Abstract
Rebalancing of the RANKL/OPG system seems to be an effective treatment strategy in postmenopausal osteoporosis. Here, we evaluate the knockdown of RANKL by in-vivo-delivered siRNA in a rat model of osteoporosis. Virus-like-particles (VLPs) derived from polyoma JC virus were used for delivering RANKL siRNA in ovariectomized (OVX) rats. 48 rats were ovariectomized and treated with either 17β-estradiol (E2), VLPs containing RANKL siRNA (siRANKL), or VLPs containing non-cognate siRNA (siCtrl). All OVX groups were subdivided into the prophylaxis group (PG) and the therapy group (TG). The PG received treatment directly after being OVX for 10 weeks. The TG received treatment 5 weeks after being OVX for 5 weeks. Rats were sacrificed 10 weeks after being OVX. Bone and blood samples were analyzed. E2 and siRANKL showed a significant knockdown of RANKL mRNA. A protein knockdown was observed with E2 and siRANKL in the TG but not in the PG. No distinct improvements in biomechanical and morphological properties of the bones were observed after siRANKL treatment. In the PG, E2 protected the bone structure. We demonstrated successful mRNA and protein knockdown by VLP-mediated RNAi in vivo. Knockdown of membranous RANKL did not result in significant improvements of bone properties in this model of early-stage postmenopausal osteoporosis.
Keywords: RANKL, osteoporosis, VLP, RNA interference
Introduction
Postmenopausal osteoporosis is an underestimated, widespread disease. Due to demographic trends, the prevalence of osteoporosis will further increase in the next decades.1, 2 The treatment of postmenopausal osteoporosis is still a challenge, and better understanding of the underlying principles of various treatment options is needed.
In postmenopausal osteoporosis, trabecular bone (e.g., distal radius, femoral neck, vertebrae, proximal tibiae) is primarily affected because of its higher bone turnover with respect to cortical bones. Cortical bone also shows bone loss, but only over a longer period of time.3
In recent years, several studies suggested a major role for the receptor activator of nuclear factor κB (NF-κB) ligand/osteoprotegerin (RANKL/OPG) system to regulate bone resorption and bone metabolism.4, 5, 6 RANKL is produced among other cells by osteoblasts. Binding of RANKL to its receptor RANK on osteoclast precursors induces their differentiation into osteoclasts and thereby increases bone resorption.7, 8 OPG is another important protein in this regulatory loop, which is also expressed by osteoblasts and is able to block RANKL by acting as a soluble decoy receptor.9, 10 Thus, OPG decreases bone resorption, while RANKL promotes it.
Several ways of influencing the RANKL/OPG balance have been described in the literature. In recent years, the monoclonal RANKL antibody denosumab was approved for treatment of postmenopausal osteoporosis, as well as bone tumors, and showed a decrease in bone loss in several clinical studies.11, 12, 13, 14, 15 Upon systemic delivery, denosumab non-specifically inhibits RANKL in the whole organism. This can result in severe systemic side effects, including joint pain, myalgia, ostealgia, infectious diseases of the respiratory and the urogenital systems, gastrointestinal dysfunction, and skin irritation.16 Furthermore there is evidence for rebound-associated vertebral fractures following the discontinuation of denosumab.17 A bone-specific inhibition of RANKL could be more effective, and side effects to the whole organism could be minimized.
Using RNAi-mediated knockdown is a promising alternative path to reduce the amount of RANKL protein. Small interfering RNA (siRNA) can be used to post-transcriptionally silence the target gene.18 One of the main hurdles of applying siRNA-mediated knockdowns in vivo is the delivery of siRNA into the body.19 A new and promising solution for this problem is the use of virus-like particles (VLPs).20, 21 VLPs are derived from the human JC polyoma virus and can be utilized to carry and deliver different types of molecules, including synthetic siRNAs.19 A tissue-specific effect can enabled by modulating the tropism through the attachment of targeting proteins to the VLP surface. Recently, we presented VLP-mediated RANKL inhibition with siRNA in healthy rats showing promising results.19
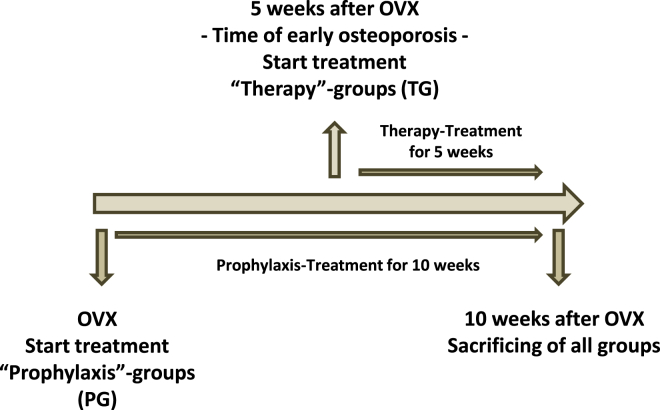
The aim of this study was to evaluate the effects of RANKL knockdown by VLP-mediated RNAi in a rat model of osteoporosis. Within this scope, we investigated the effect on biomechanical and morphological properties of bone in the early stage of postmenopausal osteoporosis. Furthermore, we intended to investigate whether there are different effects of starting RANKL knockdown before versus after the onset of osteoporosis (Figure 1). Potential poisonous side effects of long-term siRNA delivery by VLPs in vivo were also examined. We used the ovariectomized (OVX) rat, which is a standard animal model of osteoporosis.22 The entry point for VLPs into osteoblasts was the 5HT-2a receptor.23 Osteoblasts express membranous RANKL.24, 25 Thus, this study mainly evaluated the effects of membranous RANKL knockdown in osteoporosis-affected bones.
Figure 1.
Study Design
All ovariectomized groups were subdivided into prophylaxis and therapy groups. The prophylaxis group (PG) received treatment directly after ovariectomy for 10 weeks (OVX). Rats in the therapy group (TG) received treatment 5 weeks after being OVX for 5 weeks. Ten weeks after being OVX, all rats were sacrificed.
Results
qPCR Displayed RANKL Knockdown in Bones
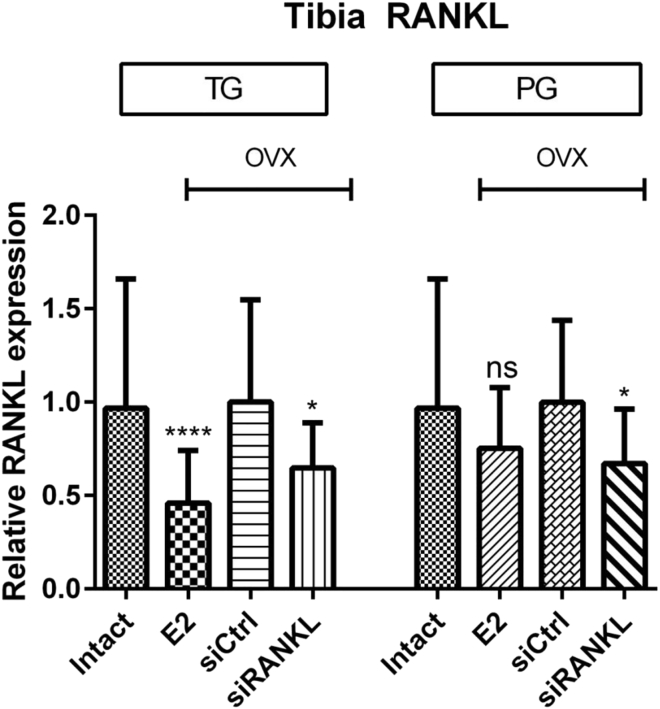
mRNA RANKL expression levels of homogenized rat tibiae were measured by qRT-PCR as described later. 17β-Estradiol (E2) and siRANKL showed a significant knockdown of RANKL in the OVX rats compared with siCtrl in the therapy group (TG) and siRANKL also in the prophylaxis group (PG) (E2 in TG: 54 ± 5%, siRANKL in TG: 35 ± 5%, E2 in PG: 25 ± 6%, siRANKL in PG: 33 ± 5%) (Figure 2). There is a significant initial effect of E2-mediated RANKL knockdown in the TG, but over the course of 10 weeks, the effect of E2 was not significantly different from that of siRANKL or intact rats (Figure 2).
Figure 2.
qPCR of RANKL mRNA in Tibiae
siRANKL showed a significant knockdown of RANKL (about 35%) in ovariectomized therapy and control groups. RANKL mRNA was also suppressed in E2-treated rats, especially in the TG. *p < 0.05 versus siCtrl; ****p < 0.0001 versus siCtrl. ns, not significant.
To further evaluate the effect of RANKL silencing on the RANKL/OPG system, we determined the expression of OPG in the tibia. In the TG, E2 administration led to a significant knockdown of OPG, whereas siRANKL showed no effect. In the PG, however, E2 did not show significant OPG regulation, whereas in 3 selected animals with the strongest RANKL knockdown, a tendency of increased OPG expression could be observed (Figure S1).
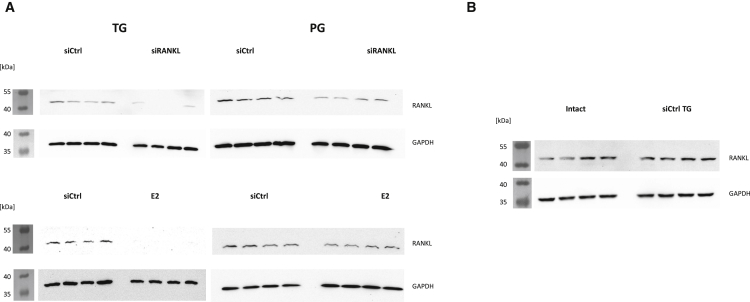
Western Blot Confirmed Knockdown of RANKL on the Protein Level
To evaluate whether the knockdown of RANKL can also be detected on the protein level, we performed western blots of tibiae from four individuals of every rat cohort (Figure 3). The TG animals showed an obvious knockdown of RANKL expression when treated with siRANKL or E2 (Figure 3). In the PG, this knockdown was not observed (Figure 3).
Figure 3.
Western Blot Analysis of RANKL Protein in Tibiae
(A) Treatment with E2 and siRANKL showed an obvious knockdown of RANKL in the TG similar to results found with qPCR analysis. In the PG, no knockdown could be shown on the protein level in contrast to qPCR results. (B) Treatment with non-cognate siRNA (siCtrl) showed no effect on protein expression.
Body Weight, Uterine Wet Weight, and Toxicity
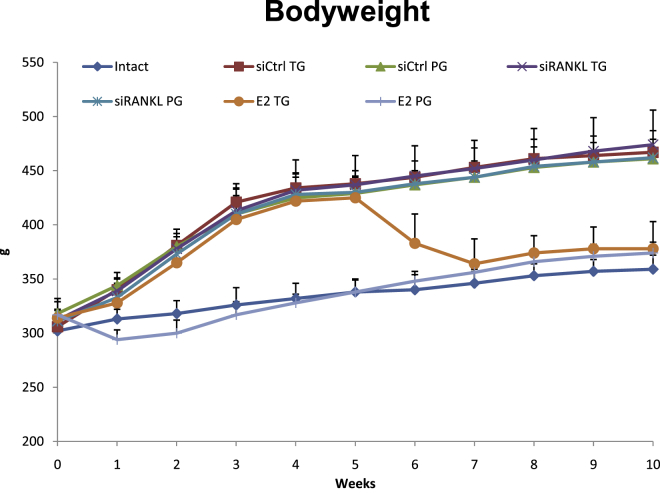
At the beginning of the study, all rats had approximately the same body weight (311.5 ± 12.8 g). The body weight increased in all groups due to normal growth. As expected, weight gain was significantly pronounced in OVX rats due to typical changes in metabolism. Treatment with E2 in the PG directly after ovariectomy prevented this distinct increase in body weight. When E2 was given 5 weeks after ovariectomy in the TG, body weight dropped in the first 2 weeks of treatment to roughly the same values as those in control animals and then followed their curve (Figure 4). Treatment with siRANKL or siCtrl showed no effects on body weight.
Figure 4.
Development of Body Weight during the Course of the Experiment
Intact rats and rats treated with E2 directly after ovariectomy showed increased body weight in the context of normal growth. Rats that underwent ovariectomy showed the typical pronounced weight increase. Rats treated with E2 5 weeks after ovariectomy (E2 TG) showed a reduction in weight in the context of E2 treatment. Treatment with virus-like particles had no effect on body weight.
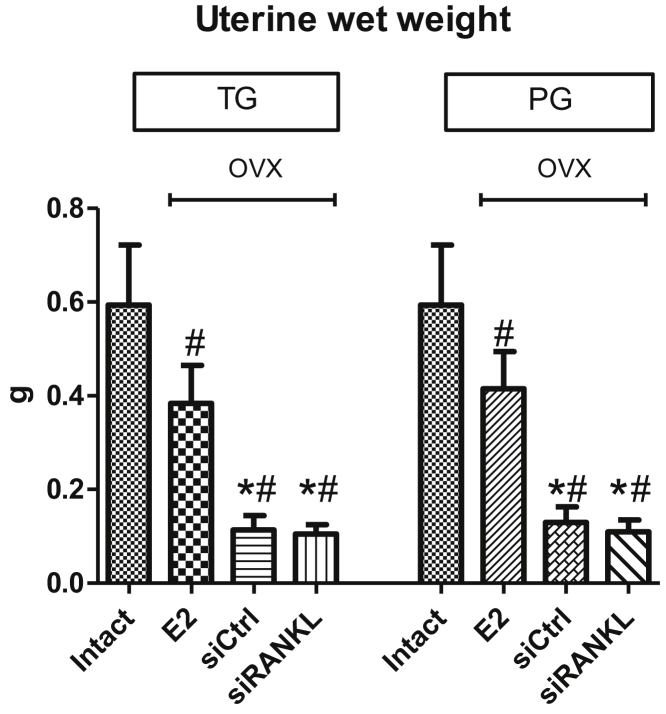
Uterine wet weight decreased significantly after ovariectomy in all OVX rats (Figure 5). The groups that received E2 displayed significantly higher uterine wet weight compared with VLP groups (Figure 5).
Figure 5.
Changes in Uterine Wet Weight
Uterine wet weight decreased after ovariectomy in rats treated with virus-like particles (VLPs) compared with uterine wet weight in intact animals. Groups that received E2 displayed significantly increased uterine wet weight compared with VLP groups. *p < 0.05 versus E2; #p < 0.05 versus intact.
There were no obvious signs of toxicity or poisonous side effects of VLPs at the injection sites. During the study, the rats showed no signs of illnesses. Food intake did not differ between the groups (data not shown). No obvious changes or side effects were found in macroscopic explorations of organs (liver, brain, kidneys, gut) after sacrifice.
Biomechanical Assessment
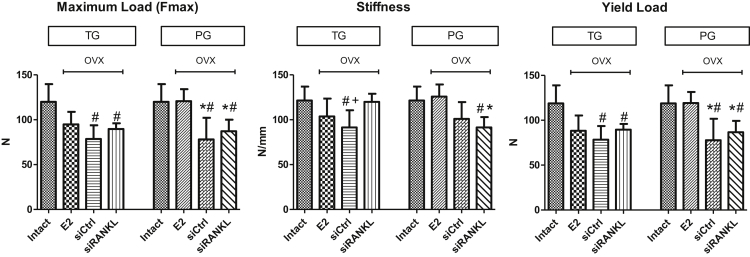
Intact rats had the highest values in almost all measured biomechanical properties (Figure 6). OVX rats treated with E2 showed significantly higher values, compared with the siCtrl group, in the PG in maximum load and yield load (Figure 6). In the TG, no significant improvements after E2 treatment were observed compared with the untreated siCtrl group.
Figure 6.
Intact Rats Showed the Highest Values in Almost All Biomechanical Properties
E2 showed significant rescue in Fmax and yield load in PG compared with the untreated siCtrl group. In the TG, no significant effects by E2 were observed. Treatment with siRANKL increased stiffness in the TG compared with siCtrl. In all other biomechanical properties, no significant changes were observed in the TG and PG following treatment with siRANKL. The effect of siRANKL in the TG was comparable to that of E2. *p > 0.05 versus E2; #p > 0.05 versus intact; +p > 0.05 versus siRANKL.
Treatment with siRANKL showed significantly increased stiffness in TG compared with siCtrl (Figure 6). In all other measured biomechanical properties, no significant changes compared with siCtrl in the PG and TG were observed. In the TG, the effect of siRANKL treatment was comparable to E2.
In Vivo pQCT
Five weeks after ovariectomy, in the TG, all OVX rats had significantly decreased bone mineral density (BMD) compared with that of intact rats, as expected (Table 1). At that time, osteopenia had already developed after ovariectomy, and treatment was not yet started. Trabecular BMD showed no significant differences between the OVX rats in the different groups. Ten weeks after ovariectomy, the BMD was still similar between E2, siRANKL, and siCtrl rats and was significantly decreased compared to BMD in intact rats (Table 1).
Table 1.
Results of In Vivo pQCT
| Time of In Vivo CT after Being OVX (Tibiae) | Intact |
E2 TG |
siCtrl TG |
siRANKL TG |
E2 PG |
siCtrl PG |
siRANKL PG |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 5 Weeks: BMD (mg/cm3) | 664.9*,** | 51.1 | 540.8*** | 39.5 | 541.3*** | 38.7 | 550.4*** | 35.7 | 677.3*,**,**** | 59.8 | 536.5*** | 17.2 | 545.8*** | 30.1 |
| 10 Weeks: BMD (mg/cm3) | 700.1*,** | 45.9 | 575.0*** | 45.9 | 551.2*** | 22.8 | 557.0*** | 35.6 | 707.5*,**,**** | 49.3 | 543.3*** | 14.4 | 558.2*** | 24.8 |
For all groups, n = 8. Intact rats and rats treated with E2 for 10 weeks (PG) had significant improved BMD. Treatment with VLP or siRANKL had no significant effect on BMD. *p < 0.05 versus Ctrl; **p < 0.05 versus siRANKL; ***p < 0.05 versus intact; ****p < 0.05 versus TG.
In the PG, E2-treated rats showed similar BMD as that of intact rats 5 and 10 weeks after ovariectomy. OVX rats treated with siRANKL and siCtrl had significantly decreased BMD compared with intact rats. There were no differences between siRANKL and siCtrl rats (Table 1).
Post-mortem Micro-computed Tomography
In the TG, E2, siRANKL, and siCtrl rats showed decreased trabecular bone compared with intact rats in 2D and 3D analyses (Figure 7). siRANKL showed no improvement compared with siCtrl. In cortical properties, no significant differences were observed between the groups (Table 2).
Figure 7.
Sagittal Sections of Tibiae in μCT Scans
Bones of intact rats and rats treated with E2 for 10 weeks (PG) have a significantly higher bone quality compared with other groups. E2 treatment for 5 weeks (TG) shows no beneficial effect. siRANKL has no effect on trabecular bone properties.
Table 2.
Post-mortem μCT of Tibiae
| Sample Size | Intact |
E2 TG |
siCtrl TG |
siRANKL TG |
E2 PG |
siCtrl PG |
siRANKL PG |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 2D Analysis of Tibiae | ||||||||||||||
| Number of N.Nd | 28.9*,** | 8.6 | 7.8*** | 3.3 | 8.6*** | 3.1 | 8.9*** | 4.3 | 29.2*,**,**** | 9.2 | 9.6*** | 7.1 | 8.5*** | 5.1 |
| % Trabecular density | 43.8*,** | 13.1 | 13.6*** | 3.3 | 11.3*** | 2.5 | 13.4*** | 3.9 | 33.3*,**,***,**** | 3.3 | 13.9*** | 5.9 | 11.6*** | 3.7 |
| Tb Wi (μm) | 176.7*,** | 63.7 | 67.8*** | 7.5 | 67.8*** | 8.2 | 73.1*** | 9.5 | 111.3*,**,***,**** | 25.1 | 67.4*** | 10.4 | 66.6*** | 9.1 |
| Trabecular bone area (mm2) | 4.4*,** | 1.4 | 1.3*** | 0.2 | 1.3*** | 0.3 | 1.4*** | 0.4 | 3.6*,**,***,**** | 0.7 | 1.5*** | 0.6 | 1.3*** | 0.5 |
| Ct Wi (μm) | 477.7 | 76.4 | 444.1 | 48.9 | 476.8 | 67.3 | 460.6 | 54.9 | 478.9 | 75.5 | 464.6 | 69.8 | 465.1 | 65.7 |
| 3D Analysis Tibiae | ||||||||||||||
| BMD [mg/cm3] | 649.5 | 142.5 | 486.9 | 94.5 | 542.4 | 88.4 | 498.6 | 112.2 | 641.6**** | 53.9 | 504.2 | 109.5 | 500.5 | 69.9 |
| % BV/TV [%] | 53.8** | 9.1 | 39.4*** | 6.8 | 44.4 | 6.2 | 40.5*** | 7.5 | 50.7*,**,**** | 4.4 | 40.4*** | 7.2 | 40.3*** | 3.8 |
| Trabecular number (Tb. N) | 187.9*,** | 60.9 | 32.6*** | 16.8 | 20.4*** | 30.2 | 26.9*** | 12.9 | 104.1*,**,***,**** | 45.8 | 39.6*** | 22.3 | 19.5*** | 8.5 |
| Trabecular volume (mm3) | 3.14*,** | 1.53 | 0.54*** | 0.24 | 0.47*** | 0.49 | 0.51*** | 0.22 | 1.83**,***,**** | 0.85 | 0.81*** | 0.38 | 0.29*** | 0.13 |
| Number of N.Nd | 223.7*,** | 72.5 | 33.6*** | 12.8 | 19.9*** | 29.9 | 22.1*** | 12.6 | 113.6*,**,***,**** | 54.5 | 39.9*** | 24.5 | 18.5*** | 9.8 |
For all groups, n = 8. *p < 0.05 versus Ctrl; **p < 0.05 versus siRANKL; ***p < 0.05 versus intact; ****p < 0.05 versus TG.
In the PG, E2 rats showed similar results in several bone properties in 2D and 3D analysis compared with intact rats (Figure 7; Table 2). siRANKL and siCtrl rats showed significantly decreased structural bone properties. There was no improvement in siRANKL compared with siCtrl (Table 2).
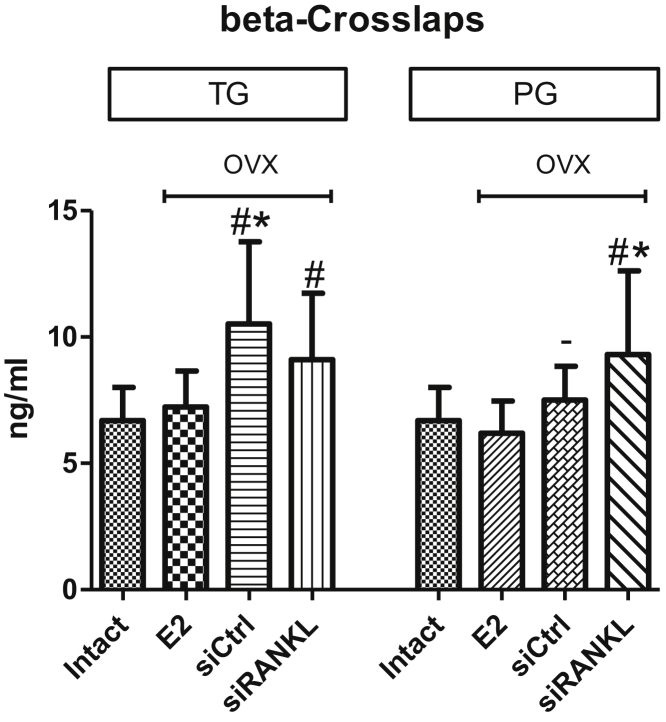
Serum Analysis of Beta-Crosslaps
In the TG, the serum levels of beta-crosslaps of intact rats and OVX rats treated with E2 were equal. OVX rats treated with siCtrl showed the highest serum levels. Treatment with siRANKL was not able to significantly decrease the enhanced levels of beta-crosslaps after ovariectomy, and the levels were similar to those of rats treated with siCtrl (Figure 8).
Figure 8.
Serum Analysis of Beta-Crosslaps
Rats treated with E2 had similar levels of beta-crosslaps as those of intact rats. Treatment with siRANKL had no effect on beta-crosslap levels after ovariectomy. siCtrl had no effect in the TG; however, in the PG, beta-crosslaps decreased after treatment. *p < 0.05 versus E2; #p < 0.05 versus intact; −p < 0.05 versus TG.
Surprisingly, in the PG, OVX rats treated with siCtrl did not display an increase in beta-crosslaps. OVX rats treated with siRANKL showed significantly increased beta-crosslaps compared with intact rats and OVX rats treated with E2 (Figure 8).
Discussion
The in vivo use of VLPs is a new and promising pathway for molecular therapy. Recently, we demonstrated, for the first time, the possibility to decrease RANKL expression in bones of rats by VLP-mediated delivery of siRNA.19 The aim of this study was to further extend this approach and to evaluate the effects of membranous RANKL knockdown by VLP-mediated RNAi in the early stage of a rat model of osteoporosis.
To evaluate the influence of VLP-based RANKL silencing, we compared the VLP-mediated knockdown of RANKL with the gold standard E2.26 The experiment was designed to have two different rat cohorts that underwent two different treatment strategies: PG and TG. In the PG, treatment started directly after ovariectomy and was given for 10 weeks. The TG treatment started 5 weeks after ovariectomy, at the early stage of osteoporosis development.
In our study, we confirmed the possibility to decrease expression of RANKL in osteoporotic bones by VLP-mediated RNA interference. The mRNA RANKL expression was significantly decreased up to 35% in both the TG and PG. The control groups treated with gold standard E2 also showed a significant knockdown of RANKL mRNA in TG, while knockdown was less pronounced and not significant in the PG. Intact rats showed no difference in RANKL mRNA levels compared with OVX rats treated with siCtrl. With a western blot technique, we could prove a knockdown of RANKL protein mediated by siRANKL and E2. Again, in the PG, after treatment over a time period of 10 weeks, we did not observe clear differences in RANKL protein expression in E2-treated groups. Furthermore, we found a decreased expression of OPG mRNA in spot samples of E2 rats in the TG and a tendency of increasing OPG mRNA after treatment with siRANKL for 10 weeks (PG).
There are studies showing an increase of RANKL mRNA after ovariectomy compared with intact rats.27, 28 This is in contrast to our results. The reason for these contradicting reports is likely caused by different experimental setups. Ikeda et al. investigated their model already 3 weeks after ovariectomy. Interestingly, they demonstrated increased levels of RANKL mRNA mainly in chondrocytes in hypertrophic growth plate and only minor expression in trabecular endosteum of femora.27 In samples of cortical bone, expression of RANKL mRNA was not increased, and when compared with young 8-week-old intact rats, it was even suppressed.27 In other studies, rats were much older (almost 1 year old) and had been used as breeder rats previously.28 In the present study, we investigated the expression of RANKL in spot samples of the whole tibiae of 5-month-old OVX rats. Our results confirm the findings of our previous study, where we also did not see any significant increase in RANKL mRNA after ovariectomy compared with sham-operated or intact rats.29, 30
In the present study, we demonstrated a significant decrease in RANKL mRNA in OVX rats treated with E2 compared with siCtrl rats in the TG. This is consistent with results in previous in vitro studies.31 In our study, the effect was more pronounced in the TG than in the PG. In western blot analyses, we also detected a significant knockdown of RANKL in the TG. However, when E2 was given for 10 weeks (PG), a knockdown of RANKL could not be demonstrated. Reasons for this effect are still not clear. We hypothesize a rebound-pathway-promoted synthesis of RANKL in response to long-term knockdown. Similar to knockdown of RANKL mRNA and protein by E2, we proved a knockdown of RANKL in bones by siRANKL. The knockdown was similar in the TG and PG on the mRNA level. In western blot analyses, we also demonstrated a knockdown of RANKL protein in the TG after 5 weeks, but not after 10 weeks (PG), of treatment. These results are consistent with a separated pathway, which induces a rebound effect of RANKL synthesis. Furthermore, these results confirm an E2-like effect on RANKL expression in osteoporotic bone by VLP-mediated RANKL RNA interferemce.
To evaluate the influence of RANKL knockdown in osteoporotic bones, we performed several biomechanical and morphological tests. The tests confirmed E2 as a gold standard to prevent osteoporosis development. We demonstrated distinctly superior results of E2 treatment for bone properties, especially when given for 10 weeks after ovariectomy (PG). When given for 5 weeks, similar effects on bones could not be demonstrated, even when a systemic effect of E2 was shown by increased uterine wet weight, decreased body weight, and decreased levels of beta-crosslaps. This is in contrast with several previous studies. However, there have been also reports that could not show significant effects of E2 after 6 weeks of treatment.32
The RANKL knockdown by siRANKL showed only a few beneficial effects on osteoporotic bones in the early stage of osteoporosis. Effects on bone did not differ between 5 weeks or 10 weeks of treatment. There can be several reasons for this result.
First, the knockdown of RANKL was only moderate, with 35% efficiency. This might have been too low to induce significant effects on the bones. Furthermore, as our approach targets formation of RANKL at the mRNA level, we do not alter the level of preexistent RANKL on the surface. Thus, the effect of knockdown probably develops slowly with a turnover of membrane proteins.
Second, there is evidence that even bone loss in early osteoporosis in rodents is mainly due to decreased osteoanabolic effects by osteoblasts and not due to increased bone loss by osteoclast activity.33 This may, in part, explain combined observations of preservation of bone stiffness in the TG siRANKL with decreased yield load. Missing bone formation after ovariectomy would lead to a relative lack of bone mass over time, as observed in our computed tomography (CT) analyses. In combination with decreased osteoclastic resorption and, therefore, decreased net remodeling, a relative increase in mineralization of bone tissue would be expected, which would lead to relatively higher stiffness of the tissue. This would be consistent with our observations.
Third, a knockdown of membranous RANKL could be compensated via circulating soluble RANKL expressed by other cells of mesenchymal lineage, endothelial cells, and T cells.6, 34, 35 We could not detect significant amounts of soluble RANKL in our bone samples at the end of treatment (Figure S2), but there may have been pronounced differences at the time closer to ovariectomy, where, presumably, most of the bone loss happened. In the present study, we used the 5-HT2a receptor as a cellular uptake receptor for VLPs. It is present in humans and rats and is involved in bone formation.23, 36 We used this key receptor because of good knockdown results of RANKL in bones in a previous feasibility study.19 Cells that produce soluble RANKL may, however, not possess the 5HT2a receptor, and, therefore, our treatment would not efficiently target this source of RANKL. This may be also the reason for still-elevated beta-crosslap levels in serum after siRANKL treatment compared with E2 treatment in OVX rats. However, reasons for decreased levels of beta-crosslaps in the siCtrl PG are still not clear from the author’s point of view. In contrast, treatment with monoclonal antibodies like denosumab targets all existing RANKL at the time of treatment, leading to a stronger effect on bone but also stronger side effects.14, 37 It may be interesting to design VLPs with a more specific tropism for mesenchymal stem cells, endothelial cells, and T cells to discriminate the relative importance of different contributing cell systems.
For unimpeachable bone specificity, a novel cellular uptake receptor needs to be created. Considering the study’s results, the need of absolute bone specificity in the case of RANKL knockdown in osteoporosis-affected bone seems to be questionable.
In conclusion, we confirmed a knockdown of proteins in bones by VLP-mediated RNA interference. A long-term use of VLPs in rats showed no obvious signs of toxicity. VLP is a promising carrier of nucleic acids for delivery in vivo. A 35% knockdown of membranous RANKL in osteoporosis-affected bones showed no significant rescue of morphological or biomechanical properties in the early stage of osteoporosis.
Materials and Methods
VLP Production and Loading
VLPs derived from capsid protein VP1 of human JC polyoma virus (JCV VP1) were produced and loaded as described elsewhere.19 In brief, the codon-optimized JC VP1 DNA was transferred as a BamHI/HindIII and SphI/NcoI amplicon into the 2× pFBDM-vector-based expression system for baculoviral expression.38 The VP1 gene containing baculovirus was generated as described by Fitzgerald et al., and expression of VP1 was performed in Hi5 cells according to the manufacturer’s manual (Invitrogen, Carlsbad, CA, USA).38
Viral-capsid-containing supernatant was harvested by centrifugation (30 min, 10,000 × g) and filtered through a 0.45-μm filter. Viral capsids were precipitated by the addition of polyethylene glycol (PEG) 8000 at room temperature to a final concentration of 7.5% (w/v). Precipitate was separated by centrifugation (30 min, 10,000 x g) and was resuspended in 20 mM HEPES (pH 7.4) containing 150 mM NaCl, 15 mM EGTA, and DTT each (dissociation buffer). After 2 hr at room temperature on a tumbling shaker, the remaining precipitate was removed by centrifugation (30 min, 21,000 × g at 4°C). Afterward, the VP1-containing supernatant was dialyzed (molecular weight cut-off [MWCO] = 6–8 kDa) against 5 L of 20 mM HEPES (pH 7.4) containing 150 mM NaCl and 1 mM CaCl2 (reassembly buffer) at 4°C under constant stirring overnight. Dialyzed viral-capsid-containing samples were centrifuged (30 min, 21,000 × g at 4°C) and further purified via size-exclusion chromatography using a Sephacryl S-300 HR gel filtration column connected to an ÄKTA Avant system. The column was equilibrated with reassembly buffer. Viral capsids were eluted within the void volume of the column and were concentrated using a Vivaspin column with MWCO of 30 kDa (Sartorius, Germany) at 4°C to the desired concentration of 0.8–1 mg/mL. Empty viral capsids were stored at −80°C until use.
For transduction, the desired amount of VP1 was incubated in dissociation buffer at room temperature for 30 min. Per 25 μg of VP1, 7 μL of a 20-μM siRANKL (J-094995-09 and J-094995-10, GE Healthcare) or control RNA (D-001100-01, GE Healthcare; or Cy3-labeled control, AM17120, Thermo Fisher Scientific, Waltham, MA, USA) was added and incubated for another 30 min. VP1 was dialyzed against 5 L of reassembly buffer at 4°C under constant stirring overnight.
Animals and Injections
All procedures on animals were approved by the local institutional animal care and use committee (district authorities of Oldenburg, Germany). The experiment was performed with 56 3-month-old female Sprague-Dawley rats (Fa. Harlan-Winkelmann, Borchen, Germany).22 All animals were maintained according to the German animal protection laws and received a soy-free diet throughout the experiment (ssniff Special Diet, Soest, Germany).
One group (intact, n = 8) received no ovariectomy and no special pharmacological treatment. The remaining rats underwent ovariectomy and were separated into 3 different groups (n = 8 per group). One group was treated with E2 in an orally administered dosage of 10 mg/kg food according to 0.6 mg/kg body weight per day. This group represented the gold standard for anti-osteoporotic treatment.26 A second group was treated with VLPs and non-cognate siRNA intraperitoneal (i.p.) injection (150 μg VLP containing 11.2 μg RNA per injection or 0.5 mg/kg body weight per week). Rats in this group represent the untreated OVX control group (siCtrl). The third group received VLPs loaded with functional RANKL siRNA (150 μg VLP containing 11.2 μg RNA per injection or 0.5 mg/kg body weight, i.p. injection, 1× per week) to decrease RANKL expression (siRANKL).
The aim of the study was to evaluate the treatment effects before and after the onset of osteoporosis in OVX rats. Therefore, treatment was initiated 5 weeks after ovariectomy, a time at which osteopenia is already present (in the TG) (Figure 1). Therapy was performed for up to additional 5 weeks. In the second part of our study, we investigated the effects of prophylaxis (in the PG) (Figure 1). In this part, all OVX rats received their treatment after ovariectomy for 10 weeks (Figure 1).
At the end of the experimental period (10 weeks after ovariectomy), all rats were sacrificed using CO2 anesthesia. Serum and tibiae were removed for analyses. We used the left tibiae for micro-computed tomography (μCT) and biomechanical testing. The right tibiae were used for gene expression and protein expression analyses. Serum samples were used for β-crosslap measurements. Tibiae for μCT and biomechanical tests, as well as serum samples, were stored at −20°C until analysis. For gene and protein expression analysis, bone samples were stored at −80°C.
qPCR
Total RNA was isolated as described in our previous publication.19 Tibiae of all animals were homogenized using the Mikro-Dismembrator S (Sartorius, Goettingen, Germany) and mixed with β-mercaptoethanol. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Due to high viscosity of the samples, a prolonged homogenization time (10 min) after TRIzol mixing was used. RNA was measured using the Qubit System (Life Technologies, Carlsbad, CA, USA), and reverse transcription was performed with 2,000 ng total RNA using the SensiFastcDNA Synthesis Kit (Bioline, Taunton, MA, USA). Real-time qPCR was performed on the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using QuantiTect Primer Assays (QIAGEN, Hilden, Germany). Relative expression of RANKL (primer QT00195125) was calculated via the ΔΔCt method, using beta-2-microglobulin (QT00176295) as a housekeeping gene.39
Western Blot
SDS gel electrophoresis was performed according to standard protocols. Proteins were extracted from bone material by the addition of 25 mM Tris (pH 7), containing 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 1 mM NP-40 (radioimmunoprecipitation assay buffer); separated by standard SDS gel electrophoresis; and transferred onto a nitrocellulose membrane followed by blocking in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.2% Tween 20 [pH 7.4]) containing 5% skimmed milk powder. Primary antibody (anti-RANKL [ab22113] or anti-GAPDH [ab8245], Abcam, Cambridge, MA, USA) was diluted in the same buffer (anti-RANKL, 1:1,000; and anti-GAPDH, 1:4,000) and incubated with the membrane for 16 hr at 4°C (anti-RANKL) or for 2 hr at room temperature (anti-GAPDH). Membrane was washed 3 times with TBST, and secondary antibody (anti-mouse or anti-rabbit, 1:10,000, Life Technologies, Carlsbad, CA, USA) was added for 1 hr at room temperature. After 3 washes with TBST, horseradish peroxidase (HRP) substrate (Luminata Forte, Merck, Darmstadt, Germany) was added, and signals were evaluated using an ECL machine (Chemocam Imager, INTAS, Göttingen, Germany).
Serum Analysis
The enzyme immune assay RatLaps CTX-I (AC-06F1, Immunodiagnostic Systems Holdings, Boldon Colliery, UK) was used to measure β-crosslap levels in serum samples. The analyses were performed according to the manufacturer’s instructions.
μCT
In Vivo pQCT
In vivo quantitative μCT (pQCT) was performed 5 and 10 weeks after ovariectomy. The rats were anesthetized with isoflurane; afterward, BMD of tibia metaphyses was scanned using the XCT Research SA (Stratec Medizintechnik, Pforzheim, Germany), as described earlier.40, 41 Analyses of tibiae were done using XCT-6.20C software (Stratec Medizintechnik, Pforzheim, Germany).
Post-mortem μCT
To measure BMD, bone volume density (BV/TV), and other morphological bone properties of tibiae, we used the Quantum FX μCT (Caliper Life Sciences, Hopkinton, MA, USA). The following scan parameters were used: 70 kVp and 200 μA, resulting in a 40 × 40 × 40 μm3 voxel size. In every scan, a phantom block with several known mineral densities was included. 3D Osteo Analyze software (developed in our laboratory) was used to calculate bone parameters according to the American Society for Bone and Mineral Research (ASBMR).
To obtain additional morphological data, we performed structural analyses on 2D images of μCT scans. Four images of sagittally cut tibiae were analyzed with the MetaMorph Basic Acquisition Software (Leica Mikrosysteme Vertrieb, Wetzlar, Germany). Collected data were trabecular nodes (N.Nd), trabecular thickness (Tb Wi), cortical thickness (Ct Wi), trabecular density, and trabecular bone area.
Biomechanical Assessment
A standardized bending test of tibiae was designed according to the procedure described by Stürmer et al.42 We utilized a Zwick mechanical testing device (type 145 660 Z020/TND, Zwick, Ulm, Germany) to perform a resistance test of the proximal tibia. The defrosted bones were fixed to the testing machine, and a stamp was lowered. The stamp had a driving force of 1 N, with a speed of 5 mm/min. The test results were obtained with a relative accuracy of 0.2%–0.4% over the range of 2–500 N. The test machine stopped automatically when the line of the curve declined more than 10 N. Throughout the test process, the compressive force was measured every 0.1 mm by the testXpert software.
We analyzed the maximum load (Fmax), stiffness (S), and yield load (yL). Maximum load is the maximum force that the bone can withstand. Stiffness represents the elasticity of the bone and is defined as a slope of the linear part of the force-deformation curve. The yield load represents the inflection point demarcating elastic and plastic deformation.
Statistical Analysis
Differences between groups were analyzed by one-way ANOVA with a Tukey-Kramer post hoc test (GraphPad Prism, La Jolla, CA, USA). Results were considered significantly different when p values were <0.05. Data are presented as the mean and SD.
Author Contributions
D.B.H., K.O.B., D.D., N.L.-D., M.K., and S. Schneider conducted the experiments; D.B.H., J.G., A.F.S., and S. Sehmisch designed the experiments; D.B.H., S. Schneider, and J.G. wrote the paper; D.B.H., K.O.B., J.G., S. Sehmisch, D.D.: data analysis and interpretation; D.B.H., J.G., A.F.S., and M.K. edited and revised the manuscript.
Conflicts of Interest
All authors have no conflicts of interest.
Acknowledgments
The authors are grateful to R. Castro-Machguth, A. Witt, E. Eckermann-Felkl, A. Backhaus, and A. Schuder for technical support. We acknowledge support by the Open Access Publication Funds of the Goettingen University.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.06.001.
Supplemental Information
References
- 1.Duquet N. [Osteoporosis: treatment and pharmaceutical care] J. Pharm. Belg. 2014;(2):14–24. [PubMed] [Google Scholar]
- 2.Osterkamp R. [Population developments in Germany until 2050] Chirurg. 2005;76:10–18. doi: 10.1007/s00104-004-0971-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Zhou X., Fujita H., Onozuka M., Kubo K.Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013;2013:213234. doi: 10.1155/2013/213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World J. Orthop. 2013;4:207–217. doi: 10.5312/wjo.v4.i4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce B.F., Xing L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 6.Findlay D.M., Atkins G.J. Relationship between serum RANKL and RANKL in bone. Osteoporos. Int. 2011;22:2597–2602. doi: 10.1007/s00198-011-1740-9. [DOI] [PubMed] [Google Scholar]
- 7.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 8.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Lüthy R., Nguyen H.Q., Wooden S., Bennett L., Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H., Shima N., Nakagawa N., Mochizuki S.I., Yano K., Fujise N., Sato Y., Goto M., Yamaguchi K., Kuriyama M. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 11.Cumming K., Hoyle G.E., Hutchison J.D., Soiza R.L. Prevalence, incidence and etiology of hyponatremia in elderly patients with fragility fractures. PLoS ONE. 2014;9:e88272. doi: 10.1371/journal.pone.0088272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bone H.G., Bolognese M.A., Yuen C.K., Kendler D.L., Wang H., Liu Y., San Martin J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2008;93:2149–2157. doi: 10.1210/jc.2007-2814. [DOI] [PubMed] [Google Scholar]
- 13.Papapoulos S., Lippuner K., Roux C., Lin C.J., Kendler D.L., Lewiecki E.M., Brandi M.L., Czerwiński E., Franek E., Lakatos P. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos. Int. 2015;26:2773–2783. doi: 10.1007/s00198-015-3234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., Peacock M., Miller P.D., Lederman S.N., Chesnut C.H., AMG 162 Bone Loss Study Group Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 15.Genant H.K., Engelke K., Hanley D.A., Brown J.P., Omizo M., Bone H.G., Kivitz A.J., Fuerst T., Wang H., Austin M., Libanati C. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone. 2010;47:131–139. doi: 10.1016/j.bone.2010.04.594. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency. (2015). Zusammenfassung des EPAR für die Öffentlichkeit Prolia [Summary of the European Public Assessment Report for the Public on Prolia] (EMA/681041/2015). http://www.ema.europa.eu/docs/de_DE/document_library/EPAR_-_Summary_for_the_public/human/001120/WC500093527.pdf.
- 17.Popp A.W., Zysset P.K., Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos. Int. 2016;27:1917–1921. doi: 10.1007/s00198-015-3458-6. [DOI] [PubMed] [Google Scholar]
- 18.Siomi H., Siomi M.C. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann D.B., Böker K.O., Schneider S., Eckermann-Felkl E., Schuder A., Komrakova M., Sehmisch S., Gruber J. In vivo siRNA delivery using JC virus-like particles decreases the expression of RANKL in rats. Mol. Ther. Nucleic Acids. 2016;5:e298. doi: 10.1038/mtna.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petry H., Goldmann C., Ast O., Lüke W. The use of virus-like particles for gene transfer. Curr. Opin. Mol. Ther. 2003;5:524–528. [PubMed] [Google Scholar]
- 21.Chang C.F., Wang M., Ou W.C., Chen P.L., Shen C.H., Lin P.Y., Fang C.Y., Chang D. Human JC virus-like particles as a gene delivery vector. Expert Opin. Biol. Ther. 2011;11:1169–1175. doi: 10.1517/14712598.2011.583914. [DOI] [PubMed] [Google Scholar]
- 22.Kalu D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 23.Galli C., Macaluso G., Passeri G. Serotonin: a novel bone mass controller may have implications for alveolar bone. J. Negat. Results Biomed. 2013;12:12. doi: 10.1186/1477-5751-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrado A., Maruotti N., Cantatore F.P. Osteoblast role in rheumatic diseases. Int. J. Mol. Sci. 2017;18:E1272. doi: 10.3390/ijms18061272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tat S.K., Pelletier J.P., Lajeunesse D., Fahmi H., Duval N., Martel-Pelletier J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: influence of osteotropic factors. Bone. 2008;43:284–291. doi: 10.1016/j.bone.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambacciani M., Vacca F. Postmenopausal osteoporosis and hormone replacement therapy. Minerva Med. 2004;95:507–520. [PubMed] [Google Scholar]
- 27.Ikeda T., Utsuyama M., Hirokawa K. Expression profiles of receptor activator of nuclear factor kappaB ligand, receptor activator of nuclear factor kappaB, and osteoprotegerin messenger RNA in aged and ovariectomized rat bones. J. Bone Miner. Res. 2001;16:1416–1425. doi: 10.1359/jbmr.2001.16.8.1416. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Dong X.L., Leung P.C., Wong M.S. Differential mRNA expression profiles in proximal tibia of aged rats in response to ovariectomy and low-Ca diet. Bone. 2009;44:46–52. doi: 10.1016/j.bone.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Komrakova M., Stuermer E.K., Tezval M., Stuermer K.M., Dullin C., Schmelz U., Doell C., Durkaya-Burchhardt N., Fuerst B., Genotte T. Evaluation of twelve vibration regimes applied to improve spine properties in ovariectomized rats. Bone Rep. 2014;7:172–180. [Google Scholar]
- 30.Hoffmann D.B., Sehmisch S., Hofmann A.M., Eimer C., Komrakova M., Saul D., Wassmann M., Stürmer K.M., Tezval M. Comparison of parathyroid hormone and strontium ranelate in combination with whole-body vibration in a rat model of osteoporosis. J. Bone Miner. Metab. 2017;35:31–39. doi: 10.1007/s00774-016-0736-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q.P., Yang L., Li X.P., Xie H., Liao E.Y., Wang M., Luo X.H. Effects of 17β-estradiol on adiponectin regulation of the expression of osteoprotegerin and receptor activator of nuclear factor-κB ligand. Bone. 2012;51:515–523. doi: 10.1016/j.bone.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Kavuncu V., Sahin S., Baydas G., Ilhan N., Ozercan I., Yasar A., Pekkutucu I., Ilhan N., Ozercan R. A comparison of estrogen and two different doses of calcitonin in ovariectomized rats. Yonsei Med. J. 2003;44:508–516. doi: 10.3349/ymj.2003.44.3.508. [DOI] [PubMed] [Google Scholar]
- 33.Seitz S., Keller J., Schilling A.F., Jeschke A., Marshall R.P., Stride B.D., Wintermantel T., Beil F.T., Amling M., Schütz G. Pharmacological estrogen administration causes a FSH-independent osteo-anabolic effect requiring ER alpha in osteoblasts. PLoS ONE. 2012;7:e50301. doi: 10.1371/journal.pone.0050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwood N.J., Kartsogiannis V., Quinn J.M., Romas E., Martin T.J., Gillespie M.T. Activated T lymphocytes support osteoclast formation in vitro. Biochem. Biophys. Res. Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 35.Collin-Osdoby P., Rothe L., Anderson F., Nelson M., Maloney W., Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J. Biol. Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K., Hirai T., Ishibashi Y., Izumo N., Togari A. Modulation of osteoblast differentiation and bone mass by 5-HT2A receptor signaling in mice. Eur. J. Pharmacol. 2015;762:150–157. doi: 10.1016/j.ejphar.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Bone H.G., Chapurlat R., Brandi M.L., Brown J.P., Czerwinski E., Krieg M.A., Mellström D., Radominski S.C., Reginster J.Y., Resch H. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J. Clin. Endocrinol. Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgerald D.J., Berger P., Schaffitzel C., Yamada K., Richmond T.J., Berger I. Protein complex expression by using multigene baculoviral vectors. Nat. Methods. 2006;3:1021–1032. doi: 10.1038/nmeth983. [DOI] [PubMed] [Google Scholar]
- 39.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Sehmisch S., Erren M., Rack T., Tezval M., Seidlova-Wuttke D., Richter J., Wuttke W., Stuermer K.M., Stuermer E.K. Short-term effects of parathyroid hormone on rat lumbar vertebrae. Spine. 2009;34:2014–2021. doi: 10.1097/BRS.0b013e3181afe846. [DOI] [PubMed] [Google Scholar]
- 41.Sehmisch S., Komrakova M., Kottwitz L., Dullin C., Schmelz U., Stuermer K.M. Effects of urocortin on spine? Results from the rat osteopenia model. Osteologie. 2015;2:99–106. [Google Scholar]
- 42.Stürmer E.K., Seidlová-Wuttke D., Sehmisch S., Rack T., Wille J., Frosch K.H., Wuttke W., Stürmer K.M. Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: effect of estradiol, testosterone, and raloxifene. J. Bone Miner. Res. 2006;21:89–96. doi: 10.1359/JBMR.050913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.