Summary
As one of the most serious diseases in grape, downy mildew caused by Plasmopara viticola is a worldwide grape disease. Much effort has been focused on improving susceptible grapevine resistance, and wild resistant grapevine species are important for germplasm improvement of commercial cultivars. Using yeast two‐hybrid screen followed by a series of immunoprecipitation experiments, we identified voltage‐dependent anion channel 3 (VDAC3) protein from Vitis piasezkii ‘Liuba‐8’ as an interacting partner of VpPR10.1 cloned from Vitis pseudoreticulata ‘Baihe‐35‐1’, which is an important germplasm for its resistance to a range of pathogens. Co‐expression of VpPR10.1/VpVDAC3 induced cell death in Nicotiana benthamiana, which accompanied by ROS accumulation. VpPR10.1 transgenic grapevine line showed resistance to P. viticola. We conclude that the VpPR10.1/VpVDAC3 complex is responsible for cell death‐mediated defence response to P. viticola in grapevine.
Keywords: grapevine, Plasmopara viticola, PR10, ROS, voltage‐dependent anion channel, Vitis pseudoreticulata
Introduction
Grape is one of the world's oldest cultivated fruit crops, with the increase in population and rising standards of living, consumption of grapes and grape products (notably wine) continues to grow. Increasing production areas and efficiency makes commercial grapes the second highest value fruit crop in the world, according to FAO report (2016). Downy mildew is one of the most serious biotrophic parasite diseases in grapes, it is caused by Plasmopora viticola and mainly infects the green tissues (Ingram, 1981). P. viticola is the foremost threat to the wine grape industry in most parts of the world (Vercesi et al., 1999). Chinese wild grape varieties are important gene resources for improving disease resistance of susceptible cultivars, and a number of studies have shown that genes derived from Chinese wild grapes play important roles in grape disease resistance (Han et al., 2016; Xu et al., 2014; Yu et al., 2013a). Many important grape cultivars are severely compromised by downy mildew, so improvements in disease resistance are urgent need for grapevine breeders. Wild grapes present abundant genetic sources of disease resistance for grape breeding. For example, resistance against downy mildew found in wild American species has been successfully integrated into commercial grape cultivars (Eibach et al., 2009; Gessler et al., 2011; Jiao et al., 2016).
Pathogenesis‐related (PR) proteins served as various roles of defence in plants (Loon and Kammen, 1968; van Loon et al., 2006). Increasing evidences show that PR10 (pathogenesis‐related protein 10) has distinct functions in plant developmental, the secondary metabolism and in bacteriostatic action (Choi et al., 2012; Hashimoto et al., 2004; Liu and Ekramoddoullah, 2006; McGee et al., 2001; Zhou et al., 2002). PR10s are highly conserved among a range of plants, and there are seventeen PR10s in Vitis vinifera (Lebel et al., 2010), although the exact biological functions of PR10 have not yet been clarified, many studies show PR10 proteins are induced by biological stresses, at the same time PR10 proteins enhance the resistance to viruses (Loon, 1997), bacteria (Breda et al., 1996) and fungi (Swoboda et al., 1995). In grape, VpPR10.2 shows nuclease activity and contributes to host resistance to P. viticola (He et al., 2013). Besides grapevine, avirulent Xanthomonas campestris pv. vesicatoria (Xcv) induced PR10 associated with the hypersensitive response in pepper and Nicotiana benthamiana, overexpression of pepper PR10 and LRR1 in Arabidopsis thaliana showed resistance to Hyaloperonospora arabidopsidis and the cytosolic LRR/PR10 complex in pepper is responsible for cell death‐mediated defence reaction (Choi et al., 2012).
There are two layers of immune defence systems in plants: the first layer involves pattern‐recognition receptors which detect the pathogen/microbe‐associated molecular patterns called PAMP/pattern‐triggered immunity (PTI). These offer a broad spectrum of resistance in terms of a nonspecific immune response (Dodds and Rathjen, 2010; Jones and Dangl, 2006). To overcome the pathogen invasion, plants use another layer of defence called effector‐triggered immunity (ETI) (Koistinen et al., 2002; Wang et al., 2009). Resistance (R) proteins recognize pathogens and induce hypersensitive response (HR), and HR reaction induces programmed cell death, which limits the ability of the pathogenic bacteria to reproduce (Bittel and Robatzek, 2007).
Voltage‐dependent anion channel (VDAC) proteins are among the most abundant proteins found in the mitochondrial outer membrane (Benz, 1994; Colombini, 1979), and these are important for regulating material and energy exchange between mitochondria and the cytoplasm. Apoptosis factors also exist in mitochondria, such as cytochrome c, the apoptosis factors released into the cytoplasm to induce cell death (Kroemer et al., 2007). In plants, VDACs also mediate PCD resulting from biotic and abiotic stresses (Benz, 1994; Colombini, 1979; Sangmin et al., 2009). The expression of AtVDAC1, AtVDAC2, AtVDAC3 and AtVDAC4 is up‐regulated in response to pst DC3000. This suggests that VDACs play an active role response to biotic stress (Robert et al., 2012). It has been shown that AtVDAC3 specifically binds to the kinesin motor protein KP1 to control seed germination at low temperatures (Yang et al., 2011). AtVDAC3 can also interact with Atthioredoxinm2, the overexpression of AtVDAC3 increased H2O2 accumulation, while overexpression of AtTrxm2 weakened H2O2 generation, and hence, they play opposing roles in stress responses involving ROS signalling (Zhang et al., 2015). Recent studies have shown the influential role of metacaspases in plant PCD, including pathogen infection (Hao et al., 2007; Hoeberichts et al., 2003), stimulation of H2O2 (He et al., 2008) and plant development (Suarez et al., 2004). Plant metacaspases are thought to be distantly related to animal caspases and can be classified as type I and type II (Lam, 2004; Uren et al., 2000). Studies have also shown that two metacaspases AtMCl and AtMC2 of Arabidopsis play positive and negative regulating roles, respectively, in plant cell death (PCD) (Coll et al., 2010). Although some progress has been made in characterizing the metacaspases (Lam and Zhang, 2012; Tsiatsiani et al., 2011; Vercammen et al., 2007; Zhang and Lam, 2011), a detailed overview of their biochemical properties is still lacking.
In our previous study, VpPR10.1 was cloned from Chinese wild grape V. pseudoreticulata and VpPR10.1 contributes to defence reactions in grape (Xu et al., 2014). In this study, we show that VpPR10.1 transgenic grapevine have elevated ROS production after P. viticola inoculation. We demonstrate the ability of VpPR10.1 promoting immunity to P. viticola through a pathway involving with VpVDAC3. They work synergistically in a cell death‐like defence response to enable resistance to P. viticola in grape.
Results
VpPR10.1 interacts with VpVDAC3
We previously obtained the VpPR10.1 from V. pseudoreticulata ‘Baihe‐35‐1’, a wild grape that shows resistance to multiple grapevine diseases (Wan et al., 2007; Wang et al., 1995; Xu et al., 2014). We used yeast two‐hybrid system to screen interacting partners to identify the resistance mechanism of VpPR10.1. Target proteins were baited from a library construct from resistant Chinese wild grape V. piasezkii ‘Liuba‐8’ after P. viticola infection. Among fifty‐six positive clones we obtained (Table S1), four of these positive clones contained the same cDNA that encodes VpVDAC3 (voltage‐dependent anion channel), which was selected as the target protein (Figure 1a). To test the interaction in living plants, bimolecular fluorescence complementation (BiFC) assay (Bracha et al., 2004; Walter et al., 2004) was carried out. N‐terminal (NE) and C‐terminal (CE) fragments of yellow fluorescent protein (YFP) were connected to VpPR10.1 and VpVDAC3, and coupled constructions were expressed in tobacco protoplasts. Fluorescence was detected in the combination of VpPR10.1‐NE/VpVDAC3‐CE and VpVDAC3‐NE/VpPR10.1‐CE, but not in the control samples (Figure 1b). As shown in Figure 1c, recombinant protein GST‐VpPR10.1 but not unmodified protein GST was bond by MBP‐VpVDAC3. Next, we tested the interaction between VpPR10.1 and VpVDAC3 using CO‐IP. We found that 3Flag‐VpVDAC3 co‐precipitated with VpPR10.1‐GFP but not with the control GFP (Figure 1d). Taken overall, we conclude that VpPR10.1 interacts directly with VpVDAC3.
Figure 1.
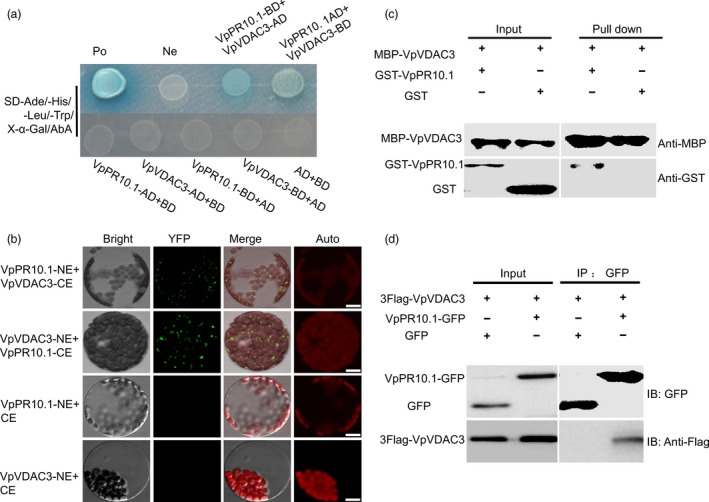
VpPR10.1 interacts with VpVDAC3. (a)Yeast two‐hybrid assay. pGBKT7 or pGADT7 plasmid containing VpPR10.1 and VpVDAC3 was transformed into Y2H Gold. Combinations of the (AD/T) with BD/p53 and BD/Lam were used as positive and negative controls. (b) Bimolecular fluorescence complementation (BiFC) assay in vivo. Merged fluorescent and visible light images were taken. Bars = 10 μm. Auto, chloroplast auto‐fluorescence. (c) Pull‐down assay. The presence or absence of each protein in the final mixture is indicated as + or −, respectively. (d) Co‐IP and immunoblotting (IB) of GFP/Flag‐VpVDAC3 and VpPR10.1‐GFP/Flag‐VpVDAC3 were co‐expressed in Nicotiana benthamiana leaves. Flag antibodies were used for the detection of immunoprecipitated VpPR10.1‐GFP and Flag‐VpVDAC3.
The localization of VpPR10.1 and VpVDAC3 in vivo
Our previous research found that VpPR10.2 is located in the host cell dynamically (He et al., 2013). In our study, we found that VpPR10.1‐GFP diffusely localized in the cytoplasm of N. benthamiana protoplasts (Figure 2a). At the same time, VpVDAC3 targeting to mitochondria was confirmed by co‐staining with Mito‐tracker Red which served as a mitochondrial‐specific reagent. To gain insight into the complex interaction, cherry‐VpPR10.1 and VpVDAC3‐GFP were co‐transformed (Figure 2b), VpVDAC3‐GFP can colocalized with VpVDAC3‐GFP. As an independent component of potential co‐localization of VpPR10.1 and VpVDAC3, we extracted mitochondria and analysed by Western blotting. As seen in Figure 2c, VpVDAC3 and VpPR10.1 were both detected in mitochondrial fractions.
Figure 2.
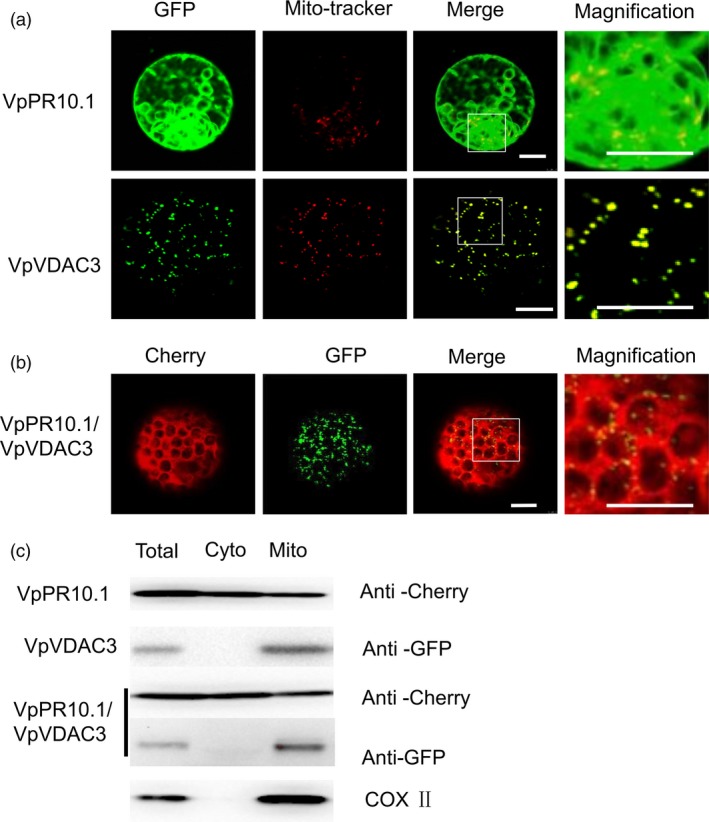
Subcellular localization of VpPR10.1 and VpVDAC3. The visible fluorescence indicates gene localization in Nicotiana benthamiana protoplasts. (a) Localization of VpPR10.1‐GFP, VpVDAC3‐GFP. Mito‐Tracker Red (Invitrogen) was stained to mark mitochondria. Bars = 10 μm. (b)Co‐localization of the VpPR10.1 and VpVDAC3 in N. benthamiana protoplasts transformed with the couple constructs of cherry‐VpPR10.1 and VpVDAC3‐GFP. Bars = 10 μm. (c) Mitochondria were extracted from transformed protoplasts and detected by immunoblotting. Total, no separated protein; Cyto, nonmitochondrial protein; Mito, mitochondrial enriched protein. COX II was present as mitochondrial marker protein.
Transient co‐expression of VpPR10.1 accelerated ROS production induced by VpVDAC3
There is a consensus view that excessive ROS in cells can lead to damage and is closely associated with cell death (Carlisle et al., 2000; Kim et al., 2006; Son et al., 2010). VDAC has been identified as an important mediator of apoptosis closely related to oxidative signals (Madesh and Hajnóczky, 2001). Overexpression of VDAC1 strengthens the ROS production and triggers apoptosis (Simamura et al., 2006). DCFH‐DA becomes irreversibly fluorescent by a distinctive reaction with ROS (Choi et al., 2010; Kim et al., 2003, 2006). To assess the effect of VpVDAC3 on ROS, we separated the protoplast from the transient expressed leaves of indicated genes and visualized ROS using DCFH‐DA. The Agrobacterium tumefaciens carried recombinant plasmids (Flag‐VpVDAC3, Flag‐VpPR10.1 and Flag‐VpVDAC3/Flag‐VpPR10.1) were transiently expressed in N. benthamiana before the separation of protoplasts. In the protoplast fluorescent‐dye assay, fluorescence indicates the production of ROS (Figure 3a). An increase in fluorescence was observed in protoplasts containing VpVDAC3, and this effect was enhanced by the co‐expression of VpPR10.1. This indicates VpPR10.1 enhanced the ability of VpVDAC3’ to induce ROS production. We also treated protoplasts with DPI as a NADPH oxidase inhibitor, fluorescence in the DPI‐pretreated protoplasts was lower than that in the untreated ones, and DPI decreased the ROS accumulation in the protoplasts (Figure 3b). These results suggest that NADPH oxidase is involved in the ROS burst that caused by VpVDAC3.
Figure 3.
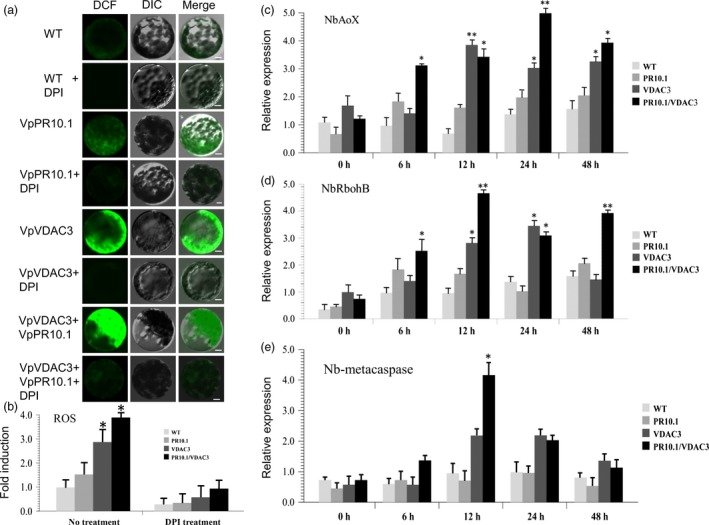
ROS Detection of Transient Expression of VpVDAC3, VpPR10.1, VpVDAC3/VpPR10.1 in N. benthamiana. Before the separation of protoplasts, Flag‐VDAC3, Flag‐VpPR10.1 and Flag‐VpVDAC3/Flag‐VpPR10.1 were transiently expressed by Agrobacterium infiltration in N. benthamiana leaves. (a) Protoplasts isolated from the indicated transient expression leaves, and protoplast lines were marked by DCFH‐DA. The green fluorescence represents intracellular ROS level; (b) Each sample was accompanied by pretreatment of ROS inhibitor DPI. Bars = 10 μm. (c, d, e) Quantification of RT‐PCR analysis of ROS‐related genes NbAOX, NbRbohB, NbAPX; values represent means and SEs of three biological replicates. * and ** indicate P < 0.05 and P < 0.01, compared to GFP control (t‐test).
Also, ROS‐related genes were confirmed by RT‐PCR after infiltration, as AOX plays an important role during the response to stress, we examined the NbAOX transcript levels. As shown in (Figure 3c), co‐expression of VpPR10.1 and VpVDAC3 resulted in induction of NbAOX by 3.5‐fold compared with the negative control (GFP). NbRboh expression was also strongly induced by VpPR10.1 and VpVDAC3 co‐expression (Figure 3d), and the ascorbate peroxidase NbAPX was up‐regulated too (Figure 3e).These results indicate VpPR10.1 positively regulate the ROS activity through VpVDAC3 in plant cells.
Depolarization of mitochondria membrane by transient expression of VpVDAC3/VpPR10.1 in N. benthamiana
Loss of mitochondrial membrane potential is an early step in apoptosis, and we used a marker of mitochondrial membrane potential dye JC‐1 to evaluate the effect of VDAC or PR10 overexpression on mitochondrial membrane potential in N. benthamiana. Protoplasts were isolated from the transient expressed N. benthamiana leaves, protoplast lines were marked by JC‐1, and JC‐1 changes colour from red/orange to green as the membrane potential decreases (Cossarizza et al., 1993; Salvioli et al., 1997). In Figure 4, the green fluorescence represents mitochondrial membrane potential loss, and VpVDAC3/VpPR10.1 together induce higher decreases of mitochondrial membrane potential.
Figure 4.
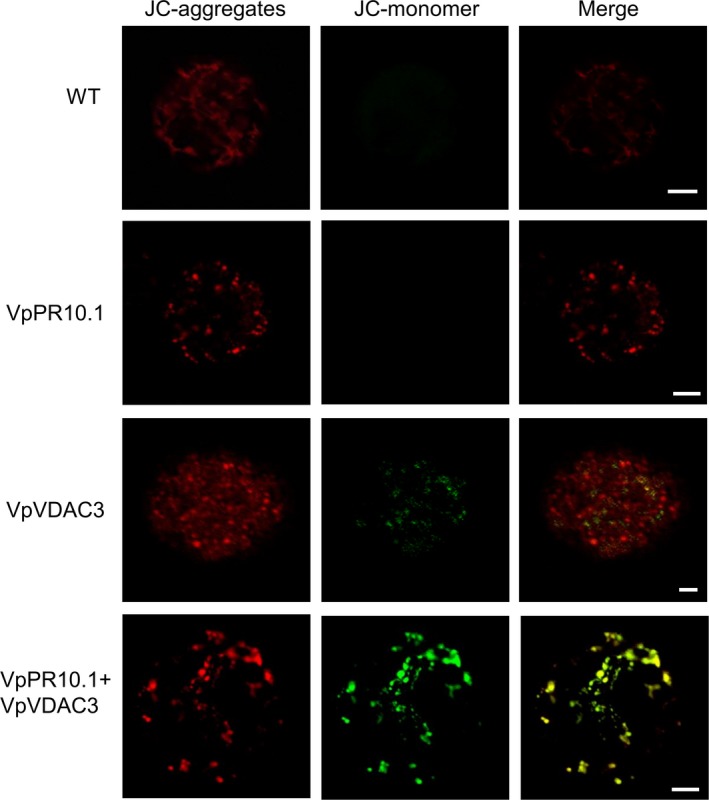
Depolarization of Mitochondria by Transient Expression VpVDAC3/VpPR10.1 in Nicotiana benthamiana. Mitochondrial depolarization. Living protoplasts were separated from N. benthamiana leaves that transiently expressed indicated genes. Decreased mitochondria membrane polarization results in the formation of JC‐1 aggregate to monomer which results in shifts of red/orange to green. Bars = 10 μm.
Transient co‐expression of VpPR10.1 and VpVDAC3 promotes ROS production and cell death in N. benthamiana
Overexpression of VDAC triggers cell death in a diverse range of organisms including yeast, rice, fish, tobacco, mouse and humans (Godbole et al., 2003, 2013; Shoshan‐Barmatz et al., 2010; Zaid et al., 2005). To determine the biological function of VpVDAC3 and VpPR10.1, we evaluated the effect of co‐expression on cell death (Figure 5a). Expression of VpPR10.1 and VpVDAC3 was confirmed by Western blot (Figure 5b) and 3,3‐diaminobenzidine (DAB) staining (Figure 5a). Transient expression of VpPR10.1 did not trigger an obvious reaction in N. benthamiana (Figure 5a). However, co‐expression of VpPR10.1/VpVDAC3 triggered enhanced cell death than VpVDAC3 (Figure 5a). The sites of leaves infiltrated by VpPR10.1/VpVDAC3 showed enhanced H2O2 than VpVDAC3 alone too (Figure 5a), and VpPR10.1 positively regulated the cell death responses induced by VpVDAC3. To confirm that cell death in N. benthamiana leaves after infiltration, we assayed the leaves for electrolyte leakage (Figure S1). The conductivity of the Bax was always higher than that of each gene or complex overexpression, and VpPR10.1/VpVDAC3 took the second place.
Figure 5.
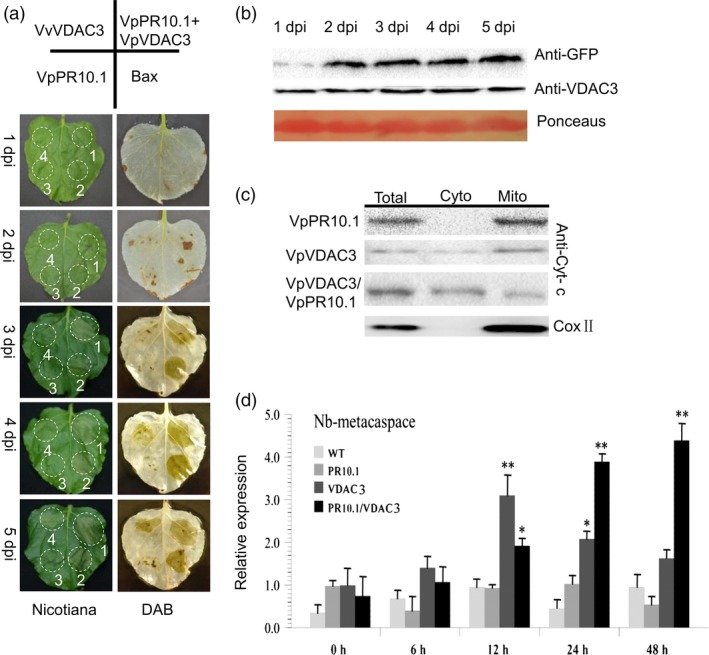
Transient Expression of VpVDAC3 or VpVDAC3/VpPR10.1 in Nicotiana benthamiana induces cell death. Phenotypic and physiological analyses of VpPR10.1 and VpVDAC3 following DAB staining. (a) Phenotypes of VpVDAC3, VpPR10.1, VpVDAC3/VpPR10.1 and Bax were all injected into one N. Benthamiana leaves. The bluish colour of DAB staining represents accumulated H2O2. (b) Visualization of VpVDAC3, VpPR10.1 protein by Western blotting in Agro‐infiltration N. benthamiana leaves. Ponceaus staining stand for control loading. (c) Western blot for detecting cytochrome c after expression with indicator gene in N. Benthamiana leaves. Total, nonseparated protein; Cyto, nonmitochondrial protein; Mito, mitochondrial enriched protein. COX II was present as mitochondrial marker protein. (d) Quantification of RT‐PCR analysis of cell death inducing gene Nb‐MCA1 expression. Values mean ± SE (n = 3) of three independent biological repeats. Asterisks indicate significant difference between each gene and GFP control, *P < 0.05; **P < 0.01 (t‐test).
Cytochrome c is exclusively located in the mitochondria and serves as an electron shuttle signal inducing mitochondrial‐mediated apoptosis (Circu and Aw, 2010; Esmaeili et al., 2016; Lei et al., 2006), so we used antibody recognizing cytochrome c to examine the release after transient expressed with VpPR10.1 or VpVDAC3. In VpPR10.1 overexpressed leaves, antibody against cytochrome c signal appeared both in the total protein and in the mitochondrial enriched fraction. Meanwhile, in VpVDAC3 overexpression and co‐expression samples, cytochrome c was also detected in the cytoplasm(Figure 5c). Metacaspase is an important component in cell death (Coll et al., 2010; Kim et al., 2003, 2006). As an additional means to assess cell death, we evaluated the expression of NbMCA1 in N. benthamiana (Figure 5d). Compared to the GFP control, the co‐expression of VpPR10.1 and VpVDAC3 induced more than a fourfold induction after 48 h of agro‐infiltration.
Generation and expression pattern analysis of VpPR10.1 transgenic grapevine
To explore function of VpPR10.1 in grapevine, we overexpressed VpPR10.1 in susceptible cultivar V. vinifera cv. ‘Thompson Seedless’, and under the normal grow conditions, features of transgenic grapevine line 6905 showed no significant differences with nontransgenic control (Figure 6a). We also used laser scanning confocal microscopy to check GFP fluorescence directly, in leaves of line 6905 and nontransgenic control (Figure 6b). Line 6905 showed strong GFP fluorescence located in whole cells including stoma. Proteins from transgenic grapevine line 6905 and WT were extracted to detect the antibody against GFP. Western blot showed clearly that the transgenic grapevine line 6905 exhibited expression of VpPR0.1‐GFP (Figure 6c).
Figure 6.
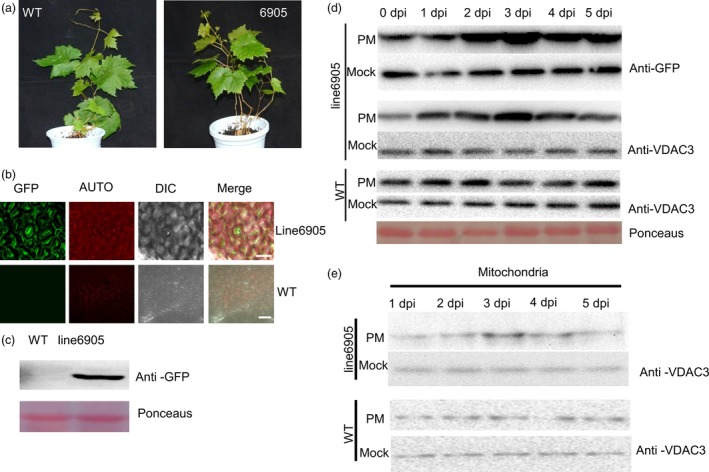
Characterization and Expression Pattern Analysis of VpPR10.1 Transgenic Grapevine. (a) Phenotypic study of transgenic line 6905 and wild type. VpPR10.1 transgenic line 6905 and WT grown in the feeding block for 40 days (left, ‘Thompson Seedless’, right, transgenic line 6905). Bars = 1 cm. (b) Direct GFP fluorescence observation of grape leaf abaxial surface from transgenic line 6905 and WT using confocal microscope. Bars = 50 μm. (c) Western blot showing expression levels of insert gene VpPR10.1 with GFP tag in transgenic line 6905 and WT. Ponceaus indicates control loading. (d) Expression patterns of VpPR10.1 and VpVDAC3 in transgenic line 6905 under infection of Plasmopara viticola. PM, P. viticola induction; Mock, inoculated with double distilled water. Ponceaus indicates control loading. (e) Expression patterns of VpVDAC3 in enriched mitochondria from transgenic line 6905 under post of P. viticola. Protein was detected by Western blot using anti‐VDAC3 as described in the experiment and procedure section.
GFP antibodies and the antibody against VpVDAC3 were used in immunoblot analysis of grapevine leaves inoculated with P. viticola (Figure 6d). The immunoblot results indicate that upon P. viticola inoculation, both VpPR10.1 and VpVDAC3 were accumulated in line 6905. To confirm the function of VpVDAC3 under pathogen attack, we evaluated the expression of VpVDAC3 in extracted mitochondrial proteins (Figure 6e). Compared to no obvious change in mock, VpVDAC3 also accumulated in the mitochondrial extracted from transgenic grapevine line 6905 inoculated with P. viticola.
VpPR10.1‐GFP transgenic grapevine line show enhanced resistance against P. viticola
Plasmopara viticola development in transgenic grapevine line and nontransgenic control was visualized by scanning electron microscopy (Liu et al., 2015). In VpPR10.1 transgenic line 6905, stomatal blockage was appeared in leaf discs as early as 2 dpi (Figure 7a). This is a resistance characteristic consistent with previous observation (Gindro et al., 2003; Trouvelot et al., 2008). By 3 dpi, differences in hyphal and sporangiophore growth between transgenic line 6905 and WT were observed (Figure 7a). Hyphae, primary hyphae and sporangia were detected in both VpPR10.1 transgenic line 6905 and WT at 4 dpi. At five days after inoculation, the sporangia were shed from the sporangiophores of both genotype lines. Development of P. viticola in the highly resistant V. piasezkii ‘Liuba‐8’ was obviously inhibited (Figure 7a), while the suppression of P. viticola in the transgenic line 6905 was also apparent. Compared with Liuba‐8 and WT (Figure 7b), sporangia density in ‘Liuba‐8’ (1.8) was lower than that in the transgenic line 6905 (45.2), in susceptible WT the sporangia density was 67.4/mm2 (Figure 7b). The number of haustoria per hyphae at 48 hpi in the transgenic line 6905 (3.5) was lower than that in susceptible grape WT (5.2), but still higher than that in Liuba‐8 (1.6) (Figure 7c).
Figure 7.
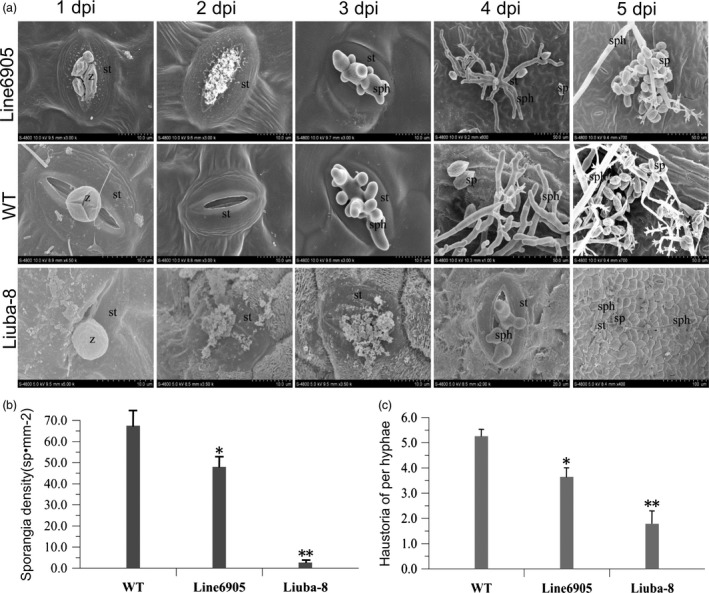
Overexpression of VpPR10.1 limits the development of Plasmopara viticola. The sporangia of P. viticola were attached to the back of leaf discs surface from WT and the overexpressing VpPR10.1 line 6905. P. viticola highly resistant Vitis piasezkii ‘Liuba‐8’ was used as positive control. (a) Scanning electron microscopy observation of VpPR10.1 transgenic line 6905 and WT leaves inoculated with P. viticola from 1 to 5 dpi. Images represented three independent experiments, z, zoospores encystment; st, stomata; sp, sporangia; sph, sporangiophores; hy, hyphae. (b) Average sporangia density of P. viticola from different genotypes at 5 dpi. Error bars indicate SE (n = 3) from three independent biological replicates (*P < 0.05 and **P < 0.01, t‐test). (c) Number of haustoria per hyphae on a total of 20 infected discs from different grapes at 48 hpi. Error bars indicate SE from three independent biological replicates (*P < 0.05 and **P < 0.01, t‐test).
To determine whether the observed VpPR10.1‐mediated resistance to P. viticola was related to ROS accumulation, H2O2 production was evaluated using DAB staining. In VpPR10.1 transgenic line 6905, distinct DAB deposits appeared at 3 dpi (Figure 8a). By 4 dpi, altered hyphae could be seen in the most strongly stained zones. VpPR10.1 overexpression grapevine line 6905 showed enhanced ROS production after P. viticola inoculation. These results suggest in VpPR10.1 transgenic line, P. viticola development was restricted by ROS accumulation. At the same time, cytochrome c was detected in the cytoplasm as early as 2 dpi in line 6905, while in nontransgenic grape, it was detected after 4 dpi (Figure 8b). Taken together, these results suggest that VpPR10.1 and VpVDAC3 responded to the invasion of P. viticola. We also recorded the development of actin of P. viticola (Schmidlin et al., 2008) by RT‐PCR, and P. viticola in the VpPR10.1 transgenic line 6905 was repressed compared to WT (Figure 8c).We also evaluated the expression of ROS‐related genes by RT‐PCR, as shown in Figure 8d, in the VpPR10.1 transgenic line, inoculation with P. viticola induced 14‐fold higher expression of VvAOX compared to the WT(Figure 8d), and at the same time, the transcriptional level of VvAPX was also increased (Figure 8e). Expression of Vvmetacaspase5 was also strongly induced by P. viticola in line 6905 (Figure 8f), we overexpressed Vvmetacaspase4,5,6 fused with GFP in N. benthamiana, and each resulted in a different level of ROS accumulation and cell death (Figure S2). From these results, we infer the overexpression of VpPR10.1 in susceptible grapevines increased the original resistance to P. viticola.
Figure 8.
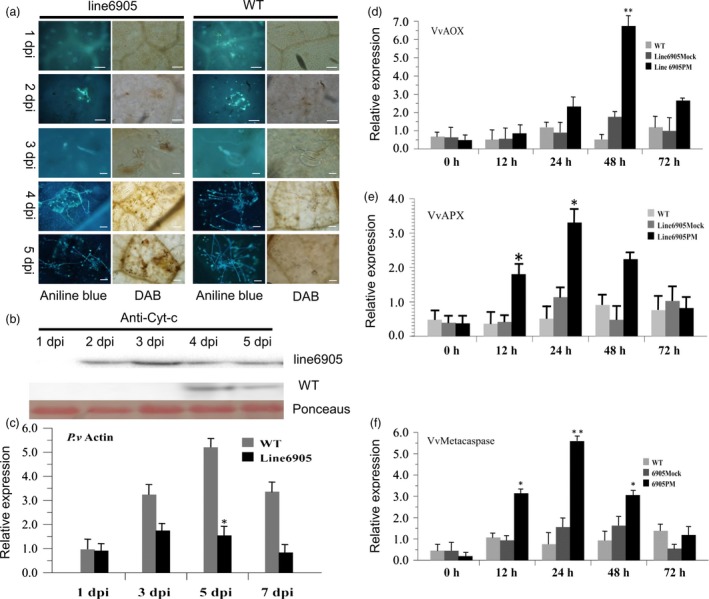
VpPR10.1 overexpression enhances H2O2 accumulation during infection. (a) Fluorescence micrographs of oomycete development were stained with aniline blue (left); H2O2 accumulation was stained with DAB. Bar = 50 μm. (b) Western blot for detecting the cytochrome c in cytoplasm after inoculation with Plasmopara viticola for 1–5 dpi in transgenic line 6905; ponceaus indicates control loading. (c) RT‐PCR was carried out to evaluate the actin gene of P. viticola in VpPR10.1 transgenic line 6905 and wild‐type Vitis vinifera ‘Thompson Seedless’. Vitis 18s rRNA was used as reference gene (*P < 0.05) (t‐test). (d, e) The expression level of ROS‐related gene VvAOX, VvAPX in transgenic line 6905 and WT after inoculation with P. viticola at indicate times. Each value is the mean ± SE of three independent biological determinations. Asterisks indicate significant difference from mock control, *P < 0.05; **P < 0.01 (t‐test). (f) The expression level of apoptosis‐related gene (type II metacaspase) Vvmetacaspase5 (Zhang et al., 2013) in transgenic line 6905 and WT after inoculation with P. viticola at indicated times. Asterisks indicate significant difference from the mock control, *P < 0.05, **P < 0.01(t‐test).
Discussion
Cultivated grape originates from the European grape (Vitis vinifera), the disease resistance properties available within V. vinifera are limited, and their disease susceptibility is not really compatible with its dominant position as major fruit crop. Great harm from downy mildew caused by P. viticola seriously affects both the yield and the quality of grapes (Brewer and Milgroom, 2010). In this study, we found that the VpPR10.1 transgenic line showed increased resistance to P. viticola (Figures 7 and 8). In previous research, VpPR10.1 inhibited the development of ascomycete Alternaria alternate, and when recombinant VpPR10.1 or the mutations are incubated with tobacco BY2s suspension cells, resulting in degrees of PCD and DNA degradation (Xu et al., 2014), it is possible that VpPR10.1 mediates antifungal activity in grape host cells.
PR10 contributes to resistance in pepper through the interaction with LRR1, and the cytoplasmic localization of PR10/LRR1 is responsible for the induction of cell death (Choi et al., 2012). In our research, VpPR10.1 together with mitochondrial located VDAC (Figure 2) enables defence responses in grapevine (Figures 7 and 8). PR10s may participate in different pathways to regulate resistance. Decoding the activation of defence responses is key step to understanding how plant immunity systems work (Postma et al., 2016). Reactive oxygen species are important transducers of induced immune responses even cell death (Jaleel et al., 2009; Miller et al., 2010; Van Breusegem et al., 2001). In our study, VpPR10.1 positively regulates VpVDAC3‐mediated cell death along with the generation of ROS (Figures 3 and 5). PR10 may take part in the defence responses linked with cell death.
The subcellular location VpVDAC3 in N. benthamiana protoplasts suggests that it is located in mitochondria (Figure 2), which function in transporting through the inner and outer membranes (Colombini, 2004;) . Predicatively located in the outer membrane of mitochondria, VDAC takes part in the transfer of molecules and macromolecules through the mitochondrial membranes (Benz, 1994; Colombini, 1979). Evidences suggest that VDAC is a unique protein participating in mitochondria‐mediated cell death (Abu‐Hamad et al., 2006; Schein et al., 1976). Studies also show clearly that apoptosis induced by VDAC exists in yeast, rice, fish and murine (Ghosh et al., 2007; Godbole et al., 2003; Zaid et al., 2005). We found that overexpression of VpVDAC3 in N. benthamiana triggered cell death (Figure 5a), and that metacaspase‐like gene was induced expression (Figure 5d). Plant metacaspase plays important roles in PCD, and the underlying mechanisms remain obscure (Cambra et al., 2010; Hoeberichts et al., 2003).
Voltage‐dependent anion channel is important in receiving signals and activating the cell death‐like responses, and release of cytochrome c in mitochondria is one of the key steps in apoptosis (Pinto et al., 2010). Previous study showed that hexokinase promotes voltage‐dependent anion channel closure and prevents mitochondria‐mediated apoptotic cell death (Azoulay‐Zohar et al., 2004), VIGS of mitochondria‐associated hexokinase Hxk1 of N. benthamiana resulted in PCD and cytochrome c release, and caspase‐9‐like and caspase‐3‐like proteolytic showed strongly activities (Kim et al., 2006). Our research shows the co‐expression of VpPR10.1 and VpVDAC3 in N. benthamiana also results in the release of cytochrome c (Figure 5c).
Many earlier studies focused on the overexpression of VDAC in plant disease resistance as well as abiotic stress (Al Bitar et al., 2003; Desai et al., 2006; Lee et al., 2009; Tateda et al., 2009; Wandrey et al., 2004; Yang et al., 2011; Zhang et al., 2015). Here, we find that VpPR10.1 interacts with VpVDAC3 (Figure 1), and VpVDAC3 was induced by P. viticola in grapevine, the results enriched the functional research of VDAC. In previous studies, VDAC has been shown to enhance mitochondria‐triggered cell death in Arabidopsis and tobacco (Shoshan‐Barmatz et al., 2010). In this study, we confirm the interactions between VpPR10.1 and VpVDAC3 to activate defence responses in grape. In contrast with previous findings, VpPR10.1 enhances VpVDAC3 triggered resistance as a positive modulator in grape. The transient co‐expression of VpVDAC3 with VpPR10.1 intensifies the cell death response (Figure 5a). Although showed no apparent phenotype, transgenic grapevine line 6905 exhibited a higher resistance to P. viticola (Figure 6, 7 and 8). The transgenic line of VpPR10.1 also presents disease‐associated cell death upon inoculation with P. viticola, which is accompanied by ROS generation and cytochrome c release (Figure 8a and 8b).
During plant‐pathogen interactions, the production of ROS plays a critical role in HR‐like defence responses (Torres et al., 2006). The most successful ROS studied are NADPH oxidases. As one of NADPH‐related genes in plants, Rbohs plays important roles in plant stress responses (Kaur et al., 2014). Besides NADPH, mitochondria also provide complementary sources of ROS and the reaction to oxidative stress (Xie and Chen, 1999).The mitochondrial pathway functions together with the generation of ROS lead to oxidative damage to whole cell (Song et al., 2009).There are other features of ROS, for example H2O2, which directly kills pathogen, inducing plant toxins (Doke, 1983), causing hypersensitive response (Delledonne et al., 2001) and operating resistance (Dat et al., 1998).
Many studies show overmuch ROS result in cell death may act as a defence role involving in plant stress responses (Torres et al., 2006). For pathology, there is much evidence that ROS act as signal transducers (Chen et al., 1993). Even so, the route of signal transduction by ROS still remains unclear. Vanacker et al. (2000) found that in disease resistant leaves, areas infected with hyphae suffer rapid accumulations of H2O2 and the formation of mastoid deposits, which are not found in susceptible varieties.
Voltage‐dependent anion channels involved in H2O2 accumulation has been confirmed, and during pathogen infections, H2O2 was accumulated in N. benthamiana overexpressed VDAC and was reduced in N. benthamiana lack VDAC (Tateda et al., 2009). AtVDAC3 from A. thaliana was shown to be involved in ROS generation (Zhang et al., 2015). Nevertheless, the mechanism of ROS production via VDACs is undefined, in our attempt to confirm the possible ROS induction of VpVDAC3, we transiently expressed VpVDAC3 in N. benthamiana. The overexpression of VpVDAC3 in N. benthamiana caused ROS accumulation and increased related gene expression thus leading to cell death (Figures 3, 5 and 8). Furthermore, when we exposed P. viticola to VpPR10.1 transgenic grapes, ROS were generated (Figure 8a) and VpPR10.1 and VpVDAC3 mediate the resistance to P. viticola, which involved in the burst of ROS together with cytochrome c release (Figure 8b).
VDAC oligomeric structure is still not clear, and the N‐terminal oligomerization of VDAC is greatly enhanced upon apoptosis and directly associated with cytochrome c release (Keinan et al., 2010; Shoshan‐Barmatz et al., 2010). In this research, we tested the interaction between different truncated VpVDAC3 fragments and VpPR10.1, and the result indicated that N‐terminal and C‐terminal VpVDAC3 showed interaction with VpPR10.1 (Figure S3). As N‐terminus is the oligomeric site of VDAC in animal, we suggest that VpPR10.1 may have the same function to promote the ability of VpVDAC to enhance the opening of MPTP, finally induce cytochrome c release and subsequent cell death.
VDACs may affect cellular redox metabolism by producing metabolites, but the precise mechanisms of VDACs in fungal resistance remain unclear (Homblé et al., 2012; Kusano et al., 2009; Takahashi and Tateda, 2013; Tikunov et al., 2010; Zhang et al., 2015). Many studies focus on the cell death induced by cytochrome c release and caspase activation (Kluck et al., 1997; Li et al., 1997; Liu et al., 1996; Yang et al., 1997), and the release of cytochrome c is especially responsible for the mitochondria‐mediated apoptotic pathway. We successfully identified the interaction between VpPR10.1 and VpVDAC3 (Figure 1). The overexpression of VpPR10.1 from resistant grape V. pseudoreticulata ‘Baihe‐35‐1’ in WT (nontransgenic Thompson Seedless) enhanced the resistance of the transgenic plant by causing ROS accumulations following downy mildews infection (Figure 8). Combining the above findings, we propose a model for the synergistic action of VpPR10.1 and VpVDAC3 in grapevine (Figure S4). Once pathogen infection occurs, pathogen‐related proteins of the host cell along with VpVDAC3 induce defence reactions, resulting in release of cytochrome c via the VDAC channel and the accumulation of ROS. Nevertheless, the accumulation of ROS may cause oxidative damage to mitochondrial and cellular proteins and the cell death‐like resistance response finally came up. Together these are responsible for destroying the infected cell, as well as promoting the HR‐like cell death and the defence action. These findings facilitate molecular breeding efforts to improve downy mildew resistance in grapevine.
In the previous study, the pepper pathogenesis‐related protein 10 (PR10) was identified as a leucine‐rich repeat protein 1 (LRR1) interacting partner, and the LRR1/PR10 complex formed in the cytoplasm is responsible for cell death‐mediated defence (Choi et al., 2012). In this study, we identify VpVDAC3 as interaction partner of VpPR10.1 to regulate defence response to P. viticola (Figure 5 and 8). The VpPR10.1/VpVDAC3 complex is also responsible for cell death. These findings come up with the possibility that PR10.1 may be involved in cell death‐related immunity through forming different complexes with different pathways in plants. These results enrich our understanding of the function of PR10. However, much more detailed study is needed for the molecular mechanisms underlying how VpPR10.1 biochemically affects VpVDAC3 in the future.
Experimental procedures
Plant materials and growth conditions
Grapevine and N. benthamiana were planted in an illuminated incubator with a day/night cycle of 25 °C 14‐h light/20 °C 10‐h dark. The transformation of grapevine was referred to the description previously published (Zhou et al., 2014).
Yeast two‐hybrid assay
The Y2H Gold system was conducted under instructions of the manufacturer's (Clontech, Dalian, China, http://www.clontech.com/. The P. viticola induced prey cDNA library of V. piasezkii ‘Liuba‐8’ that one of Chinese wild grapevine was constructed according to the user's manual of Make Your Own ‘Mate & Plate TM’ Library System (Clontech). VpVDAC3 and VpPR10.1 were cloned and recombined into the pGADT7 and pGBKT7 to constructed BD/VpPR10.1, AD/VpVDAC3, BD/VpVDAC3 and AD/VpPR10.1. Logically, the conjugated constructs were transformed into Y2H Gold according to the Yeastmaker™ Yeast Transformation System 2 User Manual. The positive strains were selected on SD/‐Leu/‐Trp/‐Ade/‐His medium containing 50 μg/mL X‐α‐Gal and 150 ng/mL aureobasidin A (AbA). Combinations of (AD/T) with BD/p53 and BD/Lam were, respectively, served as positive and negative controls.
BIFC analysis
For the BIFC constructs, both the coding regions of genes were cloned into the plasmid pUC‐SPYNE to build VPR10.1‐YFPNE and VpVDAC3‐YFPNE and into pUC‐SPYCE to form VPR10.1‐YFPCE and VpVDAC3‐YFPCE, resulting in pSPYNE‐VpPR10.1, VpVDAC3‐pSPYCE and vice versa as described by Walter (Walter et al., 2004). Corresponding BIFC plasmids and negative controls were co‐expressed in N. benthamiana protoplasts. Twenty hours after transformation, the protoplasts were visualized and captured using an Olympus FV1000 LCS confocal laser scanning microscope (Olympus).
Co‐IP
For CO‐IP, VpPR10.1 and VpVDAC3 were cloned into p35S, GFP (VpPR10.1‐GFP) and p35S, 3Flag (3Flag‐VpVDAC3) vector. Agrobacterium strain GV3101 containing the constructs was infiltrated into N. benthamiana leaves. 0.7 g flash‐frozen homogenized N. benthamiana leaf tissue was suspended in 1.5 mL IP buffer (Choi et al., 2012), followed by incubation with monoclonal anti‐GFP (ABclonal) overnight at 4 °C with gentle shaking. The immune complexes were incubated with prewashed monoclonal anti‐GFP agarose (Sigma‐Aldrich) for 5–6 h at 4 °C and were centrifuged and washed with immunoprecipitation buffer (Choi et al., 2012). Resuspend proteins were separated by 10% SDS‐PAGE. Anti‐GFP mouse monoclonal antibody (ABclonal) and flag‐specific monoclonal antibodies (Santa Cruz) were both probed at 1,2000 dilution to detect goal proteins. IPKine™ HRP Goat Anti‐Mouse IgG HCS (http,//www.abbkine.com/) was used as a secondary antibody.
Pull‐down
Recombinant proteins GST‐VpPR10.1 and MBP‐VpVDAC3 were purified with glutathione resin (Novagen, Germany) and amylose resin (NEB, England) according to methods described previously (Xu et al., 2014). Proteins GST‐VpPR10.1 and MBP‐VpVDAC3 were individually dialysed in protein dialysis buffer [50 mm Tris‐HCl (pH7.4), 200 mm NaCl, 1 mm EDTA, 1 mm DTT, 50% glycerol], and purified bait protein MBP‐VpVDAC3 (MBP as control) was added to 25 μL amylose resin (beads) and incubated in ice for 1 h. Then, prey protein GST‐VpPR10.1 (GST as control) was loaded onto the beads. After the pull‐down assay for 5 h at 4 °C, the beads were precipitated and washed with buffer [50 mm Tris‐HCl (pH7.4), 200 mm NaCl, 1 mm EDTA, 0.05% Nonidet P‐40]. Proteins detained on the beads were released and denatured by boiling the bead pellet with 5 × SDS‐PAGE loading buffer, then detected by GST and MBP antibody, respectively.
Plasmopara viticola infection and scanning electron microscopy observation
Plasmopara viticola was prepared as a sporangial suspension. The inoculation was according to the description of Liu (Liu et al., 2015), and leaf pieces were collected from 1 dpi to 5 dpi and immediately fixed with 4% (v/v) glutaraldehyde in 0.2 m pH 6.8 phosphate buffer saline. After this, they were dehydrated by aqueous ethanol series (30%, 50%, 70%, 80%, 90% and 100%) for 30 min each. The samples were observed with Hitachi S‐4800 FE‐SEM. To evaluate the susceptibility, the number of haustorias of each hypha in three different genotype was quantified at 48 hpi (hours postinoculation) and the sporangia density of each mm2 was counted and calculated at 5 dpi.
Histochemical staining and microscopy
DAB staining was carried out as previously described (Daudi and O'Brien, 2012). Aniline blue staining was performed according to Liu et al. (2015), and Olympus BX‐51 was used for microscopic observation of the P. viticola in grapevine.
Agrobacterium‐mediated transient expression
VpPR10.1 and VpVDAC3 were cloned into pCambia2300‐GFP and 3Flag ‐pCambia1307, respectively. Agrobacterium strain GV3101 carrying the constructs were infiltrated into fully expanded N. benthamiana leaves, and Bax was also transiently expressed as comparison (Du and Hwang, 2011; Lacomme and Santa Cruz, 1999).
Protein extraction and western blot
The infiltrated leaves were immediately harvested in liquid nitrogen and ground to homogeneous powder. Equal volumes of PPEB buffer (1.0 m Tris‐HCl, pH 8.0, 10% SDS, 50% glycerol, 5% mercaptoethanol) were used to extract the denatured proteins in leaves of N. benthamiana or grapevine. 700–800 mg of leaf powder was used. Followed boiling for 10 min, samples were centrifuged at 4 °C for 20 min at 14 000 g. The BCA Protein Assay Kit (CWbiotech, Beijing, China, CW0014) was used to quantify the protein. The supernatant was mixed with 5 × SDS buffer and then re‐boiled for 5 min followed fractionation before 10% SDS‐PAGE. Images were captured using ChemiDoc™ XRS+ Software. For the preparation of VpVDAC3 antibody, PET32a‐VpVDAC3 construct was transformed into E. coli BL21 strain for protein induction. The purified fusion protein was used to immune a New Zealand white rabbit to produce antibody against VpVDAC3 protein in Proteintech Group, Inc.
Grapevine transformation
VpPR10.1 was constructed into the pCambia2300‐GFP vector and was transformed into Agrobacterium strain GV3101. Then, VpPR10.1 was transformed into somatic embryos of V. vinifera. cv. ‘Thompson Seedless’ system established previously (Zhou et al., 2014).
Nicotiana benthamiana protoplast separation and gene localization
Protoplasts were isolated from healthy, nonsenescent, fully expanded leaves of N. benthamiana as described by Yoo et al. (2007). For the subcellular localization assay, fluorescence was visualized using an FV1000MPE Olympus laser confocal microscope.
Mitochondrial fractionation
The mitochondrial fractions were processed following the instructions of Plant Mitochondria Purification Kit (TIANDZ, Hangzhou, China), 4.5 g fresh N. benthamiana leaves was used, and mitochondria were collected in the precipitate. Finally, the mitochondria were re‐suspended in mitochondrial store buffer and stored in a −80 °C ultra‐low temperature freezer.
Detection of reactive oxygen species and mitochondria depolarization
Hydrogen peroxide was stained with DAB, and ROS assay kits (Beyotime) were used to determine the ROS levels. After infiltration of individual genes for 60 h in N. benthamiana, protoplasts were isolated. After incubated with DCFH‐DA at 37 °C for 20 min at a concentration of 2 μm, protoplast samples pretreated with DPI act as control. For JC‐1 dye, protoplasts were incubated with 10 mg/mL JC‐1 for 30 mins at 25 °C. The fluorescence of DCF and JC‐1 was visualized and captured using a Carl Zeiss LSM 510 confocal, and 20 N. benthamiana protoplasts were captured.
RT‐PCR analysis
RNA was extracted using the plant RNA kit (OMEGA R6827‐01). For RT‐PCR, the SYBR Premix ExTM TaqII kit (Takara, Dalian,China, http//www.takara.com.cn/ was used, and RT‐PCR was carried out in an iCycler iQ 5 thermal cycler (Bio‐Rad), using a two‐step protocol described by Han et al. (2016). Pv‐actin (He et al., 2013) was evaluated by RT‐PCR between WT and VpPR10.1 transgenic line 6905 after inoculation with P. viticola. Grapevine 18s rRNA was used as internal reference (*P < 0.05, t‐test).
Supporting information
Figure S1 Quantification of Conductivity in Different Genes.
Figure S2 Transient Expression of Vvmetacaspases in Nicotiana benthamiana induce ROS accumulation and cell death. Phenotypic and physiological analyses of following (A)Upper two lines, DAB stain of Bax, Vvmetacaspase4, 5, 6 and GFP were all injected in one N. Benthamiana leave in 1 dpi, 3 dpi, 5 dpi and 7 dpi. The bluish colour of DAB staining represents the accumulated H2O2. Bottom two lines, trypan blue stain of Bax, Vvmetacaspase4, 5, 6 and GFP injected in one N. Benthamiana leave. (B) Checking of Vvmetacaspase4, 5, 6 and GFP protein by Western blotting in Agro‐infiltration(od = 0.75) N. benthamiana leaves. Ponceaus staining stand for control loading.
Figure S3 Truncate VpVDAC3 Interaction with VpPR10.1.
Figure S4 Proposed Model of VpPR10.1 Mediated Resistance Response by Interaction with VpVDAC3.
Table S1 The summary of positive clones obtained from Chinese wild grape V. piasezkii Liuba‐8 after P. viticola infection cDNA library using VpPR10.1 as bait.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 31272125, 31471844) and National Science and Technology Major Project. The authors declare no conflict of interest.
References
- Abu‐Hamad, S. , Sivan, S. and Shoshan‐Barmatz, V. (2006) The expression level of the voltage‐dependent anion channel controls life and death of the cell. Proc. Natl Acad. Sci. USA, 103, 5787–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Bitar, F. , Roosens, N. , Smeyers, M. , Vauterin, M. , Van Boxtel, J. , Jacobs, M. and Homblé, F. (2003) Sequence analysis, transcriptional and posttranscriptional regulation of the rice vdac family. Biochim. Biophys. Acta. 1625, 43–51. [DOI] [PubMed] [Google Scholar]
- Azoulay‐Zohar, H. , Israelson, A. , Salah, A.H. and Shoshan‐Barmatz, V. (2004) In self‐defence, hexokinase promotes voltage‐dependent anion channel closure and prevents mitochondria‐mediated apoptotic cell death. Bio Protoc. 377, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz, R. (1994) Permeation of hydrophilic solutes through mitochondrial outer membranes, review on mitochondrial porins. Biochem. Biophys. Acta. 1197, 167–196. [DOI] [PubMed] [Google Scholar]
- Bittel, P. and Robatzek, S. (2007) Microbe‐associated molecular patterns (MAMPs) probe plant immunity. Curr. Opin. Plant Biol. 10, 335–341. [DOI] [PubMed] [Google Scholar]
- Bracha, D.K. , Shichrur, K. , Katz, A. , Oliva, M. , Angelovici, R. , Yalovsky, S. and Ohad, N. . (2004) Detection of protein–protein interactions in plants using bimolecular fluorescence complementation. Plant J. 40, 419–427. [DOI] [PubMed] [Google Scholar]
- Breda, C. , Sallaud, C. , Turk, J. , Buffard, D. , de Kozak, I. , Esnault, R. and Kondorosi, A. (1996) Defense reaction in Medicago sativa, a gene encoding a class 10 pr protein is expressed in vascular bundles. Mol. Plant Microbe Interact. 9, 713–719. [DOI] [PubMed] [Google Scholar]
- Brewer, M.T. and Milgroom, M.G. (2010) Phylogeography and population structure of the grape powdery mildew fungus, Erysiphe necator, from diverse Vitis species. BMC Evol. Biol. 10, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambra, I. , Garcia, F.J. and Martinez, M. (2010) Clan CD of cysteine peptidases as an example of evolutionary divergences in related protein families across plant clades. Gene, 449, 59–69. [DOI] [PubMed] [Google Scholar]
- Carlisle, D.L. , Pritchard, D.E. , Singh, J. , Owens, B.M. , Blankenship, L.J. , Orenstein, J.M. and Patierno, S.R. (2000) Apoptosis and P53 induction in human lung fibroblasts exposed to chromium (VI), effect of ascorbate and tocopherol. Toxicol. Sci. 55, 60–68. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Silva, H. and Klessig, D.F. (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science, 262, 1883–1885. [DOI] [PubMed] [Google Scholar]
- Choi, J. , Huh, S.U. , Kojima, M. , Sakakibara, H. , Paek, K.‐H. and Hwang, I. (2010) The cytokinin‐activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1‐dependent salicylic acid signaling in Arabidopsis . Dev. Cell, 19, 284–295. [DOI] [PubMed] [Google Scholar]
- Choi, D.S. , Hwang, I.S. and Hwang, B.K. (2012) Requirement of the cytosolic interaction between pathogenesis‐related protein 10 and leucine‐rich repeat protein 1 for cell death and defense signaling in pepper. Plant Cell, 24, 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu, M.L. and Aw, T.Y. (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, N.S. , Vercammen, D. , Smidler, A. , Clover, C. , Van, B.F. , Dangl, J.L. and Petra, E. (2010) Arabidopsis type i metacaspases control cell death. Science, 330, 1393. [DOI] [PubMed] [Google Scholar]
- Colombini, M. (1979) A candidate for the permeability pathway of the outer mitochondrial membrane. Nature, 279, 643–645. [DOI] [PubMed] [Google Scholar]
- Colombini, M. (2004) VDAC, The channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 256, 107–115. [DOI] [PubMed] [Google Scholar]
- Cossarizza, A. , Baccaranicontri, M. , Kalashnikova, G. and Franceschi, C. (1993) A new method for the cytofluorometric analysis of mitochondrial membrane potential using the J‐aggregate forming lipophilic cation 5, 5′, 6, 6′‐tetrachloro‐1, 1′, 3, 3′‐tetraethylbenzimidazolo‐carbocyanine iodide (JC‐1). Biochem. Biophys. Res. Comm. 197, 40–45. [DOI] [PubMed] [Google Scholar]
- Dat, J.F. , Foyer, C.H. and Scott, I.M. (1998) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 118, 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi, A. and O'Brien, J.A. (2012) Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio‐Protocol. 2, e263. [PMC free article] [PubMed] [Google Scholar]
- Delledonne, M. , Zeier, J. , Marocco, A. and Lamb, C. (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl Acad. Sci. 98, 13454–13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, M. , Mishra, R. , Verma, D. , Nair, S. , Sopory, S. and Reddy, M. (2006) Structural and functional analysis of a salt stress inducible gene encoding voltage dependent anion channel (VDAC) from pearl millet (Pennisetum glaucum). Plant Physiol. Biochem. 44, 483–493. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity, towards an integrated view of plant‐pathogen interactions. Nat. Rev. Genet. 11, 539. [DOI] [PubMed] [Google Scholar]
- Doke, N. (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23, 345–357. [Google Scholar]
- Du, S.C. and Hwang, B.K. (2011) Proteomics and functional analyses of pepper abscisic acid‐responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell, 23, 823–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibach, R. , Zyprian, E. , Töpfer, R. , Peterlunger, E. , Gaspero, G.D. and Cipriani, G. (2009) The use of molecular markers for pyramidizing resistance genes in grapevine breeding. Vitis, 46, 120–125. [Google Scholar]
- Esmaeili, M.A. , Abagheri‐Mahabadi, N. , Hashempour, H. , Farhadpour, M. , Gruber, C.W. and Ghassempour, A. (2016) Viola plant cyclotide vigno 5 induces mitochondria‐mediated apoptosis via cytochrome c release and caspases activation in cervical cancer cells. Fitoterapia, 109, 162. [DOI] [PubMed] [Google Scholar]
- Gessler, C. , Pertot, I. and Perazzolli, M. (2011) Plasmopara viticola, a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44. [Google Scholar]
- Ghosh, T. , Pandey, N. , Maitra, A. , Brahmachari, S.K. and Pillai, B. (2007) A role for voltage‐dependent anion channel vdac1 in polyglutamine‐mediated neuronal cell death. PLoS ONE, 2, 2741–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindro, K. , Pezet, R. and Viret, O. (2003) Histological study of the responses of two Vitis vinifera cultivars (resistant and susceptible) to Plasmopara viticola infections. Plant Physiol. Biochem. 41, 846–853. [Google Scholar]
- Godbole, A. , Varghese, J. , Sarin, A. and Mathew, M.K. (2003) VDAC is a conserved element of death pathways in plant and animal systems. Biochem. Biophys. Acta. 1642, 87–96. [DOI] [PubMed] [Google Scholar]
- Godbole, A. , Dubey, A.K. , Reddy, P.S. , Udayakumar, M. and Mathew, K.M. (2013) Mitochondrial VDAC and hexokinase together modulate plant programmed cell death. Protoplasma, 250, 875–884. [DOI] [PubMed] [Google Scholar]
- Han, L. , Kai, W. , Hui, M. , Xiang, G. , Li, Z. , Wang, Y. , Liu, G. et al (2016) Identification and Characterization of Erysiphe necator‐Responsive MicroRNAs in Chinese Wild Vitis pseudoreticulata by High‐Throughput Sequencing. Front Plant Sci. 7, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, L. , Goodwin, P.H. and Hsiang, T. (2007) Expression of a metacaspase gene of Nicotiana benthamiana after inoculation with colletotrichum destructivum or pseudomonas syringae pv. tomato, and the effect of silencing the gene on the host response. Plant Cell Rep. 26, 1879. [DOI] [PubMed] [Google Scholar]
- Hashimoto, M. , Kisseleva, L. , Sawa, S. , Furukawa, T. , Komatsu, S. and Koshiba, T. (2004) A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol. 45, 550–559. [DOI] [PubMed] [Google Scholar]
- He, R. , Drury, G.E. , Rotari, V.I. , Gordon, A. , Willer, M. and Farzaneh, T. (2008) Metacaspase‐8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis . J. Biol. Chem. 283, 774. [DOI] [PubMed] [Google Scholar]
- He, M. , Xu, Y. , Cao, J. , Zhu, Z. , Jiao, Y. , Wang, Y. , Guan, X. et al (2013) Subcellular localization and functional analyses of a PR10 protein gene from Vitis pseudoreticulata in response to Plasmopara viticola infection. Protoplasma, 250, 129–140. [DOI] [PubMed] [Google Scholar]
- Hoeberichts, F.A. , Have, A.T. and Woltering, E.J. (2003) A tomato metacaspase gene is upregulated during programmed cell death in, botrytis cinerea ‐infected leaves. Planta, 217, 517–522. [DOI] [PubMed] [Google Scholar]
- Homblé, F. , Krammer, E.‐M. and Prévost, M. (2012) Plant VDAC, facts and speculations. Biochim. Biophys. Acta. 1818, 1486–1501. [DOI] [PubMed] [Google Scholar]
- Ingram, D.S. (1981) Physiology and biochemistry of host–parasite interaction In The Downy Mildews (Spencer D.M., ed), pp. 143–163. London: Academic Press. [Google Scholar]
- Jaleel, C.A. , Riadh, K. , Gopi, R. , Manivannan, P. , Inès, J. , Al‐Juburi, H.J. , Zhao, C. et al (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 31, 427–436. [Google Scholar]
- Jiao, Y. , Xu, W. , Duan, D. , Wang, Y. and Nick, P. (2016) A stilbene synthase allele from a Chinese wild grapevine confers resistance to powdery mildew by recruiting salicylic acid signalling for efficient defense. J. Exp. Bot. 67, 5841–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kaur, G. , Sharma, A. , Guruprasad, K. and Pati, P.K. (2014) Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol. Adv. 32, 551–563. [DOI] [PubMed] [Google Scholar]
- Keinan, N. , Tyomkin, D. and ShoshanBarmatz, V. (2010) Oligomerization of the mitochondrial protein vdac is coupled to the induction of apoptosis. Mol. Cell. Biol. 30, 5698–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. , Ahn, J.W. , Jin, U.H. , Choi, D. , Paek, K.H. and Pai, H.S. (2003) Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J. Biol. Chem. 278, 19406–19415. [DOI] [PubMed] [Google Scholar]
- Kim, M. , Lim, J.H. , Chang, S.A. , Park, K. , Kim, G.T. , Kim, W.T. and Pai, H.S. (2006) Mitochondria‐associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana . Plant Cell, 18, 2341–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck, R.M. , Bossy‐Wetzel, E. , Green, D.R. and Newmeyer, D.D. (1997) The release of cytochrome c from mitochondria, a primary site for Bcl‐2 regulation of apoptosis. Science, 275, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Koistinen, K.M. , Hassinen, V.H. , Gynther, P.A.M. , Lehesranta, S.J. , Keinänen, S.I. , Kokko, H.I. and Kärenlampi, S.O. (2002) Birch PR‐10c is induced by factors causing oxidative stress but appears not to confer tolerance to these agents. New Phytol. 155, 381–391. [DOI] [PubMed] [Google Scholar]
- Kroemer, G. , Galluzzi, L. and Brenner, C. (2007) Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163. [DOI] [PubMed] [Google Scholar]
- Kusano, T. , Tateda, C. , Berberich, T. and Takahashi, Y. (2009) Voltage dependent anion channels, their roles in plant defense. Plant Cell Rep. 28, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Lacomme, C. and Santa Cruz, S. (1999) Bax‐induced cell death in tobacco is similar to the hypersensitive response. Proc. Natl Acad. Sci. 96, 7956–7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, E. (2004) Controlled cell death, plant survival and development. Nat. Rev. Mol. Cell Biol. 5, 305. [DOI] [PubMed] [Google Scholar]
- Lam, E. and Zhang, Y. (2012) Regulating the reapers, activating metacaspases for programmed cell death. Trends Plant Sci. 17, 487–494. [DOI] [PubMed] [Google Scholar]
- Lebel, S. , Schellenbaum, P. , Walter, B. and Maillot, P. (2010) Characterisation of the Vitis vinifera pr10 multigene family. BMC Plant Biol. 10, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.M. , Hoang, M.H.T. , Han, H.J. , Kim, H.S. , Lee, K. , Kim, K.E. , Kim, D.H. et al (2009) Pathogen inducible voltage‐dependent anion channel (AtVDAC) isoforms are localized to mitochondria membrane in Arabidopsis . Mol. Cells, 27, 321–327. [DOI] [PubMed] [Google Scholar]
- Lei, X. , Chen, Y. , Du, G. , Yu, W. , Wang, X. and Qu, H. (2006) Gossypol induces bax/bak‐independent activation of apoptosis and cytochrome c release via a conformational change in bcl‐2. FASEB J. 20, 2147–2149. [DOI] [PubMed] [Google Scholar]
- Li, P. , Nijhawan, D. , Budihardjo, I. , Srinivasula, S.M. , Ahmad, M. , Alnemri, E.S. and Wang, X. (1997) Cytochrome c and dATP‐dependent formation of Apaf‐1/caspase‐9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Liu, J.J. and Ekramoddoullah, A.K.M. (2006) The family 10 of plant pathogenesis‐related proteins: their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 68, 3–13. [Google Scholar]
- Liu, X. , Kim, C.N. , Yang, J. , Jemmerson, R. and Wang, X. (1996) Induction of apoptotic program in cell‐free extracts, requirement for dATP and cytochrome c. Cell, 86, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Wang, L. , Zhu, J. , Chen, T. , Wang, Y. and Xu, Y. (2015) Histological responses to downy mildew in resistant and susceptible grapevines. Protoplasma, 252, 259–270. [DOI] [PubMed] [Google Scholar]
- Loon, L.C.V. (1997) Induced resistance in plants. & the role of pathogenesis‐related proteins. Eur. J. Plant Pathol. 103, 753–765. [Google Scholar]
- Loon, L.C.V. and Kammen, A.V. (1968) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN”‐I. Phytochemistry, 7, 1727–1735. [Google Scholar]
- Madesh, M. and Hajnóczky, G. (2001) VDAC‐dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 155, 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, J.D. , Hamer, J.E. and Hodges, T.K. (2001) Characterization of a PR10 pathogenesis‐related gene family induced in rice during infection with Magnaporthe grisea . Mol. Plant Microbe Interact. 14, 877–886. [DOI] [PubMed] [Google Scholar]
- Miller, G. , Suzuki, N. , Ciftci‐Yilmaz, S. and Mittler, R. (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant, Cell Environ. 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Pinto, D.V. , Guarino, F. , Guarnera, A. , Messina, A. , Reina, S. , Tomasello, F.M. , Vanessa, P. et al (2010) Characterization of human VDAC isoforms: a peculiar function for VDAC3?. Biochim. Biophys. Acta, 1797, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Postma, J. , Liebr, T.W. , Bi, G. , Evrard, A. , Bye, R.R. , Mbengue, M. , Kuhn, H. et al (2016) Avr4 promotes Cf ‐4 receptor‐like protein association with the BAK1/SERK3 receptor‐like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 210, 627–642. [DOI] [PubMed] [Google Scholar]
- Robert, N. , D'Erfurth, I. , Marmagne, A. , Erhardt, M. , Allot, M. , Boivin, K. , Gissot, L. et al (2012) Voltage‐dependent‐anion‐channels (vdacs) in Arabidopsis have, a dual localization in the cell but show a distinct role, in mitochondria. Plant Mol. Biol. 78, 431–446. [DOI] [PubMed] [Google Scholar]
- Salvioli, S. , Ardizzoni, A. , Franceschi, C. and Cossarizza, A. (1997) JC‐1, but not DiOC6 (3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells, implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 411, 77–82. [DOI] [PubMed] [Google Scholar]
- Sangmin, L. , Myhanhthi, H. , Han, H.J. , Hosoo, K. , Kyunghee, L. , Kyungeun, K. and Woosik, C. (2009) Pathogen inducible voltage‐dependent anion channel (AtVDAC) isoforms are localized to mitochondria membrane in Arabidopsis . Mol. Cells, 27, 321–327. [DOI] [PubMed] [Google Scholar]
- Schein, S.J. , Colombini, M. and Finkelstein, A. (1976) Reconstitution in planar lipid bilayers of a voltage‐dependent anion‐selective channel obtained from paramecium mitochondria. J. Membr. Biol. 30, 99–120. [DOI] [PubMed] [Google Scholar]
- Schmidlin, L. , Poutaraud, A. , Claudel, P. , Mestre, P. , Prado, E. , Santos‐Rosa, M. and Hugueney, P. (2008) A stress‐inducible resveratrol O‐methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 148, 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan‐Barmatz, V. , Pinto, V.D. , Zweckstetter, M. , Raviv, Z. , Keinan, N. and Arbel, N. (2010) VDAC, a multi‐functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 31, 227–285. [DOI] [PubMed] [Google Scholar]
- Simamura, E. , Hirai, K. , Shimada, H. , Koyama, J. , Niwa, Y. and Shimizu, S. (2006) Furanonaphthoquinones cause apoptosis of cancer cells by inducing the production of reactive oxygen species by the mitochondrial voltage‐dependent anion channel. Cancer Biol. Ther. 5, 1523–1529. [DOI] [PubMed] [Google Scholar]
- Son, Y.O. , Hitron, J.A. , Wang, X. , Chang, Q. , Pan, J. , Zhang, Z. , Liu, J. et al (2010) Cr(VI) induces mitochondrial‐mediated and caspase‐dependent apoptosis through reactive oxygen species‐mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 245, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X.S. , Wang, Y.J. , Mao, W.H. , Shi, K. , Zhou, Y.H. , Nogués, S. and Yu, J.Q. (2009) Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiol. Plant. 135, 246–257. [DOI] [PubMed] [Google Scholar]
- Suarez, M.F. , Filonova, L.H. , Smertenko, A. , Savenkov, E.I. , Clapham, D.H. and Von, A.S. (2004) Metacaspase‐dependent programmed cell death is essential for plant embryogenesis. Curr. Biol. 14, 339–340. [DOI] [PubMed] [Google Scholar]
- Swoboda, I. , Jilek, A. , Ferreira, F. , Engel, E. , Hoffmann, K. , Scheiner, O. , Kraft, D. et al (1995) Isoforms of Bet v 1, the major birch pollen allergen, analyzed by liquid chromatography, mass spectrometry, and cDNA cloning. J. Biol. Chem. 270, 2607–2613. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. and Tateda, C. (2013) The functions of voltage‐dependent anion channels in plants. Apoptosis, 18, 917–924. [DOI] [PubMed] [Google Scholar]
- Tateda, C. , Yamashita, K. , Takahashi, F. , Kusano, T. and Takahashi, Y. (2009) Plant voltage‐dependent anion channels are involved in host defense against Pseudomonas cichorii and in Bax‐induced cell death. Plant Cell Rep. 28, 41–51. [DOI] [PubMed] [Google Scholar]
- Tikunov, A. , Johnson, C.B. , Pediaditakis, P. , Markevich, N. , Macdonald, J.M. , Lemasters, J.J. and Holmuhamedov, E. (2010) Closure of VDAC causes oxidative stress and accelerates the Ca(2 + )‐induced mitochondrial permeability transition in rat liver mitochondria. Arch. Biochem. Biophys. 495, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A. , Jones, J.D. and Dangl, J.L. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouvelot, S. , Varnier, A.L. , Allègre, M. , Mercier, L. , Baillieul, F. , Arnould, C. and Pugin, A. (2008) A β‐1,3 Glucan Sulfate Induces Resistance in Grapevine against Plasmopara viticola Through Priming of Defense Responses, Including HR‐like Cell Death. Mol. Plant Microbe Interact. 21, 232–243. [DOI] [PubMed] [Google Scholar]
- Tsiatsiani, L. , Van, B.F. , Gallois, P. , Zavialov, A. , Lam, E. and Bozhkov, P.V. (2011) Metacaspases. Cell Death Differ. 18, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren, A.G. , O'Rourke, K. , Aravind, L. , Pisabarro, M.T. , Seshagiri, S. , Koonin, E.V. and Vishva, M.D. (2000) Identification of paracaspases and metacaspases, two ancient families of caspase‐like proteins, one of which plays a key role in malt lymphoma. Mol. Cell, 6, 961–967. [DOI] [PubMed] [Google Scholar]
- Van Breusegem, F. , Vranová, E. , Dat, J.F. and Inzé, D. (2001) The role of active oxygen species in plant signal transduction. Plant Sci. 161, 405–414. [Google Scholar]
- van Loon, L.C. , Rep, M. and Pieterse, C.M. (2006) Significance of inducible defense‐related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Vanacker, H. , Carver, T.L. and Foyer, C.H. (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper‐sensitive response in the barley‐powdery mildew interaction. Plant Physiol. 123, 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen, D. , Declercq, W.V. , Enabeele, P. and Breusegem, F.V. (2007) Are metacaspases caspases. J. Cell Biol. 179, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi, A. , Tornaghi, R. , Sant, S. , Burruano, S. and Faoro, F. (1999) A cytological and ultrastructural study on the maturation and germination of oospores of Plasmopara viticola, from overwintering vine leaves. Mycol. Res. 103, 193–202. [Google Scholar]
- Walter, M. , Chaban, C. , Schütze, K. , Batistic, O. , Weckermann, K. , Näke, C. , Blazevic1, D. et al (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wan, Y. , Schwaninger, H. , He, P. and Wang, Y. (2007) Comparison of resistance to powdery mildew and downy mildew in Chinese wild grapes. Vitis, 46, 132–136. [Google Scholar]
- Wandrey, M. , Trevaskis, B. , Brewin, N. and Udvardi, M.K. (2004) Molecular and cell biology of a family of voltage‐dependent anion channel porins in Lotus japonicus . Plant Physiol. 134, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Liu, Y. , He, P. , Chen, J. , Lamikanraz, O. and Lu, J. (1995) Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis, 34, 159–164. [Google Scholar]
- Wang, L. , Tsuda, K. , Sato, M. , Cohen, J.D. , Katagiri, F. and Glazebrook, J. (2009) Arabidopsis CaM binding protein CBP60 g contributes to MAMP‐induced SA accumulation and is involved in disease resistance against Pseudomonas syringae . PLoS Pathog. 5, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z. and Chen, Z. (1999) Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol. 120, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T.F. , Zhao, X.C. , Jiao, Y.T. , Wei, J.Y. , Wang, L. and Xu, Y. (2014) A pathogenesis related protein, VpPR‐10.1, from Vitis pseudoreticulata, an insight of its mode of antifungal activity. PLoS ONE, 9, e95102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Liu, X. , Bhalla, K. , Kim, C.N. , Ibrado, A.M. , Cai, J. , Peng, T.I. et al (1997) Prevention of apoptosis by Bcl‐2, release of cytochrome c from mitochondria blocked. Science, 275, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Yang, X.Y. , Chen, Z.W. , Xu, T. , Qu, Z. , Pan, X.D. , Qin, X. , Ren, D.T. et al (2011) Arabidopsis kinesin KP1 specifically interacts with VDAC3, a mitochondrial protein, and regulates respiration during seed germination at low temperature. Plant Cell, 23, 1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S.D. , Cho, Y.‐H. and Sheen, J. (2007) Arabidopsis mesophyll protoplasts, a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Xu, W. , Wang, J. , Wang, L. , Yao, W. , Xu, Y. and Yao, W. (2013a) A core functional region of the RFP1 promoter from Chinese wild grapevine is activated by powdery mildew pathogen and heat stress. Planta, 237, 293–303. [DOI] [PubMed] [Google Scholar]
- Zaid, H. , Abu‐Hamad, S. , Israelson, A. , Nathan, I. and Shoshan‐Barmatz, V. (2005) The voltage‐dependent anion channel‐1 modulates apoptotic cell death. Cell Death Differ. 12, 751–760. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Gong, P. , Wei, R. , Li, S. , Zhang, X. , Yu, Y. and Wang, Y. (2013) The metacaspase gene family of vitis vinifera l.: characterization and differential expression during ovule abortion in stenospermocarpic seedless grapes. Gene 528, 267. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. and Lam, E. (2011) Sheathing the swords of death, post‐translational modulation of plant metacaspases. Plant Signal Behavior. 6, 2051–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Takano, T. , Liu, S. and Zhang, X. (2015) Arabidopsis mitochondrial voltage‐dependent anion channel 3 (AtVDAC3) protein interacts with thioredoxin m2. FEBS Lett. 589, 1207–1213. [DOI] [PubMed] [Google Scholar]
- Zhou, X.J. , Lu, S. , Xu, Y.H. , Wang, J.W. and Chen, X.Y. (2002) A cotton cDNA (GaPR10) encoding a pathogenesis related 10 protein with in vitro ribonuclease activity. Plant Sci. 162, 629–636. [Google Scholar]
- Zhou, Q. , Dai, L. , Cheng, S. and He, J. (2014) A circulatory system useful both for long‐term somatic embryogenesis and genetic transformation in Vitis vinifera L. cv. Thompson seedless. Plant Cell, Tissue Organ Cult. 118, 157–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Quantification of Conductivity in Different Genes.
Figure S2 Transient Expression of Vvmetacaspases in Nicotiana benthamiana induce ROS accumulation and cell death. Phenotypic and physiological analyses of following (A)Upper two lines, DAB stain of Bax, Vvmetacaspase4, 5, 6 and GFP were all injected in one N. Benthamiana leave in 1 dpi, 3 dpi, 5 dpi and 7 dpi. The bluish colour of DAB staining represents the accumulated H2O2. Bottom two lines, trypan blue stain of Bax, Vvmetacaspase4, 5, 6 and GFP injected in one N. Benthamiana leave. (B) Checking of Vvmetacaspase4, 5, 6 and GFP protein by Western blotting in Agro‐infiltration(od = 0.75) N. benthamiana leaves. Ponceaus staining stand for control loading.
Figure S3 Truncate VpVDAC3 Interaction with VpPR10.1.
Figure S4 Proposed Model of VpPR10.1 Mediated Resistance Response by Interaction with VpVDAC3.
Table S1 The summary of positive clones obtained from Chinese wild grape V. piasezkii Liuba‐8 after P. viticola infection cDNA library using VpPR10.1 as bait.


