Graphical abstract
Keywords: Antifungal activity, Butenafine, Microwave-assisted synthesis, Multigram-scale synthesis, Schiff base, Trichophyton rubrum
Abstract
The incidence of fungal infections is considered a serious public health problem worldwide. The limited number of antimycotic drugs available to treat human and animal mycosis, the undesirable side effects and toxicities of the currently available drugs, and the emergence of fungal resistance emphasizes the urgent need for more effective antimycotic medicines. In this paper, we describe a rapid, simple, and efficient synthetic route for preparation of the antifungal agent butenafine on a multigram scale. This novel synthetic route also facilitated the preparation of 17 butenafine analogues using Schiff bases as precursors in three steps or less. All the synthesized compounds were evaluated against the yeast, Cryptococcus neoformans/C. gattii species complexes and the filamentous fungi Trichophyton rubrum and Microsporum gypseum. Amine 4bd, a demethylated analogue of butenafine, and its corresponding hydrochloride salt showed low toxicity in vitro and in vivo while maintaining inhibitory activity against filamentous fungi.
Introduction
Fungal infections can range in severity depending on the patient age, the infection site, and the disease-causing agent. These factors affect the type of medical treatment employed to combat fungi. Superficial fungal infections caused by dermatophytes, known as dermatophytosis [1], [2], [3], have debilitating effects, and they negatively impact the quality of human or animal life [1], [4]. The main causative agents of dermatophytosis belong to the genera Microsporum, Trichophyton, and Epidermophyton, with T. rubrum being the most prevalent species worldwide [1]. Dermatophytosis is often recalcitrant to treatment mainly due to the poor penetration of antifungals at the site of infection but also because of drug resistance mechanisms employed by the infectious agent [1], [5]. Cutaneous fungal infections can be originated via direct inoculation of the fungi (primary cutaneous mycosis), or they can result from the systemic hematogenous spread of the pathogen (secondary cutaneous mycosis). Early diagnosis and treatment are very important, especially in immunocompromised individuals, as these agents can also cause invasive infections due to mucosal or cutaneous barrier disruption and metabolic dysfunction or due to neutrophil defects in the number and function and in cell-mediated immunity [6], [7]. Invasive fungal infections (IFIs) occur when fungi invade deep tissues, leading to prolonged illness and high mortality (>50% in some cases). These infections are more common in immunocompromised or other high-risk hospitalized patients, including those with hematologic or other malignancies, and in those who have undergone hematologic stem-cell or solid-organ transplants and who therefore receive immunosuppressive therapy. In recent years, there has been an increase in the number of IFIs due to an increase in the number of immunocompromised people, to the emergence of antifungal resistant species, and to the prophylactic use of antifungals. Certain fungi are known to cause IFIs including yeasts of the genera Candida and Cryptococcus [8], [9]. Previously, C. albicans was the main species of the genus Candida that was known to cause IFIs: however during recent years, non-albicans species, such as C. parapsilosis, C. glabrata, C. tropicalis and more recently and C. auris, have gained interest as etiologic agents of these infections. The resistance of these species to azoles and echinocandins has become a severe clinical challenge [10], [11], [12]. The Cryptococcus neoformans/Cryptococcus gattii species complex is responsible for almost all cryptococcal infections, which are the most common life-threatening fungal infections in patients with HIV in many regions of the world. Despite the lack of consensus regarding the nomenclature of the Cryptococcus species, it is believed that differences exist in their susceptibility to the most commonly used antifungals (amphotericin B, 5-FC and azole derivatives) [13], [14]. Worldwide, infections caused by these pathogens account for an estimated 223,000 cases of cryptococcal meningitis per year among people with HIV/AIDS, resulting in approximately 180,000 deaths per year [15].
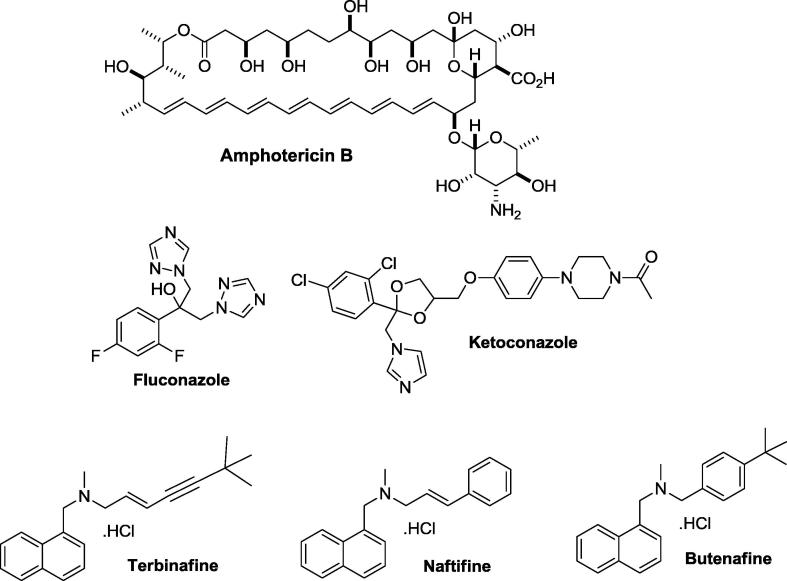
Typically, fungal infections are treated with polyenes, including amphotericin B, azoles, such as fluconazole and ketoconazole, allylamines, such as terbinafine and naftifine and butenafine, which is the only benzylamine-containing compound commonly used to treat fungal infections (Fig. 1) [1], [16], [17], [18]. Butenafine is structurally similar to terbinafine, and its antifungal activity is attributed to its ability to directly damage fungal cell membranes by disrupting the early stages of ergosterol biosynthesis via inhibition of the enzyme squalene epoxidase [19]. The inhibition of this enzyme compromises the plasma membrane, leading to the toxic accumulation of squalene in the fungal cell membrane, which culminates in fungal death [20], [21], [22].
Fig. 1.
Chemical structures of antifungal agents: amphotericin B, fluconazole, ketoconazole, terbinafine, naftifine and butenafine.
Due to antifungal properties of butenafine, many strategies have been reported in the literature to prepare this benzylamine compound. The majority of the synthetic strategies involve a bimolecular nucleophilic substitution reaction (SN2 reaction), employing N-methyl-1-(naphthalen-1-yl)methanamine hydrochloride as the nucleophile and 1-(tert-butyl)-4-(chloromethyl)benzene or 1-(bromomethyl)-4-(tert-butyl)benzene as the electrophile. These reactions occur in the presence of a base, and the desired product is obtained after 3–5 h. Subsequently, the conversion of the free base of butenafine to its corresponding hydrochloride salt furnishes the desired benzylamine in a 73–86% yield (2 steps) [23], [24], [25], [26]. Although the above methodologies involve only two steps, the use of toxic solvents such as toluene and dimethylformamide (DMF) and the use of catalysts make the process less attractive.
In 2014, Beydoun and co-workers described a “one-pot” synthesis of butenafine with a 60% yield that employed a non-commercially available catalyst formed from Ru(triphos)(tmm) (5 mol%) and trifluoromethanesulfonyl imide (HNTf2) (10 mol%) [27]. A year later, Fu and co-workers described the synthesis of butenafine, in which a boronic acid-catalyzed amide condensation was followed by the B(C6F5)3-catalyzed reduction of the amide and the direct reductive N-methylation with formic acid. Butenafine was thus obtained after two steps, with a 91% yield [28]. In general, all the synthetic strategies described for the synthesis of butenafine use an expensive and/or non-commercially available catalyst as well as a prolonged reaction time, or they require prior manipulation of the starting materials [25], [27], [28], [29], [30], [31].
As butenafine is a potent compound, many reports have been published describing the synthesis and biological evaluation of its analogues. In general, the analogues have demonstrated potent antifungal activity, while the synthetic routes result in moderate yields of the desired compounds [32], [33], [34], [35].
Previous work from our research group described the design, synthesis, and antifungal activities of a series of Schiff bases [36], [37], [38]. Schiff bases are some of the most widely used organic compounds. They serve as pigments and dyes, catalysts, intermediates/precursors in organic synthesis, and polymer stabilizers [36], [39]. Indeed, the importance of Schiff bases as precursors for organic synthesis is well established, as they have been used in numerous chemically diverse reactions, including the addition of organic metallic reagents or hydrides to convert C N into C—C bonds, the hetero Diels-Alder reaction to obtain heterocyclic compounds, and the Staudinger reaction for the preparation of β-lactams [40].
Herein, we describe a strategy to the synthesize butenafine using a Schiff bases precursors and to subsequently produce its hydrochloride salt. In addition, 17 butenafine analogues were prepared from 6 Schiff bases, and 11 amines and their corresponding hydrochloride salts were generated. Butenafine and its analogues were evaluated for their antifungal activity as well as for toxicity.
Material and methods
Chemistry
General procedures
The reagents were obtained from chemical suppliers. Benzaldehyde was purified using the method previously described by Kieboom [41], all the amines were distilled by fractional distillation, and the solvents were purified by simple distillation. The reaction progress was monitored by thin-layer chromatography and gas chromatography-mass spectrometry using a Shimadzu CGMS-QP2010 Ultra instrument. Column chromatography was performed with silica gel 60 (70–230 mesh), and hexane/ethyl acetate/triethylamine (50:50:0.1) served as the eluent for all the phenyl amines. The melting point was measured using a MQAPF-302 apparatus, and the values were not corrected. NMR spectra were obtained on a Bruker AVANCE DPX 200. The data were reported as follows: chemical shift multiplicities [s (singlet), br s (broad singlet), d (doublet), dd (double doublet) or m (multiplet)], coupling constants (hertz), and integration. Chemical shifts were reported in parts per million (ppm), relative to tetramethylsilane (TMS) for 1H spectra and relative to residual solvent peaks for 13C spectra. Copies of 1H NMR and 13C NMR spectrum for all synthesized compounds is available as Supplementary Material. High-resolution mass spectra were obtained using a mass spectrometer with an electrospray ionization source (ESI-MS) on a Shimadzu LC-ITTOF instrument. The infrared spectra were recorded as KBr plates by Fourier transform spectrometry on a Bruker Alpha spectrometer or a Perkin Elmer spectrometer.
Supplementary material.
General procedure for the synthesis of Schiff bases (3)
The requisite amine (1.0 mmol) and aldehyde (1.0 mmol) in ethanol (4 mL) were stirred under microwave radiation (MW) in a DISCOVER CEM® reactor using the following conditions: temperature 80 °C, maximum power 200–250 Watts, hold time 2 min, and run time 2–8 min, with vigorous stirring in an open tube. After the complete consumption of the starting materials, the reaction mixture was concentrated under reduced pressure to achieve the desired Schiff bases (3).
N-Benzyl-1-phenylmethanimine (3ac)
Yield 98%. Light yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.38 (s, 1H, HC N), 7.78 (dd, J = 6.6 and 2.9, 2H), 7.42–7.24 (m, 8H), 4.81 (s, 2H, CH2). 13C NMR (50 MHz, CDCl3): δ (ppm) = 162.2 (C N), 139.5 (C), 136.3 (C), 131.0 (CH), 128.8 (2CH), 128.7 (2CH), 128.5 (2CH), 128.2 (2CH), 127.2 (CH), 65.2 (CH2). IR (KBr): (cm−1) = 3062 and 3027 (v benzyl = CH), 2871 and 2839 (vs CH2), 1642 (v C N of ArCH N—Ar), 1602, 1580 and 1495 (v C C benzyl), 1451 (δs CH2). HRMS (ESI): m/z observed: 196.1099; C14H13N [M+H]+ requires: 196.1126; error (ppm): 1.4. Data for 3ac are in accordance with those reported elsewhere [42].
N-(4-(tert-Butyl)-1-)phenylmethanimine (3bc)
Yield 100%. White solid (Mp 40.7–41.1 °C). 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.38 (s, 1H, HC N), 7.80–7.77 (m, 2H), 7.43–7.35 (m, 5H), 7.27 (d, J = 8.4, 2H), 4.79 (s, 2H, CH2), 1.31 (s, 9H, 3CH3). 13C NMR (50 MHz, CDCl3): δ (ppm) = 162.0 (C N), 150.1 (C), 136.5 (C), 136.4 (C), 131.0 (CH), 128.8 (2CH), 128.5 (2CH), 127.9 (2CH), 125.6 (2CH), 65.0 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3057 and 3026 (v = CH benzyl), 2956 (vas CH3), 2871 and 2806 (vs CH2), 1644 (v C N of ArCH N—Ar), 1462 (δas CH3). HRMS (ESI): m/z observed: 252.1742; C18H21N [M+H]+ requires: 252.1752; error (ppm): 0.4. Data for 3bc are in accordance with those reported elsewhere [43].
N-((Naphthalen-1-yl)methylene)(phenyl)methanimine (3ad)
Yield 99%. Dark yellow thick oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 9.04 (s, 1H, HC = N), 8.95 (d, J = 7.8, 1H), 7.96–7.86 (m, 3H), 7.64–7.47 (m, 3H), 7.43–7.23 (m, 5H), 4.95 (s, 2H, CH2).13C NMR (50 MHz, CDCl3): δ (ppm) = 161.9 (C N), 139.7 (C), 134.0 (C), 131.7 (C), 131.5 (C), 131.3 (CH), 129.3 (CH), 128.8 (CH), 128.7 (2CH), 128.2 (2CH), 127.4 (CH), 127.2 (CH), 126.2 (CH), 125.4 (CH), 124.6 (CH), 66.2 (CH2). IR (KBr): (cm−1) = 3085, 3058 and 3028 (v = CH benzyl), 2870 and 2827 (vs CH2), 1641 (v C N of ArCH N-Ar), 1619 (v C C naphthyl), 1589 (v C C benzyl), 1452 (δs CH2). HRMS (ESI): m/z observed: 246.1259; C18H15N [M+H] + requires: 246.1282; error (ppm): 0.9. Data for 3ad are in accordance with those reported elsewhere [44].
(4-tert-Butylphenyl)-N-((naphthalen-1-yl)methylene)methanimine (3bd)
Yield 100%. Brown oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 9.02 (s, 1H, HC = N), 8.96 (d, J = 8.2, 1H), 7.95–7.84 (m, 3H), 7.58–7.44 (m, 3H), 7.42–7.27 (m, 4H), 4.91 (s, 2H, CH2), 1.32 (s, 9H, 3CH3). 13C NMR (50 MHz, CDCl3): δ (ppm) = 161.7 (C = N), 150.1 (C), 136.7 (C), 134.0 (C), 132.0 (C), 132.0 (C), 131.3 (CH), 129.2 (CH), 129.0 (CH), 127.9 (2CH), 127.4 (CH), 126.2 (CH), 125.6 (2CH), 125.4 (CH), 124.6 (CH), 66.0 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3088 and 3054 (v = CH benzyl), 2960 (vas CH3), 2903 (vas CH2), 2867 (vs CH2), 1642 (v C N of ArCH N-Ar), 1511 (v C C naphthyl), 800 (γ C—H, benzene 1,4-disubstituted), 773 (γ C—H, naphthyl). HRMS (ESI): m/z observed: 302.1833; C22H23N [M+H] + requires: 302.1908; error (ppm): 2.5.
N-Benzyl-1-(naphthalen-2-yl)methanimine (3ae)
Yield 87%. Beige solid (Mp 83,9–84,7 °C; Lit. Mp 84–86 °C [45]). 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.52 (s, 1H, HC = N), 8.06–8.02 (m, 2H), 7.90–.81 (m, 3H), 7.54–7.45 (m, 2H), 7.37–7.22 (m, 5H), 4.87 (s, 2H, CH2), 13C NMR (50 MHz, CDCl3): δ (ppm) = 162.2 (C = N), 139.5 (C), 135.0 (C), 134.0 (C), 133.3 (C), 130.3 (CH), 128.8 (CH), 128.7 (CH), 128.7 (CH), 128.2 (CH), 128.1 (CH), 127.3 (CH), 127.2 (CH), 126.7 (CH), 124.1 (CH), 65.3 (CH2). IR (KBr): (cm−1) = 3053 e 3030 (v = CH benzyl), 2865 (vs CH2), 1634 (v C N of ArCH N-Ar), 1601 (v C C naftyl), 1582 (v C C benzyl), 1451 (δs CH2), 748 (γ C—H, naftyl). HRMS (ESI): m/z observed: 246.1282; C22H23N [M+H] + requires: 246.1273; error (ppm): 0.4. Data for 3ae are in accordance with those reported elsewhere [45].
(4-tert-Butylphenyl)-N-((naphthalen-2-yl)methylene)methanamine (3be)
Yield 96%. Beige solid (Mp 79.3–80.7 °C). 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.53 (s, 1H, HC N), 8.07 (s, 1H), 8.02 (d, J = 1.6, 1H), 7.91–7.82 (m, 3H), 7.52–7.48 (m, 2H), 7.41–7.29 (m, 4H), 4.85 (s, 2H, CH2), 1.32 (s, 9H, 3CH3). 13C NMR (50 MHz, CDCl3): δ (ppm) = 162.0 (C N), 150.1 (C), 136.5 (C), 134.9 (C), 134.1 (C), 133.3 (C), 130.3 (CH), 128.8 (CH), 128.6 (CH), 128.1 (CH), 128.0 (2CH), 127.3 (CH), 126.6 (CH), 125.7 (2CH), 124.2 (CH), 65.1 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3053 and 3025 (v = CH benzyl), 2961 (vas CH3), 2869 (vs CH2), 1638 (v C N of ArCH N-Ar), 1512 (v C C naphthyl), 827 (γ C-H, benzene 1,4-disubstituted), 752 (γ C—H, naphthyl). HRMS (ESI): m/z observed: 302.1887; C22H23N [M+H] + requires: 302.1908; error (ppm): 0.7.
General procedure for the synthesis of amines (4)
Each Schiff base 3 (0.5 mmol) was dissolved in methanol (2.5 mL), and NaBH4 (0.8 mmol) was added. After stirring at room temperature for 0.5–1.0 h, the Schiff base starting material was consumed. Chloroform (30.0 mL) was added, and the organic phase was washed with brine. The aqueous layer was then extracted with chloroform (3.0 × 10.0 mL) and the combined organic extracts were dried over Na2SO4, filtered, and then concentrated under reduced pressure to produce the amine 4.
Dibenzylamine (4ac)
Yield 80%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.35–7.23 (m, 10H), 3.81 (s, 4H, 2CH2), 1.89 (s, 1H, NH).13C NMR (50 MHz, CDCl3): δ (ppm) = 140.4 (2C, C), 128.6 (4C, CH), 128.4 (4C, CH), 127.2, (2CH), 53.3 (2CH2). IR (KBr): (cm−1) = 3327 (v NH of Ar-NH-R), 3085, 3062 and 3027 (v = CH benzyl), 2814 (vs CH3), 1603 and 1453 (v C C benzyl), 1362 (v CAr-N). HRMS (ESI): m/z observed: 198.1251; C14H15N [M+H]+ requires: 198.1282; error (ppm): 1.6. Data for 4ac are in accordance with those reported elsewhere [46].
(4-tert-Butylphenyl)-N-benzylmethanamine (4bc)
Yield 94%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.38–7.23 (m, 9H), 3.82 (s, 2H, CH2), 3.78 (s, 2H, CH2), 1.94 (s, 1H, NH), 1.31 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.1 (C), 140.4 (C), 137.3 (C), 128.6 (2CH), 128.4 (2CH), 128.1 (2CH), 127.2 (CH), 125.5 (2CH), 53.4 (CH2), 52.9 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3329 (v NH of Ar-NH-R), 3086, 3060 and 3027 (v = CH benzyl), 2961 (vasCH3), 2868 (vs CH3, 1495 and 1454 (v C C benzyl), 1394 and 1363 (δs CH3 of C(CH3)3), 1269 (v CAr-N). HRMS (ESI): m/z observed: 254.1831; C18H23N [M+H]+ requires: 254.1908; error (ppm): 3.0. Data for 4bc are in accordance with those reported elsewhere [47].
N-((Naphthalen-1-yl)methyl)(phenyl)methanamine (4ad)
Yield 98%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.09–8.04 (m, 1H), 7.87–7.82 (m, 1H), 7.76 (d, J = 7.8, 1H), 7.53–7.22 (m, 9H), 4.23 (s, 2H, CH2), 3.90 (s, 2H, CH2), 2.03 (s, 1H, NH). 13C NMR (50 MHz, CDCl3): δ (ppm) = 140.3 (C), 135.8 (C), 134.1 (C), 132.0 (C), 128.9 (CH), 128.6 (2CH), 128.5 (2CH), 128.0 (CH), 127.3 (CH), 126.4 (CH), 126.3 (CH), 125.8 (CH), 125.6 (CH), 123.9 (CH), 53.8 (CH2), 50.9 (CH2). IR (KBr): (cm−1) = 3324 (v NH of Ar-NH-R), 3059 and 3028 (v = CH benzyl), 1510 (v C C naphthyl), 1452 (v C C benzyl), 1331 (v CAr-N). HRMS (ESI): m/z observed: 248.1394; C18H17N [M+H]+ requires: 248.1439; error (ppm): 1.8. Data for 4ad are in accordance with those reported elsewhere [48].
(4-tert-Butylphenyl)-N-((naphthalen-1-yl)methyl)methanamine (4bd)
Yield 96%. Brown oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.09–8.04 (m, 1H), 7.87–7.82 (m, 1H), 7.76 (d, J = 8.0, 1H), 7.51–7.41 (m, 4H), 7.39–7.28 (m, 4H), 4.24 (s, 2H, CH2), 3.88 (s, 2H, CH2), 2.12 (s, 1H, NH), 1.32 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.2 (C), 137.3 (C), 135.9 (C), 134.1 (C), 132.0 (C), 128.9 (CH), 128.2 (2CH), 128.0 (CH), 126.3 (CH), 126.2 (CH), 125.8 (CH), 125.6 (CH), 125.5 (2CH), 123.9 (CH), 53.5 (CH2), 50.9 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3323 (v NH of Ar-NH-R), 3051 (v = CH benzyl), 2960 (vasCH3), 2866 (vs CH3), 1597 and 1457 (v C C benzyl), 1511 (v C C naphthyl), 1395 and 1362 (δs CH3 of C(CH3)3), 1267 (v CAr-N). HRMS (ESI): m/z observed: 304.2003; C22H25N [M+H] + requires: 304.2065; error (ppm): 2.0. Data for 4bd are in accordance with those reported elsewhere [49].
N-Benzyl-1-(naphthalen-2-yl) methanamine (4ae)
Yield 82%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.83–7.77 (m, 4H), 7.50–7.42 (m, 3H), 7.36–7.22 (m, 5H), 3.96 (s, 2H), 3.84 (s, 2H), 2.00 (s, 1H).13C NMR (50 MHz, CDCl3): δ (ppm) = 140.0 (C), 137.5 (C), 133.6 (C), 132.9 (C), 128.7 (CH), 128.5 (CH), 128.3 (CH), 127.9 (CH), 127.9 (CH), 127.3 (CH), 126.8 (CH), 126.8 (CH), 126.2 (CH), 125.8 (CH), 53,2 (CH2), 53,2 (CH2). IR (KBr): (cm−1) = 3327 (v NH de Ar-NH-R), 3055 e 3025 (v = CH benzyl), 1601 e 1452 (v C C benzyl), 1508 (v C C naphtyl), 1359 (v CAr-N). HRMS (ESI): m/z observed:248.1405; C22H25N [M+H] + requires: 248.1439; error (ppm): 1.3.
Data for 4ae are in accordance with those reported elsewhere [48].
(4-tert-Butylphenyl)-N-((naphthalen-2-yl)methyl)methanamine (4be)
Yield 99%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.83–7.79 (m, 4H), 7.51–7.42 (m, 3H), 7.39–7.27 (m, 4H), 3.98 (s, 2H, CH2), 3.81 (s, 2H, CH2), 2.10 (s, 1H, NH), 1.32 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.2 (C), 137.8 (C), 137.2 (C), 133.6 (C), 132.9 (C), 128.3 (CH), 128.2 (2CH), 127.9 (CH), 127.9 (CH), 126.8 (CH), 126.8 (CH), 126.2 (CH), 125.8 (CH), 125.6 (2CH), 53.4 (CH2), 52.9 (CH2), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3328 (v NH of Ar-NH-R), 3054, 3023 (v = CH benzyl), 2960 (vas CH3), 2866 (vs CH3), 1602 and 1458 (v C C benzyl), 1509 (v C C naphthyl), 1393 and 1362 (δs CH3 of C(CH3)3), 1269 (v CAr-N). HRMS (ESI): m/z observed: 304.1989; C22H25N [M+H] + requires: 304.2065; error (ppm): 2.5.
General procedure for the synthesis of N-methylamines (5)
Each amine 4 (0.5 mmol) was dissolved in 1,4-dioxane (3.0 mL), except for compound 4ac, which was dissolved in water and was then heated at 65–70 °C. Formaldehyde (1.5 mmol, 37% in water), acetic acid (4.0 mmol) and zinc (1.5 mmol) were added, and the reaction mixture was stirred for 4–7 h. After, the reaction was complete, pH was adjusted to 8.5 using a 1 N NaOH solution. The mixture was washed with brine, and the aqueous phase was extracted with chloroform (2 × 30.0 mL). The combined organic extracts were concentrated under a vacuum, and the residue was purified by column chromatography, to give the N-methylamines 5.
N-Benzyl-N-methyl(phenyl)methanamine (5ac)
Yield 74%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.39–7.23 (m, 10H), 3.52 (s, 4H, 2CH2), 2.18 (s, 3H, NCH3). 13C NMR (50 MHz, CDCl3): δ (ppm) = 139.5 (2C, C), 129.1 (4C, CH), 128.4 (4C, CH), 127.1, (2CH), 62.1 (2CH2), 42.4 (NCH3). IR (KBr): (cm−1) = 3062 (v = CH benzyl), 2876 (vs CH3 of NCH3), 1601 (v C C benzyl), 1366 (δs, CH3 of NCH3). HRMS (ESI): m/z observed: 212.1422; C15H17N [M+H] + requires: 212.1439; error (ppm): 0.8.
Data for 5ac are in accordance with those reported elsewhere [50].
(4-tert-Butylphenyl)-N-benzyl-N-methylmethanamine (5bc)
Yield 79%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.39–7.23 (m, 9H), 3.52 (s, 2H, CH2), 3.51 (s, 2H, CH2), 2.19 (s, 3H, NCH3), 1.31 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.1 (C), 139.4 (C), 136.2 (C), 129.2 (2CH), 128.9 (2CH), 128.4 (2CH), 127.1 (C), 125.3 (2CH), 62.0 (CH2), 61.7 (CH2), 42.4 (NCH3), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3068, 3061 and 3027 (v = CH benzyl), 2870 (vs CH3 ofNCH3), 2874 (vs CH3), 1603 (v C C benzyl), 1513 (v C C naphthyl), 1393 and 1363 (δs CH3 of C(CH3)3), 1313 (δs, CH3 of NCH3). HRMS (ESI): m/z observed: 268.1996; C19H25N [M +H] + requires: 268.2065; error (ppm): 2.6.
N-Benzyl-N-1-(naphthalen-1-yl) methanamine (5ad)
Yield 27%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.27–8.22 (m, 1H), 7.85–7.81(m, 1H) 7.76 (d, 1H, J = 7,6) 7.51–7,43 (m, 3H), 7.39–7.20 (m, 6H), 3.93 (s, 2H), 3.95 (s, 2H), 2.19 (s, 3H13C NMR (50 MHz, CDCl3): δ (ppm) = 139.6 (C), 135.2 (C), 134.1 (C), 132.7 (C), 129.3 (CH), 128.6 (CH), 128.4 (CH), 128.1 (CH), 127.6 (CH), 127.2 (CH), 125.9 (CH), 125.8 (CH), 125.3 (CH), 125.1 (CH), 62.6 (CH2), 60.7 (CH2), 42.5 (CH3) IR (KBr): (cm−1) = 354 (v CH benzyl), 2923 (v CH2), 2862 (v CH3, N-CH3), 1588 (v C C benzyl), 1501 (v naphthyl) HRMS (ESI): m/z observed: 262.1540; C19H19N [M+H] + requires: 262.1595; error (ppm): 2.1. Data for 5ad are in accordance with those reported elsewhere [51].
Butenafine (5bd)
Yield 60%. Yellow oil. 1H NMR (200 MHz, CDCl3): δ (ppm) = 8.25–8.20 (m, 1H), 7.85–7.80 (m, 1H), 7.75 (d, J = 7.8, 1H), 7.49–7.42 (m, 3H), 7.38–7.22 (m, 5H), 3.98 (s, 2H, CH2), 3.57 (s, 2H, CH2), 2.20 (s, 3H, NCH3), 1.31 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.0 (C), 136.5 (C), 135.3 (C), 134.1 (C), 132.7 (C), 129.0 (2CH), 128.6 (CH), 128.1 (CH), 127.6 (CH), 125.8 (CH), 125.7 (CH), 125.3 (3CH), 125.1 (CH), 62.3 (CH2), 60.7 (CH2), 42.6 (NCH3), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3048 (v = CH benzyl), 2960 (vas CH3), 2868 (vs CH3 of NCH3), 1597 (v C C benzyl), 1511 (v C C naphthyl), 1395 and 1363 (δs CH3 of C(CH3)3), 1336 (δs, CH3 of NCH3). HRMS (ESI): m/z observed: 318.2141; C23H27N [M+H] + requires: 318.2221; error (ppm): 2.5. Data for 5bd are in accordance with those reported elsewhere [52].
N-Benzyl-N-methyl-1-(naphthalen-2-yl)methanamine (5ae)
Yield 72%. Mp 36.1–37.0 °C (white solid). 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.84–7.76 (m, 4H), 7.54 (dd, 1H, J = 8.4, J = 1.6), 7.47–7.22 (m, 7H), 3.67 (s, 2H), 7.51–7.43 (m, 3H), 3.93 (s, 2H), 3.95 (s, 2H), 2.19 (s, 3H).13C NMR (50 MHz, CDCl3): δ (ppm) = 139.5 (C), 137.2 (C), 133.6 (C), 133.0 (C), 129.2 (CH), 128.5 (CH), 128.1 (CH), 127.9 (CH), 127.9 (CH), 127.6 (CH), 127.5 (CH), 127.2 (CH), 126.1 (CH), 125.7 (CH), 62.2 (CH2), 62.2 (CH2), 42.5 (CH3). IR (KBr): (cm−1) = 3083, 3050, 3028 (v = CH benzyl), 2952 (vas CH3), 2868 (vs CH3 de N-CH3), 1600 (v C C benzyl), 1500 (v C C naphtyl), 1335 (δs, CH3 de N-CH3). HRMS (ESI): m/z observed: 262.1577; C23H27N [M+H]+ requires: 262.1595; error (ppm): 0.7. Data for 5ae are in accordance with those reported elsewhere [53].
(4-tert-Butylphenyl)-N-methyl-N-((naphthalen-2-yl)methyl)methanamine (5be)
Yield 73%. Mp 36.0–36.8 °C (white solid). 1H NMR (200 MHz, CDCl3): δ (ppm) = 7.83–7.77 (m, 4H), 7.54 (dd, J = 8.4 and 1.4, 1H), 7.47–7.41 (m, 2H), 7.38–7.28 (m, 4H), 3.66 (s, 2H, CH2), 3.54 (m, 2H, CH2), 2.22 (s, 3H, NCH3), 1.31 (s, 9H, 3CH3).13C NMR (50 MHz, CDCl3): δ (ppm) = 150.0 (C), 137.3 (C), 136.3 (C), 133.6 (C), 133.0 (C), 128.9 (2CH), 128.1 (CH), 127.9 (CH), 127.9 (CH), 127.6 (CH), 127.5 (CH), 126.1 (CH), 125.7 (CH), 125.3 (2CH), 62.2 (CH2), 61.8 (CH2), 42.6 (NCH3), 34.7 (C), 31.6 (3CH3). IR (KBr): (cm−1) = 3051 (v = CH benzyl), 2962 (vas CH3), 2876 (vs CH3 of N-CH3), 1622 (v C C benzyl), 1521 (v C C naphthyl), 1395 and 1363 (δs CH3 of C(CH3)3), 1328 (δs, CH3 of N-CH3). HRMS (ESI): m/z observed: 318.2147; C23H27N [M+H]+ requires: 318.2221; error (ppm): 2.3. Data for 5be are in accordance with those reported elsewhere [52].
Synthesis of amine hydrochlorides salts
Amine 4 or 5 was dissolved in ethyl ether, and HCl gas (generated from CaCl2 and a HCl 37% solution in which 1.0 g of CaCl2 was used per mL of the 37% HCl solution) was introduced. After 2–5 min, salt formation was complete, and the solvent was evaporated to achieve the desired hydrochloride salts.
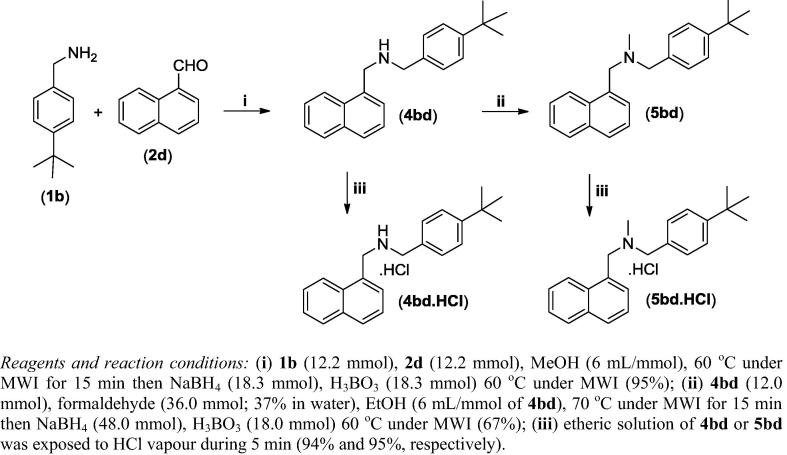
Multigram scale synthesis of butenafine (5bd)
The reaction between 1-naphthaldehyde (12.2 mmol) and N-benzyl-tert-butylamine (12.2 mmol) in methanol (73 mL) was conducted under microwave radiation (MWI) in a DISCOVER CEM® reactor under the following conditions: temperature 60 °C, maximum power 250 Watts, hold time 2 min, and run time 15 min, with vigorous stirring in an open tube. Next, NaBH4 (18.3 mmol) and H3BO3 (18.3 mmol) were added, and the reaction mixture was again heated at 60 °C under microwave radiation using the same conditions described above. After stirring in the microwave for 15 min, the solvent was removed, and the obtained mixture was dissolved in dichloromethane (DCM) and was extracted with a saturated aqueous solution of K2CO3. The organic layer was dried over Na2SO4 filtered, and concentrated to give amine 4bd in a 95% yield. In the next step, 4bd (12.0 mmol) and formaldehyde (36.0 mmol, 37% aqueous solution) were dissolved in 70 mL of ethanol, and the reaction mixture was heated under MW (conditions: temperature 70 °C, maximum power 250 Watts, hold time 2 min, and run time 15 min, with vigorous stirring in an open tube). Next, NaBH4 (48.0 mmol) and H3BO3 (18.0 mmol) were added, and the reaction mixture was again heated at 70 °C under MW using the same conditions described previously. The solvent was evaporated, and the mixture obtained was dissolved in DCM and extracted with a saturated K2CO3 solution. The organic phase was dried over Na2SO4 filtered, and concentrated in vacuo. After purification by column chromatography using hexane/ethyl acetate (3:1) as the eluent, butenafine (5bd) was obtained, with a 69% of yield.
Biologic activities
Susceptibility test
To investigate the antifungal activity of butenafine and its analogues, were determined the MIC using a broth microdilution assay following the CLSI guidelines with some modifications. The tested fungi were the yeasts C. neoformans ATCC28957 and C. gattii ATCC 32608 as well as the filamentous fungi T. rubrum (ATCC 40051) and M. gypseum (clinical isolate). Standard RPMI 1640 medium (Himedia, Brazil) at 34.54 g/L buffered with 0.165 M MOPS (Sigma-Aldrich, St. Louis, MO, USA) was used to prepare the 96-well flat-bottomed microdilution plates. For dermatophytes, the media was supplemented with 2% glucose. The test compounds were diluted in DMSO, with concentrations ranging from 64 to 0.125 µg/mL. The inoculum concentration was 0.4–5 × 104 CFU/mL for filamentous fungi and 1–5 × 103 CFU/mL for yeasts, which corresponds to two-fold the tested concentrations [54]. The MIC was determined visually as the concentration that results in 100% inhibition of fungal growth compared to the control (non-treated fungi).
Toxicity tests
Hemolysis assessment
The protocol used to test the compound’s ability to cause hemolysis of human erythrocytes (Rockland Immunochemicals, Limerick, PA, USA) was adapted from Rajamuthiah [55]. Briefly, in a 96-well plate, 50 μL of a phosphate buffered saline (PBS) solution containing 2% human erythrocytes was added to 50 μL of a PBS solution containing the serially diluted compound. The plate was incubated with butenafine and its analogues at concentration ranging from 0.0625 to 32.0 µg/mL at 37 °C for 1 h. Triton X-100 was included as a positive control. The plate was then centrifuged at 500×g for 5 min, and 50 μL of the supernatant from each well of the assay plate was transferred to a new 96-well plate. Hemolysis was confirmed by both visual observation and by measuring the absorbance at 540 nm. The compounds were evaluated in triplicates.
Cytotoxicity assay with HepG2 cells
The protocol for measuring cytotoxicity was previously described by Kwon and co-workers [56]. HepG2 cells (ATCC HB 8065; ATCC, Manassas, VA, USA) were maintained by successive passages in Dulbecco’s modified Eagle medium (DMEM; Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum, 25 mM D-glucose, 2 mM L-glutamine, 1 mM sodium pyruvate and 1% penicillin/streptomycin at 37 °C in 5% CO2. To assess cytotoxicity HepG2 cells were cultured at 70–80% confluence in culture media (100 μL/well) using 96-well plates. Serially diluted butenafine and analogues (0.125–8.0 µg/mL) were incubated with the cells at 37 °C and 5% CO2 for 24 h. Then, 10 μL of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) solution (Roche, Mannheim, Germany) was added to each well, and the plate was incubated for an additional period. WST-1 reduction was detected by reading the absorbance at 490 nm, using a Vmax microplate reader (Molecular Device, Sunnyvale, CA, USA). The percentage of surviving cells was determined as follows: survival rate (%) = (Asample − Ab)/(Ac − Ab) X 100, where Asample = Sample Absorbance, Ab = Blank (medium + wst) and Ac = Negative Control (cells + medium + wst). The compounds were evaluated in triplicate.
Toxicity assessment using the Galleria mellonella as a model
To investigate the in vivo toxicity of butenafine and its analogues, were used G. mellonella as a model. Sixth instar larvae (Vanderhorst Wholesale, St. Mary, OH, USA) were used in these experiments. The selected larvae were 2 to 2.5 cm long were light in color and weighed between 250 mg and 350 mg. The selected larvae were first disinfected with ethanol and were then injected through the last, left pro-leg with 10 µL of butenafine and analogues at a dose of 1 mg/Kg or 5 mg/kg using a Hamilton syringe [57]. The larvae were transferred to a new Petri dish and were stored in the dark at 37 °C. The larvae were observed daily, and the number of dead larvae was recorded. Control groups for this assay included a group that did not receive any injections to monitor the overall quality of the larvae during the experiment and a PBS injection group to ensure that death was not due to trauma. Killing curves were plotted, and statistical analysis was performed using Kaplan-Meier survival estimates and Graph-Pad Prism, version 5.00, for Windows (GraphPad Software, San Diego, CA, USA).
Results and discussion
Synthesis of butenafine and its analogues
Were report herein a facile synthetic process to generate butenafine (5bd; Table 1) and its analogues (see Table 1 and Scheme 1 for reference) that requires three or fewer steps. In the first step, the Schiff base 3 (Table 1) were prepared by promoting the condensation of an aldehyde (benzaldehyde, 1-naphthaldehyde or 2-naphthaldehyde) and an amine (benzylamine or 4-tert-butylbenzylamine) (Table 1). Five Schiff bases were prepared in up to a 96% yield using microwave irradiation (MWI) at 80 °C for 2–8 min and using ethanol as the solvent (Table 1). The main advantage of MWI over conventional heating is that the former is rapid and uniform, allowing the Schiff bases to be obtained by means that are rapid and clean. The next step involved reduction of the Schiff base 3 using NaBH4 as the reducing agent and using methanol as the solvent (Table 1). These reactions occurred in 20–30 min, furnishing the desired amines 4 with good to excellent yields (80–99%; Table 1) and with high purity. The classic methods for generating N-methylamines include the use of methyl iodide or dimethyl sulfate in the presence of various inorganic bases; however, these methods have several disadvantages [58]. Methyl iodide has a very low boiling point, causing air emission problems; it is also highly toxic, with an LD50 of 76 mg/kg when dosed orally in rats and is potentially carcinogenic [59]. Likewise, dimethyl sulfate is also highly toxic, with an LD50 of 205 mg/kg when dosed orally in rats and is potentially carcinogenic, mutagenic, and teratogenic [60], [61]. To overcome these issues, amine 4 was treated with formaldehyde and metal zinc in acetic acid for 4–7 h to achieve the desired N-methylamines in reasonable yields (69–79%) (Table 1). The use of formaldehyde and metal zinc in acetic acid is an efficient, selective, and cheap methylation protocol that avoids the toxic methylating agents used in the classical methods [62].
Table 1.
Synthesis of butenafine, its analogues and corresponding hydrochloride salts.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Ar1 | Ar2 | Yield (%)a |
||||
| Schiff base | Amine | Amine.HClb | N-Methylamine | N-Methylamine.HClb | |||
| 1 | a | c | 3ac (98) | 4ac (80) | 4ac.HCl (95) | 5ac (74) | 5ac.HCl (94) |
| 2 | b | c | 3bc (1 0 0) | 4bc (94) | 4bc.HCl (1 0 0) | 5bc (79) | 5bc.HCl (96) |
| 3 | a | d | 3ad (99) | 4ad (98) | 4ad.HCl (93) | 5ad (27) | 5ad.HCl (96) |
| 4 | b | d | 3bd (1 0 0) | 4bd (96) | 4bd.HCl (94) | 5bd (60) | 5bd.HCl (95) |
| 5 | a | e | 3ae (87) | 4ae (82) | 4ae.HCl (99) | 5ae (72) | 5ae.HCl (97) |
| 6 | b | e | 3be (96) | 4be (99) | 4be.HCl (1 0 0) | 5be (73) | 5be.HCl (98) |
*Reagents and reaction conditions: (i) 1 (1.0 mmol), 2 (1.0 mmol), EtOH (4 mL/mmol), 80 °C under MWI, 2–8 min. (ii) 3 (0.5 mmol), NaBH4 (0.8 mmol), MeOH (5 mL/mmol of 3), rt, 20–30 min. (iii) 4 (0.5 mmol) in dioxane (6 mL/mmol of 4), formaldehyde (1.5 mmol; 37% in water), acetic acid (4.0 mmol), zinc (1.5 mmol), 65–70 °C, 5–7 h.
Yield for purified compound.
For the preparation of hydrochloride salt, the etheric solution of 4 or 5 was exposed to HCl vapour during 2–5 min. The desirable salts were obtained after the ether being removed in vacuum.
Scheme 1.
Multigram-scale synthesis of butenafine base (5bd), butenafine hydrochloride (5bd.HCl) and their precursors (4bd and 4bd.HCl).
Finally, conversion of the amine 4 and the N-methylamine 5 to their corresponding hydrochloride salts was achieved by subjecting the amine to HCl gas (Table 1). The hydrochloride salts of the amine 4 and the N-methylamine 5 were obtained with excellent yields (up to 93%) (Table 1).
In summary, using our synthetic approach, butenafine (5bd) and its hydrochloride salt 5bd.HCl were synthesized with overall yields of 66% and 63% respectively. In addition, our synthetic strategy allowed us to prepare 17 butenafine analogues (6 Schiff bases, 11 amines and their corresponding hydrochloride salts (Table 1).
All the synthesized compounds were fully characterized using infrared (IR), proton (1H) and carbon 13 (13C) nuclear magnetic resonance (NMR) and mass spectrometry. All the data are in accordance with the proposed structures for the Schiff bases, amines and N-methylamines, and certain characteristics were observed for each class of compounds. For example, the 1H NMR spectra of the Schiff base 3 showed characteristic signals for the azomethine protons (—CH N—) at δ8.38–9.02 ppm. Likewise, stretching of the C N bond was observed in the IR spectra of the Schiff base 3 in the region 1638–1644 cm−1. Meanwhile, the 13C NMR spectra for the amine 4 showed characteristic signals at δ 50.9–53.3 ppm, corresponding to the new —CH2— carbon, while the signals for the new N-CH3 carbon of the N-methylamine 5 were observed at δ 42.4–42.6 ppm.
An alternative synthetic route was also pursued to enable multigram scale synthesis of butenafine (5bd) and its hydrochloride salt (5bd.HCl) (Scheme 1). The first step utilized a single vessel for the condensation of 1-naphthaldehyde and 4-tert-butylbenzylamine in methanol under MWI for 15 min, followed by reduction of the formed Schiff base with NaBH4 and H3BO3, again under MWI for 15 min (Scheme 1). After this two-step one-pot process, the amine 4bd was obtained, with a 95% yield. Next, amine 4bd was treated with formaldehyde in ethanol under MWI for 15 min. Then, NaBH4 and H3BO3 were added, and the reaction mixture was heated under MWI for another 15 min. Butenafine (5bd) was obtained with a 67% yield after purification (Scheme 1). The corresponding hydrochloride salts of amine 4bd and butenafine (5bd) were obtained using the same reaction conditions described in Table 1. Overall, both synthetic strategies (Table 1 and Scheme 1) were efficient in generating butenafine (5bd) and its hydrochloride salt 5bd.HCl with similar yields, as described in the literature; however, we achieved the synthesis of these substances in a rapid manner without using toxic solvents and/or without an expensive catalyst or reagent. In this multigram scale approach, were verified that the combination of the reductant NaBH4 and the catalyst H3BO3 showed better results than metal zinc. Furthermore, NaBH4 is compatible with microwaves techniques and allows for a “one-pot” process.
Biologic activities of butenafine and its analogues
In vitro antifungal activities of butenafine and its analogues
To investigate the in vitro antifungal activity of the newly generated butenafine and its analogues (Table 1), the minimal inhibitory concentration (MIC; Table 2) was determined using a broth microdilution assay following the CLSI guidelines with some modifications (M38-A2 and M27-A3). The MIC of the compounds against the yeasts, C. neoformans ATCC 28957 and C. gattii ATCC 32608 as well as the filamentous fungi, Trichophyton rubrum ATCC 40051 and Microsporum gypseum (clinical isolate) was assayed.
Table 2.
Minimal inhibitory concentration (MIC)a values (in µg/mL) of butenafine and its analogues against Cryptococcus neoformans, Cryptococcus gattii, Trichophyton rubrum and Microsporum gypseum.
| Compound | MIC values (µg/mL) |
|||
|---|---|---|---|---|
| M. gypseumb | T. rubrumc | C. neoformansd | C. gattiie | |
| 3ac | >64 | >64 | >64 | >64 |
| 4ac | >64 | >64 | >64 | >64 |
| 4ac.HCl | >64 | >64 | >64 | >64 |
| 5ac | >64 | >64 | >64 | >64 |
| 5ac.HCl | >64 | >64 | >64 | >64 |
| 3bc | >64 | >64 | >64 | >64 |
| 4bc | >64 | >64 | 64 | 64 |
| 4bc.HCl | 64 | 32 | 64 | 32 |
| 5bc | 32 | 4 | 64 | >64 |
| 5bc.HCl | 16 | 0.5 | 32 | 32 |
| 3ad | >64 | >64 | >64 | >64 |
| 4ad | >64 | >64 | >64 | >64 |
| 4ad.HCl | >64 | >64 | >64 | >64 |
| 5ad | >64 | >64 | >64 | >64 |
| 5ad.HCl | >64 | >64 | >64 | >64 |
| 3bd | >64 | >64 | >64 | >64 |
| 4bd | 2 | 0.5 | 16 | 8 |
| 4bd.HCl | 2 | 1 | 16 | 8 |
| 5bd | <0.125 | <0.125 | 1 | 1 |
| 5bd.HCl | <0.125 | <0.125 | 0.5 | 1 |
| 3ae | >64 | >64 | >64 | >64 |
| 4ae | >64 | >64 | >64 | >64 |
| 4ae.HCl | >64 | 32 | >64 | >64 |
| 5ae | >64 | >64 | >64 | >64 |
| 5ae.HCl | >64 | >64 | >64 | >64 |
| 3be | >64 | >64 | >64 | >64 |
| 4be | 4 | 8 | 8 | 4 |
| 4be.HCl | 8 | 4 | 8 | 4 |
| 5be | 0.5 | 0.25 | 16 | 32 |
| 5be.HCl | >64 | >64 | 16 | 32 |
MIC was visually determined as the concentration giving 100% inhibition of fungal growth compared with the control growth (non-treated fungi).
Clinical isolate.
ATCC number 40051.
ATCC number 28957.
ATCC number 32608.
Butenafine analogues that exhibited antifungal activity (MIC ≥ 64 µg/mL) were more efficient at inhibiting the growth of filamentous fungi compared to the two strains of yeast. Amine 4bd and its corresponding hydrochloride salt 4bd.HCl showed the lowest MIC values against filamentous fungi followed by amine 4be and its salt 4be.HCl (Table 2). It is noteworthy that butenafine (5bd; Table 2, entry 19) and its corresponding HCl salt (5bd.HCl; Table 2, entry 20) were at least 2- and 512-fold more potent against the filamentous fungi T. rubrum and M. gypseum than their regioisomers amine 5be (Table 2, entry 24) and salt 5be.HCl (Table 2, entry 25). Although 5be generated the lowest MIC values (Table 2; entry 24) among the butenafine analogues, its hydrochloride salt 5be.HCl was ineffective (MIC > 64 µg/mL) at inhibiting the growth of the fungi T. rubrum and M. gypseum with MIC values less than 32 µg/mL. This value is within the MIC range found in other studies for both dermatophytes. Thvedt and colleagues evaluated the antifungal activity of chiral benzyl-N-methyl-1-(naphthalen-1-yl)ethanamines and determined MIC values that ranged from 0.125 µg/mL to >32 µg/mL against the yeast C. neoformans and the dermatophytes T. rubrum and T. mentagrophytes [63].
Butenafine (5bd) and its hydrochloride salt 5bd.HCl served as positive controls in our study and demonstrated the lowest MIC values against all the tested fungi. The MIC values against filamentous fungi were <0.125 μg/mL; thus even at the lowest test concentration, was not detect any fungal growth. The analogues 3ac, 4ac, 4ac.HCl, 5ac, 5ac.HCl, 3ad, 4ad, 4ad.HCl, 5ad, 5ad.HCl and 3bd demonstrated no relevant antifungal activity against the tested fungi.
Even though the most significant growth inhibition was achieved against filamentous fungi, butenafine and some of its analogues were also able to reduce the growth of C. neoformans and C. gattii (Table 2). Butenafine (5bd) and its hydrochloride salt 5bd.HCl were the most potent at inhibiting the growth of C. neoformans and C. gattii, followed by the analogues/salts 4be/4be.HCl, 4bd/4bd.HCl, and 5be/5be.HCl (Table 2). Considering the in vitro antifungal activity of butenafine all its analogues, it is noteworthy that both the presence of a para-tert-butyl group and a 1-substituted naphthyl group is important for potency in this class of antifungal agents (see Table 2).
In vitro toxicity effect of butenafine and its analogues
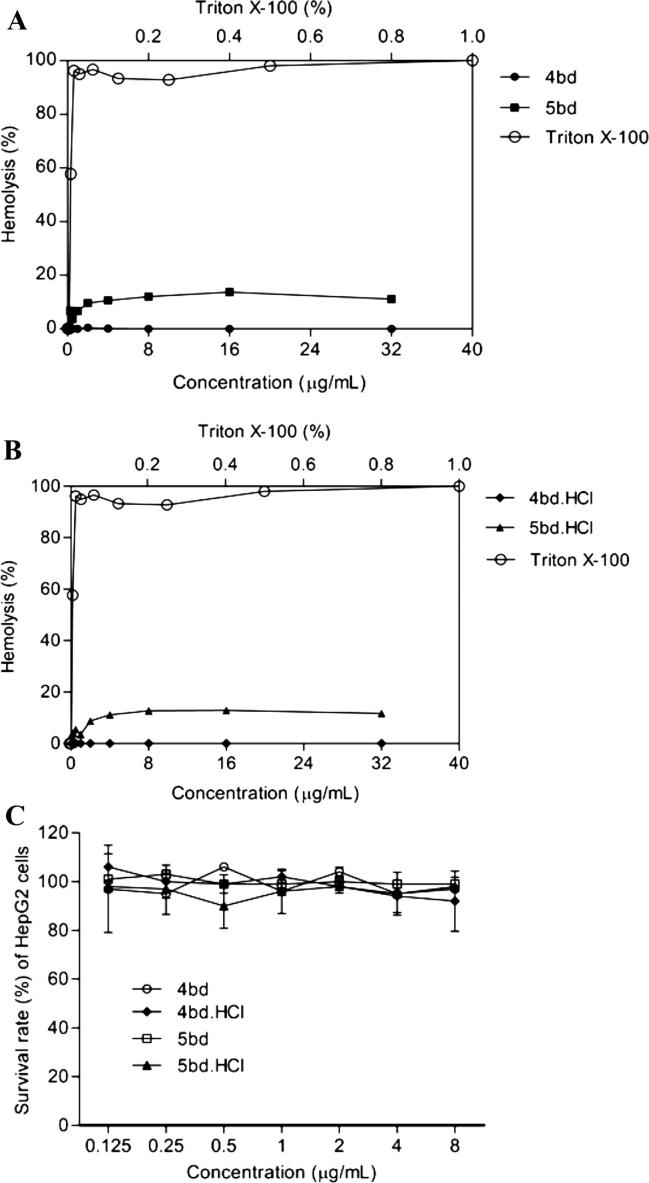
To move a compound forward as an antimicrobial agent, was evaluated the toxicity of the compounds to determine if there were any deleterious effects on to mammalian cells. In vitro toxicity of butenafine (5bd), its hydrochloride salt (5bd.HCl) and the most potent analogues against filamentous fungi, 4bd (free-base) and 4bd.HCl (HCl-salt), was evaluated by determining if the compounds elicited hemolysis of human blood cells or if they altered the survival of liver cells (HepG2) (Fig. 2, panels A, B and C). Human red blood cells were treated with serial dilutions of butenafine and its analogues (0.0625–32.0 µg/mL) for 1 h. Cells treated with serial dilutions of Triton X-100 (0.0019–1% solution) served as the positive control (Fig. 2, panels A and B). Butenafine (5bd) and its hydrochloride salt (5bd.HCl) were slightly hemolytic at higher concentrations of but neither its analogue 4bd nor 4bd.HCl lysed red blood cells compared to the Triton X-100 control. The positive control Triton X-100 caused a high rate of hemolysis at concentrations of 0.0078% or higher. The highest percentage of hemolysis caused by the investigational compounds was less than 14%, which indicates low toxicity, as were considered 10% hemolysis as the limit for detection of toxic effects [57]. Thus, the LD50 of the compounds for human red blood cells were >32 μg/mL, which is greater than the observed antifungal MIC values.
Fig. 2.
In vitro of toxicity of butenafine (5bd), its analogue (4bd) and corresponding hydrochloride salts (4bd.HCl and 5bd.HCl). Hemolytic activity of butenafine (5bd), its analogue (4bd) and corresponding hydrochloride salts (4bd.HCl and 5bd.HCl) (0.0625–32.0 µg/mL) and of Triton X-100 (0.0019–1% – positive control) on human erythrocytes (A and B; respectively). Cytotoxicity of butenafine (5bd), its analogue (4bd) and corresponding hydrochloride salts (4bd.HCl and 5bd.HCl) to HepG2. The survival rates of HepG2 cells were measured after treatment with serially diluted concentrations (0.125–8.0 µg/mL) of butenafine compounds (C). Cell viability was measured spectrophotometrically by detecting degradation of WST-1 dye into formazan by viable cells, which produces an intense color.
The cytotoxicity of 5bd and 5bd.HCl as well as the most potent analogues against filamentous fungi, 4bd and 4bd.HCl, was also evaluated using the human liver carcinoma-derived HepG2 cell line. HepG2 cells were treated with serial dilutions of the drug using a concentration range of 0.125–8.0 µg/mL, and cellular viability was measured. The cells treated with butenafine and its analogues were almost 100% viable at all the drug concentrations tested (Fig. 2, panel C), indicating a compound LD50> 8 μg/mL.
In vivo toxicity of butenafine and its analogues
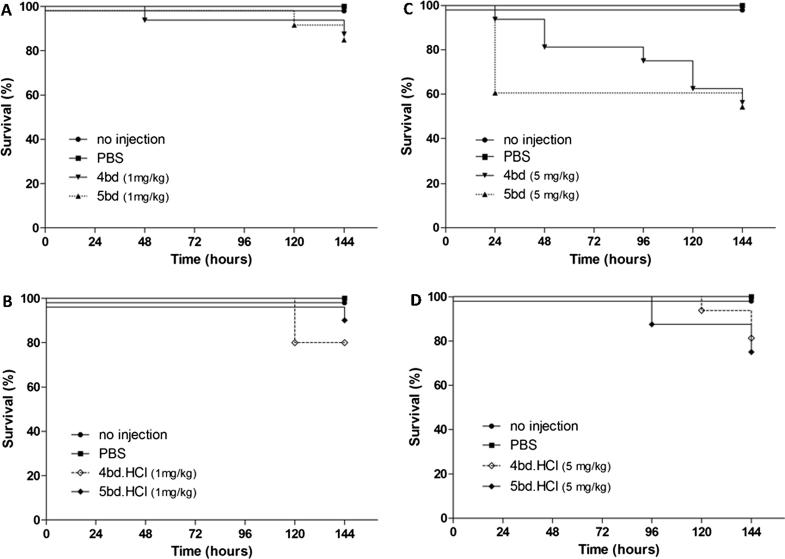
Larvae of the greater wax moth (Galleria mellonella) were used as an invertebrate model system to evaluate the toxicity of butenafine (5bd), its hydrochloride salt (5bd.HCl) and its most potent analogues, 4bd (free-base) and 4bd.HCl (HCl-salt). Such a model provides meaningful data at low cost and does not require the same ethical considerations as mammalian models [64]. In addition, the G. mellonella model satisfies many basic requirements of a useful mammalian model, such as having an immune system with a similar structure and function as that of mammals as well as the presence of both cellular and humoral defenses [65], [66]. The larvae mortality rate of butenafine and its analogues was less than 20%, even after prolonged exposure (144 h), when compounds were dosed 1 mg/kg (Fig. 3, panels A and B). However, 40% of the larvae experienced mortality within 24 h after administration of butenafine (5bd) at 5 mg/kg (Fig. 3, panel C). The larvae treated with demethylated analogue 4bd showed a time-dependent survival behavior, with almost 40% mortality observed 144 h after being treated with this analogue at a dose of 5 mg/kg (Fig. 3, panel C). No relevant reduction (≤20%) in larvae was observed for 5bd.HCl or 4bd.HCl when these substances were dosed at 5 mg/kg (Fig. 3, panel D). In summary, all the tested substances presented low toxicity in the G. mellonella model, except for butenafine (5bd) when dosed at 5 mg/kg, which significantly reduced larvae survival (Fig. 3, panel C).
Fig. 3.
Survival of G. mellonella larvae treated with butenafine (5bd), its analogue (4bd) and corresponding hydrochloride salts (4bd.HCl and 5bd.HCl) at 1 mg/kg (A and B) or 5 mg/kg (C and D). No injection; stands for non-treated larvae. PBS; stands for larvae treated with the vehicle.
Conclusions
A short synthetic route to obtain the antifungal butenafine and 17 butenafine analogues was developed. The synthetic approach using Schiff bases as precursors yielded the desired products in multigram scales. All the synthesized compounds were evaluated for their in vitro antimicrobial activity against fungi of clinical interest. The most active compound, 4bd (and its hydrochloride salt 4bd.HCl), is a simple demethylated analogue of butenafine that can be prepared in only two steps from commercial sources. The low toxicity of this analogue and toxicity of butenafine (5bd) and its hydrochloride salt (5bd.HCl) was demonstrated in two mammalian cell types (hemolysis of human blood cells and survival of the liver cells line HepG2) and in an invertebrate model system (G. mellonella). Therefore, compound 4bd (and its salt) represents a promising antifungal agent as an alternative to butenafine that benefits from a short and simple synthetic route and that has the potential to reduce the burning sensation that is a reported side effect of butenafine.
Conflict of interest
The authors report no conflicts of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
The authors are thankful for the financial support provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Ângelo de Fátima, Cleide Viviane Buzanello Martins, Daniel de Assis Santos, Maria Aparecida Resende-Stoianoff and Rossimiriam Pereira de Freitas are supported by research fellowships from CNPq. Danielle Leticia da Silva and Thais Furtado Ferreira Magalhães were supported by research fellowships provided by the Brown-Brazil Initiative. Dr. Fuchs and Mylonakis received provisions through a grant from the Brown-Brazil Initiative. The authors thank Esther da Silva Dias for her contribution in the previous project that motivated us to pursue the development of new butenafine analogues as antifungal agents.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Peres N.T., Maranhao Fc Fau - Rossi A., Rossi A Fau - Martinez-Rossi N.M., Martinez-Rossi N.M. Dermatophytes: host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–667. doi: 10.1590/s0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 2.Hay R. Superficial fungal infections. Medicine. 2013;41:716–718. [Google Scholar]
- 3.Hube B., Hay R., Brasch J., Veraldi S., Schaller M. Dermatomycoses and inflammation: the adaptive balance between growth, damage, and survival. J Med Mycol. 2015;25:e44–e58. doi: 10.1016/j.mycmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Rotta I., Otuki M.F., Sanches A.C.C., Correr C.J. Eficácia de antifúngicos tópicos em diferentes dermatomicoses: uma revisão sistemática com metanálise. Rev Assoc Med Bras. 2012;58:308–318. [PubMed] [Google Scholar]
- 5.Ghannoum M.A., Chaturvedi V., Espinel-Ingroff A., Pfaller M.A., Rinaldi M.G., Lee-Yang W., et al. Intra- and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytes. J Clin Microbiol. 2004;42:2977–2979. doi: 10.1128/JCM.42.7.2977-2979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci M., Varon A.G., Garnica M., Akiti T., Barreiros G., Trope B.M., et al. Increased incidence of invasive fusariosis with cutaneous portal of entry, Brazil. J Emerg Infect Dis. 2013;19:1567–1572. doi: 10.3201/eid1910.120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badiee P., Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 2014;139:195–204. [PMC free article] [PubMed] [Google Scholar]
- 8.Enoch D.A., Yang H., Aliyu S.H., Micallef C. The changing epidemiology of invasive fungal infections. Methods Mol Biol. 2017;1508:17–65. doi: 10.1007/978-1-4939-6515-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Kontoyiannis D.P. Invasive mycoses: strategies for effective management. Am J Med. 2012;125:S25–S38. doi: 10.1016/j.amjmed.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Vaezi A., Fakhim H., Khodavaisy S., Alizadeh A., Nazeri M., Soleimani A., et al. Epidemiological and mycological characteristics of candidemia in Iran: a systematic review and meta-analysis. J Mycol Med. 2017;27:146–152. doi: 10.1016/j.mycmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J.F., et al. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob Agents Chemother. 2017;61:e01056–e1117. doi: 10.1128/AAC.01056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezai M.S., Vaezi A., Fakhim H., Soleimani A., Mohammad Jafari H., Mohseni S., et al. Successful treatment with caspofungin of candiduria in a child with Wilms tumor; review of literature. J Mycol Med. 2017;27:261–265. doi: 10.1016/j.mycmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Hagen F., Lumbsch H.T., Arsic Arsenijevic V., Badali H., Bertout S., Billmyre R.B., et al. Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus Genus. MSphere. 2017;30:e00238–e317. doi: 10.1128/mSphere.00238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badali H., Alian S., Fakhim H., Falahatinejad M., Moradi A., Mohammad Davoudi M., et al. Cryptococcal meningitis due to Cryptococcus neoformans genotype AFLP1/VNI in Iran: a review of the literature. Mycoses. 2015;58:689–693. doi: 10.1111/myc.12415. [DOI] [PubMed] [Google Scholar]
- 15.Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M., et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira P.R., Resende S.M., de Oliveira F.C., de Oliveira A.C. Ceratite fúngica. Arq Bras Oftalmol. 2001;64:75–79. [Google Scholar]
- 17.Gregorí Valdés B.S. Estructura y actividad de los antifúngicos. Rev Cubana Farm. 2005;39 1–1. [Google Scholar]
- 18.Millikan L.E. Current concepts in systemic and topical therapy for superficial mycoses. Clin Dermatol. 2010;28:212–216. doi: 10.1016/j.clindermatol.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Iwatani W., Arika T., Yamaguchi H. Two mechanisms of butenafine action in Candida albicans. Antimicrob Agents Chemother. 1993;37:785–788. doi: 10.1128/aac.37.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer D.L., Weiss J., Rodriguez D.A., Hebert A.A., Swinehart J.M. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Am Acad Dermatol. 1997;37:231–235. doi: 10.1016/s0190-9622(97)80130-2. [DOI] [PubMed] [Google Scholar]
- 21.Nahm W.K., Orengo I., Rosen T. The antifungal agent butenafine manifests anti-inflammatory activity in vivo. J Am Acad Dermatol. 1999;41(2):203–206. doi: 10.1016/s0190-9622(99)70049-6. [DOI] [PubMed] [Google Scholar]
- 22.Mingeot-Leclercq M.-P., Gallet X., Flore C., Van Bambeke F., Peuvot J., Brasseur R. Experimental and conformational analyses of interactions between butenafine and lipids. Antimicrob Agent Chemother. 2001;45:3347–3354. doi: 10.1128/AAC.45.12.3347-3354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters J.A.M., Posthumus T.A.P., Van Vliet NP, Zeelen F.J., Johnson W.S. Biomimetic polyene cyclizations. Total synthesis of dl-19-nor-4-pregnen-20-one. Asymmetric induction by the initiating center. J Org Chem. 1980;45:2208–2214. [Google Scholar]
- 24.Takahashi M., Tanaka M., Sakamoto E., Imai M., Funakoshi K., Sakai K., et al. Application of Rh-catalyzed cyclization to the formation of a chiral quaternary carbon. Chem Pharm Bull. 2000;48:1822–1825. doi: 10.1248/cpb.48.1822. [DOI] [PubMed] [Google Scholar]
- 25.Yueheng L, Lifang M, Tao J. Method of preparing aminopropylene kind antifungus medicine. CN1597660A, 2005.
- 26.Sass D.C., de Oliveira K.T., Constantino M.G. Synthesis of homoallylic oxygenated α-methylene-γ-butyrolactones: a model for preparing biologically active natural lactones. Tetrahedron Lett. 2008;49:5770–5772. [Google Scholar]
- 27.Beydoun K., Ghattas G., Thenert K., Klankermayer J., Leitner W. Ruthenium-catalyzed reductive methylation of imines using carbon dioxide and molecular hydrogen. Angew Chem Int Ed. 2014;53:11010–11014. doi: 10.1002/anie.201403711. [DOI] [PubMed] [Google Scholar]
- 28.Fu M.-C., Shang R., Cheng W.-M., Fu Y. Boron-catalyzed N-alkylation of amines using carboxylic acids. Angew Chem Int Ed. 2015;54:9042–9046. doi: 10.1002/anie.201503879. [DOI] [PubMed] [Google Scholar]
- 29.Arai K, Arita M, Sekino T, Komoto N, Hirose S. Benzylamine derivatives, process for production thereof, and use thereof. EP0221781 A2; 1987.
- 30.Maeda T., Takase M., Ishibashi A., Yamamoto T., Sasaki K., Arika T., et al. Synthesis and antifungal activity of butenafine hydrochloride (KP-363), a new benzylamine antifungal agent. Pharmac Soc Jpn. 1991;111(2):126–137. doi: 10.1248/yakushi1947.111.2_126. [DOI] [PubMed] [Google Scholar]
- 31.Ling P, Wang S, He Y, AihuaLiu, Zhang L, Tang X. Method for preparing butenafine hydr ochloride. CN101077858 A, 2007.
- 32.Stanetty P., Koller H., Pürstinger G., Grubner S. Synthese neuer 7-benzofuranmethanamine als heterocyclische analoga des squalenepoxidasehemmers butenafine. Arch Pharm Chem Life Sci. 1993;326:351–358. [Google Scholar]
- 33.Salmoiraghi I., Rossi M., Valenti P., Da Re P. Allylamine type xanthone antimycotics. Arch Pharm Chem Life Sci. 1998;331:225–227. doi: 10.1002/(sici)1521-4184(199806)331:6<225::aid-ardp225>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Krauss J., Stadler M., Bracher F. Synthesis and structure-activity relationships of novel benzylamine-type antifungals as butenafine-related antimycotics. Arch Pharm Chem Life Sci. 2017;350 doi: 10.1002/ardp.201600342. e1600342 (1-20) [DOI] [PubMed] [Google Scholar]
- 35.Castellano S., La Colla P., Musiu C., Stefancich G. Azole antifungal agents related to naftifine and butenafine. Arch Pharm Chem Life Sci. 2000;333:162–166. [PubMed] [Google Scholar]
- 36.da Silva C.M., da Silva D.L., Martins C.V., de Resende M.A., Dias E.S., Magalhaes T.F., et al. Synthesis of aryl aldimines and their activity against fungi of clinical interest. Chem Biol Drug Des. 2011;78:810–815. doi: 10.1111/j.1747-0285.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 37.Magalhães T.F.F., da Silva C.M., de Fátima Â., da Silva D.L., Modolo L.V., Martins C.V.B., et al. Hydroxyaldimines as potent in vitro anticryptococcal agents. Lett Applied Microbiol. 2013;57:137–143. doi: 10.1111/lam.12086. [DOI] [PubMed] [Google Scholar]
- 38.Gasparto A.K., Baltazar L.M., Gouveia L.F., da Silva C.M., Byrro R.M., Rachid M.A., et al. 2-(benzylideneamino)phenol: a promising hydroxyaldimine with potent activity against dermatophytoses. Mycopathologia. 2015;179:243–251. doi: 10.1007/s11046-014-9850-5. [DOI] [PubMed] [Google Scholar]
- 39.Dhar D.N., Taploo C.L. Schiff bases and their applications. J Sci Ind Res. 1982;41:501–506. [Google Scholar]
- 40.Qin W., Long S., Panunzio M., Biondi S. Schiff bases: a short survey on an evergreen chemistry tool. Molecules. 2013;18:12264. doi: 10.3390/molecules181012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrin DD, Amarengo WLF. Purification of Laboratory Chemicals. vol. 107. 3rd ed. Oxford: Kieboom, A. P. G; 1988.
- 42.Marinescu L.G., Pedersen C.M., Bols M. Safe radical azidonation using polystyrene supported diazidoiodate(I) Tetrahedron. 2005;61:123–127. [Google Scholar]
- 43.Baker J.W., Nathan W.S., Shoppee C.W. 430. The mechanism of aromatic side-chain reactions with special reference to the polar effects of substituents. Part VI. The effects of p-alkyl substituents on prototropy in the methyleneazomethine system. J Chem Soc. 1935 1847. [Google Scholar]
- 44.Ushakov D.B., Plutschack M.B., Gilmore K., Seeberger P.H. Factors influencing the regioselectivity of the oxidation of asymmetric secondary amines with singlet oxygen. Chem Eur J. 2015;21:6528–6534. doi: 10.1002/chem.201500121. [DOI] [PubMed] [Google Scholar]
- 45.Joly G.D., Jacobsen E.N. Thiourea-catalyzed enantioselective hydrophosphonylation of imines: practical access to enantiomerically enriched α-amino phosphonic acids. J Am Chem Soc. 2004;126:4102–4103. doi: 10.1021/ja0494398. [DOI] [PubMed] [Google Scholar]
- 46.Miriyala B., Bhattacharyya S., Williamson J.S. Chemoselective reductive alkylation of ammonia with carbonyl compounds: synthesis of primary and symmetrical secondary amines. Tetrahedron. 2004;60:1463–1471. [Google Scholar]
- 47.Ayesa S., Samuelsson B., Classon B. A One-pot, solid-phase synthesis of secondary amines from reactive alkyl halides and an alkyl azide. Synlett. 2008;2008:97–99. [Google Scholar]
- 48.Giovenzana G.B., Imperio D., Penoni A., Palmisano G. Reductive amination with zinc powder in aqueous media. Beilstein J Org Chem. 2011;7:1095–1099. doi: 10.3762/bjoc.7.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arika T, Yokoo M, Amamiya K, Maeda T. Antifungal composition. EP0310122 (A1); 1991.
- 50.Hanada S., Ishida T., Motoyama Y., Nagashima H. The ruthenium-catalyzed reduction and reductive N-alkylation of secondary amides with hydrosilanes: practical synthesis of secondary and tertiary amines by judicious choice of hydrosilanes. J Org Chem. 2007;72:7551–7559. doi: 10.1021/jo070591c. [DOI] [PubMed] [Google Scholar]
- 51.Molander G.A., Beaumard F. Cross-coupling of mesylated phenol derivatives with potassium ammonio- and amidomethyltrifluoroborates. Org Lett. 2011;13:1242–1245. doi: 10.1021/ol200128y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda T, Yamamoto TT, Takase mituo, Sasaki K, Arika T, Yokoo M, et al. Amine derivatives, processes for preparing the same and fungicides containing the same. EP0164697 (A2); 1984.
- 53.Dahn H., Solms U., Zoller P. Über die hydrogenolyse benzylähnlicher gruppen in tertiären aminen. 3. mitteilung über hydrogenolyse. Helv Chim Acta. 1952;35:2117–2131. [Google Scholar]
- 54.Santos D.A., Barros M.E.S., Hamdan J.S. Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J Clin Microbiol. 2006;44:98–101. doi: 10.1128/JCM.44.1.98-101.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajamuthiah R., Fuchs B.B., Conery A.L., Kim W., Jayamani E., Kwon B., et al. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS One. 2015;10:e0124595. doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon B., Kumar P., Lee H.K., Zeng L., Walsh K., Fu Q., et al. Aberrant cell cycle reentry in human and experimental inclusion body myositis and polymyositis. Hum Mol Genet. 2014;23:13. doi: 10.1093/hmg/ddu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer D., Li Y., Ahlemeyer B., Krieglstein J., Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 58.Jiang X., Tiwari A., Thompson M., Chen Z., Cleary T.P., Lee T.B.K. A Practical method for N-methylation of indoles using dimethyl carbonate. Org Proc Res Dev. 2001;5:604–608. [Google Scholar]
- 59.Johnson M.K. Metabolism of iodomethane in the rat. Biochemical J. 1966;98:38–43. doi: 10.1042/bj0980038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ono Y. Dimethyl carbonate for environmentally benign reactions. Catal Today. 1997;35:15–25. [Google Scholar]
- 61.Rippey J.C.R., Stallwood M.I. Nine cases of accidental exposure to dimethyl sulphate—a potential chemical weapon. Emerg Med J. 2005;22:878–879. doi: 10.1136/emj.2004.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.da Silva R.A., Estevam I.H.S., Bieber L.W. Reductive methylation of primary and secondary amines and amino acids by aqueous formaldehyde and zinc. Tetrahedron Lett. 2007;48:7680–7682. [Google Scholar]
- 63.Krane Thvedt T.H., Kaasa K., Sundby E., Charnock C., Hoff B.H. Chiral N-benzyl-N-methyl-1-(naphthalen-1-yl)ethanamines and their in vitro antifungal activity against Cryptococcus neoformans, Trichophyton mentagrophytes and Trichophyton rubrum. Eur J Med Chem. 2013;68:482–496. doi: 10.1016/j.ejmech.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 64.Megaw J, Thompson TP, Lafferty RA, Gilmore BF. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 11;139:197–201. [DOI] [PubMed]
- 65.Hoffmann J.A. Innate immunity of insects. Curr Op Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 66.Gibreel T.M., Upton M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus. J Antimicrob Chemother. 2013;68:2269–2273. doi: 10.1093/jac/dkt195. [DOI] [PubMed] [Google Scholar]