Abstract
Transcranial direct current stimulation (tDCS) is an innovative technique recently shown to improve language outcomes even in neurodegenerative conditions such as primary progressive aphasia (PPA), but the underlying brain mechanisms are not known. The present study tested whether the additional language gains with repetitive tDCS (over sham) in PPA are caused by changes in functional connectivity between the stimulated area (the left inferior frontal gyrus (IFG)) and the rest of the language network.
We scanned 24 PPA participants (11 female) before and after language intervention (written naming/spelling) with a resting-state fMRI sequence and compared changes before and after three weeks of tDCS or sham coupled with language therapy. We correlated changes in the language network as well as in the default mode network (DMN) with language therapy outcome measures (letter accuracy in written naming).
Significant tDCS effects in functional connectivity were observed between the stimulated area and other language network areas and between the language network and the DMN. TDCS over the left IFG lowered the connectivity between the above pairs. Changes in functional connectivity correlated with improvement in language scores (letter accuracy as a proxy for written naming) evaluated before and after therapy.
These results suggest that one mechanism for anodal tDCS over the left IFG in PPA is a decrease in functional connectivity (compared to sham) between the stimulated site and other posterior areas of the language network. These results are in line with similar decreases in connectivity observed after tDCS over the left IFG in aging and other neurodegenerative conditions.
Keywords: Primary progressive aphasia (PPA), Transcranial direct current stimulation (tDCS), Resting-state fMRI (rsfMRI), Functional connectivity, Neurodegenerative diseases
Highlights
-
•
A brain mechanism for tDCS over the left IFG in PPA is proposed.
-
•
Functional connectivity changes only between the stimulated and temporal areas.
-
•
Changes in functional connectivity correlate with language therapy outcomes.
-
•
This is the first study providing evidence for tDCS mechanisms in PPA.
1. Introduction
Several research studies have investigated the effects of transcranial direct current stimulation (tDCS) as an augmentative technique to improve language interventions in post-stroke aphasia (Hamilton et al., 2011; Holland and Crinion, 2012; Schlaug et al., 2011) and, more recently, in primary progressive aphasia (PPA) as documented by our and other groups (see reviews by Sebastian et al., 2016; Tippett et al., 2015). Understanding the brain mechanisms supporting successful intervention is important for scientific and clinical reasons. Such understanding may lead to targeted clinical trials and discovery of adjunct therapies, particularly important in neurodegenerative conditions with no other pharmacological treatment options, as is the case in PPA. Early investigations indicate that tDCS may work physiologically by altering cell membrane potentials and thus affecting the synaptic conductivity of neurons (Stagg, 2014; Stagg et al., 2009; Stagg and Johansen-Berg, 2013). Since tDCS changes synaptic conductivity and possibly brain connectivity, functional connectivity alterations due to tDCS may be a potential brain mechanism for behavioral effects (Bachtiar et al., 2015; Stagg et al., 2014). The present study tests this hypothesis for tDCS enhanced effects in written language outcomes in a neurodegenerative syndrome (PPA).
Primary progressive aphasia is a neurodegenerative syndrome in which language is the first cognitive faculty that deteriorates. The most common underlying diseases are Alzheimer's disease, frontotemporal lobar degeneration, corticobasal degeneration, or progressive supranuclear palsy. Three variants are currently identified based on language functions compromised—at least at disease/symptom onset—that may reflect the functions of brain areas that show initial atrophy: the non-fluent (nfvPPA), the semantic (svPPA) and the logopenic (lvPPA) PPA variants (Gorno-Tempini et al., 2011). Recently the language deficits in each PPA variant have been characterized. It needs to be noted that the two studies that looked at spelling deficits in the three PPA variants found that the number of errors in PPA does not depend on the variant (Sepelyak et al., 2011), although the type of errors may be distinct in some variants (Shim et al., 2012). There is no disease-modifying medication, but language therapy can help in many cases. Other groups and ours have reported case studies of language interventions (Beeson et al., 2011; Croot et al., 2009; Henry et al., 2013; Jokel et al., 2014; Newhart et al., 2009; Rapp and Glucroft, 2009; Tsapkini and Hillis, 2013) that have shown that participants can improve in naming of treated words but without much generalization or sustained gains. Recently, two independent groups, including ours (Cotelli et al., 2014; Tsapkini et al., 2014), reported on the additional beneficial effects of tDCS for both oral and written word production, respectively. Subsequent studies have reported similar findings (Gervits et al., 2015; Teichmann et al., 2016).
Written and oral naming deficits are among the first and most disruptive language symptoms in PPA (Sepelyak et al., 2011) and negatively impact the quality of life. Writing has become increasingly important in today's society in which substantial communication happens through email, texting, etc. In this context, writing is a good compensatory mechanism for alternative communication in cases of oral language decline and an ideal target for treatment (Hillis et al., 2002). Word representations in either a written or spoken modality may be accessed from either modality or the semantic (word meaning) system (Best and Nickels, 2000; Croot et al., 2009; Ellis, 1993). The implication—which is the basis of several treatment studies for non-neurodegenerative (Kiran, 2005; Kiran et al., 2001, Kiran et al., 2009) as well as neurodegenerative (Beeson and Egnor, 2006; Henry et al., 2013) language deficits—is that access to lexical and sublexical routes from one modality may contribute to word retrieval in the other (Henry et al., 2012). Thus, treatments stimulating residual knowledge across the semantic, phonological, and orthographic domains have resulted in cross-domain improvements (DeDe et al., 2003; Hillis, 1989). For example, a combination of spelling treatment with spoken repetition (Beeson and Egnor, 2006; DeDe et al., 2003; Hillis, 1989) improved written and spoken production even in participants with semantic impairments. In the present study, we used the Copy and Recall Treatment (CART + Repetition) that has been successfully implemented (Beeson and Egnor, 2006) to maximize treatment effects. Repetition has been shown to have synergetic effects for both oral and written naming (Beeson and Egnor, 2006). Each patient may have different spelling deficits, although particular spelling deficits have not been undisputedly associated with particular variants (Sepelyak et al., 2011; Shim et al., 2012).
Although beneficial effects of tDCS in PPA treatment have been shown, the underlying brain mechanisms have not been identified. Recent work in tDCS has identified possible mechanisms by which tDCS induces change in the brain, including altering functional connectivity networks as measured by resting-state fMRI (rsfMRI) in healthy controls (Meinzer et al., 2013; Pena-Gomez et al., 2012; Polania et al., 2011; Schlaug et al., 2008; Sehm et al., 2012; Stagg and Nitsche, 2011). There are very few studies that have directly documented such effects in post-stroke language rehabilitation using tDCS (Marangolo et al., 2013, Marangolo et al., 2016; Meinzer et al., 2013, Meinzer et al., 2015). To our knowledge, no studies have looked at rsfMRI functional connectivity change due to tDCS in PPA. Likewise, there have been no studies examining functional connectivity change induced by tDCS or language therapy even in more common dementias, such as Alzheimer's disease (AD), as shown in recent comprehensive reviews on rsfMRI (Fox et al., 2014). If changes in functional connectivity underlie the benefits of tDCS at the neural level, we hypothesized that functional connectivity would change particularly between the stimulated area and other areas of the language network in the tDCS condition compared to the sham condition. With regard to the direction of change, existing data did not allow us to predict whether functional connectivity between nodes in the language network would increase or decrease due to stimulation. Some studies in healthy controls (Meinzer et al., 2012) and post-stroke aphasia (Marangolo et al., 2016) have found that functional connectivity between language network nodes can increase tDCS effects. However, other studies in healthy aging populations (Meinzer et al., 2013) and in mild cognitive impairment (Meinzer et al., 2015) found decreases in functional connectivity with tDCS that correlated with improved cognitive outcomes.
2. Methods
2.1. Participants
Twenty-four participants with primary progressive aphasia took part in this study. All had a history of at least two years of progressive language deficits with no other etiology (e.g., stroke, tumors, etc.), atrophy predominantly in the left hemisphere and not primary memory deficits. Diagnosis was based on differential diagnosis and three types of evidence: neuropsychological and language testing, MRI, and clinical assessment, according to the recently revised criteria (Gorno-Tempini et al., 2011). Participants were all right-handed and native speakers of English. The study was approved by the Johns Hopkins Hospital Institutional Review Board. All participants provided informed consent for research participation. See Table 1 for demographic and clinical information on the participants. As shown in Table 1, participants in the two groups were closely matched with regard to disease severity as well as the language component of severity based on the revised fronto-temporal dementia clinical dementia rating (FTD-CDR) used to rate severity in primary progressive aphasia (Knopman et al., 2008). To calculate severity, three raters independently scored each item for each participant based on interaction with the participant and family, language and cognitive testing, and questionnaires. They then convened to discuss and produce a consensus score. Groups were also matched at baseline in language and cognitive task scores (Table 2). In addition, the two groups had similar numbers of participants with each PPA variant. In the tDCS group, there were 5 with lvPPA, 3 with nfvPPA, and 4 with svPPA; in sham, there were 3 with lvPPA, 5 with nfvPPA, and 4 with svPPA.
Table 1.
Patient demographics. For age, years post onset, severity, total treatment sessions and left IFG volume (in mm3), values shown are Mean (Standard Deviation). P-values are from Welch two sample t-tests for continuous outcomes and Fisher's exact test for categorical outcomes. R-squared (R sq.) is obtained from simple linear regression of the change in letter accuracy against each covariate. Language severity is based on the language subset from the FTD-CDR scale. Total severity refers to the sum of boxes, including language and behavior as added in Knopman et al., 2008. The two groups (tDCS vs. sham) were matched in all measures.
| Combined (n = 24) | tDCS (n = 12) | Sham (n = 12) | P-value | R sq. | |
|---|---|---|---|---|---|
| Sex | 11 F, 13 M | 5 F, 7 M | 6 F, 6 M | 0.999 | 0.012 |
| PPA variant | 8 L, 8 N, 8 S | 5 L, 3 N, 4 S | 3 L, 5 N, 4 S | 0.873 | 0.099 |
| Age | 67.2 (6.5) | 65.2 (7.0) | 69.1 (5.6) | 0.153 | 0.068 |
| Years post onset | 4.9 (3.0) | 5.5 (3.5) | 4.3 (2.4) | 0.339 | 0.040 |
| Language severity (FTD-CDR) | 1.9 (0.8) | 1.9 (0.9) | 1.8 (0.7) | 0.801 | 0.003 |
| Total with language severity 0.5 | 2 | 2 | 0 | – | – |
| Total with language severity 1 | 5 | 1 | 4 | – | – |
| Total with language severity 2 | 12 | 6 | 6 | – | – |
| Total with language severity 3 | 5 | 3 | 2 | – | – |
| Total severity (FTD-CDR) | 7.5 (4.9) | 7.0 (4.5) | 8.1 (5.4) | 0.569 | 0.122 |
| Total treatment sessions | 13.4 (1.9) | 13.3 (1.9) | 13.5 (2.0) | 0.836 | 0.003 |
| Left IFG volume (*1000) | 10.4 (1.8) | 10.7 (1.6) | 10.2 (2.0) | 0.537 | 0.146 |
Table 2.
Means (standard deviations) for baseline tasks grouped by first-phase condition.
| Task | tDCS first | Sham first | F(1, 22) | P-value |
|---|---|---|---|---|
| Letter fluency (FAS, sum of categories, words generated in 1 min) | 17.83 (11.98) | 13.67 (13.04) | 0.665 | 0.424 |
| Semantic fluency (fruits/animals/vegetables, sum of categories, words generated in 1 min) | 13.92 (12.57) | 12.36 (8.04) | 0.122 | 0.731 |
| Object naming (Boston Naming Test, 30 total) | 12.82 (11.67) | 12.92 (10.80) | 0.000 | 0.983 |
| Action naming (Hopkins Assessment for Naming Actions, 35 total) | 13.91 (10.74) | 15.33 (10.00) | 0.109 | 0.745 |
| Digit span forward (9 total) | 4.29 (2.26) | 4.58 (2.05) | 0.109 | 0.744 |
| Digit span backward (9 total) | 2.92 (2.09) | 2.71 (1.72) | 0.071 | 0.792 |
| Spatial span forward (9 total) | 4.23 (1.23) | 2.85 (1.80) | 4.270 | 0.053 |
| Spatial span backward (9 total) | 4.00 (1.57) | 3.10 (1.79) | 1.510 | 0.234 |
| Sentence anagrams (Johns Hopkins University, 10 total) | 5.40 (3.72) | 6.09 (4.21) | 0.158 | 0.696 |
| Object semantics (Pyramids and Palm Trees, 15 total) | 12.73 (2.61) | 14.00 (1.41) | 2.165 | 0.156 |
| Action semantics (Kissing and Dancing, 15 total) | 12.82 (2.18) | 12.00 (3.57) | 0.430 | 0.519 |
| Sentence repetition (National Alzheimer's Coordinating Center, 37 words total) | 29.50 (8.64) | 26.91 (10.55) | 0.374 | 0.548 |
| Syntactic comprehension (SOAP, 40 total) | 27.60 (7.95) | 27.36 (8.00) | 0.005 | 0.947 |
| Verbal learning (Rey Auditory Verbal Learning Test, Delayed Recall, 15 total) | 3.83 (3.30) | 2.75 (2.63) | 0.791 | 0.384 |
| Spelling words (Johns Hopkins Dysgraphia Battery, % correct) | 88.82 (18.18) | 79.06 (17.01) | 1.614 | 0.219 |
| Spelling non-words (Johns Hopkins Dysgraphia Battery, % correct) | 74.71 (24.25) | 69.86 (23.54) | 0.206 | 0.655 |
2.2. Overall design
Participants belonged to a larger cohort who received tDCS and sham (plus language therapy) in a double-blinded, within-subject crossover design (see Clinical Trial Identifier: NCT02606422 on clinicaltrials.gov). This is an analysis of the first phase results of the current clinical trial, focusing on the association between gains in written naming (the primary outcome measure) and changes in connectivity in participants who initially received tDCS versus those who initially received sham. The crossover design used in the larger study was introduced for recruitment purposes because PPA is a condition without any disease-modifying treatment available. Given the complexity of modeling a possible carryover effect with the additional effect of neurodegeneration and the unknown effect of an adequate wash-out period in neurodegenerative diseases, we focus on data only from the first phase of stimulation in a between-subject design. Half the patients in the present study were randomly assigned to the sham condition and half to tDCS. The tDCS phase consisted of anodal tDCS over the left inferior frontal gyrus (IFG) and speech-language therapy, while the sham phase consisted of a sham procedure explained below (Gandiga et al., 2006) paired with speech-language therapy. We used anodal tDCS over the left IFG. This decision was based on preliminary behavioral results from our previous study with the same montage (Tsapkini et al., 2014) as well as other previous studies in aging and neurodegenerative conditions that had also looked at resting-state connectivity and used anodal stimulation over the left IFG to enhance age-associated cognitive effects (Meinzer et al., 2013). Furthermore, the left IFG has been identified by two recent meta-analyses of task-fMRI studies as a main area associated with the central components of writing (Planton et al., 2013; Purcell et al., 2011), the brain basis of orthographic long-term memory (Rapp and Dufor, 2011) as well as the neural substrate of the mechanism of phoneme-to-grapheme conversion (DeMarco et al., 2017). Therefore, it was an appropriate brain target for both lexical and sublexical spelling processes that may be disrupted in PPA.
Each condition consisted of 15 sessions of daily therapy, in which patients received either tDCS or sham simultaneous with the start of language therapy. (Limitations working with an elderly population and other co-morbidities introduced some variability in the number of sessions; the exact number of sessions for each group is reported in Table 1.) The language therapy involved oral and written picture naming/spelling therapy, and was based on previously successful treatments of PPA patients by Beeson and Egnor following a spell-study-spell procedure in similar studies (Beeson and Egnor, 2006; Rapp and Glucroft, 2009) as well as in our previous tDCS study on spelling rehabilitation in PPA (Tsapkini et al., 2014). The scientific premise behind this therapy that has shown to yield beneficial effects in PPA, even in behavioral-only therapies, was that by training all components of the naming system (semantics, orthography and phonology) we target each word by multiple modalities and processing routes and thus facilitate treatment outcomes (Beeson and Egnor, 2006). There were two sets of materials: trained items (practiced at each session of either tDCS or sham stimulation), and untrained items (not practiced but only tested before treatment and at follow-up points). MRI and behavioral data were collected before the start of treatment and immediately after the treatment phase. Each participant was trained in a set of 10–30 words according to the level of language severity and was also evaluated at each follow-up time on a matched set of untrained words that was not practiced. There were two phases of stimulation in the crossover design mainly for recruitment purposes. We report on the immediate effects of the first phase for trained items only since our behavioral study has revealed differential effects of treatment for each variant for untrained items but not for trained items in the first phase of stimulations. The focus of the present paper is on the changes in functional connectivity related to improvements, which were seen only in spelling trained words.
2.3. Written word production/spelling intervention
We adapted the basic design of a spell-study-spell procedure used in studies to an oral and written naming paradigm (Beeson and Egnor, 2006) or to a similar spelling paradigm (Rapp and Glucroft, 2009). In particular, we used the same picture for oral and written naming. For each word produced incorrectly or not produced we prompted with semantic cues, and then provided the correct word to be studied for spelling and oral repetition. Each patient was given a set of 10–30 words for practice during the treatment.
In detail, each training trial, the patient was shown a picture on the computer, asked to orally name the object, and then write it down. If the patient could not name the object, he was asked to “talk” about the picture, what it is, what it does, etc. (i.e., to provide three properties of the pictured item) to check and reinforce semantic knowledge as in semantic feature analysis. If he could still not name the word, he was provided with the correct word. If he made an error, he was given corrective feedback and repeated opportunities to correctly say the object name. Likewise, if the patient wrote the word incorrectly, the clinician would provide a model of the correct spelling in a spell-study-spell procedure, rehearsing the letters one-by-one in a letter-by-letter manner and reinforcing learning by copying.
2.3.1. Behavioral outcomes measures
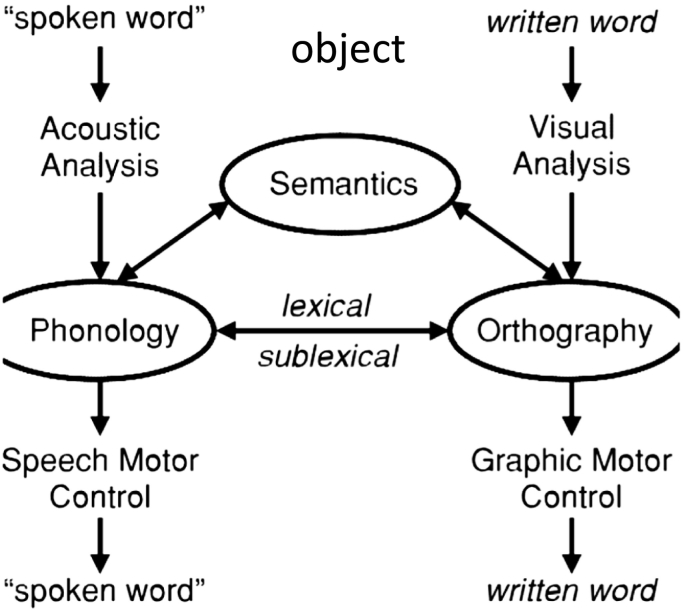
The data analyzed in the present study are part of the data collected in our larger study (ClinicalTrials.gov Identifier: NCT02606422) that included changes in oral and written naming measures for trained and untrained items. The primary outcomes for the present study were changes in written naming because writing has the potential to be a good compensatory mechanism for alternative communication in cases of oral language decline in some PPA variants, as exemplified in the introduction. Fig. 1 shows the relationship between spoken and written word production mechanisms (Ellis and Young, 1988). Although the modules of orthography and phonology may interact with each other, and this is the basis of many treatments including ours, they still constitute independent systems and are involved differentially in different tasks. For example, written naming and reading may be performed without necessarily accessing the phonology of the word and oral naming may be performed without accessing the orthography of the word. In the present study we looked at the effects of tDCS within the orthographic system itself. The proxy we used to measure performance in written naming was letter accuracy instead of whole-word accuracy because we wanted to capture more subtle changes at the graphemic level as well as the letter-sound correspondences, i.e., to capture changes in both the lexical and sublexical representation of each word. In the present study we did not use composite scores to measure language improvements for two reasons: (1) if tDCS is claimed to have a trained-task specific effect then we should directly measure outcomes specific to the task; and (2) composite scores usually measure a variety of tasks, but if a particular brain area is a neural substrate for a certain cognitive or language function, but not another, and only that function is affected by tDCS, then the effects may be diluted in the presence of all the other functions measured together. Therefore, composite language or cognitive scores (such as MMSE or total language batteries' scores) may be more comprehensive for science communication purposes but are not very informative of the exact language or cognitive processes affected.
Fig. 1.
Interactive model of lexical processing.
2.4. tDCS methods
We targeted the left inferior frontal gyrus (IFG) for anodal stimulation, using a Soterix CT 1 × 1 device. The center of the site of stimulation corresponded to the F7 electrode, using the EEG 10–20 electrode position system (Homan et al., 1987); however, electrode patches were big, 5 × 5 = 25 cm2, and thus extended the region of stimulation beyond the left IFG. The reference electrode, the cathode, was placed on each participant's right cheek, shown to successfully isolate the targeted area (Hummel et al., 2006; Tsapkini et al., 2014). Stimulation was delivered at an intensity of 2 mA (estimated current density 0.08 mA/cm2; estimated total charge 0.048C/cm2) for a maximum of 20 min in the tDCS condition and 30 s in the sham condition. The stimulator was not connected to a mainline power source and could not produce an excess of 4 mA of current. We used non-metallic, conductive rubber electrodes covered by saline-soaked sponges (NaCl concentration 0.09%) to minimize the potential for chemical reactions at the interface of the skin and electrodes. In both conditions, the electrical current was increased in a ramp-like fashion at the onset of the stimulation, eliciting a transient tingling sensation on the scalp that usually disappeared after 30 s. TDCS continued sending current, while sham ramped down to 0 mA. This procedure has been shown to successfully blind participants to their treatment condition (Gandiga et al., 2006) and was overall very well-tolerated. Additionally, the electrical current flow was modeled by a group with expertise in this type of modeling (see Supplementary Fig. 1; Courtesy of Dr. Marom Bikson).
2.5. Resting-state fMRI methods
MRI scans were obtained at the Kennedy Krieger Institute at Johns Hopkins University, using a 3 T Philips Achieva MRI scanner equipped with a 32-channel head coil. Resting-state fMRI (rsfMRI) data were acquired for approximately 9 min (210 time-point acquisitions) before, after, and two months post-intervention. We used a 2D EPI sequence with SENSE partial-parallel imaging acceleration to obtain an in-plane resolution of 3.3 × 3.3 mm2 (64 × 64 voxels; TR/TE = 2500/30 ms; flip angle = 75°; SENSE acceleration factor = 2; SPIR for fat suppression, 3 mm slice thickness). The data were co-registered with structural scans into the same anatomical space. Structural scans, acquired axially with a scan time of 6 min (150 slices), used a T1-weighted MPRAGE sequence with 3D inversion recovery, magnetization-prepared rapid gradient, isotropic with a resolution of 1 × 1 × 1 mm3 (FOV = 224 × 224 mm2; TR/TE = 8.1/3.7 ms; flip angle = 8°; SENSE acceleration factor = 2).
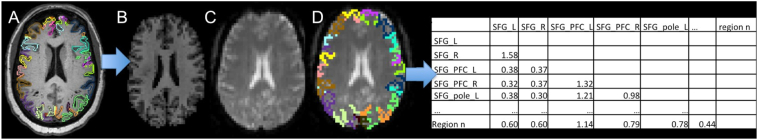
Using MRICloud, a cloud-platform for automated image parcellation approach (atlas-based analysis (ABA)), the MPRAGE scan was parcelled into 283 structures (Mori et al., 2016). In detail, each participant's high resolution MPRAGE was segmented by using a multi-atlas fusion label algorithm (MALF) and large deformation diffeomorphic metric mapping, LDDMM (Ceritoglu et al., 2013; Miller et al., 2005; Tang et al., 2013). This highly accurate diffeomorphic algorithm, associated with multiple atlases, minimizes the mapping inaccuracies due to atrophy or local shape deformations. All analyses were performed in native space. To control for relative regional atrophy, volumes for each ROI were normalized by the total intracerebral volume (total brain tissue without myelencephalon and cerebrospinal fluid). The resting-state fMRI was also processed in MRICloud and analyzed in a seed-by-seed manner. The image processing was described in our previous publication (Faria et al., 2012) including routines imported from the SPM connectivity toolbox for coregistration, motion, and slice timing correction; physiological nuisance correction using CompCor (Behzadi et al., 2007); and motion and intensity TR outlier rejection using “ART” (https://www.nitrc.org/projects/artifact_detect/). The MRICloud pipeline follows well established steps for rsfMRI processing: after exclusion of “outlier” TRs, detected by ART routine (parameters: 2 standard deviations for motion and 4 standard deviations for intensity, more severe than the default of 9), the movement matrix combined with the physiological nuisance matrix is used in the deconvolution regression for the remaining TRs. These two steps for motion correction (outlier rejection and regression of motion parameters) ensure the minimization of the motion effect. The parcels resultants from the high resolution T1 segmentation were brought to the resting state dynamics by co-registration. Time-courses of 19 cortical and deep gray matter ROIs (thirteen from the language network and six from the DMN) were extracted and the correlation among them, as well as the Fisher z-transformed scores, were calculated (see Fig. 2).
Fig. 2.
Schematic representation of the structure-based analysis performed. The T1-high resolution and the respective parcellation map (A, only cortical parcels represented) is co-registered (B) to the rsfMRI (C), therefore bringing the structural labels to this latter space (D), from where the fisher z-correlations between ROIs are extracted (E).
In the present study, we examined changes in functional connectivity in the language network, as identified in task-based fMRI studies of spoken and written word production, to determine whether functional connectivity change is a mechanism for tDCS effects in PPA. Thirteen ROIs were predefined to comprise the Language Network: the pars opercularis, pars orbitalis, and pars triangularis of the left and right inferior frontal gyrus (IFG_opercularis_L, IFG_opercularis_R, IFG_orbitalis_L, IFG_orbitalis_R, IFG_triangularis_L, IFG_triangularis_R), left middle temporal gyrus (MTG_L), left supramarginal gyrus (SMG_L), left superior temporal gyrus (STG_L), left inferior temporal gyrus (ITG_L), left fusiform gyrus (FuG_L), pole of the left middle temporal gyrus (MTG_L_pole) and pole of the left superior temporal gyrus (STG_L_pole). We also included the right IFG (IFG_opercularis_R, IFG_orbitalis_R, IFG_triangularis_R), since current distribution in traditional tDCS has been shown to affect areas contralateral to the stimulated areas (Brunoni et al., 2012). Six ROIs were included to comprise the DMN: the left angular gyrus (AG_L), right angular gyrus (AG_R), left middle frontal gyrus (dorsal prefrontal cortex) (MFG_DPFC_L), right middle frontal gyrus (dorsal prefrontal cortex) (MFG_DPFC_R), left posterior cingulate gyrus (PCC_L) and right posterior cingulate gyrus (PCC_R) (Andrews-Hanna et al., 2007; Greicius et al., 2003).
2.6. Statistical analyses
2.6.1. tDCS effects on letter accuracy
For all participants, behavioral data including language treatment test scores (letter accuracy, i.e., percentage of letters corresponding to sounds making up words) were collected before and after the written naming/spelling treatment intervention, with percentage of correct letters as the primary outcome. Percentages were used because the number of items was different in each patient according to the severity of the deficit. As an example, some participants were given ten words to learn and some were given 30 words. We report the percentage point change in percent correct out of total stimuli. For example, if they improved from 10/100 letters (10%) to 30/100 letters (30%), they showed a change of 20 percentage points. Spelling was scored on a rule-based system, in which each letter was given one point if correct, and points were subtracted if letters were deleted, added, substituted, transposed, or moved (see scoring system from Goodman and Caramazza, 1985). Each item was entered into a database and scored. A second person reviewed the scores and noted any discrepancies in scoring based on the same rule system, which were then discussed to generate a consensus score. Raters were identical in their scoring for 95–98% of letters. Welch two sample t-tests were conducted on the difference for tDCS vs. sham groups in absolute change in percentage correct from before and after treatment. Although we report one type of outcome in the present study—letter accuracy—this corresponds to the correct letters of words that could be lexically retrieved correctly without using cues. We did not observe any case of modality specific naming deficit in our cohort of participants, indicating that those who could retrieve the name of the word in written modality could also retrieve it orally. Therefore, it is in reality a composite score of naming and spelling performance.
2.6.2. tDCS effects on brain connectivity
Pearson's correlation coefficients were Fisher z-transformed (Fisher, 1915) for the stimulated area (left IFG) as well as its right hemisphere homologue. We also included other common language network ROIs, in particular for written and oral language production in the left hemisphere (IFG, SMG, STG, MTG, ITG and FuG) according to recent studies (Gitelman et al., 2005; Purcell et al., 2011; Purcell and Rapp, 2013), as well as the right and left hemisphere areas of the DMN (Greicius et al., 2003) since it has also been involved in post-therapy changes of functional connectivity after speech language therapy (Marcotte et al., 2013). The coordinates of ROI centers used are listed in Table 3.
Table 3.
Centers of the ROIs in the Montreal Neurological Institute (MNI) coordinate system. Center location of each ROI is averaged over 24 subjects.
| ROIs | L/R | Network | Coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| IFG pars opercularis | L | Language | −46.23 | 9.75 | 16.81 |
| IFG pars opercularis | R | Language | 46.80 | 15.85 | 15.43 |
| IFG pars orbitalis | L | Language | −43.15 | 29.10 | −4.28 |
| IFG pars orbitalis | R | Language | 43.56 | 31.59 | −5.78 |
| IFG pars triangularis | L | Language | −45.90 | 23.57 | 11.70 |
| IFG pars triangularis | R | Language | 46.51 | 27.42 | 8.56 |
| FuG | L | Language | −33.15 | −45.86 | −18.95 |
| ITG | L | Language | −48.71 | −25.41 | −26.15 |
| MTG | L | Language | −55.62 | −38.22 | −4.03 |
| MTG pole | L | Language | −40.21 | 11.30 | −32.59 |
| SMG | L | Language | −52.46 | −35.48 | 29.59 |
| STG | L | Language | −53.57 | −28.56 | 5.35 |
| STG pole | L | Language | −42.08 | 9.63 | −20.00 |
| AG | R | DMN | 44.28 | −57.30 | 36.63 |
| AG | L | DMN | −42.98 | −60.49 | 36.51 |
| MFG DPFC | L | DMN | −34.93 | 44.05 | 14.00 |
| MFG DPFC | R | DMN | 35.24 | 46.20 | 13.26 |
| PCC | L | DMN | −7.38 | −52.41 | 24.65 |
| PCC | R | DMN | 8.61 | −50.47 | 25.08 |
For each pair of the ROIs, we evaluated the average treatment effect (ATE) of tDCS on interregional correlations. Denoting the correlation (Fisher z-transformed) between a certain pair of ROIs after intervention as Y and the treatment condition as S (S = 1 for tDCS, S = 0 for sham), the target of inference, i.e., ATE (denoted by θ), is:
where E[X|W = w] is the expectation of random variable X given variable W at level w.
In order to obtain efficient and consistent estimation of the ATE, we employed a semi-parametric method assembled with automated covariate selection (Tsiatis et al., 2008), which simultaneously lowered the estimated standard error, eliminated subjectivity in model selection and ensured consistency and asymptotic normality. Specifically, for each pair of the ROIs (i.e., intra-language network, DMN and inter-Language-DMN network), we used the z-transformed correlation coefficients before intervention, baseline demographic variables (i.e., age and sex), clinical variables including years post-onset, the patient's PPA variant, language severity and total severity of dementia using the clinical dementia rating (FTD-CDR) score, and the volume of left IFG before intervention as covariates. We conducted forward model selection using Akaike information criterion (AIC) (Akaike, 1998) separately for the tDCS and sham groups before combining them to estimate the ATE. We applied the standard sandwich estimator approach (Freedman, 2006) to calculate the standard error with adjustment for the small sample size. The Wald statistic was then attained for hypothesis testing. P-values were adjusted to control for the family-wise error rate (FWER) in multiple testing following the Holm procedure (Holm, 1979). We report the estimated ATE, standard error, Wald test statistic, raw and adjusted P-values, and 95% confidence interval for all pairs of the ROIs (see Results Section 3), i.e., the pairs in the language network, DMN and the pairs between the DMN and language network (inter-Language-DMN).
2.6.3. Association between changes in letter accuracy and changes in brain connectivity
Associations between the behavioral improvements and the functional connectivity changes were tested. For each pair of the ROIs with an unadjusted P-value < 0.05 in the treatment effect analysis, the before-and-after behavioral difference was regressed against the before-and-after functional connectivity correlation difference. t-Tests were then performed on the model coefficients (see Results Section 3).
3. Results
3.1. tDCS tolerability
Overall, tDCS was well-tolerated by participants in the study. When debriefed at the end of the study they were at chance when guessing the stimulation condition and did not report any side effects except for tingling or itching sensations in the beginning of each session. Participants were asked to report their general pain levels 1–2 times during each session with the Wong-Baker FACES Pain Rating Scale (www.WongBakerFACES.org). The maximum pain rating per session was averaged across sessions and participants. The tDCS mean rating was 2.63 (standard deviation 3.27, range 0–10); the sham mean rating was 2.26 (standard deviation 2.22, range 0–10). No episodes of intolerability occurred, nor did any adverse effects.
3.2. tDCS effects on letter accuracy
3.2.1. Improvement of behavioral scores for tDCS vs sham
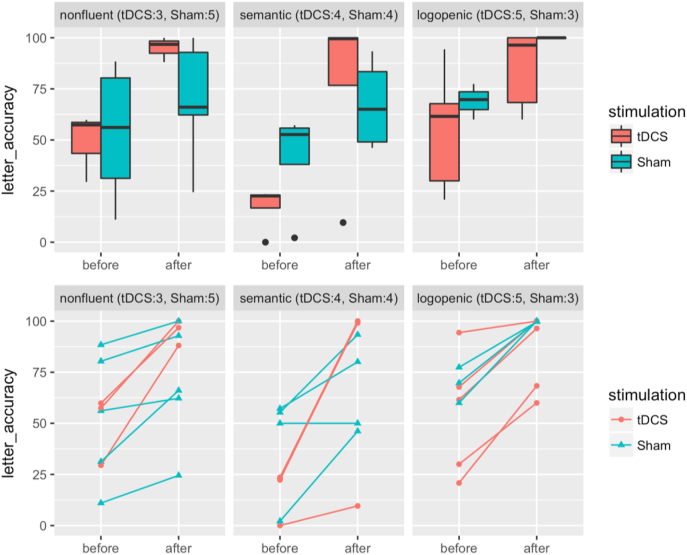
In both the tDCS (combination of current stimulation and language therapy) and sham (language therapy only) groups, significant improvements in percentage of correct letters in trained words were observed after treatment (Table 4, Fig. 3; Trained tDCS group mean improvement: 44.03, P-value: 6.33 × 10−5, sham group mean improvement: 23.00, P-value: 1.82 × 10−4). Additional behavioral improvement in the tDCS group as compared to the sham group was identified by a Welch two sample t-test (additional mean improvement for trained items: 21.03, P-value: 0.020). At the individual level, all patients improved except for one patient in the sham group whose performance did not change. In the present study we focus on the relationship between connectivity and treatment gains for tDCS versus sham, rather than the behavioral effects of tDCS versus sham.
Table 4.
Letter accuracy measurements. Mean ± Standard Deviation of behavioral scores in trained items before and after intervention and the pre-post changes are reported for each treatment group. P-values from one sample t-tests for changes within treatment groups are listed in the rows labeled “P-values for additional gain”. Mean and 95% confidence interval of additional gain for tDCS over sham are reported in the fourth column. P-values from Welch two sample t-tests for additional gain of tDCS over sham are listed in the fifth column.
| Trained | tDCS (n = 12) | Sham (n = 12) | Additional gain tDCS over sham | P-value for additional gain tDCS over sham |
|---|---|---|---|---|
| Before | 40.83 ± 26.85 | 53.24 ± 26.61 | – | – |
| After | 84.86 ± 27.29 | 76.25 ± 25.93 | – | – |
| Change | 44.03 ± 24.44 | 23.00 ± 14.44 | 21.03 [3.80, 38.25] | 0.020 (df = 17.85) |
| P-values for changes | 6.33e-05 | 1.82e-04 | – | – |
Fig. 3.
Box plots and scatter plots for behavioral scores by treatment group and variants. Y-axes are for absolute letter accuracy in % and X-axes are for time points. Different variants—nonfluent, semantic and logopenic—are separated in three columns. Treatment groups are color-coded as red for tDCS and blue for Sham. In the scatter plots, individuals are further coded as circles for tDCS and triangles for Sham. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. tDCS effects on brain connectivity
3.3.1. Effects of tDCS on correlations between the language network ROIs
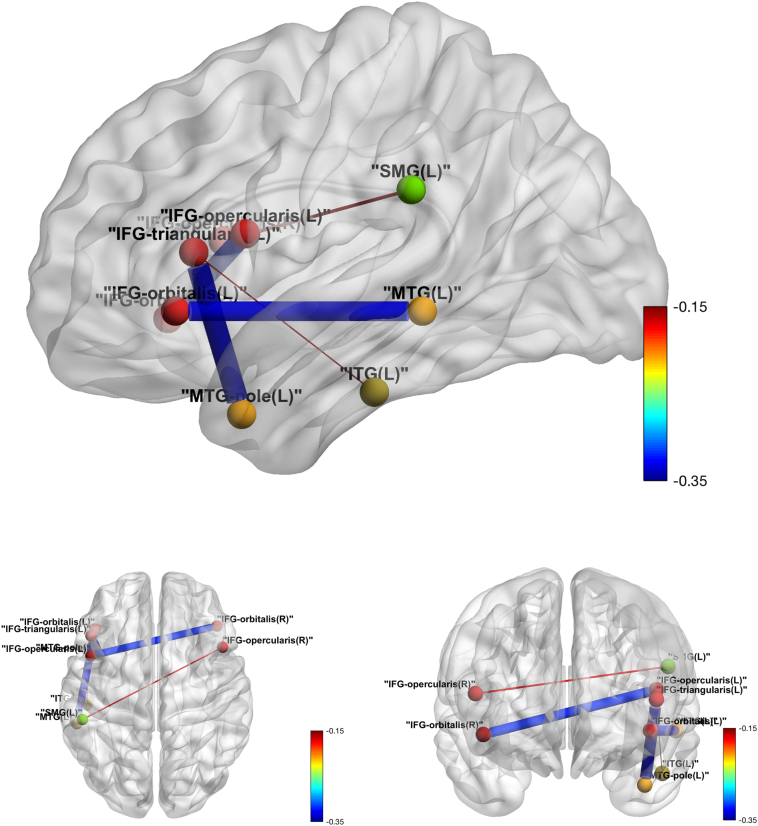
Among the 78 ROI pairs comprising the 13 predefined language-related regions, significant negative tDCS effects were detected at a significance level of 0.05 in five pairs before adjusting for multiple testing: IFG_orbitalis_L and MTG_L, IFG_triangularis_L and MTG_pole_L, IFG_opercularis_L and IFG_orbitalis_R, IFG_triangularis_L: ITG_L and IFG_opercularis_R: SMG_L. The tDCS effect on functional connectivity between IFG_orbitalis_L and MTG_L also survived the FWER correction for multiple testing (adjusted P-value: 0.008) with an estimated tDCS effect of −0.33 (standard error 0.09 and 95% confidence interval [−0.50, −0.16]). In all five pairs, a negative tDCS effect on ROI correlations was observed (see Table 5). Fig. 4 illustrates these five significant pairs.
Table 5.
Identified intra Language Network ROI pairs with significant tDCS effects on inter-regional correlation before family-wise error rate (FWER) correction. Pairs are identified by asymptotic two-sided Wald tests on average treatment effect (ATE) at significance level 0.05 before adjusting for multiple comparisons. Estimates of ATE, standard error (SE), Wald statistics (Stat.), P-values, FWER corrected P-values (Adj. P.), 95% confidence intervals (CI) are reported. Results are ranked by test statistics.
| ROI pairs | Estimate (SE) | Stat. | P-value | Adj. P. | CI |
|---|---|---|---|---|---|
| IFG_orbitalis_L: MTG_L | −0.33 (0.09) | −3.891 | 0.000 | 0.008 | [−0.50, −0.16] |
| IFG_triangularis_L: MTG_L_pole | −0.35 (0.10) | −3.368 | 0.001 | 0.058 | [−0.56, −0.15] |
| IFG_opercularis_L: IFG_orbitalis_R | −0.32 (0.12) | −2.622 | 0.009 | 0.665 | [−0.57, −0.08] |
| IFG_triangularis_L: ITG_L | −0.15 (0.07) | −2.221 | 0.026 | 1.000 | [−0.29, −0.02] |
| IFG_opercularis_R: SMG_L | −0.17 (0.08) | −2.086 | 0.037 | 1.000 | [−0.33, −0.01] |
Fig. 4.
Intra-language network tDCS effects over sham in functional connectivity. Edge thickness is proportional to the absolute value of the tDCS effect, and the color map shows the value of the tDCS effect.
3.3.2. No effects of tDCS on correlations between the DMN ROIs
In the predefined DMN, significant tDCS effect was not observed on the correlation between any ROIs at a significance level of 0.05 before any multiple testing adjustment.
3.3.3. Effects of tDCS on functional connectivity between the language network and the DMN
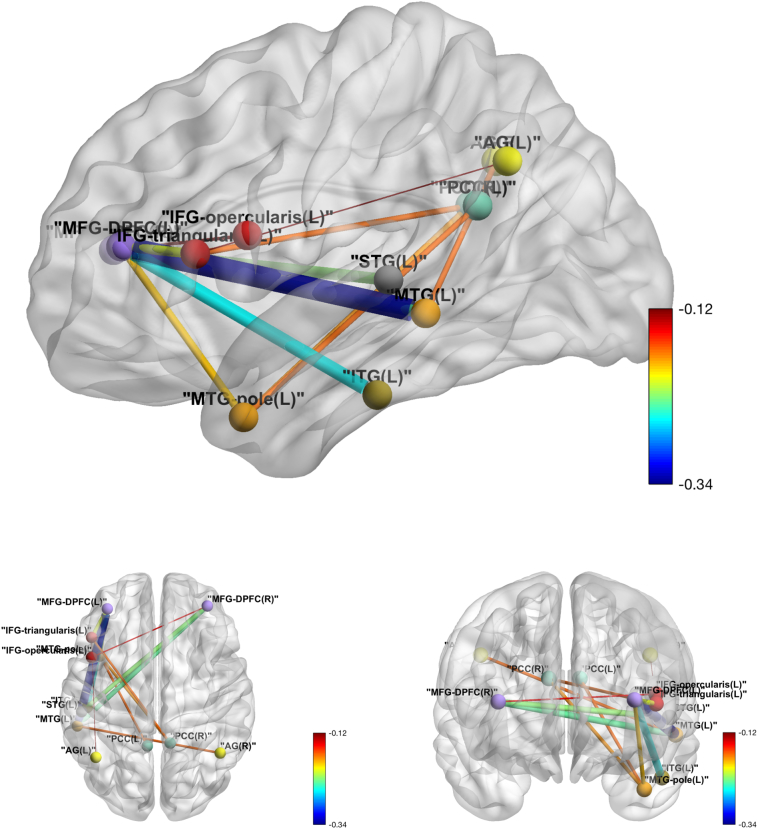
Among the 78 pairs involving both the Language Network and the DMN, relatively lower correlation in the tDCS group contrasted to the sham group was identified in twelve pairs at a significance level of 0.05, with only one pair (MFG_DPFC_L and MTG_L) surviving the FWER adjustment (see Table 6 for exact values and Fig. 5 for visualization).
Table 6.
Identified inter-Language-DMN ROI pairs with significant tDCS effects on inter-regional correlation before family-wise error rate (FWER) correction. Pairs are identified by asymptotic two-sided Wald tests on average treatment effect (ATE) at significance level 0.05 before adjusting for multiple comparisons. Estimates of ATE, standard error (SE), Wald statistics (Stat.), P-values, FWER corrected P-values (Adj. P.), 95% confidence intervals (CI) are reported. Results are ranked by test statistics.
| ROI pairs | Estimate (SE) | Stat. | P-value | Adj. P. | CI |
|---|---|---|---|---|---|
| MFG_DPFC_L: MTG_L | −0.34 (0.09) | −3.742 | 0.000 | 0.014 | [−0.52, −0.16] |
| MFG_DPFC_L: ITG_L | −0.26 (0.08) | −3.368 | 0.001 | 0.058 | [−0.41, −0.11] |
| MFG_DPFC_L: IFG_triangularis_L | −0.21 (0.07) | −3.153 | 0.002 | 0.123 | [−0.34, −0.08] |
| MFG_DPFC_R: STG_L | −0.23 (0.09) | −2.732 | 0.006 | 0.472 | [−0.40, −0.07] |
| MFG_DPFC_R: IFG_opercularis_L | −0.14 (0.06) | −2.433 | 0.015 | 1.000 | [−0.26, −0.03] |
| MTG_L_pole: PCC_L | −0.17 (0.07) | −2.428 | 0.015 | 1.000 | [−0.31, −0.03] |
| IFG_triangularis_L: PCC_R | −0.17 (0.07) | −2.359 | 0.018 | 1.000 | [−0.31, −0.03] |
| MTG_L_pole: PCC_R | −0.18 (0.08) | −2.359 | 0.018 | 1.000 | [−0.34, −0.03] |
| AG_R: MTG_L | −0.17 (0.07) | −2.353 | 0.019 | 1.000 | [−0.30, −0.03] |
| MFG_DPFC_R: MTG_L | −0.24 (0.12) | −2.100 | 0.036 | 1.000 | [−0.47, −0.02] |
| MFG_DPFC_L: MTG_L_pole | −0.19 (0.09) | −2.093 | 0.036 | 1.000 | [−0.36, −0.01] |
| IFG_triangularis_L: AG_L | −0.12 (0.06) | −1.979 | 0.048 | 1.000 | [−0.25, −0.00] |
Fig. 5.
Inter-Language-DMN network tDCS effects over sham in functional connectivity. Edge thickness is proportional to the absolute value of the tDCS effect, and the color map shows the value of the tDCS effect.
3.4. Association between changes in functional connectivity and letter accuracy
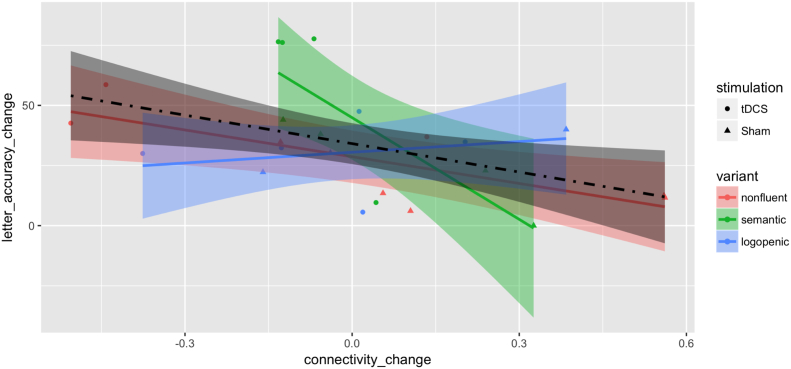
We further investigated, for those pairs with a significant tDCS effect, whether the change in functional connectivity was associated with the improvement in behavior (language scores) for trained items. We found a significant association between the behavioral improvement and the correlation change in the pair of IFG_triangularis_L and ITG_L using a t-test at significance level 0.05 (t(22) = −2.58, P-value = 0.017), which was one of the five significant pairs in the Language Network. No significant association was found in any other pair in the Language Network or inter-Language-DMN pairs (see Table 7, Fig. 6).
Table 7.
Letter accuracy changes regressed against correlation changes with intercept on previously identified pairs. Type of the ROI pair (L: intra Language Network; I: inter-Language-DMN; D: DMN); estimate, standard error (SE), T-statistic, P-value and 95% confidence interval (CI) of the linear coefficient of correlation change; the R-squared (R sq.) of the linear model are reported. Within networks, ROI pairs are ranked by test statistics in previous test on average treatment effect on inter-regional correlation.
| ROI pairs | Type | Estimate (SE) | T stat. | P-value | CI | R sq. |
|---|---|---|---|---|---|---|
| IFG_orbitalis_L: MTG_L | L | −29.01 (17.68) | −1.64 | 0.115 | [−65.67, 7.66] | 0.109 |
| IFG_triangularis_L: MTG_L_pole | L | −23.46 (15.55) | −1.51 | 0.146 | [−55.72, 8.80] | 0.094 |
| IFG_opercularis_L: IFG_orbitalis_R | L | 5.27 (16.33) | 0.32 | 0.750 | [−22.25, 39.18] | 0.015 |
| IFG_triangularis_L: ITG_L | L | −39.43 (15.30) | −2.58 | 0.017 | [−71.16, −7.70] | 0.232 |
| IFG_opercularis_R: SMG_L | L | −39.36 (22.83) | −1.73 | 0.099 | [−86.68, 7.96] | 0.119 |
| MFG_DPFC_L: MTG_L | I | −16.96 (17.36) | −0.98 | 0.339 | [−52.96, 19.03] | 0.042 |
| MFG_DPFC_L: ITG_L | I | −26.25 (19.99) | −1.31 | 0.203 | [−67.70, 15.21] | 0.073 |
| MFG_DPFC_L: IFG_triangularis_L | I | 17.59 (21.78) | 0.81 | 0.428 | [−27.58, 62.76] | 0.029 |
| MFG_DPFC_R: STG_L | I | −0.42 (21.93) | −0.02 | 0.985 | [−45.90, 45.05] | 0.000 |
| MFG_DPFC_R: IFG_opercularis_L | I | −15.74 (18.68) | −0.84 | 0.409 | [−54.47, 22.99] | 0.031 |
| MTG_L_pole: PCC_L | I | −1.03 (23.00) | −0.04 | 0.965 | [−48.73, 46.67] | 0.000 |
| IFG_triangularis_L: PCC_R | I | −19.51 (16.41) | −1.19 | 0.247 | [−53.54, 14.53] | 0.060 |
| MTG_L_pole: PCC_R | I | 0.26 (22.43) | 0.01 | 0.991 | [−46.25, 46.78] | 0.000 |
| AG_R: MTG_L | I | 7.14 (29.23) | 0.24 | 0.809 | [−53.48, 67.76] | 0.003 |
| MFG_DPFC_R: MTG_L | I | 23.46 (20.10) | 1.17 | 0.256 | [−18.23, 65.15] | 0.058 |
| MFG_DPFC_L: MTG_L_pole | I | −25.48 (19.93) | −1.28 | 0.215 | [−66.81, 15.86] | 0.069 |
| IFG_triangularis_L: AG_L | I | −22.10 (24.48) | −0.90 | 0.377 | [−72.87, 28.67] | 0.036 |
| MTG_L_pole: PCC_R | I | 0.26 (22.43) | 0.01 | 0.991 | [−46.25, 46.78] | 0.000 |
Fig. 6.
Scatter plot by treatment groups and PPA variants for change (after minus before) of absolute letter accuracy in % against change in connectivity for the IFG_triangularis_L: ITG_L pair. Variants are color-coded as red for nonfluent, green for semantic and blue for logopenic. Treatment assignments are coded as circles for tDCS and triangles for Sham. Fitted lines and pointwise confidence bands are plotted for each variant with the corresponding coded color and for all patients with the dot-dashed line and the color black. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our goal was to identify changes in functional connectivity associated with tDCS in individuals with PPA and to determine how these changes may relate to therapy gains. Three results are worth noting. First, consistent with previous papers, the behavioral data indicate that tDCS coupled with language therapy was associated with greater gains in letter accuracy in trained words than sham coupled with language therapy. More importantly for our hypotheses, the effects of tDCS on resting-state fMRI functional connectivity were significant only between pairs that included the stimulated area(s), i.e., parts of the left IFG targeted in the intervention. Resting-state fMRI connectivity between the left frontal stimulated area(s) and temporal areas of the language network was observed to be lower in the active tDCS condition than in sham, and this difference was statistically significant. Finally, the changes in functional connectivity between the left IFG orbitalis and the temporal cortex were also associated with the improvement in behavioral (language) treatment outcomes, i.e., in letter accuracy as a proxy for written naming. There was no significant connectivity change comparing tDCS and sham within the default mode network (DMN). There were also a few inter-network tDCS effects on functional connectivity. Only one pair between an area of the DMN (the left DLPFC) and an area of the language network (the left MTG) survived multiple comparisons but no areas were related to behavioral outcomes. Though other studies have demonstrated decreases in functional connectivity due to frontal tDCS in aging (Meinzer et al., 2012, Meinzer et al., 2013) and mild cognitive impairment (Meinzer et al., 2015), to our knowledge, this is the first study to document such changes (1) after repeated anodal tDCS over the left frontal areas resulting in lower functional connectivity of the stimulated areas, (2) in a neurodegenerative condition and in particular in PPA, and (3) with positive correlation of functional connectivity changes with therapy gains.
4.1. Lower functional connectivity as a mechanism for anodal tDCS over the left IFG?
Our results align well with studies that showed that one left-IFG tDCS session resulted in decreased functional connectivity between frontal and temporal areas in healthy aged controls (Meinzer et al., 2012, Meinzer et al., 2013) and those with mild cognitive impairment (Meinzer et al., 2015). In the present study we extended this premise and showed that after repetitive stimulation these effects are strengthened and significantly correlate with therapy gains. Although no other studies compare the effects of repeated and consecutive tDCS vs. sham in neurodegeneration, the present and above mentioned studies in aging and MCI, contradict some previous findings showing increases in functional connectivity within the trained network or in the DMN after or during one session of tDCS in healthy young controls (Keeser et al., 2011; Pena-Gomez et al., 2011). Therefore, given the conflicting results on the directionality of the effects of non-invasive stimulation on intrinsic connectivity, it is just as possible that the stimulation would decrease connectivity. Some possible mechanisms for this effect would be that tDCS introduces noise at the site of stimulation and “uncouples” it from its other network couplings.
In language rehabilitation without tDCS, a previous study on post-stroke language rehabilitation (Marcotte et al., 2013) had shown that functional connectivity within the language network during task-fMRI increased in the same hemisphere after successful rehabilitation. In fact, in our study, we did not replicate this result, despite finding an insignificant increase in functional connectivity within the language network in the sham condition. To our knowledge, the only other study that has looked at the effects of tDCS on functional connectivity using resting-state fMRI during language (or rather speech) rehabilitation is a recent study by Marangolo and colleagues (Marangolo et al., 2016) that applied 15 consecutive sessions of bilateral tDCS (anodal over the left IFG and cathodal over the right IFG) along with speech therapy treating post-stroke speech apraxia in nine participants in a cross-over design. The study showed that improved speech outcomes were correlated with increases in functional connectivity between the stimulated area and other areas of the language network in the left hemisphere. In our study, as well, all functional connectivity differences after intervention involved the stimulated area, but consecutive tDCS induced lower, relative to sham, functional connectivity between frontal and temporal areas in the language network, rather than increases as in the Marangolo and colleagues study.
A possible explanation for the changes in connectivity in our study versus the previous post-stroke aphasia studies is the type of participants. In the Marcotte and colleagues, as well as the Marangolo and colleagues studies (Marangolo et al., 2016; Marcotte et al., 2012), participants were chronic post-stroke aphasic patients who may have had damage in the left IFG and possibly its tracts to other areas. Also, lesions due to stroke may have induced changes in functional connectivity, and the BOLD signal in the left IFG may have decreased from the ischemic infarct. Therefore, the effects and implications of this intervention may be particular to tDCS but also to participants' neurological condition (aging and neurodegeneration versus recovering from stroke).
Another explanation of our findings may be related to the baseline functional connectivity in PPA as well as in aging and other forms of neurodegeneration. Studies of functional connectivity in AD have shown decreased posterior connectivity in typical AD with episodic memory deficits (Caffo et al., 2010; Greicius et al., 2004; Seeley et al., 2007; Supekar et al., 2008) but increased anterior connectivity in logopenic or early-onset AD (Lehmann et al., 2015) as well as in normal aging (Keeser et al., 2011; Meinzer et al., 2013). As shown in the above studies on these populations, functional connectivity in aging and neurodegeneration may be particularly altered; tDCS may regulate the baseline abnormalities in connectivity between frontal and temporal brain areas as shown in a previous study even after one tDCS application (Meinzer et al., 2013).
Here we would like to comment on the particular functional couplings that were found to change in the language network. The first observation is that all pairs that showed changes in functional connectivity that correlated with language effects of tDCS over sham involved the stimulated area. Although our analysis did not allow us to look at directionality of effects, the specificity and focality of these pairs in the language network confirms that they probably resulted from the stimulation, as the current flow model also suggests (see Supplementary Fig. 1). The couplings themselves shed light on possible mechanisms of current flow in tDCS and point towards structural connectivity explanatory accounts of tDCS effects. In particular, the pair IFG_opercularis_L: IFG_orbitalis_R is connected through the corpus callosum; the pair IFG_triangularis_L: MTG_pole corresponds to the uncinate fasciculus; the pairs IFG_opercularis_L: SMG_L and IFG_orbitalis_L: MTG_L (which survived multiple comparisons) could reflect the structural connectivity attributed to the arcuate fasciculus; and the pair IFG_triangularis_L: ITG_L could reflect the structural connectivity of the inferior longitudinal fasciculus or the inferior occipito-frontal fasciculus. The latter has been recently found to connect directly the posterior temporal areas and the orbito-frontal region (Catani et al., 2003), crucial for semantic processing since it elicits semantic paraphasias when stimulated directly in direct electric stimulation studies intra-operatively (Mandonnet et al., 2007). Although this hypothesis needs to be confirmed by structural connectivity analyses, it is consistent with previous studies in other functional networks, in particular in the DMN, that showed that resting-state functional connectivity reflects structural connectivity (Greicius et al., 2009). Additionally, we and other groups have routinely found that the left ITG is an area responsible for naming in both lesion studies as well as in PPA (Race et al., 2013; Tsapkini et al., 2011).
It is worth commenting briefly on the lack of significant changes in the DMN observed in our study. This finding is in line with the previously mentioned tDCS studies with one or multiple applications that did not look for or did not find effects of tDCS in the DMN (Marangolo et al., 2016; Meinzer et al., 2013), and the study by Marcotte and colleagues on regular language rehabilitation that found upregulation of DMN activity after language rehabilitation in post-stroke aphasia (Marcotte et al., 2013). Our results taken together with these studies confirm that tDCS over the left frontal cortex coupled with language therapy does not induce changes in the DMN and therefore the effects that we see are language-specific and not domain-general changes, pointing towards the specificity of tDCS effects. In the present study, we additionally found changes in the inter-network connectivity between the language network and DMN but these did not survive multiple comparisons correction. This may indicate that, although the effects of tDCS may not be specific to the DMN, by altering one network (the language network), the inter-network communication may also be altered. However, larger studies are needed to confirm these preliminary findings.
4.2. Variant effects and additional outcomes
Despite the fact that we have 24 participants (which is more than previous treatment studies using resting-state methodology in neurodegenerative diseases), this number may not be enough in PPA given the variability of the syndrome and underlying pathologies (Gorno-Tempini et al., 2011; Seeley et al., 2007). Although there is no evidence that pathology itself may play a role in moderating the effects of tDCS, and given that spelling deficits are common in all three variants, we have taken several measures to mitigate the possible effects of these limitations. First, we randomized the treatment condition (tDCS vs. sham) within each variant. Randomization and patient flow resulted in the following numbers in each variant in each treatment condition: lvPPA: 3 sham, 5 tDCS, nfvPPA: 5 sham, 3 tDCS, and svPPA: 4 sham, 4 tDCS. Second, we matched the initial language performance of those who received tDCS combined with language intervention and those who received sham combined with language intervention, so participants in each condition were at matched stages of disease progression. In addition, Fig. 6 illustrates the effects of tDCS on functional connectivity stratified by variant, as well as individual results. Studies with adequate power to look at the effects of tDCS in each variant may elucidate the effect of variant in the functional connectivity changes induced by tDCS.
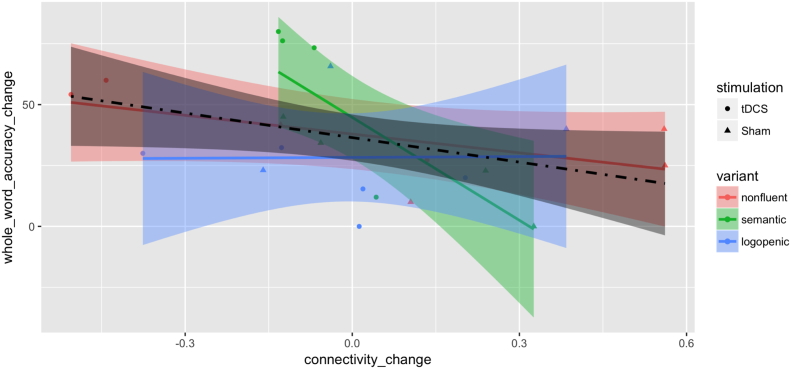
The primary outcome for measuring language performance in our study was the letter accuracy of therapy items, which could have potential limitations in assessing generalization of both the tDCS effect on language performance and the association between the connectivity change and performance improvement. Another measurement is the whole-word accuracy of therapy items, a crude estimate for some participants but still an indicator of generalization of results. Whole-word accuracy results were similar to letter-accuracy results for non-logopenic participants (15 in total) for both: (A) tDCS effect on language performance (additional mean improvement for non-logopenic subjects: 27.36, Welch two sample t-test P-value: 0.037, df: 9.74; additional mean improvement for all: 8.46, Welch two sample t-test P-value: 0.391, df: 19.10), and, (B) the association between the connectivity change on IFG_triangularis_L and ITG_L and the performance improvement (Fig. 7) (whole-word accuracy changes regressed on connectivity changes on IFG_triangularis_L and ITG_L, for non-logopenic subjects estimated linear coefficient: -41.40, SE: 17.37, T-statistic: −2.38, P-value: 0.032, 95% confidence interval: [−78.64, −4.16]; for all estimated linear coefficient: -33.57, SE: 16.71, T-statistic: −2.009, P-value: 0.058, 95% confidence interval: [−68.31, 1.18]). Such similarity suggests potential generalization despite the need of more sufficient data to support more accurate analysis by variant.
Fig. 7.
Scatter plot by treatment groups and PPA variants for change (after minus before) of absolute whole-word accuracy in % against change in connectivity between the IFG_triangularis_L: ITG_L pair. Variants are color-coded as red for nonfluent, green for semantic and blue for logopenic. Treatment assignments are coded as circles for tDCS and triangles for Sham. Fitted lines and pointwise confidence bands are plotted for each variant with the corresponding coded color and for all participants with the dot-dashed line and the color black. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.3. Limitations of the present study
Given that our design only addressed the additional effects of tDCS during language therapy in PPA, we did not compare changes of connectivity without any intervention, i.e., only due to disease progression or aging. Other studies, however, that have included aging populations and neurodegenerative diseases confirmed the initial abnormalities in resting-state neural networks observed in the present study and the possibility to be modified by tDCS (Caffo et al., 2010; Meinzer et al., 2013).
The findings are tied to our style of processing and analysis. While anatomically derived ROIs enjoy variance reduction via averaging over voxels within the region, voxel based approaches, such as seed correlations, can interrogate less structured hypotheses at the expense of increased multiplicity concerns. In addition, decomposition methods, such as principal and independent component analysis, can perform dimension reduction and investigate promising directions of variations (networks). Another concern is the focus on static connectivity (correlations over the entire scan). Recent efforts in dynamic connectivity have raised the possibility of its importance in the study of connectivity. Stimulation may impact dynamic connectivity as much as or more than static connectivity, and this modulation may be essential for the study of interactions with behavior.
Finally, the present study may be limited by the spatial resolution of the electrode patches used to deliver tDCS. TDCS generally provides reduced spatial specificity compared to more targeted approaches such as transcranial magnetic stimulation (TMS). TDCS was chosen as a therapeutic tool because it is has an excellent safety record, it is easily tolerated, and, because it is also small and inexpensive, it has potential for future implementation at the speech therapist's office. Due to the 5 × 5 cm size of the sponge electrodes, tDCS may have stimulated surrounding brain regions in addition to the intended target. For this reason we added the DMN as a control network and showed that findings were specific to the language network. Future investigations to assess the effectiveness of TMS as a therapeutic tool for PPA are warranted.
5. Conclusions
The present study has important implications for both clinical practice and our understanding of the brain mechanisms involved in repeated tDCS in neurodegenerative syndromes. Isolating the additional effect of tDCS over the left IFG from language therapy itself allowed us to investigate the brain mechanism of this particular tDCS application. The positive correlation between changes in functional connectivity in fronto-temporal areas of the language network with the therapy outcomes confirmed that these changes in functional connectivity are a possible mechanism underlying tDCS effects in neurodegenerative disorders, as implied by previous studies after one tDCS application (Meinzer et al., 2013).
The following is the supplementary data related to this article.
Simulation of current flow over the left IFG as targeted by 5 × 5 cm sponges. (Image courtesy of Dr. Marom Bikson).
Declarations of interest
None.
Acknowledgements
This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to KT. AH was supported by NIH (NIDCD) through awards R01 DC05375, R01 DC011317 and P50 DC014664. We are grateful to our participants for their unfailing commitment and interest in our study. We also thank referring physicians.
References
- Akaike H. Selected Papers of Hirotugu Akaike. Springer; 1998. Information theory and an extension of the maximum likelihood principle; pp. 199–213. [Google Scholar]
- Andrews-Hanna J.R., Snyder A.Z., Vincent J.L., Lustig C., Head D., Raichle M.E., Buckner R.L. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar V., Near J., Johansen-Berg H., Stagg C.J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. elife. 2015;4 doi: 10.7554/eLife.08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P.M., Egnor H. Combining treatment for written and spoken naming. J. Int. Neuropsychol. Soc. 2006;12:816–827. doi: 10.1017/S1355617706061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P.M., King R.M., Bonakdarpour B., Henry M.L., Cho H., Rapcsak S.Z. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. J. Mol. Neurosci. 2011;45:724–736. doi: 10.1007/s12031-011-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best W., Nickels L. From theory to therapy in aphasia: where are we now and where to next? Neuropsychol. Rehabil. 2000;10:231–247. [Google Scholar]
- Brunoni A.R., Nitsche M.A., Bolognini N., Bikson M., Wagner T., Merabet L., Edwards D.J., Valero-Cabre A., Rotenberg A., Pascual-Leone A., Ferrucci R., Priori A., Boggio P.S., Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffo B.S., Crainiceanu C.M., Verduzco G., Joel S., Mostofsky S.H., Bassett S.S., Pekar J.J. Two-stage decompositions for the analysis of functional connectivity for fMRI with application to Alzheimer's disease risk. NeuroImage. 2010;51:1140–1149. doi: 10.1016/j.neuroimage.2010.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Donato R., Ffytche D.H. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Ceritoglu C., Tang X., Chow M., Hadjiabadi D., Shah D., Brown T., Burhanullah M.H., Trinh H., Hsu J.T., Ament K.A. Computational analysis of LDDMM for brain mapping. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M., Manenti R., Petesi M., Brambilla M., Cosseddu M., Zanetti O., Miniussi C., Padovani A., Borroni B. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J. Alzheimers Dis. 2014;39:799–808. doi: 10.3233/JAD-131427. [DOI] [PubMed] [Google Scholar]
- Croot K., Nickels L., Laurence F., Manning M. Impairment- and activity/participation-directed interventions in progressive language impairment: clinical and theoretical issues. Aphasiology. 2009;23(125–160):36p. [Google Scholar]
- DeDe G., Parris D., Waters G. Teaching self-cues: a treatment approach for verbal naming. Aphasiology. 2003;17:465–480. [Google Scholar]
- DeMarco A.T., Wilson S.M., Rising K., Rapcsak S.Z., Beeson P.M. Neural substrates of sublexical processing for spelling. Brain Lang. 2017;164:118–128. doi: 10.1016/j.bandl.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A.W. Psychology Press; 1993. Reading, Writing and Dyslexia: A Cognitive Analysis. [Google Scholar]
- Ellis A.W., Young A.W. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Human Cognitive Neuropsychology. [Google Scholar]
- Faria A.V., Joel S.E., Zhang Y., Oishi K., van Zjil P.C., Miller M.I., Pekar J.J., Mori S. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. NeuroImage. 2012;61(3):613–621. doi: 10.1016/j.neuroimage.2012.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- Fox M.D., Buckner R.L., Liu H., Chakravarty M.M., Lozano A.M., Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D.A. On the so-called “Huber sandwich estimator” and “robust standard errors”. Am. Stat. 2006;60:299–302. [Google Scholar]
- Gandiga P.C., Hummel F.C., Cohen L.G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gervits F., Ash S., Diloyan M., Morgan B., Coslett H., Grossman M., Hamilton R. Transcranial direct current stimulation for the treatment of primary progressive aphasia. Neurology. 2015;84 doi: 10.1016/j.bandl.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R., Nobre A.C., Sonty S., Parrish T.B., Mesulam M.M. Language network specializations: an analysis with parallel task designs and functional magnetic resonance imaging. NeuroImage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Goodman R.A., Caramazza A. Johns Hopkins University. ed.; Baltimore, MD: 1985. The Johns Hopkins University Dysgraphia Battery. [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R.H., Chrysikou E.G., Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118:40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.L., Beeson P.M., Alexander G.E., Rapcsak S.Z. Written language impairments in primary progressive aphasia: a reflection of damage to central semantic and phonological processes. J. Cogn. Neurosci. 2012;24:261–275. doi: 10.1162/jocn_a_00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.L., Rising K., DeMarco A.T., Miller B.L., Gorno-Tempini M.L., Beeson P.M. Examining the value of lexical retrieval treatment in primary progressive aphasia: two positive cases. Brain Lang. 2013;127:145–156. doi: 10.1016/j.bandl.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E. Efficacy and generalization of treatment for aphasic naming errors. Arch. Phys. Med. Rehabil. 1989;70:632–636. [PubMed] [Google Scholar]
- Hillis A.E., Tuffiash E., Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J. Cogn. Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Holland R., Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012;26:1169–1191. doi: 10.1080/02687038.2011.616925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979:65–70. [Google Scholar]
- Homan R.W., Herman J., Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Hummel F.C., Voller B., Celnik P., Floel A., Giraux P., Gerloff C., Cohen L.G. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokel R., Graham N.L., Rochon E., Leonard C. Word retrieval therapies in primary progressive aphasia. Aphasiology. 2014;28:1038–1068. [Google Scholar]
- Keeser D., Meindl T., Bor J., Palm U., Pogarell O., Mulert C., Brunelin J., Moller H.J., Reiser M., Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S. Training phoneme to grapheme conversion for patients with written and oral production deficits: a model-based approach. Aphasiology. 2005;19:53–76. [Google Scholar]
- Kiran S., Thompson C.K., Hashimoto N. Training grapheme to phoneme conversion in patients with oral reading and naming deficits: a model-based approach. Aphasiology. 2001;15:855–876. [Google Scholar]
- Kiran S., Sandberg C., Abbott K. Treatment for lexical retrieval using abstract and concrete words in persons with aphasia: effect of complexity. Aphasiology. 2009;23:835–853. doi: 10.1080/02687030802588866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Kramer J.H., Boeve B.F., Caselli R.J., Graff-Radford N.R., Mendez M.F., Miller B.L., Mercaldo N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Madison C., Ghosh P.M., Miller Z.A., Greicius M.D., Kramer J.H., Coppola G., Miller B.L., Jagust W.J., Gorno-Tempini M.L. Loss of functional connectivity is greater outside the default mode network in nonfamilial early-onset Alzheimer's disease variants. Neurobiol. Aging. 2015;36:2678–2686. doi: 10.1016/j.neurobiolaging.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E., Nouet A., Gatignol P., Capelle L., Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain J. Neurol. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Marangolo P., Fiori V., Paola M.D., Cipollari S., Razzano C., Oliveri M., Caltagirone C. Differential involvement of the left frontal and temporal regions in verb naming: a tDCS treatment study. Restor. Neurol. Neurosci. 2013;31:63–72. doi: 10.3233/RNN-120268. [DOI] [PubMed] [Google Scholar]
- Marangolo P., Fiori V., Sabatini U., De Pasquale G., Razzano C., Caltagirone C., Gili T. Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: preliminary data from aphasia. J. Cogn. Neurosci. 2016;28(5):724–738. doi: 10.1162/jocn_a_00927. [DOI] [PubMed] [Google Scholar]
- Marcotte K., Adrover-Roig D., Damien B., de Preaumont M., Genereux S., Hubert M., Ansaldo A.I. Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia. 2012;50:1776–1786. doi: 10.1016/j.neuropsychologia.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Marcotte K., Perlbarg V., Marrelec G., Benali H., Ansaldo A.I. Default-mode network functional connectivity in aphasia: therapy-induced neuroplasticity. Brain Lang. 2013;124:45–55. doi: 10.1016/j.bandl.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Meinzer M., Antonenko D., Lindenberg R., Hetzer S., Ulm L., Avirame K., Flaisch T., Floel A. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J. Neurosci. 2012;32:1859–1866. doi: 10.1523/JNEUROSCI.4812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Antonenko D., Flaisch T., Flöel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013;33:12470–12478. doi: 10.1523/JNEUROSCI.5743-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Phan M.T., Ulm L., Volk C., Flöel A. Transcranial direct current stimulation in mild cognitive impairment: behavioral effects and neural mechanisms. Alzheimers Dement. 2015;11:1032–1040. doi: 10.1016/j.jalz.2014.07.159. [DOI] [PubMed] [Google Scholar]
- Miller M.I., Beg M.F., Ceritoglu C., Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wu D., Ceritoglu C., Li Y., Kolasny A., Vaillant M.A., Faria A.V., Oishi K., Miller M.I. MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput. Sci. Eng. 2016;18:21–35. [Google Scholar]
- Newhart M., Davis C., Kannan V., Heidler-Gary J., Cloutman L., Hillis A.E. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23:823–834. [Google Scholar]
- Pena-Gomez C., Sala-Lonch R., Junque C., Clemente I.C., Vidal D., Bargallo N., Falcon C., Valls-Sole J., Pascual-Leone A., Bartres-Faz D. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimulat. 2011;5(3):252–263. doi: 10.1016/j.brs.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Gomez C., Sole-Padulles C., Clemente I.C., Junque C., Bargallo N., Bosch B., Molinuevo J.L., Valls-Sole J., Pascual-Leone A., Bartres-Faz D. APOE status modulates the changes in network connectivity induced by brain stimulation in non-demented elders. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planton S., Jucla M., Roux F.E., Demonet J.F. The “handwriting brain”: a meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex J. Devoted Study Nerv. Syst. Behav. 2013;49:2772–2787. doi: 10.1016/j.cortex.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Polania R., Paulus W., Nitsche M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2011;33(10):2499–2508. doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell J.J., Rapp B. Identifying functional reorganization of spelling networks: an individual peak probability comparison approach. Front. Psychol. 2013;4:964. doi: 10.3389/fpsyg.2013.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell J.J., Turkeltaub P.E., Eden G.F., Rapp B. Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol. 2011;2:239. doi: 10.3389/fpsyg.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race D.S., Tsapkini K., Crinion J., Newhart M., Davis C., Gomez Y., Hillis A.E., Faria A.V. An area essential for linking word meanings to word forms: evidence from primary progressive aphasia. Brain Lang. 2013;127:167–176. doi: 10.1016/j.bandl.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B., Dufor O. The neurotopography of written word production: an FMRI investigation of the distribution of sensitivity to length and frequency. J. Cogn. Neurosci. 2011;23:4067–4081. doi: 10.1162/jocn_a_00109. [DOI] [PubMed] [Google Scholar]
- Rapp B., Glucroft B. The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology. 2009;23:236–265. doi: 10.1080/02687030801943054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G., Renga V., Nair D. Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G., Marchina S., Wan C.Y. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol. Rev. 2011;21:288–301. doi: 10.1007/s11065-011-9181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R., Tsapkini K., Tippett D.C. Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: current knowledge and future clinical applications. NeuroRehabilitation. 2016;39:141–152. doi: 10.3233/NRE-161346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Allman J.M., Carlin D.A., Crawford R.K., Macedo M.N., Greicius M.D., Dearmond S.J., Miller B.L. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis. Assoc. Disord. 2007;21:S50–S57. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- Sehm B., Schafer A., Kipping J., Margulies D., Conde V., Taubert M., Villringer A., Ragert P. Dynamic modulation of intrinsic functional connectivity by transcranial direct current stimulation. J. Neurophysiol. 2012;108:3253–3263. doi: 10.1152/jn.00606.2012. [DOI] [PubMed] [Google Scholar]
- Sepelyak K., Crinion J., Molitoris J., Epstein-Peterson Z., Bann M., Davis C., Newhart M., Heidler-Gary J., Tsapkini K., Hillis A.E. Patterns of breakdown in spelling in primary progressive aphasia. Cortex J. Devoted Study Nerv. Syst. Behav. 2011;47:342–352. doi: 10.1016/j.cortex.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H., Hurley R.S., Rogalski E., Mesulam M. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia. 2012;50:1929–1935. doi: 10.1016/j.neuropsychologia.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J. Magnetic resonance spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. NeuroImage. 2014;86:19–27. doi: 10.1016/j.neuroimage.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Johansen-Berg H. Studying the effects of transcranial direct-current stimulation in stroke recovery using magnetic resonance imaging. Front. Hum. Neurosci. 2013;7:857. doi: 10.3389/fnhum.2013.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Nitsche M.A. Physiological basis of transcranial direct current stimulation. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Best J.G., Stephenson M.C., O'Shea J., Wylezinska M., Kincses Z.T., Morris P.G., Matthews P.M., Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Amadi U., Gudberg C.A., Ilie A.S., Sampaio-Baptista C., O'Shea J., Woolrich M., Smith S.M., Filippini N., Near J., Johansen-Berg H. Local GABA concentration is related to network-level resting functional connectivity. elife. 2014;3 doi: 10.7554/eLife.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Menon V., Rubin D., Musen M., Greicius M.D. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Oishi K., Faria A.V., Hillis A.E., Albert M.S., Mori S., Miller M.I. Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M., Lesoil C., Godard J., Vernet M., Bertrand A., Levy R., Dubois B., Lemoine L., Truong D.Q., Bikson M. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann. Neurol. 2016;80:693–707. doi: 10.1002/ana.24766. [DOI] [PubMed] [Google Scholar]
- Tippett D.C., Hillis A.E., Tsapkini K. Treatment of primary progressive aphasia. Curr. Treat. Options Neurol. 2015;17 doi: 10.1007/s11940-015-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K., Hillis A.E. Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behav. Neurol. 2013;26:55–66. doi: 10.3233/BEN-2012-110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K., Vindiola M., Rapp B. Patterns of brain reorganization subsequent to left fusiform damage: fMRI evidence from visual processing of words and pseudowords, faces and objects. NeuroImage. 2011;55:1357–1372. doi: 10.1016/j.neuroimage.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K., Frangakis C., Gomez Y., Davis C., Hillis A.E. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology. 2014;28:1112–1130. doi: 10.1080/02687038.2014.930410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiatis A.A., Davidian M., Zhang M., Lu X. Covariate adjustment for two-sample treatment comparisons in randomized clinical trials: a principled yet flexible approach. Stat. Med. 2008;27:4658–4677. doi: 10.1002/sim.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Simulation of current flow over the left IFG as targeted by 5 × 5 cm sponges. (Image courtesy of Dr. Marom Bikson).