Abstract
Cell-free microRNAs in plasma provide circulating biomarkers for lung cancer. Most techniques for analysis of miRNAs require a large plasma volume to purify a sufficient RNA yield followed by complicated downstream processing. Small differences in the multiple procedures often cause large analytical variations and poor diagnostic values of the plasma biomarkers. Here we investigate whether directly quantifying plasma miRNAs without RNA purification could diagnose lung cancer. FirePlex assay was directly applied to 20 μl plasma of 56 lung cancer patients and 28 cancer free controls for quantifying 11 lung tumor–associated miRNAs. FirePlex assay is easier, less expensive and time-consuming for quantification of plasma miRNAs compared with conventional reverse transcription PCR with an equivalent analytic performance. From the lung tumor–associated miRNAs, a prediction model based on two miRNAs (miRs-205-5p and -210-3p) was developed, producing 78.6% sensitivity and 89.3% specificity for identifying lung cancer. The diagnostic value was independent of stage of lung tumor, and patients’ age and sex (all P > 0.05). Furthermore, based on the same two miRNAs, additional prediction models were developed with 75.0% sensitivity and 89.3% specificity for diagnosis of lung squamous cell carcinoma, and 82.2% sensitivity and 89.3% specificity for lung adenocarcinoma. The direct plasma assay can improve the efficacy of miRNA assessment in a small plasma volume by reducing multiple procedure-associated analytical variables. The developed plasma miRNA biomarkers might be useful for the early detection and histological classification of lung cancer.
Introduction
Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer that is the number one cancer killer [1]. NSCLC mainly consists of two major histological subtypes: squamous cell carcinoma (SCC) and adenocarcinoma (AC) [2]. The 5-year survival rate for stage IV NSCLC is only 10%, whereas nearly 85% for stage IA NSCLC [1]. These statistics provide the primary rationale to improve the early detection of NSCLC. Furthermore, histological subtype is predictive of a differential response rate, overall survival, or toxicity profile from certain therapies. Precisely classifying subtypes of lung cancer is also clinically important for the personalized treatment of the malignancy [3], [4], [5]. MicroRNAs (miRNAs) are small molecules that have important functions in diverse biological processes, including cell proliferation, differentiation, and apoptosis [6], [7], [8]. Furthermore, dysfunction of miRNAs contributes to the development and progression of human malignancies, including lung cancer [9]. In addition, miRNA expression profiles represent molecular signatures for the classification, diagnosis, and progression of tumor, and thus could be developed as cancer biomarkers [8], [10]. Blood is one of the most easily and noninvasively accessible body fluids. The use of blood plasma for assessment of circulating miRNAs originating from primary tumors would provide a useful tool for the early detection and histological classification of lung cancer [11], [12], [13].
The most common techniques used in analysis of plasma miRNAs mainly include quantitative reverse transcription polymerase chain reaction (qRT-PCR) and microarray [14]. Being sensitive and quantitative, qRT-PCR is relatively inexpensive and flexible, and thus the preferred choice for assessment of limited number of miRNAs in relatively small experiments. Compared with qRT-PCR, microarrays can target more miRNAs for larger studies, however, have a low detection sensitivity. Since the cancer-associated miRNAs are derived from primary tumor and could be ‘diluted’ in a background of normal miRNAs, the circulating miRNAs are present in very low abundance in plasma [11], [15], [16]. In order to profile the low-abundance miRNAs in plasma, these current techniques require a large plasma volume to purify a sufficient RNA yield followed by complex downstream workflow, including reverse transcription [17]. However, small differences in the complex processes often result in large analytical variations and poor diagnostic performances of the plasma biomarkers [14], [17].
FirePlex technology is a new approach for quantification of miRNAs [18]. This technique is dependent on hydrogel particles that contain custom selected probes against target miRNAs. miRNAs bind to these probes and are then ligated to adaptor sequences for pre-detection amplification. Particles are optimized for use with common bench-top flow cytometers, allowing detection without specialized laboratory equipment. Furthermore, data analysis is relatively straightforward and does not require advanced bioinformatics skills. This technique can quantify multiple miRNAs across a range of samples without the labor-intensive workflow or large sample requirement for other techniques. Importantly, it can be used directly with crude body fluids without RNA extraction and reverse transcription. In addition, miRNA profiling by the FirePlex technology can be achieved from input of as little as 10 μL of plasma or 100 pg purified RNA [18]. Therefore, FirePlex technology for multiplex miRNA profiling may overcome the obstacles of the current techniques by eliminating the errors induced during sample purification and insufficient methods for analytical performance. The objective of the present study was to use FirePlex miRNA assay to develop plasma miRNA biomarkers for lung cancer early detection and histological classification.
Materials and Methods
Patients and Clinical Specimens
The Institutional Review Boards of University of Maryland Baltimore and Veterans Affairs Maryland Health Care System approved this study. We recruited lung cancer patients and cancer-free smokers by using the inclusion and/or exclusion criteria recommended by U.S. Preventive Services Task Force for lung cancer screening in heavy smokers [19]. We collected blood in BD Vacutainer spray-coated K2EDTA Tubes (BD, Franklin Lakes, NJ) and prepared plasma using the standard operating protocols developed by The NCI-Early Detection Research Network within 2 hours of the collection [20]. A total of 56 NSCLC patients and 28 cancer-free smokers were recruited. Among the cancer patients, 9 patients were female and 47 were male. Twelve had stage I NSCLCs, 6 with stage II, 12 with stage III, 18 with stage IV, and 7 with unknown stage. Histologically, 28 lung cancer patients were diagnosed with AC, while 28 with SCC. Of the cancer-free smokers, 2 patients were female and 28 were male. The cancer-free patients who were smokers and served as control subjects had granulomatous inflammation (n = 14), nonspecific inflammatory changes (n = 12) or lung infections (n = 2). The cancer-free smokers had been followed for at least 2 years, and none had any evidence of cancer. The demographic and clinical variables of the cohort are shown in Table 1.
Table 1.
Characteristics of NSCLC Patients and Cancer-Free Smokers
| NSCLC Cases (n = 56) | Controls (n = 28) | P-Value | |
|---|---|---|---|
| Age | |||
| 65.68 (SD 10.09) | 62.37 (SD 9.26) | 0.262 | |
| Sex | |||
| Female | 9 | 2 | 0.2 |
| Male | 47 | 26 | |
| Pack-years (median) | 52.88 | 48.75 | 0.37 |
| Stage | |||
| Stage I | 12 | ||
| Stage II | 6 | ||
| Stage III | 13 | ||
| Stage IV | 18 | ||
| Unknown stage | 7 | ||
| Histological type | |||
| Adenocarcinoma | 28 | ||
| Squamous cell carcinoma | 28 |
Abbreviations: NSCLC, non-small cell lung cancer; SD, standard deviation.
miRNA Quantification by the FirePlex Assay
Using next-generation deep sequencing to comprehensively characterize miRNA profiles in eight lung tumor tissues, we identified miRNAs whose dysregulation was associated with NSCLC [21]. From the miRNAs, we found that 11 miRNAs might be potential plasma biomarkers for lung cancer [12], [13], [21], [22], [23], [24], [25], [26]. The 11 miRNAs were analyzed in this study, including miRs-205-5p, -145, -422a,-34a-5p, -93-5p,-223-3p,-210-3p, and 628-3p, and lets-7d-5p, -7g-5p, and -7i-5p.
Plasma 20 μL was mixed with 36 μL Digest Buffer, 20 μL water and 4 μL Protease Mix and incubated at 60°C for 45 minutes with shaking. For each sample run, FirePlex Particles (35 μL) were added to a well of a 96-well filter plate and filtered. Twenty-five μL Hybe Buffer was added to each well. One ng total RNA was loaded. The plate was incubated at 37°C for 60 minutes with shaking. After rinsing twice with 1× Rinse A, 75 μL of 1× Labeling Buffer was added to each well. The plate was incubated at room temperature for 60 minutes with shaking. After two rinses with 1× Rinse B followed by one rinse with 1× Rinse A, a catch plate was added to the vacuum manifold and the filter plate put under constant vacuum. 65 μL of 95°C RNAse-free water was added twice to each well to elute the ligated sample. 30 μL of this meltoff was added to a clean PCR plate and mixed with 20 μL PCR master mix. The mixture underwent 32 cycles of PCR amplification. 60 μL of Hybe Buffer was added back to each well of the original particles followed by 20 μL of the PCR product, and the plate was incubated at 37°C for 30 minutes with shaking. After rinsing twice with 1× Rinse B followed by one rinse with 1× Rinse A, 75 μL of 1× Reporting Buffer was added to each well and the plate incubated at room temperature for 15 minutes with shaking. After rinsing twice with 1× Rinse A, 175 μL of Run Buffer was added to each well. The samples were then scanned on an EMD Millipore Guava 6HT flow cytometer (MilliporeSigma, Germany). Flow cytometry quantification data was analyzed with the FirePlex™ Analysis Workbench software as previously described [18].
RNA Isolation and qRT-PCR
Plasma 200 μL was used for RNA extraction by Trizol LS reagent (Invitrogen Carlsbad, CA) and RNeasy Mini Kit (Qiagen, Hilden, Germany) [22], [26], [27]. RT was carried out to generate cDNA by using a RT Kit (Applied Biosystems, Foster City, CA) as described in our published works [22], [26], [27]. PCR was performed to measure expressions of target genes by using a PCR kit (Applied Biosystems) on a Bio-Red IQ5 Multi-color RT-PCR Detection System (Bio-Red, Hercules, CA). Expression levels of the genes were determined using comparative cycle threshold (CT) method with miR-1228 as an internal control. The targeted genes with CT values >35 were considered to be below the detection level of qRT-PCR [28]. All reactions were run in triplicate. CT values were converted into absolute copy numbers using a standard curve from a synthetic miRNA as previously described [22], [26], [27].
Statistical Analysis
We used the area under receiver operating characteristic (ROC) curve (AUC) with the null hypothesis (H0) of 0.5 to determine sample size. 28 subjects were required in each category of cases and controls to show a minimum difference of interest between an AUC of 0.75 versus an AUC of 0.5 with 80% power at the 5% significance level. To determine if a particular miRNA signal was detectable, assay background was subtracted from the miRNA signal measured in the samples and the result was compared to a limit of detection. The detection threshold was set based on comparison of fluorescence intensity of sample wells to negative (water) wells by calculating [(3Sww)2+(4Spp)2]1/2. Sww was the well-to-well standard deviation of the mean signals, and Spp was the mean of the particle-particle standard errors. The detection threshold was set at 2.00 as previously established [18]. We generated the heatmap based on a grid of raw and background subtracted miRNA signal intensities indicated by color. We used an ANOVA to determine if the groups exhibit significantly different expression levels for a particular miRNA, and the ANOVA results were reported as an unadjusted P-value. Differential expression with P-values less than 0.05 indicated that the two groups were different with at least a 95% confidence level. Pearson's correlation analysis was utilized to assess relationship between plasma miRNA expressions and demographic characteristics of the patients and cancer-free controls. Correlation between FirePlex versus qRT-PCR output analyses was tested by using linear regression models. The precision of miRNA measurements was estimated by using coefficient of variation [CV = (SD/mean)*100] of quadruplicate measures for each sample. The ROC and AUC analyses were applied to determine sensitivity and specificity of each miRNA. We used logistic regression models with constrained parameters as in least absolute shrinkage and selection operator (LASSO) to eliminate the irrelevant genes and develop prediction models with the highest sensitivity and specificity.
Results
Directly Targeting Plasma Samples for Quantification of miRNAs
The releases of miRNAs in plasma by hemolysis of blood cells often produce nonspecific and low reproducible results [16], [29], [30], [31], [32]. To determine if the plasma had hemolysis of blood cells, we included hemolysis-associated miRNA markers (miRs-451a and-486-5) in the FirePlex miRNA Assay. All the specimens had negative results of the hemolysis miRNA markers, suggesting no hemolysis in the plasma samples. All the 11 miRNAs, including miRs-205-5p,-145, -422a,-34a-5p, -93-5p,-223-3p,-210-3p, and 628-3p, and lets-7d-5p, -7g-5p, and -7i-5p, had background-subtracted signal above the detection threshold (Figure 1). Therefore, the FirePlex miRNA Assay could quantify the 11 miRNAs in plasma.
Figure 1.
Directly targeting 84 plasma samples for quantifying 11 miRNAs. The heatmap is a grid of raw, background subtracted miRNA signal intensities indicated by color. The 11 genes have background-subtracted signal above the detection threshold (2.00) and thus are reliably quantified in plasma.
To determine the robustness of the FirePlex assay in miRNA quantification, we performed qRT-PCR in plasma samples of 20 cancer-free controls and 20 lung cancer patients for quantitation of a miRNA, miRs-34a-5p. There was no statistical difference of the CVs of miRNA levels determined by FirePlex vs. qRT-PCR in the same samples (P = 0.26) (Supplementary Figure 1), implying that FirePlex had comparable precision and repeatability compared with qRT-PCR for quantification of plasma miRNA. Furthermore, there was a close correlations of expression levels of the miRNA determined by the two platforms (Pearson r = 0.9961, P < 0.0001) (Supplementary Figure 2). In addition, the total time required for the FirePlex assay is 3-5 hours, whereas 24 hours for qRT-PCR. Altogether, the FirePlex assay is a less time-consuming technique for quantification of plasma miRNAs with an equivalent analytic performance compared with qRT-PCR.
Plasma Biomarker Panels for the Early Detection and Histological Classification of Lung Cancer
Of the 11 miRNAs that robustly detectable in the plasma samples, 10 miRNAs (miRs-93-5p, 628-3p, -422a,-34a-5p, -223-3p, -210-3p,and -205-5p, and lets-7d-5p, -7g-5p, and -7i-5p) had a different expression level in lung cancer patients vs. cancer-free individuals (All P < 0.05) (Figure 2) (Supplementary Figure 3). We used ROC to evaluate the diagnostic performance of the miRNAs for lung cancer. The 10 miRNAs showed 0.663-0.77 AUC values with 58.93-73.21% sensitivity and 60.25-82.14% specificity (Table 2), implying that the miRNAs held promise as plasma biomarkers for lung cancer.
Figure 2.
Of the 11 miRNAs tested in plasma samples of cancer-free smokers vs. lung cancer patients, 10 miRNAs have a significantly different level in the lung cancer patients vs. cancer-free smokers (all P < 0.05). The inside line denotes the median.
Table 2.
miRNAs with a Different Expression Level in Lung Cancer Patients vs. Cancer-Free Smokers
| miRNAs | AUC | Standard Deviation | P Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| mir-93-5p | 0.7691 | 0.0518 | <0.0001 | 71.43 | 71.43 |
| miR-628-3p | 0.6633 | 0.0600 | 0.0152 | 58.93 | 60.71 |
| mir-422a | 0.6773 | 0.0599 | 0.0084 | 62.5 | 60.71 |
| miR-34a-5p | 0.7165 | 0.0597 | 0.0013 | 67.86 | 67.86 |
| mir-223-3p | 0.6709 | 0.0592 | 0.0110 | 73.21 | 60.71 |
| mir-210-3p | 0.7545 | 0.0518 | 0.0002 | 64.29 | 82.14 |
| miR-205-5p | 0.7519 | 0.0543 | 0.0002 | 69.64 | 67.86 |
| lets-7d-5p | 0.6823 | 0.0368 | 0.0013 | 63.92 | 60.25 |
| let-7i-5p | 0.6678 | 0.0156 | 0.0002 | 59.66 | 61.26 |
| let-7g-5p | 0.7126 | 0.0387 | 0.0036 | 68.23 | 67.53 |
| A panel of 2 miRNAs | 0.8510 | 0.0162 | 0.0001 | 78.57 | 89.29 |
Abbreviations: AUC, area under the receiver-operator characteristic curve.
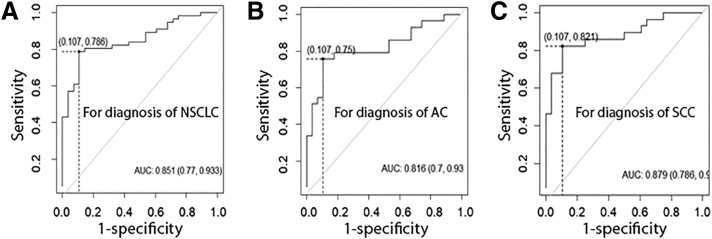
We used logistic regression models with constrained parameters as in LASSO and AUCs to optimize a marker panel for lung cancer. From the 10 genes, a logistic regression model with 2 miRNAs (miRs-205-5p and -210-3p) was developed for diagnosing lung cancer: U = -1.44 + 0.05×miR205-5p + 0.07×miR-210-3p. Incorporated analysis of the 2 biomarkers by using the logistic regression model produced a higher AUC (0.85) than did the individual biomarkers (Figure 3A) (Table 2) (P < 0.05). We calculated the distance to the perfect point (0, 1) with varying cut-offs for U, and the cut-off corresponding to the shortest distance in the AUC analysis was considered the optimal one. The optimal cut-off for the logistic regression model was U = 0.62. Any subject with U ≥ 0.62 was classified as a lung cancer case. As a result, combined analysis of the 2 miRNAs by using the logistic regression model yielded significantly higher sensitivity (78.6%) and specificity (89.3%) compared with the individual biomarkers (all P < 0.05) (Table 2). Furthermore, based on the 2 miRNAs, a second logistic regression model was developed for diagnosis of AC with AUC of 0.82: U = -1.69+ 0.05×miR-205-5p +0.03×miR-210-3p (Figure 3B). When cut-off was set at U = 0.53, this logistic regression model produced 75.0% sensitivity and 89.3% specificity for diagnosis of AC. In addition, using the same 2 miRNAs, a third logistic regression model was established for diagnosis of SCC with AUC of 0.88: U = -2.62+ 0.043×miR-205-5p +0.1×miR-210-3p (Figure 3C). When cut-off was set at U = 0.35, this logistic regression model produced 82.1% sensitivity and 89.3% specificity for diagnosis of SCC.
Figure 3.
Receiver-operator characteristic (ROC) curves of 2 plasma miRNAs (miRs-205-5p and -210-3p). The area under the ROC curve (AUC) for the biomarkers conveys the accuracy. (A), a logistic regression model based on the 2 miRNAs creates an AUC value of 0.85 with 78.6% sensitivity and 89.3% specificity for diagnosis of non-small-cell lung cancer (NSCLC). (B), a logistic regression model based on the 2 miRNAs produces 0.82 AUC with 75.0% sensitivity and 89.3 % specificity for diagnosis of lung adenocarcinoma (AC). (C), a logistic regression model based on the 2 miRNAs generates 0.88 AUC with 82.1% sensitivity and 89.3% specificity for diagnosis of lung squamous cell carcinoma (SCC).
Combined use of all the 10 miRNA genes did not produce higher sensitivity and specificity compared with the two miRNAs (miRs-205-5p and -210-3p) used in combination (P > 0.05). Furthermore, the estimated correlations among levels of the 2 genes were very low (Pearson r = -0.0219, P = 0.7720) (Supplementary Figure 4), implying that the integration of the two molecular biomarkers has complementary classification. In addition, the logistic regression models had no special association with age, gender, and smoking status of the participants (All P > 0.05). Moreover, the three logistic regression models with the same two miRNAs had similar sensitivity and specificity for diagnosis of different stages of NSCLC (Supplementary Figure 5).
Discussion
The current techniques for the assessment of plasma miRNAs require a large plasma volume followed by the complicated downstream handling. Small differences in the processing will lead to poor analytical and diagnostic values of plasma biomarkers. We propose to avoid the multiple procedures by direct analyzing plasma using the FirePlex technology for quantifying multiple miRNAs. Our head-to-head comparison of the FirePlex Assay and qRT-PCR demonstrates that the FirePlex can eliminate intra-assay variables and improve reliability of miRNA quantification by directly targeting crude plasma and minimizing the complicated workflow. Furthermore, unlike qRT-PCR requiring a large sample volume (200 μL), the FirePlex assay can simultaneously quantify multiple miRNAs from input of as little as 20 μL of plasma. In addition, without the expensive procedures including RNA isolation, RNA quantification, and RT, the FirePlex technology can be used at less cost compared with qRT-PCR. The FirePlex assay only takes 3–5 hours from sample to data, and thus requires a shorter turnaround time compared with qRT-PCR demanding 24 hours. Moreover, the data handling for the FirePlex technology is straightforward for fast analysis and interpretation, as the results are obtained by using conventional flow cytometry with simple software. Therefore, the FirePlex assay might provide an efficient means for quantification of the plasma miRNAs whose changes are the hallmark of lung cancer.
Importantly, we develop 2 miRNA biomarkers, which used in combination could diagnose lung cancer. Since the biomarkers show similar sensitivity and specificity in the early vs. advanced stages of NSCLC, it might be a useful approach for the early detection of lung cancer, a clinically challenging. Furthermore, the use of the 2 miRNA biomarkers would potentially improve personalized treatment of lung cancer by precisely classifying the subtypes. In addition, our analysis of the plasma miRNAs by simply calculating the equation with the established cut-off could be a convenient tool in the clinics. Therefore, the developed biomarkers with the easy-to-use prediction models may represent promising noninvasive diagnostic tools not only for early detection of lung cancer, but improving the efficacy of therapeutics of NSCLC.
Increased miR-205-5p expression contributes to the development and progression of NSCLC [21], [33]. We have shown that miR-205-5p is one of the miRNAs that could be used as biomarkers for the early detection of lung cancer [21], [24], [34]. miR-210-3p stimulates a hypoxic phenotype and upsurges radioresistance in NSCLCs [35]. Hypoxia-induced miR-210-3p can regulate tumor cell susceptibility to cytolytic T-lymphocyte-mediated lysis by a mechanism involving its downstream targets PTPN1, HOXA1, and TP53I11 [36]. The results created from our present study support these previous findings, and further provide evidence of using the miRNAs as potential biomarkers for diagnosis of lung cancer.
There are limitations in the present study. 1), the sample size is small. Furthermore, this single and retrospective cohort of cases and controls may produce selection bias. To diminish the bias, we will perform pivotal evaluation of the diagnostic assay in a large cohort by using prospective-specimen collection, retrospective-blinded-evaluation design developed by NCI [37]. 2), in the present study, we only assess 11 miRNAs whose changes are associated with lung cancer, from which 2 miRNA biomarkers are optimized. Although showing promise, the 2 miRNAs have moderate sensitivity and specificity that are not sufficient in routine laboratory settings. We will use this platform to evaluate more lung tumor-specific miRNAs to identify additional plasma miRNA biomarkers that can be added to the current ones so that the diagnostic efficacy of the plasma assays could be improved. Furthermore, the assessments of cell-free circulating tumor DNA or DNA methylation status of gene promoters have attracted increasing attention as potential liquid biopsy tests for lung cancer [38], [39], [40]. Our ongoing efforts are to compare the diagnostic efficiency of the plasma miRNA signature with those of the cell-free DNA biomarkers in the early detection of lung cancer. 3), given that the changes of the miRNAs are not specific for lung cancer, the patents with positive results might have other type of cancers. In future clinical trials, the patients who have positive results with the molecular analysis should undertake clinical examinations and tests to have final clinical diagnosis.
Conclusions
This study demonstrates that an approach for directly targeting less blood and skipping the multipole procedures that are associated with large analytic variations would improve the efficacy of miRNA assessment. The developed plasma miRNA biomarkers may help diagnose lung cancer at the early stage and classify the subtypes. Nevertheless, the continued development of this technology and validation of the biomarkers are required.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
The Institutional Review Boards of University of Maryland Baltimore and Veterans Affairs Maryland Health Care System approved this study. reference number is H40666.
Competing Interests
The authors declare no conflict of interest.
Funding
Grant support: This work was supported in part by NCI R21CA205746, VA Merit Award I01 CX000512, Award from the Geaton and JoAnn DeCesaris Family Foundation, UMD-UMB Research and Innovation Seed Grant, DoD-Idea Development Award, and Maryland Innovation Initiative (MII) Commercialization Program- Phase 1/2 Grant (F.J.)
Authors' Contributions
QL, YW, and FJ conducted the experiments and participated in study design, coordination, and data interpretation, and preparing the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.05.001.
Appendix A. Supplementary data
Supplementary Figures
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Geisinger KR. Histological grading in lung cancer: one system for all or separate systems for each histological type? Eur Respir J. 2016;47:720–723. doi: 10.1183/13993003.00035-2016. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik S, Mallick R, Kannisto E, Sharma R, Bshara W, Yendamuri S, Dhillon SS. MiR-205 and MiR-375 microRNA assays to distinguish squamous cell carcinoma from adenocarcinoma in lung cancer biopsies. J Thorac Oncol. 2015;10:446–453. doi: 10.1097/JTO.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gressett SM, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009;43:490–501. doi: 10.1345/aph.1L426. [DOI] [PubMed] [Google Scholar]
- 5.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 7.Galasso M, Sana ME, Volinia S. Non-coding RNAs: a key to future personalized molecular therapy? Genome Med. 2010;2:12–16. doi: 10.1186/gm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 2012;5:492–497. doi: 10.1158/1940-6207.CAPR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen J, Jiang F. Applications of MicroRNAs in the Diagnosis and Prognosis of Lung Cancer. Expert Opin Med Diagn. 2012;6:197–207. doi: 10.1517/17530059.2012.672970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329:125–136. doi: 10.1016/j.canlet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy B, Hu ZI, Cordova KN, Close S, Lee K, Becker D. Clinical Utility of Liquid Diagnostic Platforms in Non-Small Cell Lung Cancer. Oncologist. 2016;21:1121–1130. doi: 10.1634/theoncologist.2016-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiberio P, Callari M, Angeloni V, Daidone MG, Appierto V. Challenges in using circulating miRNAs as cancer biomarkers. Biomed Res Int. 2015;2015:731–738. doi: 10.1155/2015/731479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tackett MR, Diwan I. Using FirePlex( ) Particle Technology for Multiplex MicroRNA Profiling Without RNA PurificationMethods. Mol Biol. 2017;1654:209–219. doi: 10.1007/978-1-4939-7231-9_14. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 20.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Mannoor K, Gao L, Tan A, Guarnera MA, Zhan M, Shetty A, Stass SA, Xing L, Jiang F. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol Oncol. 2014;8:1208–1219. doi: 10.1016/j.molonc.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Todd NW, Zhang H, Yu L, Lingxiao X, Mei Y, Guarnera M, Liao J, Chou A, Lu CL. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Investig. 2011;91:579–587. doi: 10.1038/labinvest.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leng Q, Lin Y, Jiang F, Lee CJ, Zhan M, Fang H, Wang Y. A plasma miRNA signature for lung cancer early detection. Oncotarget. 2017;8:111902–111911. doi: 10.18632/oncotarget.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Jiang Z, Leng Q, Bai F, Wang J, Ding X, Li Y, Zhang X, Fang H, Yfantis HG. A prediction model for distinguishing lung squamous cell carcinoma from adenocarcinoma. Oncotarget. 2017;8:50704–50714. doi: 10.18632/oncotarget.17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Guarnera MA, Zhou W, Fang H, Jiang F. A Prediction Model Based on Biomarkers and Clinical Characteristics for Detection of Lung Cancer in Pulmonary Nodules. Transl Oncol. 2017;10:40–45. doi: 10.1016/j.tranon.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Liu Z, Todd NW, Zhang H, Liao J, Yu L, Guarnera MA, Li R, Cai L, Zhan M. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374–379. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Jemal A, Smith R. Reply to lung cancer deaths averted by screening should be considered in the context of tobacco control policies. Cancer. 2013;119:3420–3421. doi: 10.1002/cncr.28192. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie JL, Seah C, Brown S, Tang P, Jamieson F, Drews SJ. Use of Bordetella pertussis BP3385 to establish a cutoff value for an IS481-targeted real-time PCR assay. J Clin Microbiol. 2008;46:3798–3799. doi: 10.1128/JCM.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarry J, Schadendorf D, Greenwood C, Spatz A, van Kempen LC. The validity of circulating microRNAs in oncology: five years of challenges and contradictions. Mol Oncol. 2014;8:819–829. doi: 10.1016/j.molonc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med. 2014;18:371–390. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 34.Xing L, Todd NW, Yu L, Fang H, Jiang F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod Pathol. 2010;23:1157–1164. doi: 10.1038/modpathol.2010.111. [DOI] [PubMed] [Google Scholar]
- 35.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noel A, Pouyssegur J. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B, Mami-Chouaib F, Chouaib S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 37.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 38.Nie K, Jia Y, Zhang X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumour Biol. 2015;36:7–19. doi: 10.1007/s13277-014-2758-3. [DOI] [PubMed] [Google Scholar]
- 39.Balgkouranidou I, Chimonidou M, Milaki G, Tsaroucha E, Kakolyris S, Georgoulias V, Lianidou E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin Chem Lab Med. 2016;54:1385–1393. doi: 10.1515/cclm-2015-0776. [DOI] [PubMed] [Google Scholar]
- 40.Normanno N, Denis MG, Thress KS, Ratcliffe M, Reck M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget. 2017;8(7):12501–12516. doi: 10.18632/oncotarget.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures