Abstract
Within each synovial joint, the articular cartilage is uniquely adapted to bear dynamic compressive loads and shear forces throughout the joint's range of motion. Injury and age-related degeneration of the articular cartilage often lead to significant pain and disability, as the intrinsic repair capability of the tissue is extremely limited. Current surgical and biological treatment options have been unable to restore cartilage de novo. Before successful clinical cartilage restoration strategies can be developed, a better understanding of how the cartilage forms during normal development is essential. This review focuses on recent progress made towards addressing key questions about articular cartilage morphogenesis, including the origin of synovial joint progenitor cells and postnatal development and growth of the tissue. These advances have provided novel insight into fundamental questions about the developmental biology of articular cartilage, as well as potential cell sources that may participate in joint response to injury.
Keywords: articular cartilage, joint formation, lineage tracing, progenitor cells, cartilage development
1. Introduction
During postnatal growth, the articular cartilage undergoes a series of tremendous structural and functional changes. While the tissue is highly cellular and isotropic at birth, unique zones develop as the tissue matures. This unique zonal architecture allows the articular cartilage to withstand significant shear and compressive forces throughout a joint's range of motion (Gannon et al., 2014; Helminen et al., 2000; Mienaltowski et al., 2008). At the surface adjacent to the joint cavity, the superficial zone is composed of elongated, flattened cells oriented parallel to the articular surface. These superficial cells play a key role in maintaining frictionless joint motion through production of hyaluronate, phospholipids and Prg4/lubricin (Jay et al., 2001). The adjacent underlying intermediate/transitional zone is made of slightly larger and rounder chondrocytes oriented more randomly and separated by appreciable matrix. The largest of the cartilage zones, the deep zone, consists of very large, round chondrocytes often found aligned in vertical stacks oriented perpendicularly to the articular surface. At the base of the articular cartilage, the subchondral junction provides physical stability and link to underlying bone (Broom and Poole, 1982). Throughout the articular cartilage, an abundant extracellular matrix composed primarily of collagen II organized in fibrils, and aggrecan organized into multimeric superstructures, provides the tissue with its key tensile strength and elasticity.
During normal aging and in response to injury, some or all of these vital components are often compromised. The intrinsic repair capacity of the articular cartilage is notoriously poor, and lost cartilage is often replaced by a structurally and functionally inferior fibrous scar tissue (Mienaltowski et al., 2009). While common surgical and biological treatment techniques are often able to temporarily improve joint function and reduce pain, they fail to reproduce the native characteristics of articular cartilage and are only partially effective long-term (Huey et al., 2012). In order for more successful reparative strategies to be developed, a better understanding of normal articular cartilage development is essential. Interestingly, there are indications that immature articular cartilage has at least partial innate regeneration capacity, although this ability appears to be lost with increasing age (Calandruccio and Gilmer, 1962; Ikegawa et al., 2015; Matsuoka et al., 2015; Namba et al., 1998). Key questions regarding the origin, fate, and role of synovial joint progenitor cells which may contribute to repair have been recently addressed, although not yet fully resolved. Such knowledge could be leveraged to create novel biological and pharmacological treatments designed to exploit normal articular cartilage biology. Such strategies have been widely used in other fields, although not yet fully realized in cartilage repair (Nicholas and Kriegstein, 2010; Szabo et al., 2010; Zhou and Melton, 2008). This review focuses on recent advances in knowledge of embryonic and postnatal articular cartilage development, growth and morphogenesis, providing essential insight into not only the developmental biology of the articular cartilage, but also into potential biomedical strategies for repair.
2. Origin of synovial joint progenitor cells
Within the uninterrupted cartilaginous anlagen of developing limbs, the first explicit sign of joint development is marked by the appearance of a region of flattened, condensed cells at putative joint sites. This compact region of mesenchymal cells has been classically defined as the interzone, and early studies found that its removal from chick embryos prevented formation of limb joints over time (Holder, 1977). The histological appearance of the interzone varies by developmental stage, joint location and species. Mitrovic described the interzone in the chick as having three distinct layers, including an intermediate zone consisting of dense, flattened cells in between layers of “chondrogenic” cells (Mitrovic, 1977). The putative mouse knee has also been described as consisting of a dense intermediate compartment and two flanking outer compartments with more loosely arranged cells (Hyde et al., 2007; Jenner et al., 2014). As the joint site forms, cells within the interzone region cease expression of early cartilage markers Col2a1 and Matn1, and may be identified by increasingly restricted expression of Wnt4, Wnt9a, Dcx, Gdf5 and Erg (Guo et al., 2004; Hartmann and Tabin, 2001; Hyde et al., 2008; Hyde et al., 2007; Spater et al., 2006; Storm et al., 1994b; Iwamoto et al., 2007). Exploiting these unique gene expression patterns, several groups have developed transgenic mouse lines to gain further insight into the origin and eventual fate of these early cell populations. At early stages, Gdf5 mRNA is highly expressed in regions flanking future joint sites, within the flattened intermediate interzone, and also, although less abundantly, in the outer interzone and adjacent regions of the cartilaginous anlagen (Storm and Kingsley, 1996). We and others have utilized compound Gdf5Cre;ROSA-reporter mice to investigate the lineage of early Gdf5-expressing cell populations at future joint sites (Decker et al., 2015; Dyment et al., 2015; Koyama et al., 2008; Rountree et al., 2004). While Gdf5mRNA expression in joint tissues is highly diminished or absent by the time of birth, Gdf5Cre;R26RLacZ (Gdf5Cre+) labeled cells are found within most mouse joint tissues into maturity - including the articular cartilage, synovial lining, meniscus and intrajoint ligaments (Fig. 1 A, C, E). This suggests that cells with a Gdf5-expressing lineage are not transient, actively take part in joint tissue formation, and constitute a progenitor cell cohort endowed with joint-formation capacity. After these initial experiments, it remained unclear if the broad cell population labeled by Gdf5 was made of progenitors with multiple tissue differentiation capacity or included specific subsets of cells with unique roles in joint development.
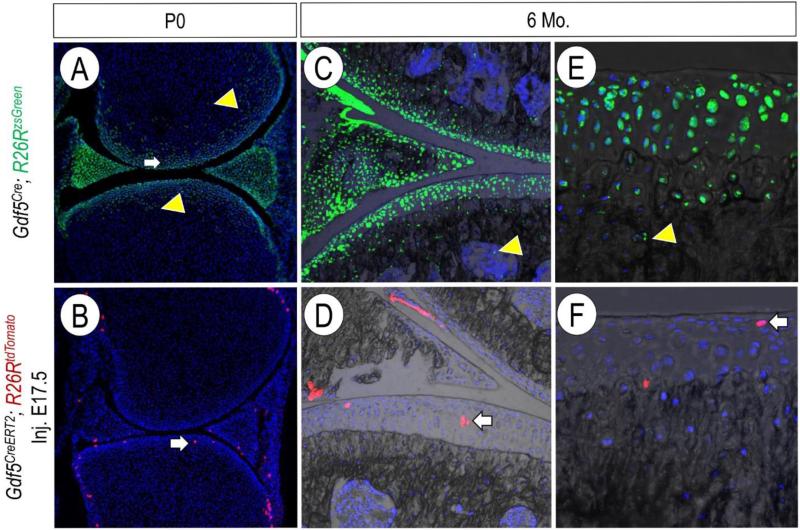
Fig. 1.
Selective labeling of Gdf5-expressing cells in Gdf5CreERT2 mice. At birth, Gdf5Cre;R26RzsGreen labeled cells are found throughout the joint, including within the articular cartilage (white arrow) and the putative secondary ossification center (yellow arrowhead) (A). After tamoxifen administration at E17.5, Gdf5CreERT2; R26RtdTomato labeled cells are less numerous, and more restricted towards the articular cartilage (white arrow) (B). Gdf5Cre;R26RzsGreen remain present in all joint tissues through 6 months of age, including within the secondary ossification center (yellow arrowhead, C) as well as the entire thickness of the articular cartilage (E). Gdf5CreERT2; R26RtdTomato labeled cells remain restricted primarily to the articular cartilage (white arrows, D-F).
More recent work from several groups has addressed these key questions, and there is increasing evidence that synovial joint tissues may arise from cell populations originally contained within, as well as those flanking the primordial cartilaginous anlagen. Notably, populations of cells within each of these regions display Gdf5 expression during early stages of joint development (Koyama et al., 2007; Rountree et al., 2004). As the interzone appears at sites previously occupied by chondrocytes, it was originally proposed that cells within the interzone were direct descendants of de-differentiated chondrocytes (Craig et al., 1987; Nalin et al., 1995). Using Col2a1Cre;R26RLacZ and Matn1Cre;R26R LacZ reporter mice, Hyde and collaborators demonstrated that cells within the cartilaginous anlagen ceased Col2a1 expression as the histological interzone was formed, later giving rise to portions of the articular cartilage, cruciate ligament and inner medial meniscus of the knee (Hyde et al., 2008; Hyde et al., 2007). Interestingly, these authors also noted that a band of chondrocytes adjacent to, but not within, the region of flattened cells constituting the intermediate histological interzone lacked Matn1 expression and gave rise to articular chondrocytes. While Col2a1 expression ceases within the intermediate interzone as the joint forms, expression of doublecortin (Dcx) is maintained. Studies on Dcx-reporter mice found that Dcx is initially expressed throughout the limb mesenchyme, and is maintained within the interzone but lost in the adjacent regions of the cartilaginous anlagen (Zhang et al., 2011). Dcx expression also overlaps that of the transcription factor Sox9, which is expressed by osteo - and chondro- progenitors in the developing limb mesenchyme (Akiyama et al., 2005; Zhang et al., 2011). Soeda and collaborators used Sox9LacZ/+ mice as well as an inducible Sox9CreERT2/+;R26R system to investigate stage dependent expression and lineage of Sox9+ cells (Soeda et al., 2010). Sox9LacZ/+ expression was found in the knee interzone prior to embryonic day 13.5 (E13.5), and was thereafter limited exclusively to the outer regions of the interzone and flanking chondrocytes. When Sox9CreERT2/+;R26R mice were injected with tamoxifen prior to E13.5, Sox9+ cells were found within the cruciate ligaments, and injection after E14.5 resulted in a marked reduction of labeled cells. Thus, the authors concluded that cells within the intermediate region of the interzone likely give rise to the cruciate ligaments. Hyde and collaborators (2008) also noted that at later stages during joint formation cells without a Col2a1 lineage appear to invade the joint to form the ligaments, indicating that invading cell populations may combine with those in the original anlagen during morphogenesis of unique joint tissues. Indeed, earlier studies had also found that DiI labeled cells flanking the putative joint sites later migrated into developing chick joints in ovo (Pacifici et al., 2006). To determine if these flanking cell populations had a separate ancestry from those within the interzone, Koyama and collaborators crossed Gdf5Cre;R26R LacZ and Indian hedgehog null (Ihh−/−) mice (Koyama et al., 2007). In the absence of Ihh, synovial joints failed to form (St-Jacques et al., 1999). Interestingly, we found that populations of Gdf5Cre+ cells did form in regions flanking, but not within, prospective joint sites and expressed joint site-associated marker genes including Erg and Tnc (Decker et al., 2014; Koyama et al., 2007). Taken together, these data suggest that populations of joint progenitor cells broadly labeled by Gdf5 are indeed of heterogeneous origin, consisting of de-differentiated chondrocytes from within the cartilaginous anlagen as well as from regions surrounding future joint sites. Further characterization of these flanking cell populations has been provided by Li and collaborators, who identified populations of Tgfbr2-expressing cells flanking the dorsal and ventral regions of digit joints (Li et al., 2012). Over time, these distinct cell populations were maintained in these local niches, eventually giving rise to cells in the groove of Ranvier, meniscal surface, synovial lining, and outer ligaments.
More recently, additional novel Cre mouse lines have been developed to investigate cell niches within developing synovial joints. In constitutively active Gdf5Cre mice, broad labeling of R26R-reporter cells is seen throughout multiple joint tissues (Fig. 1 A, C, E, Koyama et al., 2008). We recently developed a novel inducible BAC transgenic Gdf5CreERT2 mouse line, and when the mice were crossed with R26RzsGreen mice, we were able to more selectively, although less abundantly, label specific cell populations (Decker et al., 2016). For example, when tamoxifen was administered at late embryonic time points, Gdf5CreERT2+ cell labeling was more restricted to cells within the eventual articular cartilage. (Fig. 1 B, Decker et al., 2016). These positively labeled cells and/or their progeny remained in the articular cartilage through at least 6 months of age (Fig. 1 D, F).
The histological interzone, as well as immediately flanking regions within the cartilaginous anlagen, are broadly labeled by Gdf5 but may have distinct destinies. Jenner and collaborators utilized laser capture microdissection to investigate gene expression in multiple regions of the interzone (Jenner et al., 2014). These authors found that genes associated with joint formation were more evident in cells from the intermediate compartment of the histological interzone, while genes associated with cartilage maturation and hypertrophy were over-represented in outer compartment/flanking cells. They concluded that the intermediate compartment of the histological interzone gave rise to articular cartilage, while cells in outer compartments were destined for endochondral ossification and thus part of the putative secondary ossification center formation. A very recent study has provided further insight into the origin and fate of Gdf5 expressing cells surrounding the cartilaginous anlagen, as well as within the intermediate and flanking regions of the histological interzone. Shwartz and collaborators utilized a novel knock-in Gdf5CreER mouse line to perform a detailed analysis of Gdf5CreER+ cell labeling and fate during various embryonic stages (Shwartz et al., 2016). Through this approach, they were able to uniquely label sub-populations of cells by E18.5. These authors proposed that a continuous influx of cells which are Gdf5 negative, Sox9 positive and Col2a1 negative migrate into the interzone of developing joints, where they later begin to express Gdf5. Notably, the onset, timing and localization of Gdf5 expression may play a key role in lineage divergence to specific joint tissues. For example, active Gdf5 expression is maintained for longer periods of time in cells that will contribute to the meniscus and articular cartilage, but is lost early in cells that will eventually contribute to the epiphysis. Together, these exciting studies have demonstrated that the articular cartilage and other synovial joint tissues arise from a broad, heterogeneous interzone progenitor cell population, which is found within the developing anlagen and is supplemented by progenitors flanking the prospective joint sites.
3. Mechanisms Regulating Synovial Joint Formation
Although initially mesenchymal in character, joint progenitors clearly undergo time and tissue specific phenotypic changes as the joint matures. Many recent studies have provided insight into mechanisms regulating joint progenitor cell phenotype and gene expression over time. While it has long been associated as a marker for developing joints, the control, and role of, Gdf5 at putative joint sites remains under investigation. Absent or aberrant Gdf5 expression results in malformations of the developing limb, including shortening of long bones and absence of cruciate ligament development (Harada et al., 2007; Storm et al., 1994; Shwartz et al., 2016). During early stages of limb development, Gdf5 promotes cell adhesion, while likely increasing chondrocyte proliferation at later stages (Francis-West et al., 1999). Kan and collaborators found that Gdf5 expression might be at least partially regulated by Sox11, which transitions from broad expression patterns in prechondrogenic limb condensations to become increasingly restricted to putative joint sites as development progresses (Kan et al., 2013). However, Bhattaram and collaborators did not observe Sox11 expression at presumptive joint sites, but did find strong expression of Sox4 (Bhattaram et al., 2014). These authors found an increase in Gdf5 expression in Sox4fl/flPrx1Cre and Sox11fl/flPrx1Cre mutant mouse embryos, while Kan and collaborators found that overexpression of Sox11 stimulated Gdf5 expression in vitro. Gao and coworkers showed that the zinc finger transcription factors Osr1 and Osr2 were not needed for onset of Gdf5, Wnt4 and Wnt9a expression in incipient limb interzones, but in their absence the expression of those interzone genes was not sustained and was followed by fusion of limb joints over embryonic time (Gao et al., 2011). Ablation of Wnt/β-catenin in Gdf5Cre/β-catnfl/fl mice was previously shown to result in defective joint formation, highlighting its importance in regulation of embryonic joint morphogenesis. At later stages, Wnt signaling is also required to sustain the function and regulate the thickness of the joint's superficial zone (Yasuhara et al., 2011; Yuasa et al., 2009). Searching for upstream regulators of Wnt9a expression, Kan and Tabin identified c-Jun as a pivotal regulator acting at the enhancer level that when ablated, deranged both Wnt signaling and the initiation and progression of joint formation (Kan and Tabin, 2013).
Further analysis of gene expression at putative joint sites was performed by Longobardi and collaborators using laser capture-assisted gene arrays of tissue samples from E14.5 mouse embryo digits. They found that joint-forming interzone cells were characterized by low expression of chemokines, and in particular Mcp5, compared to adjacent chondrocytes constituting the putative growth plate regions (Longobardi et al., 2012). In absence of Tgfbr2, limb synovial joints did not form (Seo and Serra, 2007; Spagnoli et al., 2007). Longobardi and colleagues found that joint formation could be rescued in Tgfbr2-deficient mouse embryos by concurrent blockade of the Mcp5 receptor Ccr2. These authors concluded that joint formation is closely linked to, and coordinately regulated with, shaft development and that the TgfβRII/Mcp5 axis is an essential crossroad for this process. In their study described above, Jenner and colleagues also performed transcriptional profiling of specific regions within putative joints (Jenner et al., 2014). In the mouse knee, they similarly found that genes related to inflammation and actin cytoskeletal organization were differentially regulated in the intermediate interzone. Clearly, many distinct effectors and molecular regulators act at the local and long-range levels and participate in the regulation of interzone gene expression patterns, fate and function (Decker et al., 2014; 2015).
4. Postnatal Articular Cartilage Morphogenesis
At birth, the articular cartilage exists as a dense tissue consisting of small cells within scant matrix. As described above, the tissue undergoes tremendous structural changes over postnatal life, increasing in thickness and acquiring a distinct zonal organization. How the articular cartilage grows and acquires its important zonal organization, and whether joint progenitors persist postnatally and participate in tissue morphogenesis have remained long standing questions. Early studies suggested that a region of proliferating cells “subjacent to the gliding surface of the joint” was responsible for interstitial growth of articular cartilage and increasing thickness of the articular surface (Mankin, 1962). In this same study, Mankin and collaborators found that proliferation continued within deeper regions of the tissue and adjacent to the calcified cartilage, but ceased within the sub-superficial zone at later stages of postnatal growth. The presence of these two proliferative cell regions was confirmed by tritiated thymidine incorporation in the articular cartilage of immature rabbits (Mankin, 1962; Mankin, 1963). Later, Archer and collaborators confirmed the presence of a proliferative cell region in the superficial zone, suggesting that these cells were primarily responsible for the appositional growth and thickening of the articular cartilage postnatally (Archer et al., 1994; Hayes et al., 2001). Unlike the previous studies, however, these authors did not find regions of proliferative cells within the deeper regions of the articular cartilage. Later, Hunziker and collaborators identified a population of bromodeoxyuridine (BrdU) labeled slow-cycling cells in the superficial zone, as well as more rapidly proliferating regions of chondrocytes within the deeper zones as detected by concurrent 3H-thymidine labeling (Hunziker et al., 2007). These authors hypothesized that lateral expansion of the articular surface could be attributed to proliferation of cells within the superficial zone that would also give rise to daughter cells in a more rapidly proliferating cell population in the deeper zones leading presumably to vertical tissue growth.
Recently developed transgenic mouse models have permitted more detailed cell tracing and tracking during postnatal development. Kozhemyakina and collaborators created a novel inducible Prg4CreER knock-in mouse line. The Prg4 gene encodes diverse products, including lubricin/superficial zone protein (SZP), which is secreted by articular cartilage surface zone cells and synovial cells and is thought to play an essential role in joint lubrication (Rhee et al., 2005). In contrast with the earlier studies described above, which found proliferating cells throughout specific zones or within multiple zones, these authors found that tamoxifen administration to Prg4CreER mice at E17.5 resulted in R26RLacZ labeling in only a single layer of cells at the articular surface by birth (Kozhemyakina et al., 2015). By one month of age, the labeled cells and/or their progeny were found throughout the entire thickness of the articular cartilage, leading the authors to conclude that the early Prg4-expressing superficial cell population served as progenitors which later gave rise to cells throughout the entire tissue by appositional growth. The initial, selective labeling of a single layer of cells Prg4CreER ; R26RLacZ mice was intriguing but also perplexing, as previous studies demonstrated that Prg4 mRNA expression characterizes the entire incipient articular cartilage at late embryonic and neonatal stages (Iwamoto et al., 2007; Rhee et al., 2005). Indeed, when we repeated these experiments using these now commercially available knock-in Prg4CreER mice, R26RLacZ labeled cells were found throughout the entire thickness of the articular cartilage at birth after single tamoxifen administration at E17.5 (Decker et al., 2016). Thus, it is plausible that the patterns of reporter activation and cell progeny behavior observed and reported by Kozhemyakina may not accurately reflect those of endogenous Prg4 expressing cell populations in either wild-type or Prg4CreER ; R26RLacZ mice. To mitigate concerns over haploinsufficiency in the knock-in Prg4CreER mice, we recently developed novel BAC transgenic Prg4CreERT2 mice, which maintain both functional copies of the Prg4 gene. After tamoxifen administration at E17.5, Prg4CreERT2/R26RtdTomato labeled cells were found throughout the entire thickness of the articular cartilage at birth, recapitulating endogenous Prg4 mRNA expression patterns at the stage of injection (Decker et al., 2016). After tamoxifen administration at later stages, we found populations of Prg4CreERT2/R26RtdTomato labeled cells were increasingly restricted to the superficial zone, accurately reflecting changes in Prg4 mRNA over increasing postnatal age. While these Prg4CreERT2/R26RtdTomato labeling patterns did reflect endogenous gene expression patterns, they did not permit selective labeling and tracing of unique cell populations within the articular cartilage.
Patterns of chondrocyte proliferation during articular cartilage maturation are not well understood. We recently utilized Prg4CreERT2/R26RConfetti mice to examine spatial expansion of Prg4-labeled cell populations postnatally. In R26RConfetti mice, individual cells are traced by one of four color reporters (GFP, YFP, RFP and CFP), thus permitting simultaneous tracking of distinct cell progenies and their developmental roles. As with the single-color R26RtdTomato reporter, Prg4CreERT2/ R26RConfetti labeled cells were found throughout the entire thickness of the articular cartilage after tamoxifen administration at E17.5 and collection at P0 (Decker et al., 2016). Over time, we found that clusters of uniquely-colored cells appeared to proliferate locally during postnatal growth, later aligning to form characteristic chondrocyte “stacks” with a mosaic color pattern. These experiments were repeated using the more broadly expressed Gdf5Cre as well as ubiquitous, non-biased ROSACreER mice, confirming that embryonically-labeled chondrocyte progenitors produced only small non-migratory progenies and that non-daughter lineage cells produced the vertical chondrocyte stacks, a result at variance with previous appositional models.
In the growth plate, tremendous increases in chondrocyte volume contribute greatly to lengthening of long bones (Farnum and Wilsman, 1998). To investigate changes in articular chondrocyte volume during growth, we used multiphoton microscopy to evaluate chondrocyte size within the intact articular cartilage of mice harvested at various stages of postnatal development (Decker et al., 2016). We found that chondrocyte volume in the middle and deep layers increased by over 8 fold from birth to 2 months of age, while overall decreases in cell density reflecting an increase in extracellular matrix production occurred during this same period. Strikingly, we also observed that proliferation of uniquely labeled ROSACreER/ R26RConfetti articular chondrocytes was limited after birth. Taken together, this suggests that, rather than being driven by proliferation and apposition of daughter cells from the surface to the deep zone to create cell columns (Dowthwaite et al., 2004; Hunziker et al., 2007; Kozhemyakina et al., 2015), thickening of the articular cartilage occurs primarily through an increase in the volume of articular chondrocytes and is aided by accumulation of extracellular matrix and formation of chondrocyte stacks. However, important questions remain unanswered regarding the mechanisms responsible for regulation of articular chondrocyte volume. This phenomenon has been more extensively studied in the growth plate, where changes in volume may be attributed partially to cell swelling and largely to intrinsic cell hypertrophy (Bush et al., 2008). Chondrocyte hypertrophy in the growth plate is a complex, directional event, in which volume progressively increases with distancing from the proliferating zone (Smits et al., 2004). Interestingly, the maximum size of articular chondrocytes is only about 60% of that found in the largest chondrocytes of the growth plate, indicating that location and function may uniquely dictate maximum chondrocyte volume. (Decker et al., 2016; Ginzberg et al., 2015; Wilsman et al., 1996; Youn et al., 2006). For example, articular chondrocytes are distinctively adapted to respond to inherent biomechanical stress. Many cell types display significantly negative resting membrane potentials, permitting rapid changes in membrane permeability in response to changes in osmotic pressure (Lewis et al., 2011). However, articular chondrocytes have less negative resting membrane potential, maintained by gadolinium-sensitive cation channels and permitting response to osmotic changes induced by biomechanical forces with only minimal changes in cell volume (Lewis et al., 2011).
Structurally, the cellular stacks of cells in mature articular cartilage likely form through alignment and repositioning of neighboring cells. This is in contrast to the distinct columns of chondrocytes in the growth plate, in which each individual column arises from a common ancestor (Decker et al., 2016). At birth, collagen fibrils within the extracellular matrix of the articular cartilage display an anisotropic distribution, later undergoing changes in deposition and orientation to form highly organized structures in the mature tissue (Clark et al., 1997; Hughes et al., 2005; Youn et al., 2006). It remains unclear if such changes within the extracellular matrix directly influence chondrocyte alignment, or if additional mechanisms may play a role. In embryonic structures, cell intercalation and convergent extension have been shown to directly influence cell migration (Tada and Heisenberg, 2012). During convergent extension, tissue growth and elongation occur while cells undergo very little proliferation, as is seen in the articular cartilage (Decker et al., 2016). The planar cell polarity pathway components Vangl2 and Ror2 regulate convergent extension and alignment of newly-formed chondrocytes along the proximo-distal axis of long bone cartilaginous rods during early limb skeletogenesis and patterning, and may play a role in postnatal tissue morphogenesis as well (Gao et al., 2011; Randall et al., 2012). Clearly, much work remains to be done in investigating mechanisms directing postnatal articular cartilage morphogenesis.
5. Mature Articular Cartilage
The limited intrinsic repair capacity of mature articular cartilage is widely appreciated, and major efforts have been directed towards surgical and biological restoration of the tissue in arthritic patients (Caldwell and Wang, 2015; Johnstone et al., 2013; Mollon et al., 2013). However, substantial challenges are posed by the low cell density and avascular and aneural characteristics of mature articular cartilage. The limited ability of chondrocytes to proliferate and the essentially permanent nature of the extensive collagen matrix explain the failure of the tissue to turnover once it has reached maturity (Heinemeier et al., 2016). Several studies have demonstrated the presence of cells with progenitor potential in adult joint tissues (Candela et al., 2014a; Candela et al., 2014b; Dowthwaite et al., 2003; Grogan et al., 2009; Williams et al., 2010), and there is growing interest in further characterization and exploitation of these cell populations for repair strategies. Many key questions remain, including the origin, fate, and role of these populations in intrinsic repair.
There is increasing evidence that embryonically-derived cell populations with a Gdf5, Dkk3, and/or Prg4 lineage remain present in joint tissues at maturity (Decker et al 2016; Koyama et al., 2008; Kozhemyakina et al., 2015). Sub-populations of slow-cycling cells have been identified in several tissues, including the articular cartilage and synovium. Early studies using tritiated thymidine demonstrated that embryonically labeled cells remained in the articular surface long-term (Ohlsson et al., 1992). More recent studies have confirmed the presence of cells with a progenitor or stem character in the superficial zone of adult articular cartilage (Alsalameh et al., 2004; Candela et al., 2014a; Dowthwaite et al., 2004; Williams et al., 2010b; Yasuhara et al., 2011). At the onset of osteoarthritis, cells in the superficial zone show increased proliferation and rearrangement (Rolauffs et al., 2010), but it is unknown how these cells may respond to acute injury. In the synovium, slow cycling cells expressing characteristic mesenchymal stem cell markers undergo proliferation and chondrogenic differentiation in response to injury (Kurth et al., 2011). Using novel cell lineage tracing strategies, we have recently demonstrated that embryonically labeled cells with a Prg4-expressing lineage respond massively to acute injury (Decker et al., 2016). After creation of a focal, full-thickness chondral defect, Prg4+ cells labeled at E17.5 were found within the defect site by 7d after injury (Fig. 2 D). EdU labeling indicated that cell proliferation within the synovium was much greater than within the articular cartilage adjacent to the defect site, suggesting that Prg4+cells within the synovium may play a primary role in the early injury response. Previous studies have also suggested that the synovium may be an essential source of cells contributing to articular cartilage injury, and our new data provide additional insight into the lineage and character of these cells (Kurth et al., 2011; Miyamoto et al., 2007; Rothwell, 1990). Future techniques designed to target synovium-associated Prg4 + cells or inclusion of these cells in bioengineered constructs may provide promise for repair of damaged articular cartilage.
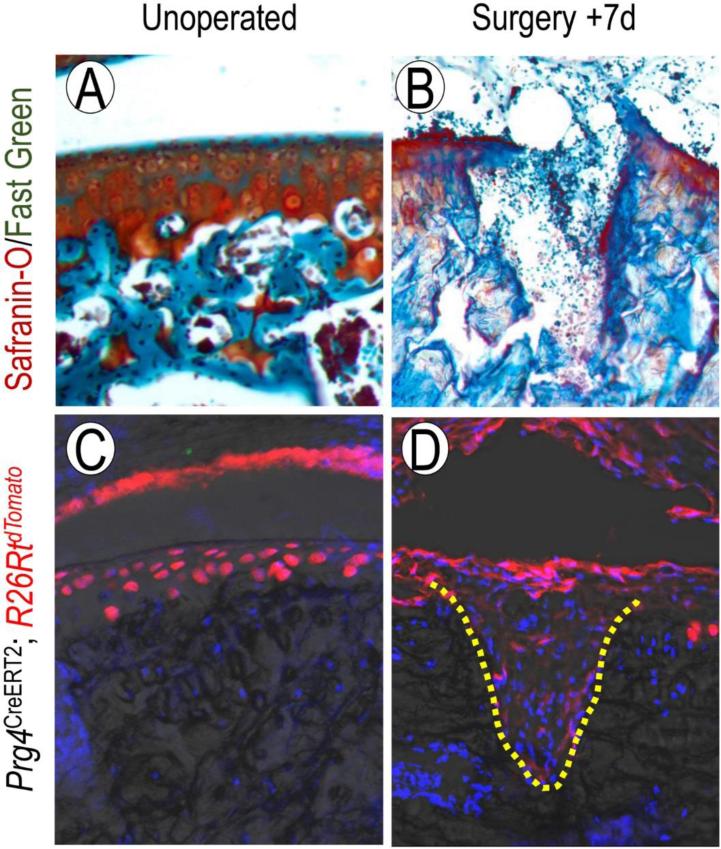
Fig. 2.
Cells with a Prg4+ lineage respond to articular cartilage injury. Safranin-O/Fast green staining of mature mouse femoral articular cartilage before (A) and 7 days (B) after creation of a full-thickness chondral defect. After tamoxifen administration to Prg4CreERT2/R26RtdTomato mice at 1month of age, labeled cells are found throughout several layers of the articular cartilage (C). One week after injury, cells within the defect site (dotted line) are Prg4+, indicating that cells with a Prg4-expressing lineage participate in the acute injury response (D).
6. Conclusion
Current clinical strategies for articular cartilage repair have failed to replicate the structure and function of innate articular cartilage. In recent years, novel genetic cell lineage tracing techniques have provided tremendous insight into the origin and morphogenesis of the articular cartilage during normal development, and this knowledge may be extremely useful for developing novel biological repair strategies. Taken together, these studies have revealed that the articular cartilage and other joint tissues have a heterogeneous origin, arising from de-differentiated chondrocytes as well as cells surrounding the cartilaginous anlagen. Postnatally, chondrocyte volume increase, matrix production, and realignment drive the growth and morphogenesis of the articular cartilage into a multifaceted tissue. While we now have a greater understanding of cell origin and fate, there is much work left to be done to decipher the molecular mechanisms responsible for guiding these processes and to find appropriate ways to manipulate them for therapeutic strategies.
Acknowledgements
Assistance and advice from Dr. Maurizio Pacifici is graciously acknowledged. Work originally carried out and summarized here was supported by NIH grant AR062908. R.S.D. is the recipient of a postdoctoral training grant (1F32AR064071) from the NIH. We express our gratitude to our several colleagues who contributed to the original studies described here, and apologize for not citing and describing the work of other relevant groups given the space limitations of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Kim J-E, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J. Anat. 1994;184:447. [PMC free article] [PubMed] [Google Scholar]
- Bhattaram P, Penzo-Méndez A, Kato K, Bandyopadhyay K, Gadi A, Taketo MM, Lefebvre V. SOXC proteins amplify canonical WNT signaling to secure nonchondrocytic fates in skeletogenesis. J. Cell. Biol. 2014;207:657–671. doi: 10.1083/jcb.201405098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom ND, Poole C. A functional-morphological study of the tidemark region of articular cartilage maintained in a non-viable physiological condition. J. Anat. 1982;135:65. [PMC free article] [PubMed] [Google Scholar]
- Bush PG, Parisinos CA, Hall AC. The osmotic sensitivity of rat growth plate chondrocytes in situ; clarifying the mechanisms of hypertrophy. J. Cell. Physiol. 2008;214:621–629. doi: 10.1002/jcp.21249. [DOI] [PubMed] [Google Scholar]
- Calandruccio RA, Gilmer WSJR. Proliferation, Regeneration, and Repair of Articular Cartilage of Immature Animals. J. Bone Joint Surg. Am. 1962;44:431–455. [Google Scholar]
- Caldwell KL, Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthr. Cart. 2015;23:351–362. doi: 10.1016/j.joca.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela ME, Cantley L, Yasuaha R, Iwamoto M, Pacifici M, Enomoto-Iwamoto M. Distribution of Slow-Cycling Cells in Epiphyseal Cartilage and Requirement of β-Catenin Signaling for Their Maintenance in Growth Plate. J. Orthop. Res. 2014a;32:661–668. doi: 10.1002/jor.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela ME, Yasuhara R, Iwamoto M, Enomoto-Iwamoto M. Resident mesenchymal progenitors of articular cartilage. Matrix Biol. 2014b;39:44–49. doi: 10.1016/j.matbio.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Norman A, Nötzli H. Postnatal development of the collagen matrix in rabbit tibial plateau articular cartilage. J. Anat. 1997;191:215–227. doi: 10.1046/j.1469-7580.1997.19120215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS, Koyama E, Pacifici M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Current Osteoporosis Reports. 2015;13:407–414. doi: 10.1007/s11914-015-0290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS, Um H, Dyment N, Cottingham N, Usami Y, Enotomi-Iwamoto M, Kronenburg M, Maye P, Koyama E, Pacifici M. Cell reposition, volume and local proliferation drive articular cartilage development, growth and response to injury. 2016 Manuscript submitted for publication. [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, Butler DL. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 2015;405:96–107. doi: 10.1016/j.ydbio.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. Skeletal Growth and Development. AAOS; Rosemont: 1998. Effects of distraction and compression on growth plate function. pp. 517–530. [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. Mechanisms of GDF5 action during skeletal development. Development. 1999;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- Gannon A, Nagel T, Bell A, Avery N, Kelly D. Postnatal changes to the mechanical properties of articular cartilage are driven by the evolution of its collagen network. Eur. Cel.l Mater. 2014;29:105–123. doi: 10.22203/ecm.v029a09. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzberg MB, Kafri R, Kirschner M. On being the right (cell) size. Science. 2015;348:1245075. doi: 10.1126/science.1245075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz M. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthr. Res. Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Takahara M, Zhe P, Otsuji M, Iuchi Y, Takagi M, Ogino T. Developmental failure of the intra-articular ligaments in mice with absence of growth differentiation factor 5. Osteoarthr. Cart. 2007;15:468–474. doi: 10.1016/j.joca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104 doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat. Embryol. (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Schjerling P, Heinemeier J, Møller MB, Krogsgaard MR, Grum-Schwensen T, Petersen MM, Kjaer M. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Science Trans. Med. 2016;8:346ra390–346ra390. doi: 10.1126/scitranslmed.aad8335. [DOI] [PubMed] [Google Scholar]
- Helminen HJ, Hyttinen MM, Lammi MJ, Arokoski JP, Lapvetelainen T, Jurvelin J, Kiviranta I, Tammi MI. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J. Bone Miner. Metab. 2000;18:245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- Holder N. An experimental investigation into the early development of the chick elbow joint. J. Embryology Experimen. Morphol. 1977;39 [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L, Archer C, Ap Gwynn I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. Eur. Cell Mate.r. 2005;9:e84. doi: 10.22203/ecm.v009a09. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartilage. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hyde G, Boot-Handford RP, Wallis GA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J. Anat. 2008;213:531–538. doi: 10.1111/j.1469-7580.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev. Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegawa N, Sasho T, Yamaguchi S, Saito M, Akagi R, Muramatsu Y, Akatsu Y, Fukawa T, Nakagawa K, Nakajima A. Identification of genes required for the spontaneous repair of partial-thickness cartilage defects in immature rats. Connect. Tissue Res. 2016;57(3):190–9. doi: 10.3109/03008207.2015.1121250. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, Nakamura T, Enomoto-Iwamoto M, Pacifici M. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev. Biol. 2007;305:40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res. 2001;19:677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- Jenner F, IJpma A, Cleary M, Heijsman D, Narcisi R, van der Spek PJ, Kremer A, van Weeren R, Brama P, van Osch GJ. Differential Gene Expression of the Intermediate and Outer Interzone layers of developing articular cartilage in murine embryos. Stem Cells Dev. 2014;23(16):1883–98. doi: 10.1089/scd.2013.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, et al. Tissue engineering for articular cartilage repair. The state of the art. Eur. Cells Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- Kan A, Ikeda T, Fukai A, Nakagawa T, Nakamura K, Chung U.-i., Kawaguchi H, Tabin CJ. SOX11 contributes to the regulation of GDF5 in joint maintenance. BMC Dev. Biol. 2013;13:4. doi: 10.1186/1471-213X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Ochiai T, Rountree RB, Kingsley DM, Enomoto-Iwamoto M, Iwamoto M, Pacifici M. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann. N. Y. Acad. Sci. 2007;1116:100–112. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, Kronenberg H, Warman ML, Lassar AB. Identification of a Prg4- expressing articular cartilage progenitor cell population in mice. Arthritis Rheum. 2015;67:1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth TB, Dell'Accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- Lewis R, Asplin KE, Bruce G, Dart C, Mobasheri A, Barrett-Jolley R. The Role of the Membrane Potential in Chondrocyte Volume Regulation. J. Cell. Physiol. 2011;226:2979–2986. doi: 10.1002/jcp.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Longobardi L, Myers TJ, Temple JD, Chandler RL, Ozkan H, Contaldo C, Spagnoli A. Joint TGF-β type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem cells and development. 2012;22:1342–1359. doi: 10.1089/scd.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. J. Bone Joint Surg. 1962;44:688–698. [Google Scholar]
- Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. J. Bone Joint Surg. 1963;45:529–540. [Google Scholar]
- Matsuoka M, Onodera T, Sasazawa F, Momma D, Baba R, Hontani K, Iwasaki N. An articular cartilage repair model in common C57Bl/6 mice. Tissue Eng. Part C Methods: Methods. 2015;21:767–772. doi: 10.1089/ten.tec.2014.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienaltowski MJ, Huang L, Frisbie DD, McIlwraith CW, Stromberg AJ, Bathke AC, Macleod JN. Transcriptional profiling differences for articular cartilage and repair tissue in equine joint surface lesions. BMC Med. Genomics. 2009;2:60. doi: 10.1186/1755-8794-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienaltowski MJ, Huang L, Stromberg AJ, MacLeod JN. Differential gene expression associated with postnatal equine articular cartilage maturation. BMC Musculoskelet. Disord. 2008;9:149. doi: 10.1186/1471-2474-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic DR. Development of the metatarsophalangeal joint of the chick embryo: Morphological, ultrastructural and histochemical studies. Am. J. Anat. 1977;150:333–347. doi: 10.1002/aja.1001500207. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Deie M, Yamasaki T, Nakamae A, Shinomiya R, Adachi N, Ochi M. The role of the synovium in repairing cartilage defects. Knee Surg. Sports Traumatol. Arthrosc. 2007;15:1083–1093. doi: 10.1007/s00167-006-0277-5. [DOI] [PubMed] [Google Scholar]
- Mollon B, Kandel RA, Chahal J, Theodoropoulos J. The clinical status of cartilage tissue regeneration in humans. Osteoarthr. Cart. 2013;21:1824–1833. doi: 10.1016/j.joca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Nalin AM, Greenlee TK, Jr., Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of types II and XI collagen genes in the joint capsule. Dev. Dyn. 1995;203:352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J. Bone Joint Surg. 1998;80:4. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Kriegstein AR. Regenerative medicine: Cell reprogramming gets direct. Nature. 2010;463:1031–1032. doi: 10.1038/4631031a. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc. Natl. Acad. Sci. U S A. 1992;89:9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Shibukawa Y, WU C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and Molecular Mechanisms of Synovial Joint and Articular Cartilage Formation. Ann. N. Y. Acad. Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RM, Shao YY, Wang L, Ballock RT. Activation of Wnt Planar cell polarity (PCP) signaling promotes growth plate column formation in vitro. J. Orthop. Research. 2012;30:1906–1914. doi: 10.1002/jor.22152. [DOI] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolauffs B, Williams JM, Aurich M, Grodzinsky AJ, Kuettner KE, Cole AA. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 2010;62:489–498. doi: 10.1002/art.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell A. Synovium transplantation onto the cartilage denuded patellar groove of the sheep knee joint. Orthopedics. 1990;13:433–442. doi: 10.3928/0147-7447-19900401-09. [DOI] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H-S, Serra R. Deletion of< i> Tgfbr2</i> in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev. Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Dy P, Mitra S, Lefebvre V. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. The Journal of Cell Biology. 2004;164:747–758. doi: 10.1083/jcb.200312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing Precursors Are the Cellular Origin of the Cruciate Ligament of the Knee Joint and the Limb Tendons. Genesis (New York, N.Y. : 2000) 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli A, O'Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K. TGF- signaling is essential for joint morphogenesis. J. Cell Biol. 2007;177:1105. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spater D, Hill TP, O'Sullivan R J, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes & Develop. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee S-J. Limb alterations in brachypodism mice due to mutations in a new member of the TGF[beta]-superfamily. Nature. 1994a;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Tada M, Heisenberg C-P. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139:3897–3904. doi: 10.1242/dev.073007. [DOI] [PubMed] [Google Scholar]
- Williams R, Khan IM, Richardson K, Nelson I, McCarthy HE, Analbelsi T. Identification and clonal characterirization of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010a;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsman NJ, Leiferman EM, Fry M, Farnum CE, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J. Orthop. Res. 1996;14:927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Invest. 2011;91:1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn I, Choi J, Cao L, Setton L, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthr. Cartilage. 2006;14:889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Cigan AD, Marrero L, Lopreore C, Liu S, Ge D, Savoie FH, You Z. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis. 2011;49:75–82. doi: 10.1002/dvg.20702. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]