Abstract
Depression in young adults is a growing concern to public health. This study aims to investigate if depression status in young adults is related to clinical and behavioral cardiovascular disease (CVD) risk factors. Cross-sectional data from a population-based sample of young Danish adults participating in the European Youth Heart Study 2009–2010 were used to examine this (n = 644, mean age 24.3 years 47% male). Measures of cardiorespiratory fitness (CRF), body composition, blood pressure, fasting levels of high and low density lipids (HDL, LDL), insulin, and glucose were obtained. Symptoms of depression were obtained using the Major Depression Inventory scale. Information on sleep disorders; drinking and smoking habits were obtained by questionnaires. Associations of depression with CVD risk factors were examined using logistic and linear regression adjusted for age and sex. Prevalence of mild-moderate-severe depression was 8.7% (5.6% males, 11.5% females). Significant sex differences were found in the association between several CVD risk factors and depression status. Women with depression had higher odds of overweight (OR = 2.2, 95%CI: 1.01–4.0), abdominal adiposity (OR = 2.5, 95%CI: 1.2–4.8), low CRF (OR = 2.5, 95%CI: 1.2–5.5), insulin resistance (OR = 2.3, 95%CI: 1.1–4.6), low HDL (OR = 2.0, 95%CI: 1.01–4.1) and high LDL (OR = 2.2, 95%CI: 1.04–4.5) compared to women without depression. Men with depression had significantly increased odds of having high blood pressure and being smokers compared to men without depression (OR: 3.1, 95%CI: 1.1–8.8 and OR: 3.0, 95%CI: 1.1–8.4, respectively). Depression symptoms in young adulthood were related to unfavorable clinical- and behavioral CVD risk factors, particularly in women.
Keywords: Depression, Cardiovascular disease risk, Behavioral risk, Young adults, EYHS, Gender
Highlights
-
•
Depression, starting already in young adulthood, is a challenge to public health.
-
•
Prevalence of mild-moderate-severe depression is high in young adults.
-
•
Already in young adulthood depression is associated to unfavorable CVD risk factors.
-
•
Associations are predominantly present in young women with depression.
-
•
Young adults with depression might need special attention to prevent later CVD.
1. Introduction
Depression is a common disorder and a growing challenge to public health (Murray et al., 2015). It often starts at young age and it is the principal cause of disability-adjusted life years lost among adolescents and young adults worldwide (Gore et al., 2011). In middle- and older adulthood depression is a major risk factor for development of cardiovascular diseases and type 2 diabetes (Van der Kooy et al., 2007; Pan et al., 2011; Nouwen et al., 2010). However, individuals with cardiovascular disease and diabetes also have increased risk of depression and a bidirectional association has been suggested (Nouwen et al., 2010; Luppino et al., 2010). Also chronic medical conditions, alongside with unhealthy lifestyle behaviors, have been shown to explain the association between depression and cardiovascular disease (CVD) mortality (Atlantis et al., 2012). While these relationships have been studied extensively in middle-aged and older adults it remains less clear if young people with depression are at greater risk of CVD or already show indications of increased clinical and behavioral CVD risk factors. One prospective cohort study (Shah et al., 2011) has shown that clinically diagnosed depression in young adulthood is associated with increased risk of coronary heart disease mortality during 15 years of follow-up. Furthermore, there is some evidence that young people with depression symptoms have poor cardiovascular health (Davidson et al., 2000; Charlson et al., 2013; Dietz and Matthews, 2011) although most studies have reported on single risk factors such as blood pressure (Meng et al., 2012). Also, some studies have suggested that young people with symptoms of depression engage more in high risk health behaviors such as smoking (Dierker et al., 2015) and excessive alcohol consumption (Edwards et al., 2014) and that the relationships between depression and CVD risk could be explained by such factors (Dietz and Matthews, 2011; Katon, 2003; Ont. Health Technol. Assess. Ser., 2013). Yet further studies are needed to clarify the extent to which young adults with depression symptoms have greater prevalence of clinical and behavioral CVD risk factors. In particular, studies in non-selected general population samples are lacking.
The objective of this study therefore was to investigate the associations between self-reported depression status and clinical CVD risk factors and health behaviors in a random population sample of Danish young adults.
2. Materials and methods
2.1. Study design and sampling of participants
This study is based on the Danish cohort of the European Youth Heart Study (EYHS). EYHS is an ongoing, international, population-based prospective observational multicenter study that addresses CVD risk factors in children and adolescents. The participants in the EYHS study was originally sampled in 1997/1998 at age 8–10 or 14–16 years of age attending 3rd or 9th grade in schools located in the municipality of Odense Denmark (the third largest community in Denmark). A detailed description of the sampling procedure and the general methods used in the EYHS can be found elsewhere (Riddoch et al., 2005). In brief the children were randomly sampled through a two-stage cluster sampling from schools stratified according to location (urban, suburban, and rural) and socioeconomic character of its uptake area (high middle, and low). The primary unit was the schools where 28 out of 35 eligible schools were randomly sampled and 25 agreed to participate. A total of 1429 children and adolescents were randomly sampled on these schools (N = 1018, 71,2% participated in 1997/1998). In 2009/10 the original sample were re-invited to participate in a new wave. The present cross sectional study is based on data obtained in 2009/2010 of n = 650 young Danish adults (mean age 24.3 years, range: 20.8–28.8 years). The eligible sample for the current analyses consisted of n = 632 to n = 644 individuals who had complete data depending on the exposure and outcome variables used in the analysis, except for questionnaire on alcohol consumption, where n = 580 had complete data.
Ninety-six percentage of the population at baseline were white (Caucasian).
The Regional Scientific Ethical Committee for Southern Denmark (ID S-20090100) approved the study and all participants provided written informed content.
2.2. Assessment of symptoms of depression
Symptoms of depression were assessed using the Major Depression Inventory (MDI) scale (Bech et al., 2001). The MDI scale consists of ten ICD-10 symptoms of depression and is based on the universe of symptoms in Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV) major depression symptoms. The 10 symptoms are based on 12 items (no. 8 and no. 10 are twofold) on a six-point Likert scale (0 to 5) and each individual item measures how much time the symptoms have been present during the past 14 days. The MDI questionnaire is self-reported and the individual is asked: The following questions ask about how have you been feeling over the last two weeks. Please put at tick in the box which is closest to how you have been feeling. How much of the time have you: 1) felt low in spirits or sad? 2) lost interest in daily activities? 3) felt lacking of energy and strength? 4) felt less self-confident? 5) had a bad conscience or feeling guilt? 6) felt life wasn't worth living? 7) had difficulty in concentrating, e.g. when reading the newspaper or watching television? 8a) felt very restless? 8b) felt subdued? 9) had trouble sleeping at night? 10a) suffered from reduced appetite? 10b) suffered from increased appetite? Zero on the scale indicates that the symptom has not been present at all and five that the symptom has been present all of the time (Bech et al., 2001). The questionnaire is available in different languages from https://www.psykiatri-regionh.dk/CCMH/Rating-scales-og-spoergeskemaer/Sider/default.aspx.
We scored and classified individuals according to published guidelines (Bech et al., 2001): 1) mild, moderate or severe depression (MDI ≥ 20) or 2) DSM-IV major depression. The MDI scale has been reported to have a high degree of agreement with the Schedule for Clinical Assessment in Neuropsychiatry tool and the Hamilton Depression Scale (Bech et al., 2001; Olsen et al., 2003). Due to a relatively low number of individuals with symptoms of depression we were unable to further sub-divide participants into mild, moderate, and severe depression for analyses. There for prevalence of Major Depression according to DSM-IV is only presented in Table 1 and not used in further analysis.
Table 1.
Depression score and prevalence rates of mild, moderate, severe and major depression.
| Data obtained 2009–2010 |
Mean MDI score (SD) | Prevalence rates (%) (95%CI) |
|
|---|---|---|---|
| Any significant depression (MDI ≥ 20) | DSM-IV major depression | ||
| Total population (n = 644) | 8.6 (7.4) | 8.7 (6.7–11.1) | 4.2 (2.9–6.1) |
| Men (n = 304) | 7.4 (6.6)a | 5.6 (3.4–8.8)a | 2.0 (0.9–4.3)a |
| Women (n = 340) | 9.7 (8.0)a | 11.5 (8.5–15.3)a | 6.2 (4.1–9.3)a |
| 21-year olds (n = 366) | 9.1 (7.5) | 9.0 (6.5–12.4) | 4.9 (3.1–7.7) |
| 27-year olds (n = 278) | 8.0 (7.2) | 8.3 (5.6–12.2) | 3.2 (1.7–6.1) |
MDI = Major Depression Inventory, DSM-IV = Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition.
Significant difference between gender.
2.3. Assessment of other covariates
Body height and body weight were assessed using standard anthropometric procedures by trained research staff, and body mass index (BMI) was calculated.
Waist circumference was measured twice with a metal anthropometric tape midway between the lower rib margin and the iliac crest, at the end of a gentle expiration. The mean value of the two measurements was used for analysis.
Resting (sitting 5 min prior to measurement) systolic and diastolic blood pressure were measured in the sitting position using the individuals left arm, with a Dinamap vital signs monitor (model XL, Critikron, Inc., Tampa, FL). Five measurements were taken at 2-min intervals. The mean of the last three measurements was used for analysis.
Blood sampling and analysis: Fasting (overnight) blood samples were taken at the beginning of the day from the antecubital vein. Blood samples were aliquoted and separated within 30 min of venepuncture and samples were stored at −80 °C until transported to a WHO-certified laboratory for analysis.
Total cholesterol, high density lipoprotein cholesterol (HDL-C), and triglyceride (TG) were measured by enzymatic methods. Glucose was analyzed by the hexokinase method. Each of these was measured on an Olympus auto analyser (model AU600, Olympus Diagnostica GmbH, Hamburg, Germany). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedwald equation (Friedewald et al., 1972).
Abdominal obesity, overweight and obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, low HDL and glucose impairment were defined according to Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (Circulation, 2002). As a measure for insulin resistance a HOMA-IR score was calculated from fasting glucose and fasting insulin ((Insulin pU/ml ∗ glucose mmol/l) / 22.5) (Matthews et al., 1985). As no internationally recognized cut points are established for insulin resistance a cut point for having altered risk of insulin resistance was set at belonging to the highest sex-specific quartile of the HOMA-IR score.
Cardiorespiratory fitness was assessed directly using a maximal exercise test on a cycle ergometer (Monark 839E, Varberg, Sweden). The test started with a five-minute warm-up at 75 W (women) or 100 W (men) and the work-load was subsequently increased by 35 W every 3 min until exhaustion (participants were verbally encouraged to continue for as long as possible as described by the American College of Sports Medicine (2010) as a good criteria measure). During the test, pulmonary oxygen uptake (VO2), expired carbon dioxide (CO2), and pulmonary ventilation were analyzed using indirect calorimetry (AMIS 2001, Innovision, Odense Denmark). The respiratory exchange ratio (RER) was estimated as expired CO2/VO2. Maximal VO2 consumption (VO2-max) was estimated as the average of VO2 during the last 15 s of the test divided by body weight. Heart rate (HR) was measured consecutively during the test using a Polar HR monitor (RS800, Kempele, Finland). Individuals were excluded if subjective assessment of exhaustion were not met (considered “not reached” by tester), unless RER >1.1 or HR >85% of age predicted HR max (220-age (years)) at the end of the test.
Data on smoking, drinking and sleeping habits were obtained from questionnaires. Participants were asked: “Do you smoke?” There were six possible categories of answers: 1) Yes, daily, 2) Yes, at least once a week, 3) Yes, at least once a month, but not every week, 4) Yes, but less than once a month, 5) No, I quit, 6) No, I never smoked. For analysis participants were defined as smokers if they had answered 1) Yes, daily, 2) Yes, at least once a week.
For alcohol consumption participants were asked to fill in how many units of alcohol they typically would consume on every day of the week. For analyses the amount of daily units were summed up and a dichotomous variable were constructed defining whether participants met the recommendations by the national guidelines (men < 21, women < 14 units per week) or not. Sleep disorder was assessed by asking: “How often do you experience bad or uneasy sleeping?” Possible answers were: 1) every or almost every night, 2) Several times a week, 3) Several times a month, 4) Several times a year, 5) Never. For analysis participants were defined as having “sleep disorder” if the answer was 1) or 2).
2.4. Statistical analysis
The associations of CVD risk factors with depression score were examined using linear or logistic regression analyses adjusted for age and sex. We examined the possibility that the associations of CVD risk with symptoms of depression would be different between men and women by including an interaction term between sex and depression including main effects as covariates. As interaction was evident for several of the CVD risk factors (waist circumference, triglycerides and glucose, p-values < 0.03) we also examined associations stratified by sex.
Although we a priori identified ethnicity as a confounding variable it was not considered in the analyses because 96% of the study population was white. Standard linear regression diagnostics were examined, including examining linearity and normality of residuals, and for most variables the examination revealed no indication of violation, but triglycerides, glucose and HOMA-IR was log transformed to meet the assumptions. For the outcomes that were log transformed we exponentiated the regression coefficients and presented them as ratio of the geometric means expressed in percentage. All statistical analyses were performed in STATA 14 with alpha = 0.05 (two-sided).
3. Results
Prevalence of mild-moderate-severe depression was 8.7% (5.6% males, 11.5% females). Prevalence of major depression was 4.2% (2.0% males, 6.2% females) (Table 1). Several mean values of single risk factors were significantly higher for individuals with symptoms of mild to severe depression compared to individuals with no symptoms (Table 2).
Table 2.
Cardiovascular risk factors of participants with and without symptoms of depression.
| Variable | Depresseda N = 56 (30,4% males) Crude mean (SD) |
Nondepresseda N = 574–588 (48.8% males) Crude mean (SD) |
Mean difference in risk factor by depression statusa |
Gender specific mean difference in risk factor by depression statusa |
||
|---|---|---|---|---|---|---|
| All N = 628–644 |
Women N = 328–340 |
Men N = 293–304 |
||||
| Mean difference (95%CI) | p-Value | Mean difference (95%CI) | ||||
| Age, years | 24.2 (3.1) | 24.3 (3.0) | Age and gender adjusted | Age adjusted differences | ||
| BMI, points | 25.2⁎ (5.2) | 24.1⁎ (3.8) | 1.3⁎ (0.2–2.4) | 0.02 | 1.7⁎ (0.3–3.1) | 0.3 (−1.5–2.1) |
| Waist circumference, cm | 81.7 (13.0) | 80.7 (10.6) | 2.5 (−0.4–5.3) | 0.09 | 4.8⁎ (1.5–8.0) | −2.4 (−7.7–2.8) |
| Systolic blood pressure, (mm Hg) | 115.6 (11.5) | 114.7 (10.4) | 2.7⁎ (0.1–5.2) | 0.04 | 1.6 (−1.3–3.8) | 4.8⁎ (0.01–9.6) |
| Diastolic blood pressure, (mm Hg) | 71.3 (7.9) | 69.4 (7.9) | 2.2⁎ (0.05–4.4) | 0.04 | 1.3 (−1.3–3.8) | 4.0⁎ (0.03–7.9) |
| Glucose, (mmol/l) Glucoselog (%) |
5.3⁎ (1.6) | 5.0⁎ (0.5) | 0.3⁎ (0.1–0.5) 0.04⁎ (0.01–0.07) |
0.001 | 0.4⁎ (0.2–0.7) 5.8⁎ (2.1–9.3) |
0.003 (−0.2–0.2) 0.2 (−4.4–4.7) |
| HOMA-IR (Insulin p μU/ml ∗ glucose mmol/l) / 22.5 HOMAlog (%) |
1.8⁎ (1.5) | 1.4⁎ (1.0) | 0.4⁎ (0.08–0.6) 19⁎ (3.0–34.6) |
0.01 | 0.4⁎ (0.03–0.7) 26.4⁎ (8.3–44.5) |
0.4 (−0.1 to 0–9) 1.7 (−27.9–31.2) |
| Total cholesterol, (mmol/l) | 4.7 (0.9) | 4.6 (0.9) | 0.10 (−0.1–0.3) | 0.8 | 0.18 (−0.1–0.5) | −0.1 (−0.5–0.3) |
| HDL, (mmol/l) | 1.4 (0.4) | 1.4 (0.4) | −0.07 (−0.2–0.02) | 0.1 | −0.1 (−0.2–0.01) | −0.01 (−0.2–0.2) |
| LDL, (mmol/l) | 2.8 (0.7) | 2.7 (0.8) | 0.08 (−0.1–0.3) | 0.5 | 0.13 (−0.13–0.4) | −0.1 (−0.4–0.3) |
| Triglycerides, (mmol/l) Triglycerideslog (%) |
1.4⁎ (0.4) | 1.2⁎ (0.6) | 0.2 (0.02–0.35)⁎ 16 (3.8–28.2)⁎ |
0.03 | 0.3⁎ (0.14-0.5) 26 (12.3–39.7) |
−0.1 (−0.4 to 0.2) −5.7 (−28.8 to 17.4) |
| Fitness, (mlO2/min/kg) | 35.2⁎ (9.2) N = 55 |
39.4⁎ (8.5) | −2.9 (−5.2 to −0.6)⁎ | 0.015 | −3.8⁎ (−6.4 to −1.1) | −0.9 (−5.2 to 3.3) |
Varying N is explained by N = 574–577 for blood samples and N = 571 fitness for non-depressed and by N = 55 for fitness for depressed.
Mild, moderate or severe depression (MDI ≥ 20).
significant difference between depressed/nondepressed (p ≤ 0.05).
Age and sex adjusted mean difference in CVD risk factors between depressed and non-depressed were significant for BMI (β = 1,3 kg/m2 95%CI: 0.2–2.4), triglycerides (β = 0.19 mmol/L, 95%CI: 0.02–0.35, triglycerideslog 16%, 95%CI: 3.8–28.2), blood pressure (systolic: β = 2.7 mm Hg, 95%CI: 0.13–5.2, diastolic: β = 2.2 mm Hg, 95%CI: 0.06–4.8), glucose (β = 0.3 mmol/L 95%CI: 0.12–0.47, glucoselog 4%, 95%CI: 1.0–7.0), logHOMA-IR (β = 19%, 95%CI: 3.0–34.6) and CRF (β = −2.9 ml/O2/min/kg, 95%CI: −5.2 to −0.6). Sex stratified analysis confirmed sex-discordant associations of depression with biological risk factors; women with depression symptoms had raised BMI, waist circumference, glucose, HOMA-IR and triglycerides and low fitness compared to women without depression. In men with depression symptoms only blood pressure was significantly raised compared to non-depressed men (Table 2).
Table 3 shows the prevalence and odds of being at risk defined by sex specific international guidelines for each risk factor (or by distribution if no international guidelines were available) including the odds of risk behavior according to smoking, drinking and having sleep disorders. Women with symptoms of depression had increased odds of overweight (BMI: OR = 2.0, 95%CI: 1.002–4.0, WC: OR = 2.5, 95%CI: 1.2–4.8), of having levels of HOMA-IR at or above the 75th sex specific percentile of (OR = 2.3, 95%CI: 1.1–4.6), raised levels of triglycerides (OR = 3.4, 95%CI: 1.6–7.4) and low CRF (OR = 2.5, 95%CI: 1.2–5.5) compared to women without symptoms of depression. For men with depression symptoms only odds of having high blood pressure was significantly increased compared to men without depression (OR = 3.1, 95%CI: 1.1–8.7).
Table 3.
Prevalence rates and odds ratios of clinical cardiovascular risk factors and risk behaviors (smoking, drinking and sleep disorders) by depression status.
| “At risk”a variable | Depressedb N = 47–56 (30,4% males) |
Nondepressedb N = 529–588 (48.8%males) |
All N = 595–644 |
Odds ratios for being “at risk”a by depression statusb |
||
|---|---|---|---|---|---|---|
| Prevalence rates % (n) (95%CI) | Women N = 328–340 Adjusted for age |
Men N = 302–304 Adjusted for age |
All adjusted for age and gender |
|||
| OR (95%CI) | ||||||
| Overweight or obesity | 41.1 (23) (29.0–54.4) | 32.8 (193) (29.1–36.7) | 33.6 (216) (30.0–37.3) | 2.0⁎ (1.002–4.0) | 1.0 (0.4–2.8) | 1.6 (0.0–2.8) |
| Abdominal adiposity | 41.1⁎ (23) (29.0–54.4) | 22.0⁎ (129) (18.8–25.6) | 23.9 (152) (20.8–27.4) | 2.5⁎ (1.2–4.8) | 1.7 (0.5–5.5) | 2.2⁎ (1.2–3.9) |
| Hypertension | 14.3 (8) (7–26) | 9.0 (50) (6.5–11.1) | 8.9 (58) (7.0–11.4) | 2.4 (0.5–11.9) | 3.1⁎ (1.1–8.7) | 2.8⁎ (1.2–6.8) |
| Impaired fasting glucose | 12.5 (7) (6.1–24.1) | 11.6 (67) (9.2–14.5) | 11.6 (74) (9.3–14.3) | 2.4 (0.8–6.8) | 0.6 (0.1–2.8) | 1.4 (0.6–3.2) |
| Insulin resistance | 37.5 (21) (25.8–50.9) | 23.7 (136) (20.4–27.4) | 25 (157) (21.6–28.4) | 2.3⁎ (1.1–4.6) | 1.3 (0.5–4.0) | 1.9⁎ (1.1–3.5) |
| Hypercholesterolemia, | 35.7 (20) (24.2–49.1) | 24.6 (142) (21.2–28.3) | 25.8 (162) (22.6–29.4) | 1.8 (0.9–3.5) | 0.7 (0.2–3.1) | 1.5 (0.8–2.7) |
| Low HDL | 35.7 (20) (24.2–49.1) | 27.2 (157) (23.7–31.0) | 28 (177) (24.7–31.6) | 2.0⁎ (1.01–4.1) | 0.9 (0.3–2.7) | 1.6 (0.9–2.8) |
| High LDL | 28.6 (16) (18.2–41.8) | 18.1 (104) (15.2–21.5) | 19.2 (120) (16.3–22.4) | 2.2⁎ (1.04–4.5) | 1.0 (0.3–3.7) | 1.8 (0.9–3.3) |
| Hypertriglyceridemia, | 23.2 (13) (13.9–36.1) | 12.7 (73) (10.2–15.6) | 13.6 (86) (11.2–16.5) | 3.4⁎ (1.6–7.4) | 0.4 (0–05-3.0) | 2.1⁎ (1.1–4.1) |
| Low fitness | 35.6 (16) (22.9–50.6) | 20.9 (115) (17.7–24.5) | 22.0 (131) (18.7–25.4) | 2.5⁎ (1.2–5.5) | 1.3 (0.4–4.3) | 2.0⁎ (1.1–3.9) |
| Regular smoker | 44.6 (25) (32.1–57.9) | 32.6 (191) (28.9–36.5) | 33.7 (216) (30.2–37.4) | 1.4 (0.7–2.9) | 3.0⁎ (1.1–8.4) | 1.9 (1.1–3.3) |
| Drinking, >recommendations | 17.1 (8) (8.7–30.7) | 17.2 (91) (14.2–20.7) | 17.1 (99) (14.2–20.4) | 0.9 (0.3–2.9) | 1.4 (0.4–4.5) | 1.1 (0.5–2.5) |
| Sleep disorder | 60.7⁎ (34) (47.3–72.7) | 13.3⁎ (78) (10.8–16.3) | 17.4 (112) (14.7–20.6) | 7.1⁎ (3.5–14.5) | 18⁎ (6.1–52.6) | 9.6⁎ (5.2–17.4) |
Varying N is explained by N = 574–577 for blood samples, N = 571 fitness N = 586 for smoking regularly, N = 587 for sleep disorder and N = 529 for risk of drinking > recommendations for the nondepressed, and by N = 55 for fitness and N = 47 for drinking disorder for depressed.
According to international guidelines; defined at risk if: Overweight or obese (≥25 BMI (kg/m2)), Abdominal adiposity (male: WC ≥ 94 cm, female: WC ≥ 80 cm), Hypertension: systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg, Glucose impairment: fasting glucose ≥ 5.6 mmol, Insulin resistance: (HOMA-IR) ≥ 75 sex-specific percentile of the population Hypercholesterolemia > 200 mg/Dl, Low HDL: cholesterol < 1.03 mmol/l (men) and < 1.29 mmol/l (women), High LDL: cholesterol > 130 mg/dl, Hypertriglyceridemia > 1.7 mmol/l, Low Fitness: lowest sex-specific quartile of fitness (mlO2/min/kg), Smoker: currently smoking every week or more, Drinking: more than national recommendations; men < 21, women < 14 units per week, Sleep disorder: having experienced restless or bad sleep several times a week/almost every night.
Mild, moderate or severe depression (MDI ≥ 20).
Significant difference between depressed/nondepressed (p ≤ 0.05).
The odds ratio of being a smoker were raised for young men with symptoms of depression compared to young men without symptoms of depression (OR = 3.0, 95%CI: 1.1–8.4) but not for women. The odds ratio of drinking more than recommend by national guidelines were not significantly raised for men and women with symptoms of depression compared to men and women without symptoms of depression, whereas both men and women had significantly higher odds of experiencing disturbed sleep compared to their counterparts with no symptoms of depression (for men OR = 18.0, 95%CI: 6.1–52.6, for women OR = 7.1, 95%CI: 3.5–14.5) (Table 3).
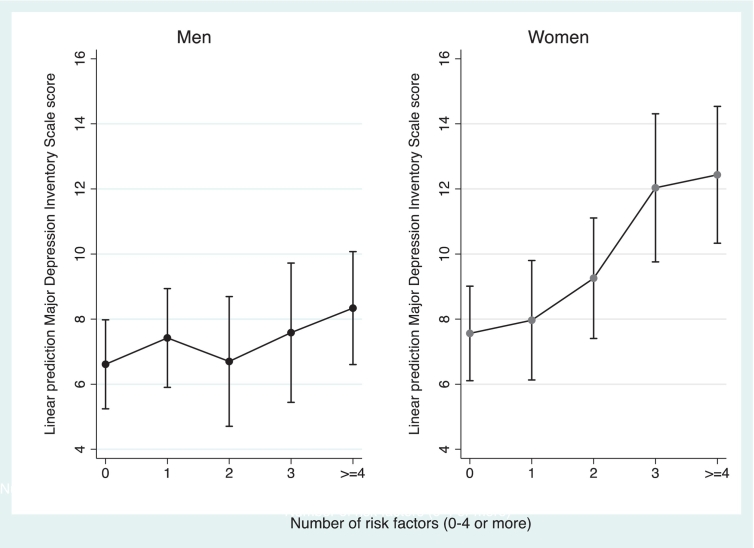
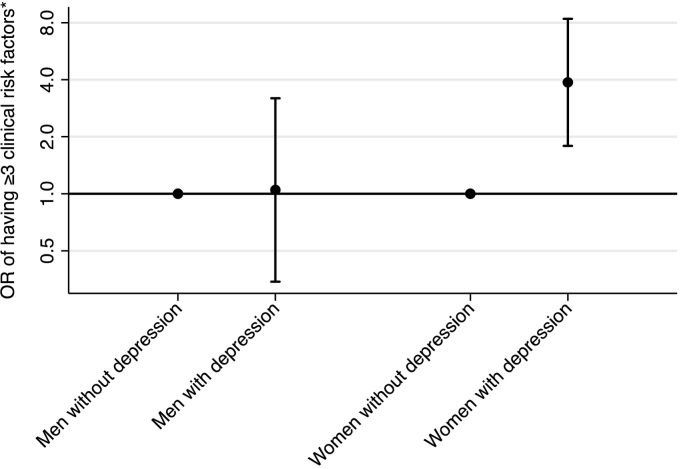
More than 60% of the depressed young adults reported having three or more risk factors elevated. This proportion was 36% among non-depressed. The association of symptoms of depression score with the number of elevated risk factors is presented in Fig. 1. Logistic regression showed that having three or more risk factors elevated was associated with greater odds of having symptoms of depression (OR = 2.7, 95%CI: 1.5–4.8). For depressed women the odds ratio for having raised levels of three or more CVD risk factors was as high as 4.0 (95%CI: 2.0–8.3) compared to women without depression. For men this difference was not significant (Fig. 2).
Fig. 1.
Association of depression score with number of raised biological cardiovascular risk factors by gender.§
Circles represent mean. Vertical bars represents 95%CI. Estimates are adjusted for age and gender. N = 340 (men) N = 344 (women). Danish data 2009–2010.
§Raised biological risk factors defined from gender specific cut-points (see Materials and methods section): body mass index, waist circumference, total cholesterol, LDL, HDL, glucose, blood pressure or fitness.
Fig. 2.
Odds of having three or more clinical cardiovascular risk factors by depression status.
*Risk factor defined by gender specific cut-points from international guidelines for each risk factor (body mass index, waist circumference, total cholesterol, LDL, HDL, glucose, blood pressure or fitness) (see Materials and methods section).
N = 644, depressed: N = 56 (30,4% males), non-depressed: N = 588 (48.8% males). Danish data 2009–2010.
4. Discussion
In this population-based study we found that Danish young adults with any significant depression had greater prevalence of several clinical- and behavioral CVD risk factors compared with non-depressed individuals. These associations were generally greater for women compared to men. Other studies have shown these associations in middle- and older adulthood, but the current study shows that this unfavorable CVD risk profile are already present in early adulthood in individuals with symptoms of depression. Our findings suggest that young people, in particular women, with depression needs special attention regarding support for life style changes to prevent later CVD and other metabolic disturbances such as type 2 diabetes.
The prevalence of depression found in this study is in line with previous findings in a Danish population (Olsen et al., 2004) aged 20–79 years. In this large cross sectional study (n = 1123), based on data obtained by postal questionnaire in 2000, prevalence of any depression (MDI ≥ 20) was estimated to 7.1% and mean score of MDI was 7.2 in the total population. But when looking at a similar age group (20–34 years) in the study by Olsen et al. (2004), prevalence rates of depression were 8.8% and mean score of MDI 8.9, which equals the prevalence rates in our study (8.7% and mean score of MDI at 8.6). Similar to our study, Olsen and colleagues observed higher prevalence rates and mean scores in women compared to men. In our study women had significantly higher prevalence of major depression compared to men. Put together these two Danish studies indicate that symptoms of depression appear more often at young age compared to older age and in women compared to men. In our study, the sex-stratified analyses are limited by the relative small numbers of cases with depression symptoms, which affects the precision of the estimated differences in CVD risk factor levels by depression status.
A study by Rhee et al. (2014) supports our findings of a sex specific relationship of depression with CVD risk factors. In this Korean study, comprising >30,000 adults (mean age years (SD): 47.5 (10.7) for depressed individuals and 47.1(10.4) for non-depressed individuals), they found that depressed women had increased odds of having metabolic syndrome but no relationship was found among men. In contrast to that, and our findings, they found that depressed men had significantly lower blood pressure than men without depression. We found that men with symptoms of depression had greater prevalence of raised blood pressure, though the confidence intervals were wide. Our results on blood pressure are supported by findings in a meta-analysis conducted by Meng et al. (2012) including n = 22,367 adults (age range in nine studies 12–75 years, most studies in middle aged 25–64 years) with a mean follow-up period of 9.6 years. They showed that depression in adulthood is independently associated to hypertension (Meng et al., 2012). However, they did not stratify the analysis by sex. In addition, our study found that hypertension is more prevalent in young men with depression symptoms compared to men without depression. This sex difference was also observed in the Cardiovascular Risk in Young Finns Study (Elovainio et al., 2005). In this study Elovainio et al. (2005) also reported that for men, but not for women, depressive symptoms during early adulthood were associated with higher levels of carotoid intima-media thickness. In a longitudinal study by Dietz and Matthews (2011), with N = 157 adolescents aged 16–21 years the association between depression and intima-media thickness was not shown. The observed association of depression and overweight/obesity in women is supported by several longitudinal studies (Luppino et al., 2010; Liem et al., 2008; Rottenberg et al., 2014). We also found higher prevalence of smoking, low fitness and sleep disturbances among young adults with depression symptoms. Though confidence intervals were large and cases with depression relatively low, these findings of behavioral risk factors are in line with findings in other studies (Olsen et al., 2004; Rottenberg et al., 2014).
A plausible explanation for the observed sex difference could be that the autonomic function is altered differently in males and females with major depression, as suggested in a cross sectional study by Voss et al. (2011) where they observed a sex-dependent relationship of major depression with autonomic cardiovascular modulation among 36 patients with major depression and 36 matched controls. The pronounced differences in the relationship of depression with CVD risk factors across sex suggests that young women with depression are at greater risk of developing CVD and type 2 diabetes compared with men.
The results of this study should be interpreted in the light of some limitations. Firstly, the cross-sectional design prevents us from pointing to any causal pathway or direction in the relationship between depression and CVD risk factors. Secondly, data on depression was self-reported and not clinically diagnosed by professionals, this might have led to an overestimation (Seldenrijk et al., 2015). Also data on smoking, drinking, and sleeping were self-reported, which makes data prone to both over and under reporting. The data on alcohol consumption was the question where most young people (12%) did not give complete answers, yet, the majority of missing answers were among the non-depressed participants (87%). Last but not least it is a limitation that the absolute number of participants with any depression was low in this cohort, which might have underpowered some of the sub-group analysis and also made it impossible to divide symptoms of depression into further sub-groups for analysis.
Strength of this study includes the population-based random sample of healthy young adults. Because depressed and non-depressed participants in our study were from the same source population the possibility of selection bias in less likely to have influenced our comparisons of CVD risk factors. To our knowledge no other studies have investigated the associations between symptom of depression and several clinical and behavioral CVD risk factors in a similar population in one study, but on single risk factors (Meng et al., 2012), proxy measures (Meng et al., 2012) for risk factors and on mortality (Shah et al., 2011) or an older population (Ont. Health Technol. Assess. Ser., 2013; Rhee et al., 2014). Furthermore, the random sample of young individuals is likely to be less confounded, than an older population with more comorbidity and medication use that may distort the relationship (Shah et al., 2011). Yet, it is unknown if our comparisons of Danish young adults with and without depression symptoms are generalizable to populations in other countries.
In summary, our study conducted in a population-based cohort of Danish young adults suggest that depression symptoms at this age is associated to several unfavorable CVD risk factors, that are known to predict early morbidity and mortality in later life (Pan et al., 2011; Shah et al., 2011; Haukkala et al., 2009). Furthermore, we found that women with depression symptoms were particularly likely to have raised levels of biological CVD risk factors, which suggest that depression may increase the risk of later CVD more strongly in women compared with men.
4.1. Perspectives and practical implications
Public health is challenged by high prevalence of depression in young adults. Depressed adults suffer more often from chronic diseases than non-depressed adults (Ont. Health Technol. Assess. Ser., 2013) and >80% of depressed adults report their depression to be untreated by a doctor (Olsen et al., 2004). At present, there is no evidence that screening and treating for depression in adult populations improves the symptoms of chronic diseases or lead to use of fewer health care services (Ont. Health Technol. Assess. Ser., 2013). In that perspective our results underline the importance of simultaneously focusing on behavioral risk factors when dealing with young people with symptoms of depression to prevent increasing CVD risk, co-morbidity and mortality (Shah et al., 2011; Atlantis et al., 2012), especially in young women.
Longitudinal studies providing evidence of causality between risk factors and depression are warranted as well as longitudinal studies evaluating interventions integrating physical and behavioral health.
Conflicts of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Financial support/funding
Funding for the study has been provided by: The Danish Council for Strategic Research (grant no. 95-103-21990), the Danish Heart Foundation and the TrygFonden Foundation.
Author's contribution
HK and AG had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to important parts of the process: Study concept and design: HK and AG. Acquisition of data: AG. Analysis and interpretation of data: HK and AG. Drafting of the manuscript: HK and AG. Critical revision of the manuscript for important intellectual content: HK, AG, NC, PLK, LBA and KF. Statistical analyses: HK and AG. Obtained funding: LBA, KF, AG.
Acknowledgement
The authors gratefully acknowledge all the participants and several researchers for their involvement in the EYHS study, for several years, here especially Niels Wedderkopp and Mathias Ried-Larsen for collecting the Danish data.
References
- American College of Sports Medicine . Third edition. Wolters Kluwer Health: Lippincott Williams & Wilkins; 2010. American College of Sports Medicine: ACSM's Health-related Physical Fitness Assessment Manual; p. 72. [Google Scholar]
- Atlantis E., Shi Z., Penninx B.J., Wittert G.A., Taylor A., Almeida O.P. Chronic medical conditions mediate the association between depression and cardiovascular disease mortality. Soc. Psychiatry Psychiatr. Epidemiol. 2012;47:615–625. doi: 10.1007/s00127-011-0365-9. [DOI] [PubMed] [Google Scholar]
- Bech P., Rasmussen N.A., Olsen L.R., Noerholm V., Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J. Affect. Disord. 2001;66:159–164. doi: 10.1016/s0165-0327(00)00309-8. [DOI] [PubMed] [Google Scholar]
- Charlson F.J., Moran A.E., Freedman G. The contribution of major depression to the global burden of ischemic heart disease: a comparative risk assessment. BMC Med. 2013;11:250. doi: 10.1186/1741-7015-11-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Davidson K., Jonas B.S., Dixon K.E., Markovitz J.H. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch. Intern. Med. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- Dierker L., Rose J., Selya A., Piasecki T.M., Hedeker D., Mermelstein R. Depression and nicotine dependence from adolescence to young adulthood. Addict. Behav. 2015;41:124–128. doi: 10.1016/j.addbeh.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz L.J., Matthews K.A. Depressive symptoms and subclinical markers of cardiovascular disease in adolescents. J. Adolesc. Health. 2011;48:579–584. doi: 10.1016/j.jadohealth.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.C., Heron J., Dick D.M. Adolescent alcohol use is positively associated with later depression in a population-based U.K. cohort. J. Stud. Alcohol Drugs. 2014;75:758–765. doi: 10.15288/jsad.2014.75.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovainio M., Keltikangas-Jarvinen L., Kivimaki M. Depressive symptoms and carotid artery intima-media thickness in young adults: the Cardiovascular Risk in Young Finns Study. Psychosom. Med. 2005;67:561–567. doi: 10.1097/01.psy.0000170340.74035.23. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gore F.M., Bloem P.J., Patton G.C. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- Haukkala A., Konttinen H., Uutela A., Kawachi I., Laatikainen T. Gender differences in the associations between depressive symptoms, cardiovascular diseases, and all-cause mortality. Ann. Epidemiol. 2009;19:623–629. doi: 10.1016/j.annepidem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Katon W.J. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol. Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Liem E.T., Sauer P.J., Oldehinkel A.J., Stolk R.P. Association between depressive symptoms in childhood and adolescence and overweight in later life: review of the recent literature. Arch. Pediatr. Adolesc. Med. 2008;162:981–988. doi: 10.1001/archpedi.162.10.981. [DOI] [PubMed] [Google Scholar]
- Luppino F.S., de Wit L.M., Bouvy P.F. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Meng L., Chen D., Yang Y., Zheng Y., Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J. Hypertens. 2012;30:842–851. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Barber R.M., Foreman K.J. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen A., Winkley K., Twisk J. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L.R., Jensen D.V., Noerholm V., Martiny K., Bech P. The internal and external validity of the Major Depression Inventory in measuring severity of depressive states. Psychol. Med. 2003;33:351–356. doi: 10.1017/s0033291702006724. [DOI] [PubMed] [Google Scholar]
- Olsen L.R., Mortensen E.L., Bech P. Prevalence of major depression and stress indicators in the Danish general population. Acta Psychiatr. Scand. 2004;109:96–103. doi: 10.1046/j.0001-690x.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- Screening and management of depression for adults with chronic diseases: an evidence-based analysisOnt. Health Technol. Assess. Ser. 2013;13:1–45. [PMC free article] [PubMed] [Google Scholar]
- Pan A., Sun Q., Okereke O.I., Rexrode K.M., Hu F.B. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.J., Kim E.Y., Kim S.H. Subjective depressive symptoms and metabolic syndrome among the general population. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;54:223–230. doi: 10.1016/j.pnpbp.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Riddoch C.E.D., Page A., Froberg K. The European Youth Heart Study - cardiovascular disease risk factors in children: rationale, aims, design and validation of methods. J. Phys. Act. Health. 2005;2:115–129. [Google Scholar]
- Rottenberg J., Yaroslavsky I., Carney R.M. The association between major depressive disorder in childhood and risk factors for cardiovascular disease in adolescence. Psychosom. Med. 2014;76:122–127. doi: 10.1097/PSY.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldenrijk A., Vogelzangs N., Batelaan N.M., Wieman I., van Schaik D.J., Penninx B.J. Depression, anxiety and 6-year risk of cardiovascular disease. J. Psychosom. Res. 2015;78:123–129. doi: 10.1016/j.jpsychores.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Shah A.J., Veledar E., Hong Y., Bremner J.D., Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch. Gen. Psychiatry. 2011;68:1135–1142. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooy K., van Hout H., Marwijk H., Marten H., Stehouwer C., Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- Voss A., Boettger M.K., Schulz S., Gross K., Bar K.J. Gender-dependent impact of major depression on autonomic cardiovascular modulation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1131–1138. doi: 10.1016/j.pnpbp.2011.03.015. [DOI] [PubMed] [Google Scholar]