–130. [PubMed] [Google Scholar]
- 10.International Council for Control of Iodine Deficiency Disorders newsletter; 40:1, 2012.
- 11.Roche Diagnostics Cobas TSH reagent packet insert Ref, V 19 English, 2010-05.
- 12.EP evaluator. Release 10. Data innovations. 〈https://www.datainnovations.com/ep-evaluator/〉. AccessedFebruary 26, 2017.
- 13.Sasse E.A., Committee N. How to Define and Determine Reference Intervals in the Clinical Laboratory: Approved Guideline. 2nd ed. Clinical Laboratory Standard Institute; United States: Wayne, PA: 2000. 〈https://www.ncbi.nlm.nih.gov/nlmcatalog/101087605〉 (accessed 22 January 2017) [Google Scholar]
- 14.Analyse-it for Microsoft Excel (version 2. 20). Analyse-it Software, Ltd. 〈http://www.analyse-it.com/〉.
- 15.Lazarus J.H., Premawardhana L.D. Screening for thyroid disease in pregnancy. J. Clin. Pathol. 2005;58:449–452. doi: 10.1136/jcp.2004.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ga¨rtner R. Thyroid diseases in pregnancy. Curr. Opin. Obstet. Gynecol. 2009;21:501–507. doi: 10.1097/GCO.0b013e328332a836. [DOI] [PubMed] [Google Scholar]
- 17.Allan W.C., Haddow J.E., Palomaki G.E. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J. Med. Screen. 2000;7:127–130. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 18.Haddow J.E., Palomaki G.E., Allan W.C. Maternal thyroid deficiency during pregnancy and subsequent Neuropsychological development of the child. New Engl. J. Med. 1999;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus J.H. Thyroid function in pregnancy. Br. Med. Bull. 2010;97(1):137–148. doi: 10.1093/bmb/ldq039. [DOI] [PubMed] [Google Scholar]
- 20.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr. Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 21.Brent G.A. Maternal thyroid function: interpretation of thyroid function tests in pregnancy. Clin. Obstet. Gynecol. 1997;40(1):3–15. doi: 10.1097/00003081-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J. Clin. Endocrinol. Metab. 2009;94:21–25. doi: 10.1210/jc.2008-1288. [DOI] [PubMed] [Google Scholar]
- 23.Groot Leslie De, Abalovich Marcos, Alexander Erik K., Amino Nobuyuki, Barbour Linda, Cobin Rhoda H., Eastman Creswell J., Lazarus John H., Luton Dominique, Mandel Susan J., Mestman Jorge, Rovet Joanne, Sullivan Scott. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010;95:E44–E48. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 24.d'Herbomez M., Forzy G., Gasser F., Massart C., Beaudonnet A., Sapin R. Clinical evaluation of nine free thyroxine assays: persistent problems in particular populations. Clin. Chem. Lab. Med. 2003;41:942–947. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 25.Roti E., Gardini E., Minelli R., Bianconi L., Flisi M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J. Endocrinol. Investig. 1991;14(1):1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
- 26.Li Chenyan, Shan Zhongyan, Mao Jinyuan, Wang Weiwei, Xie Xiaochen, Zhou Weiwei, Li Chenyang, Xu Bin, Bi Lihua, Meng Tao, Du Jianling, Zhang Shaowei, Gao Zhengnan, Zhang Xiaomei, Yang Liu, Fan Chenling, Teng Weiping. Assessment of thyroid function during first- trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J. Clin. Endocrinol. Metab. 2013;98:3678–3686. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- 27.Korevaar T.I., Medici M., de Rijke Y.B., Visser W., de Muinck Keizer-Schrama S.M., Jaddoe V.W., Hofman A., Ross H.A., Visser W.E., Hooijkaas H., Steegers E.A., Tiemeier H., Bongers-Schokking J.J., Visser T.J., Peeters R.P. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J. Clin. Endocrinol. Metab. 2013;98(9):3678–3686. doi: 10.1210/jc.2013-2005. (Epub2013 Jul 8) [DOI] [PubMed] [Google Scholar]
- 28.Marwaha R.K., Chopra S., Gopalakrishnan S., Sharma B., Kanwar R.S., Sastry A., Singh S. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115(5):602–606. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 29.Vila L., Serra-Prat M., Palomera E. Reference values for thyroid function tests in pregnant women living in Catalonia, Spain. Thyroid. 2010;20:221–225. doi: 10.1089/thy.2008.0264. [DOI] [PubMed] [Google Scholar]
- 30.Moon Hee-Won, Chung Hee-Jung, Park Chul-Min, Hur Mina, Yun Yeo-Min. Establishment of trimester–specific reference intervals for thyroid hormone in Korean pregnant. Ann. Lab. Med. 2015;35:198–204. doi: 10.3343/alm.2015.35.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker J.A., Illions E.H., Huddleston J.F. Small ridge RC. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet. Gynecol. 2005;106:1365–1371. doi: 10.1097/01.AOG.0000185475.61612.ea. [DOI] [PubMed] [Google Scholar]
- 32.La’ulu Sonia L., Roberts William L. Second-trimester reference intervals for thyroid tests: the role of ethnicity. Clin. Chem. 2007;53:1658–1664. doi: 10.1373/clinchem.2007.089680. [DOI] [PubMed] [Google Scholar]
- 33.La’ulu S.L., Roberts W.L. Ethnic differences in first-trimester thyroid reference intervals. Clin. Chem. 2011;57:913–915. doi: 10.1373/clinchem.2010.161240. [DOI] [PubMed] [Google Scholar]
- 34.Benhadi N., Wiersinga W.M., Reitsma J.B., Vrijkotte T.G., van der Wal M.F., Bonsel G.J. Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin. Endocrinol. 2007;66(6) doi: 10.1111/j.1365-2265.2007.02803.x. [DOI] [PubMed] [Google Scholar]
- 35.Alexander E.K., Pearce E.N., Brent G.A., Brown R.S., Chen H., Dosiou C., Grobman W.A., Laurberg P., Lazarus J.H., Mandel S.J., Peeters R.P., Sullivan S. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 36.Männistö T., Surcel H.M., Ruokonen A., Vääräsmäki M., Pouta A., Bloigu A., Järvelin M.R., Hartikainen A.L., Suvanto E. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21(3):291–298. doi: 10.1089/thy.2010.0337. [DOI] [PubMed] [Google Scholar]
- 37.Medici Marco, Korevaar Tim I.M., Visser W. Edward, Visser Theo J., Peeters Robin P. Thyroid function in pregnancy: what Is normal? Clin. Chem. 2015;61(5):704–713. doi: 10.1373/clinchem.2014.236646. [DOI] [PubMed] [Google Scholar]