Abstract
Antiangiogenic therapy has shown promising results in preclinical and clinical trials. However, tumor cells acquire resistance to this therapy by gaining ability to survive and proliferate under hypoxia induced by antiangiogenic therapy. Combining antiangiogenic therapy with hypoxia-activated prodrugs can overcome this limitation. Here, we have tested the combination of antiangiogenic drug sunitinib in combination with hypoxia-activated prodrug evofosfamide in neuroblastoma. In vitro, neuroblastoma cell line SK-N-BE(2) was 40-folds sensitive to evofosfamide under hypoxia compared to normoxia. In IV metastatic model, evofosfamide significantly increased mice survival compared to the vehicle (P=.02). In SK-N-BE(2) subcutaneous xenograft model, we tested two different treatment regimens using 30 mg/kg sunitinib and 50 mg/kg evofosfamide. Here, sunitinib therapy when started along with evofosfamide treatment showed higher efficacy compared to single agents in subcutaneous SK-N-BE(2) xenograft model, whereas sunitinib when started 7 days after evofosfamide treatment did not have any advantage compared to treatment with either single agent. Immunofluorescence of tumor sections revealed higher number of apoptotic cells and hypoxic areas compared to either single agent when both treatments were started together. Treatment with 80 mg/kg sunitinib with 50 mg/kg evofosfamide was significantly superior to single agents in both xenograft and metastatic models. This study confirms the preclinical efficacy of sunitinib and evofosfamide in murine models of aggressive neuroblastoma. Sunitinib enhances the efficacy of evofosfamide by increasing hypoxic areas, and evofosfamide targets hypoxic tumor cells. Consequently, each drug enhances the activity of the other.
Introduction
Since 1970, notable improvement has been observed in average survival rate and quality of life of pediatric cancer patients. The average survival rate in neuroblastoma has increased from 52% in 1975 to 79% in 2013 [1]. However, the survival rate in high-risk and metastatic neuroblastoma is still below 55% despite the advent of multimodal therapy [1]. Several newer strategies are being investigated to achieve tumor growth delay and survival rate enhancement in advanced stage and high-risk neuroblastoma.
Antiangiogenic therapies have shown promise in preclinical studies involving neuroblastoma [2], [3]. However, one major drawback of antiangiogenic therapy is the ability of tumor cells to survive in hypoxic zones [4]. Hypoxic zones, arising due to inefficient vasculature, lower the accessibility of chemotherapeutics to tumor cells residing therein and also alter tumor cell behavior to metastatic and stem-like phenotypes [5]. Antiangiogenic therapy exacerbates hypoxia due to reduction in tumor vasculature. Hypoxia-activated prodrugs can possibly overcome this limitation by distributing in hypoxic regions of tumor if used in combination with antiangiogenic therapy [5], [6]. Evofosfamide is a hypoxia-activated prodrug, which distributes into hypoxic zones and preferentially releases its cytotoxic effector within hypoxic areas, thereby selectively targeting hypoxic cells while sparing normoxic cells [7]. This pharmacodistribution is particularly designed to yield a higher benefit to risk ratio. Despite negative phase III study involving evofosfamide and doxorubicin, evofosfamide remains in development in combination with antiangiogenic agents and immune checkpoint inhibitors [7], [8], [9], [10], [11], [12], [13], [14]. Clinical trials combining evofosfamide with antiangiogenic drugs are underway [15], [16]. Developing new proof of principle leading to precision medicine is a critical step for this new class of therapeutic agents, and the present preclinical study is an effort in this direction.
Here, we tested the efficacy of combination of antiangiogenic multikinase inhibitor sunitinib with evofosfamide in preclinical models of neuroblastoma. Sunitinib has been approved for treating imatinib-resistant gastrointestinal tumors, renal cell carcinoma, and pancreatic neuroendocrine tumors and has shown efficacy in several preclinical pediatric solid tumor models [17], [18]. We have previously demonstrated the efficacy of single-agent sunitinib in preclinical models of pediatric neuroendocrine tumor neuroblastoma [2]. However, in other studies, its efficacy has been found to be limited due to antiangiogenesis-induced hypoxia, which enhanced metastatic phenotype of tumor cells [19], [20], [21]. Therefore, in this study, we considered sunitinib to be an appropriate antiangiogenic agent to be combined with evofosfamide.
In this study, we used SK-N-BE(2) cells for developing tumor xenografts in immunodeficient mice. We have previously tested in vitro and in vivo the benefit of combination of metronomic topotecan (another hypoxia generating agent) and evofosfamide on SK-N-BE(2) cells [22]. In that study, we demonstrated that the IC50 of evofosfamide was highest for SK-N-BE(2) cells under both hypoxia and normoxia, among all the neuroblastoma cell lines tested. Addition of topotecan did not significantly alter IC50 of evofosfamide. In another study, sunitinib has been reported to reduce MYCN expression in SK-N-BE(2) cells [23]. Therefore, we selected SK-N-BE(2) cells as a representative of highly aggressive and drug-resistant neuroblastoma cells to test the efficacy of combination of sunitinib and evofosfamide.
Our hypothesis is that both these agents can additively or synergistically delay tumor growth and enhance survival rates in murine models of neuroblastoma. Our hypothesis is based upon the premise that sunitinib due to its antiangiogenic activity can provide appropriate condition for evofosfamide activity, while evofosfamide can target hypoxic zones in sunitinib-treated tumors.
Materials and Methods
Materials
Evofosfamide ((1-methyl-2-nitro-1H-imidazol-5-yl) methyl N,N'-bis (2-bromoethyl) phosphorodiamidate) was provided by Threshold Pharmaceuticals (South San Francisco, CA). Sunitinib (N-(2-diethylaminoethyl)-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene) methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide) was provided by Pfizer (Groton, CT). Neuroblastoma cell line SK-N-BE(2) was purchased from American Type Culture Collection (Manassas, VA). Detection of hypoxia was done by pimonidazole immunohistochemistry (HypoxyprobeTM-1 Plus kit, Burlington, MA).
In Vitro Cytotoxicity
SK-N-BE(2) were seeded into 24-well plates (20,000 cells/well) and incubated overnight. Cells were pretreated with indicated concentrations of sunitinib for 1 hour followed by evofosfamide treatment for 2 hours in either normoxia (21% O2) or anoxia (95% N2 and 5% CO2). After evofosfamide treatment, drug-containing medium was removed and cells were washed twice. Sunitinib was added back to the cells and incubated for additional 3 days in an atmosphere of 5% CO2, 95% air, and 100% relative humidity. On day 3, the Alamar Blue assay was performed to quantify viable cells. IC50 values were calculated using the GraphPad Prism 4.0 software.
Mice Tumor Models
For subcutaneous xenograft model, 1 × 106 SK-N-BE(2) cells were injected into the subcutaneous inguinal fat pad of each of 4- to 8-week-old nonobese diabetic/severe combined immune deficient (NOD/SCID) mice. The tumor sizes were measured on daily basis until the end point or till euthanasia. The long diameter (D) and short diameter (d) were measured with calipers. Tumor volume (cm3) was calculated as V = 0.5 × D × d2. The criterion for end point was individual tumor volume exceeding 2000 mm3 or mean tumor volume exceeding 1000 mm3 in the group or animals showing signs of morbidity.
For IV metastatic models, SK-N-BE(2) cells were injected into lateral tail veins of NOD/SCID mice to generate experimental metastases, as previously described [2]. The criteria of end point were mice showing signs of morbidity, distended belly, and uneven gait. Animals were euthanized by CO2 gas chamber upon reaching end point or at the end of the treatment.
Treatment
For single-agent evofosfamide experiment, mice were randomized into control and treatment groups. For experiments involving both sunitinib and evofosfamide, animals were randomized into four groups: control (treated with vehicle), SU (treated with sunitinib); Evo (treated with evofosfamide), and SU+Evo (treated with sunitinib and evofosfamide). The dose of evofosfamide was 50 mg/kg (qd × 5 per week) by IP route. The dose of sunitinib was 30 mg/kg or 80 mg/kg (qd × 7 per week) by oral gavage.
For detecting hypoxia, 60 mg/kg pimonidazole was intraperitoneally administered to the mice 30 minutes before sacrifice.
Immunohistochemistry
Frozen tumor sections were fixed in 4% paraformaldehyde. This was followed by permeabilization with 0.05% Triton X-100 and blocking with 5% BSA in PBS. The sections were then incubated overnight with antipimonidazole FITC-conjugated IgG1 mouse monoclonal antibody (included in Hypoxyprobe-1 plus kit, dilution 1:50) and cleaved caspase-3 (Cell Signaling Technology; 9711s; 1:400). The secondary antibody (Alexa Fluor 594 conjugated) incubation, for detecting cleaved caspase-3, was done for 1 hour. The sections and the slides were mounted with Vectashield mounting medium (H1000). The microscopic examination of these tissue sections was done under a Nikon ECLIPSE Ti series fluorescence microscope, with NIS Elements (BR 3.10) software.
Data Analysis
In subcutaneous xenograft model, the conditional survival was defined as the time that an animal reached the tumor volume of 500 mm3. Kaplan-Meier plots were constructed based on the percentage of animals surviving in each group as a function of time. Median time (MT) is the time at which half the animals in the group had a tumor size less than 500 mm3. T/C is the ratio of MT in treatment group (T) to that of control group (C). The antitumor activity was evaluated as follows: T/C % = MT of treated group/MT of control group × 100. Increase in life span (% ILS) was calculated as 100 × (T/C − 1). In addition, here, relative tumor volume was calculated as the ratio of mean tumor volume at time of efficacy assessment to mean tumor volume at the start of treatment (day 1). Antitumor activity was assessed by tumor growth inhibition (TGI) and tumor growth delay (TGD). TGI was defined as (1 − ΔT/ΔC) × 100, where ΔT/ΔC is the ratio of the change in mean tumor volume of the treated group (ΔT) and of the control group (ΔC). Animals were sacrificed when individual tumor size was over 2000 mm3 or mean tumor volume exceeded 1000 mm3 in the group. TGI was determined on the last measurement when all the animals in the vehicle group still survived. Tumor growth delay TGD500 represents time (in days) taken by individual tumor to attain the volume of 500 mm3.
In vitro dose-response, in vivo tumor growth curves, and the number of pixels for immunofluorescence are presented as mean ± SD. Statistical significance for the difference in mean values of any parameter between two groups was assessed on the basis of P values using two-tailed, unpaired t test (Graphpad Prism 4.0 or 5.2). For comparing the levels of markers in immunofluorescence experiments, mean pixel value indicates the mean readings of four xenografts in each group.
Results
Evofosfamide Exhibited Higher Cytotoxicity Under Hypoxia than Normoxia
SK-N-BE(2) cells were exposed to different concentrations of sunitnib for 3 days, with and without initial treatment (2 hours) with evofosfamide, under both normoxia and anoxia. As shown in Figure 1, evofosfamide alone exhibited hypoxia-selective and concentration-dependent cytotoxicity. IC50 of evofosfamide under normoxia was 220 μM and 320 μM, alone and in combination with sunitinib, respectively. IC50 of evofosfamide under anoxia (under nitrogen) was 4.8 μM and 8.2 μM, alone and in combination with sunitinib, respectively. The difference between the IC50 values of evofosfamide used alone and in combination was not statistically significant under either normoxia or anoxia. Therefore, evofosfamide exhibits little additivity with sunitinib in in vitro cytotoxicity assays.
Figure 1.
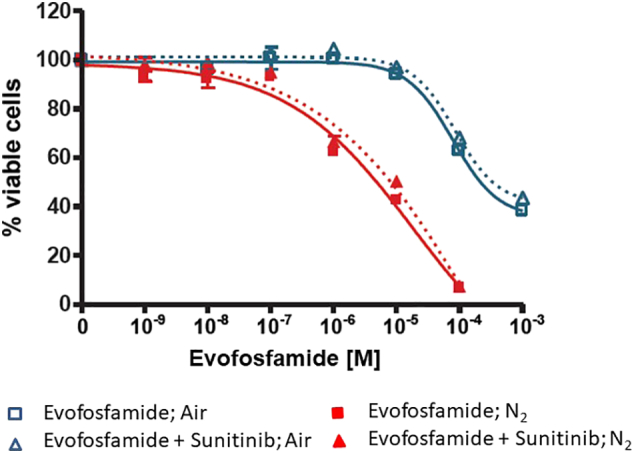
In vitro cytotoxicity of evofosfamide alone and in combination with sunitinib.
The graph shows the IC50 of evofosfamide (TH-302), alone and in combination with sunitinib (1 μM), on SK-N-BE(2) cells under normoxia (air) and anoxia (N2).
Evofosfamide Single-Agent Therapy Prolonged Mice Survival in IV Metastatic Model
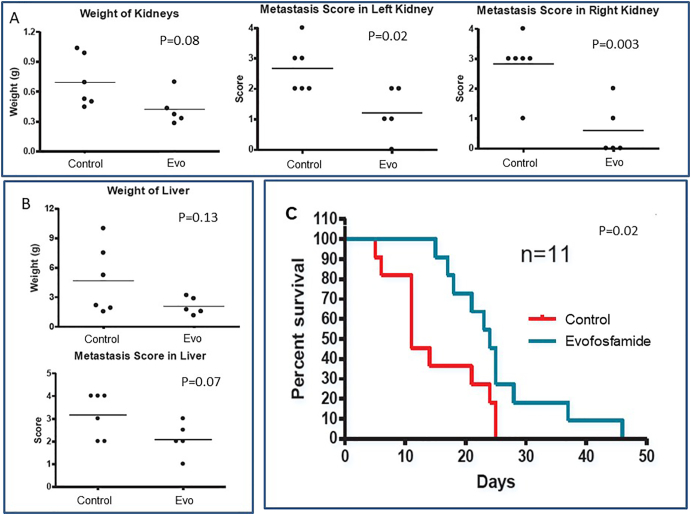
We have previously employed IV metastatic SK-N-BE(2) model for sunitinib activity profiling [2]. SK-N-BE(2) metastases appear mostly in liver and kidney in this model [2]. In this study, treatment was started 45 days after IV injection of the 100,000 SK-N-BE(2) cells. Mice were treated for 2 weeks. Fifteen days after commencement of the treatment, mice were euthanized. Evofosfamide reduced the extent of metastasis in liver and kidneys, with significant reduction in metastatic score of both the kidneys (Figure 2, A and B). In IV metastatic model survival study, 2 weeks of evofosfamide treatment led to two-fold increase in mean survival time of mice (Figure 2C).
Figure 2.
Effect of single-agent evofosfamide in IV metastatic model.
A and B show the effect of evofosfamide (Evo) treatment on liver and kidney after treatment for 14 days. The criteria assigning metastatic score in organs are: 0 (no metastasis), 1 (one or two metastases in entire organ), 2 (few scattered metastases), 3 (extensive metastases, but the organ has maintained its gross anatomic integrity), and 4 (extensive metastases affecting anatomic features of the organ). C indicates the Kaplan-Meier survival curve of mice treated with single-agent evofosfamide till the end point, where mean survival time for untreated control and evofosfamide-treated groups is 11 days and 24 days, respectively.
Combination Exhibited Higher Efficacy Than Single Agents
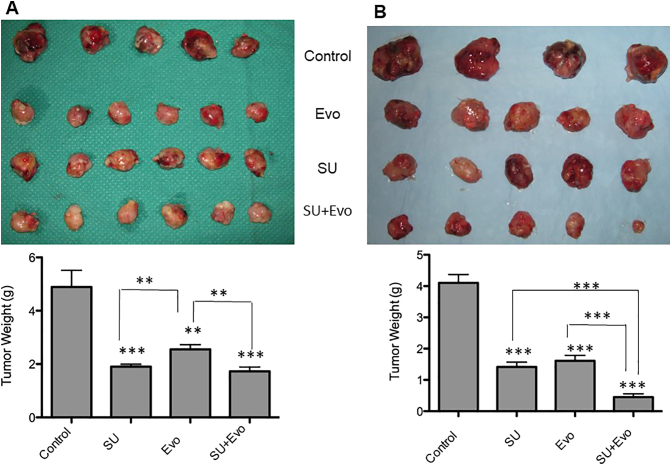
For studying the effectiveness of the evofosfamide and sunitinib combination, we conducted four experiments (CT-1, CT-2, CT-3, and CT-4). Our first experiment (CT-1) involved sunitinib (30 mg/kg) treatment and evofosfamide (50 mg/kg) in subcutaneous xenograft model. Here, when the average tumor size reached 0.5 cm in diameter, mice were randomized into four groups and assigned following treatments: control (vehicle treated), sunitinib (30 mg/kg), evofosfamide (50 mg/kg), and combination (in same doses as single agents). Sunitinib treatment was started immediately after randomization, when tumor sizes were 0.5cm, in groups assigned to sunitinib single agent and the combination. In groups assigned to evofosfamide and the combination, evofosfamide treatment was started 7 days after the start of sunitinib treatment (Figure 3A). Mice were euthanized after 4 weeks of treatment. All three treatment groups demonstrated significant delay in tumor growth compared to control group. However, the combination-treated tumors did not have significant weight difference compared to those treated with either single agent (P>.05) (Figure 3A).
Figure 3.
Tumor size and weights in xenograft model involving sunitinib (30 mg/kg) and evofosfamide (50 mg/kg).
The image on the left (A) shows the comparison of tumor sizes after treatment with sunitinib (SU) and evofosfamide (Evo) in xenograft model where treatment with sunitinib was started 7 days prior to evofosfamide treatment (CT-1). Comparison of tumor weights is shown below the image. All three treatment groups (SU, Evo, and SU+Evo) had significantly lower tumor weights compared to that of control group (P=.0005, .003, and .0005 for SU, Evo, and SU+Evo, respectively). Both SU (P=.009) and SU+Evo (P=.007) groups had significantly lower tumor weights than Evo group. The image on the right (B) shows the comparison of tumor sizes in experiment where treatments with sunitinib and evofosfamide were started simultaneously (CT-2). All three treatment groups had significantly lower tumor weights compared to control (P<.0001). In both the graphs, asterisks above the bar represent P values compared to the control, whereas those depicted along with lines represent P values between combination and respective single-agent–treated groups.
Since we failed to observe any advantage with combination therapy over either single agent in first experiment, we conducted another experiment in subcutaneous xenograft model (CT-2) where sunitinib (30 mg/kg) and evofosfamide (50 mg/kg) treatments were started simultaneously in mice assigned (Figure 3B) when the average tumor sizes reached 0.5 cm in diameter. Here, all three treatment groups had significantly lower tumor weights compared to control (P<.0001) after 4 weeks of treatment. Unlike CT-1 experiment, the combination-treated mice had significantly lower tumor weights compared to the groups treated with either sunitinib (P=.0005) or evofosfamide (P=.0008) in CT-2 experiment.
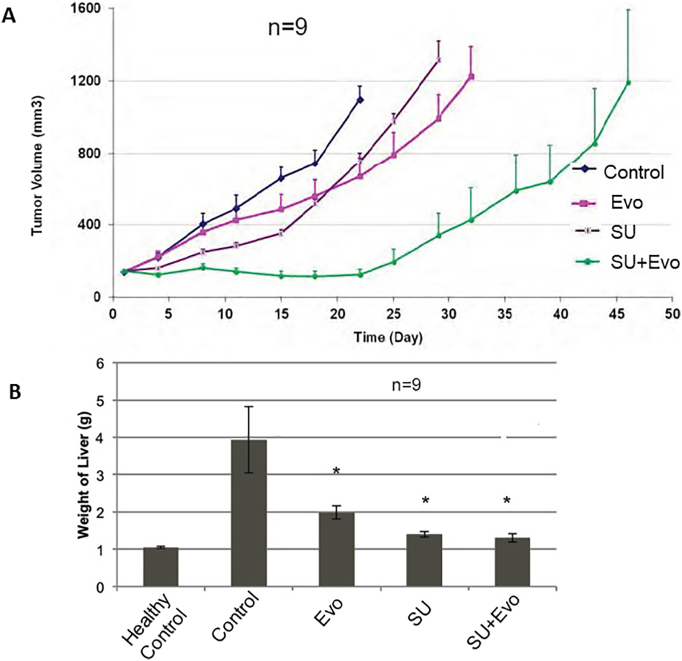
In our third experiment involving the combination (CT-3), sunitinib (80mg/kg) and evofosfamide (50 mg/kg) administration was started simultaneously in subcutaneous xenograft model (Figure 4A). Treatment was started 20 days after tumor cell inoculation, when tumor sizes were approximately 150 mm3. Here all the parameters of efficacy such as TGI, TGD, mean conditional survival time, T/C ratio, and %ILS, as measured on day 22 of the treatment, were higher for all three treatment groups compared to control group (Table 1). In the combination treatment group, T/C and %ILS were higher by three-fold and two-fold, respectively, compared to control group. Among single-agent groups, evofosfamide-treated mice had higher TGI, while TGD, MST, T/C, and %ILS were higher for sunitinib-treated mice.
Figure 4.
Effect of sunitinib (SU, 80 mg/kg) and evofosfamide (Evo, 50 mg/kg) on tumor growth delay. A represents the effect of treatment(s) on tumor growth in subcutaneous xenograft model (CT-3). B represents the effect of treatments on tumor burden in liver (indicated as mean weight of liver) in IV metastatic model (CT-4).
Table 1.
Parameters for Assessment of Efficacy of SU (80 mg/kg) and Evofosfamide (50 mg/kg) in Subcutaneous Xenograft Model
| TGI % |
TGD500 (Days) | Max Body Weight Loss (%) | MST (Days) | T/C % |
ILS % |
|
|---|---|---|---|---|---|---|
| Control | - | - | - | 15 | - | - |
| Evo | 45 | 4 | 3.5 | 15 | 100 | 0 |
| SU | 37 | 7 | 1.9 | 18 | 120 | 20 |
| SU+Evo | 100 | 23 | 9.4 | 44.5 | 297 | 197 |
In metastatic model experiment (CT-4), treatment with sunitinib (80mg/kg) and evofosfamide (50 mg/kg) was started 20 days after IV injection of tumor cells (6 × 106 cells via tail vein). Mice were euthanized after 12 days of treatment. Liver weight was used to assess the tumor burden in liver (Figure 4B). All three treatments significantly lowered the tumor burden in liver compared to untreated controls (P<.05). Combination-treated mice had significantly lower liver weights compared to vehicle-treated (P=.01) and evofosfamide-treated (P=.005) mice. The combination experiments have been summarized in Table 2.
Table 2.
List of In Vivo Experiments Conducted to Test the Efficacies of Sunitinib and Evofosfamide
| Regimen | Model | Figure | Time of Start | End | Dose (mg/kg) |
|
|---|---|---|---|---|---|---|
| Sunitinib | Evofosfamide | |||||
| Evofosfamide | Metastatic | 2 | 45 days post IV injection of tumor cells | 15 days of treatment | - | 50 |
| Combination (CT-1) | Xenograft | 3A | Sunitinib started when average tumor size reached 0.5 cm diameter; evofosfamide started 1 week after sunitinib | 4 weeks from the start of sunitinib | 30 | 50 |
| Combination (CT-2) | Xenograft | 3B | Both drugs started when average tumor size reached 0.5 cm diameter | 4 weeks | 30 | 50 |
| Combination (CT-3) | Xenograft | 4A | 20 days after the implantation of tumor cells | Till end point | 80 | 50 |
| Combination (CT-4) | IV metastatic | 4 B | 20 days post IV injection of cells | 12 days of treatment | 80 | 50 |
Sunitinib and Evofosfamide Enhanced Apoptosis and Hypoxia in Tumors
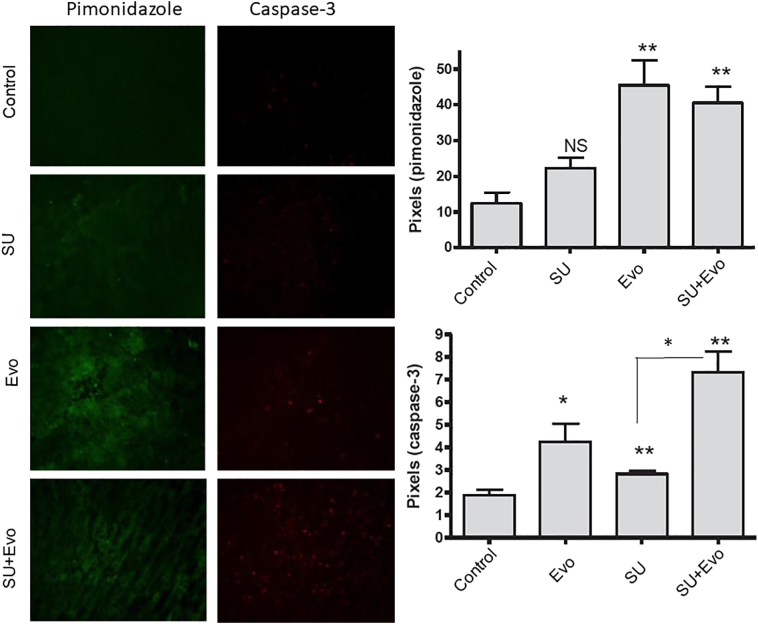
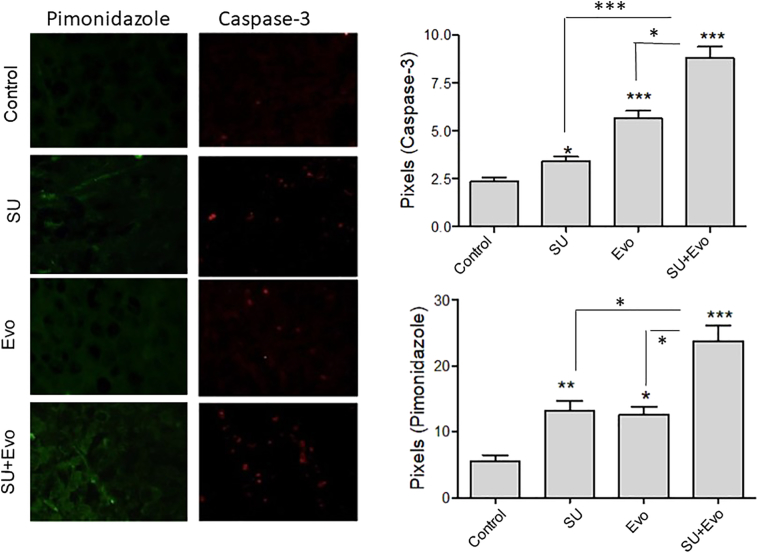
The pharmacodynamic effect of treatment with sunitinib (30 mg/kg) and evofosfamide (50 mg/kg) on tumor apoptosis and hypoxia was assessed by immunofluorescence for cleaved caspase-3 and pimonidazole, respectively (Figures 5 and 6). Immunofluorescence was performed on tumor sections obtained from subcutaneous xenograft experiments CT-1 and CT-2. In sequential regimen (CT-1), all the three treatment groups had significantly higher number of apoptotic cells compared to the controls (Figure 5). Here, combination group had higher number of apoptotic cells compared to sunitinib monotherapy group (P=.03) but not when compared to evofosfamide monotherapy group. In this group, at the time of tumor collection, both sunitinib and evofosfamide were being administered. Tumors in sunitinib and combination groups, but not evofosfamide group, had higher hypoxic zones than those in control group. However, no difference between the hypoxic zones of sunitinib and combination groups was observed. With the second regimen (CT-2), where both sunitinib and evofosfamide treatments were started simultaneously, tumors belonging to all three treatment groups showed significantly higher number of apoptotic cells (P<.05) and higher volumes of tumor hypoxia compared to untreated controls (P<.05) (Figure 6). Unlike CT-1 experiment, the combination-treated tumors exhibited significantly higher apoptosis and hypoxia than those treated with sunitinib (P=.0002) and evofosfamide (P=.02) in CT-2 experiment. Also, the combination-treated tumors had significantly higher hypoxia than those treated with sunitinib (P=.02) and evofosfamide (P=.03).
Figure 5.
Hypoxia and apoptosis in tumors treatment with sunitinib (SU) and evofosfamide (Evo).
The microscopic images of tumor sections showing caspase-3 (red) and pimonidazole (green) in tumors obtained from CT-1 experiment. The histograms below the images represent the comparison of pixels for caspase-3 and pimonidazole. In both the graphs, asterisks above the bar represent P values compared to the control, whereas those depicted along with lines represent P values between combination and respective single-agent–treated groups.
Figure 6.
Hypoxia and apoptosis in tumors treatment with sunitinib (SU) and evofosfamide (Evo).
The microscopic images of tumor sections showing caspase-3 (red) and pimonidazole (green) in tumors in CT-2 experiment. The histograms below the images represent the comparison of pixels for caspase-3 and pimonidazole. In both the graphs, asterisks above the bar represent P values compared to the control, whereas those depicted along with lines represent P values between combination and respective single-agent–treated groups.
Discussion
Hypoxia is a major negative prognostic factor in cancers [5]. Hypoxia affects tumor biology by promoting prosurvival pathways, invasive phenotype, angiogenesis, and drug resistance [5]. These hypoxic tumor zones arise due to abnormal and inefficient tumor vasculature. As a result, cells in these areas are inaccessible and resistant to conventional chemotherapy.
Though antiangiogenic therapy initially causes tumor growth delay or tumor size reduction, it eventually loses efficacy due to the enhancement of metastatic phenotype. Sunitinib has shown to enhance metastasis in preclinical studies employing glioblastoma and breast cancers [19], [20], [21]. One explanation put forth for this type of antiangiogenic escape is that hypoxic tumor response, as a result of reduced microvessel density, leads to the activation of genes involved in glycolysis and metastasis [4]. Therefore, simultaneous targeting of angiogenesis and hypoxia has been proposed [4].
The bioreductive hypoxia-activated prodrug evofosfamide has shown great promise for treating cancers in preclinical and early clinical studies [7], [9], [10], [11], [13]. Evofosfamide has demonstrated efficacy as a single agent in a panel of human tumor xenografts [9]. It is reported to enhance the cytotoxicity of cytotoxic agents in preclinical models of non–small cell lung cancer (NSCLC), colon cancer, prostate cancer, melanoma, fibrosarcoma, and pancreatic cancer [10]. Its combination with gemcitabine and nab-paclitaxel has shown significant advantage over single agents in xenograft models of pancreatic cancer [7]. Trimodal therapy involving evofosfamide, DC101, and radiation resulted in tumor dormancy for more than 3 months after cessation of therapy compared to regimens without evofosfamide which only slowed tumor growth [11]. Despite the success of early trials of evofosfamide in soft tissue sarcoma patients, the phase III randomized study in combination with doxorubicin failed to demonstrate positive outcome [13], [17]. Phase I clinical trial of the combination of evofosfamide and ipilimumab in adult solid tumors is underway [14]. Due to tumor hypoxia-enhancing effect of antiangiogenic drugs, we believe evofosfamide in combination with antiangiogenic or low-dose metronomic therapy will be more effective in delaying tumor growth [22].
SK-N-BE(2) is an aggressive, MYCN-amplified, and P53-mutated cell line obtained from bone marrow metastases of a patient who had relapsed after combined radiotherapy and chemotherapy [24]. MYCN amplification is a major determinant of disease severity [25]. A previous study has established the role of MYCN in neuroblastoma angiogenesis [26]. We have observed that SK-N-BE(2) cells exposed to 24-hour hypoxia acquired resistance to etoposide-induced apoptosis, associated with MAPK activation, bcl-2 upregulation, and proangiogenic phenotype [27]. We have also observed that resistance to prolonged antiangiogenic therapy with oral metronomic topotecan and pazopanib was associated with increased hypoxia and glycolysis in SK-N-BE(2) subcutaneous mouse xenograft model [28].
In the present study, we have tested the effectiveness of single-agent evofosfamide and its combination with sunitinib in subcutaneous xenograft and IV metastatic neuroblastoma mice models. Here, the in vitro sensitivity of SK-N-BE(2) cells to evofosfamide under nitrogen is the evidence of hypoxic activation of evofosfamide. IC50 values indicate that sunitinib did not have any significant additive or synergistic effect under either normoxia or anoxia, which proves that sunitinib does not have direct activity on tumor cells. The advantage of combination observed over single-agent evofosfamide, in vivo, can be attributed to mechanisms other than direct action of sunitinib on tumor cells, one being the inhibition of angiogenesis.
In two experiments in SK-N-BE(2) metastatic model involving single-agent evofosfamide, reduction of tumor burden in liver and kidneys and two-fold survival enhancement provide evidence of antitumor and antimetastatic activity of evofosfamide in neuroblastoma. We employed this IV metastatic model to represent minimal residual disease. Minimal residual disease constitutes microscopic tumors surviving after treatment and is a major cause of relapse in neuroblastoma. More than 50% of relapses in high-risk neuroblastoma are attributed to minimal residual disease [29]. Micrometastatic lesions are severely hypoxic, which may explain the significant activity of evofosfamide in above-mentioned metastatic models [30].
Having confirmed the activity of single-agent evofosfamide in neuroblastoma, we tested its effectiveness in combination with sunitinib using different sequences and doses of administration. Our rationale behind the first in vivo combination regimen (CT-1), where we initially treated SK-N-BE(2) xenograft-bearing mice with sunitinib alone, was that initial sunitinib treatment will inhibit angiogenesis and induce hypoxia, thus providing an optimum environment for the activity of evofosfamide. However, since the combination did not show any advantage over sunitinib, in our next experiment (CT-2), we started evofosfamide treatment concurrently with sunitinib treatment. Here, unlike the first regimen, we observed significant advantage of the combination over either single agent in terms of tumor growth delay, apoptosis, and tumor hypoxia. This difference between the findings of CT-1 and CT-2 experiments is reflected in immunofluorescence, where the combination enhanced apoptosis and tumor hypoxia compared to either single agent in tumors obtained from CT-2 experiment, while in CT-1 experiment, the combination-treated tumors failed to show higher apoptosis and hypoxia than evofosfamide. One possible reason behind lack of benefit of combination over single-agent sunitinib in CT-1 experiment is that 1-week treatment with 30 mg/kg sunitinib caused vascular normalization instead of antiangiogenesis. Vascular normalization has been reported during early stages of sunitinib therapy in mice tumor xenograft model [31]. The normalization of tumor vasculature caused by pruning of immature vessels leads to reduction in tumor hypoxia during initial stages of antiangiogenic therapy. For vascular normalization to happen, the dose and duration of antiangiogenic drug have to be within “normalization window.” In our present experiment, dose (30 mg/kg) and duration (1 week) of sunitinib could have been within the normalization window. This could have caused increased oxygenation within the tumor, leading to the loss of activity of evofosfamide. Another possible explanation for the lack of benefit of the combination over sunitinib in CT-1 experiment could be that hypoxia induced by antiangiogenic sunitinib, administered alone, stimulates proangiogenic pathways in hypoxic tumor cells. This is one of resistance mechanisms to antiangiogenic therapy [32]. When evofosfamide administration is initiated concurrently with sunitinib, evofosfamide will possibly inhibit this resistance mechanism and help in the maintaining tumor hypoxia, as observed in CT-2 experiment. Combination enhanced apoptosis and tumor hypoxia compared to either single agent in CT-2 experiment, as revealed by immunofluorescence. However, the combination-treated tumors failed to show higher apoptosis and hypoxia than evofosfamide in CT-1 experiment. In this study, we observed an increase in hypoxia upon evofosfamide treatment in both CT-1 and CT-2 experiments. Though this finding is in agreement with a previous finding where evofosfamide enhanced hypoxia in liver cancer xenografts after 14 days of treatment, other studies have reported reduction in tumor hypoxia in renal cell carcinoma and leukemia in response to evofosfamide treatment [33], [34], [35]. These contradictory findings indicate the possibility that the effect of evofosfamide on the extent of tumor hypoxia may depend upon factors such as tumor type and dose and duration of the therapy.
In mice xenograft model survival study using 80 mg/kg sunitinib and 50 mg/kg evofosfamide (CT-3), combination showed significantly higher efficacy compared to either single agent. In IV metastatic model (CT-4), mice belonging to all three treatment groups had significantly lower liver weights compared to control mice. However, we did not observe significant difference in liver weights between combination-treated and sunitinib-treated mice. This is unexpected considering the significant advantage of single-agent evofosfamide in earlier metastatic models and the benefit of the combination over either single agent in xenograft models CT-1, CT-2, and CT-3. An explanation for the lack of advantage of combination over single agents in CT-4 experiment is that the liver weights in mice treated with sunitinib and combination are close to those of healthy untreated controls. It needs to be considered that the duration of this experiment (12 days) was short compared to other experiments in this study. We may have observed differential effects between sunitinib and combination in metastatic model if this experiment was done for a longer duration.
In summary, this study demonstrates the advantage of combining antiangiogenic therapy with hypoxia-targeting therapy for treating neuroblastoma. Evofosfamide as a single agent exhibited antitumor efficacy in neuroblastoma mice models. Combination of evofosfamide with sunitinib, when started concurrently, demonstrated significant antitumor activity compared to either single agent in mice xenograft models of MYCN-amplified neuroblastoma in terms of tumor growth delay, survival enhancement, tumor apoptosis, and hypoxia. Hence, this combination can be a suitable candidate for maintenance therapy in neuroblastoma.
Footnotes
Funding Sources: James Fund for Neuroblastoma Research, Toronto, Canada.
Threshold Pharmaceutical Inc., San Francisco, CA, USA.
Curtis Chow Memorial Fund, Toronto, Canada.
References
- 1.SEER, author Surveillance, epidemiology, and end results (SEER) program web site. 1973-2012. http://www.seer.cancer.gov Available at.
- 2.Zhang L, Smith KM, Chong AL, Stempak D, Yeger H, Marrano P, Thorner PS, Irwin MS, Kaplan DR, Baruchel S. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia. 2009;11(5):426–435. doi: 10.1593/neo.09166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Mokhtari RB, Sheikh R, Wu B, Zhang L, Xu P, Man S, Oliveira ID, Yeger H, Kerbel RS. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17(17):5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre A, Harris AL. Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality. EMBO Mol Med. 2015;7(4):368–379. doi: 10.15252/emmm.201404271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 6.Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol. 2016;77(3):441–457. doi: 10.1007/s00280-015-2920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JD, Liu Q, Ahluwalia D, Li W, Meng F, Wang Y, Bhupathi D, Ruprell AS, Hart CP. Efficacy and safety of the hypoxia-activated prodrug evofosfamide (TH-302) in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol Ther. 2015;16(3):438–449. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.http://www.scripintelligence.com/researchdevelopment/Threshold-Plummets-As-Merck-Drops-evofosfamide-For-Sarcoma-361960 Press release retrieved from.
- 9.Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res. 2012;18(3):758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, Ferraro D, Wang Y, Duan JX, Ammons WS. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012;69(6):1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon C, Lee HJ, Park DJ, Lee YJ, Tap WD, Eisinger-Mathason TS, Hart CP, Choy E, Simon MC, Yoon SS. Hypoxia-activated chemotherapeutic TH-302 enhances the effects of VEGF-A inhibition and radiation on sarcomas. Br J Cancer. 2015;113(1):46–56. doi: 10.1038/bjc.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganjoo KN, Cranmer LD, Butrynski JE, Rushing D, Adkins D, Okuno SH, Lorente G, Kroll S, Langmuir VK, Chawla SP. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80(1-2):50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 13.Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S, Ganjoo KN. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol. 2014;32(29):3299–3306. doi: 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Immunotherapy study of evofosfamide in combination with Ipilimumab. 2017. https://clinicaltrials.gov/ct2 Retrieved from. (Identification No. NCT03098160)
- 15.A study to assess the safety and the efficacy of the combination of TH-302 and sunitinib in neuroendocrine pancreatic tumours. https://clinicaltrials.gov/ct2 Retrieved from. (Identification No. NCT02402062)
- 16.Dose-escalation study of TH-302 in combination with sunitinib to treat patients with advanced renal cell carcinoma, gastrointestinal stromal tumors and pancreatic neuroendocrine tumors (TH-CR-410) https://clinicaltrials.gov/ct2 Retrieved from. (Identification No. NCT01381822)
- 17.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 18.Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23(6):377–389. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wragg JW, Heath VL, Bicknell R. Sunitinib treatment enhances metastasis of innately drug-resistant breast tumors. Cancer Res. 2017;77(4):1008–1020. doi: 10.1158/0008-5472.CAN-16-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Marrano P, Wu B, Kumar S, Thorner P, Baruchel S. Combined antitumor therapy with metronomic topotecan and hypoxia-activated prodrug, evofosfamide, in neuroblastoma and rhabdomyosarcoma preclinical models. Clin Cancer Res. 2016;22(11):2697–2708. doi: 10.1158/1078-0432.CCR-15-1853. [DOI] [PubMed] [Google Scholar]
- 23.Calero R, Morchon E, Johnsen JI, Serrano R. Sunitinib suppress neuroblastoma growth through degradation of MYCN and inhibition of angiogenesis. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58(23):5396–5405. [PubMed] [Google Scholar]
- 25.Tonini GP, Boni L, Pession A, Rogers D, Iolascon A, Basso G, Cordero di Montezemolo L, Casale F, Pession A, Perri P. MYCN oncogene amplification in neuroblastoma is associated with worse prognosis, except in stage 4s: the Italian experience with 295 children. J Clin Oncol. 1997;15(1):85–93. doi: 10.1200/JCO.1997.15.1.85. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Rychahou PG, Ishola TA, Mourot JM, Evers BM, Chung DH. N-myc is a novel regulator of PI3K-mediated VEGF expression in neuroblastoma. Oncogene. 2008;27(28):3999–4007. doi: 10.1038/onc.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das B, Yeger H, Tsuchida R, Torkin R, Gee MF, Thorner PS, Shibuya M, Malkin D, Baruchel S. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1alpha through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Res. 2005;65(16):7267–7275. doi: 10.1158/0008-5472.CAN-04-4575. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Mokhtari RB, Oliveira ID, Islam S, Toledo SR, Yeger H, Baruchel S. Tumor dynamics in response to antiangiogenic therapy with oral metronomic topotecan and pazopanib in neuroblastoma xenografts. Transl Oncol. 2013;6(4):493–503. doi: 10.1593/tlo.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds CP. Detection and treatment of minimal residual disease in high-risk neuroblastoma. Pediatr Transplant. 2004;8(Suppl. 5):56–66. doi: 10.1111/j.1398-2265.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang T, Civelek AC, Zheng H, Ng CK, Duan X, Li J, Postel GC, Shen B, Li XF. (18)F-misonidazole PET imaging of hypoxia in micrometastases and macroscopic xenografts of human non–small cell lung cancer: a correlation with autoradiography and histological findings. Am J Nucl Med Mol Imaging. 2013;3(2):142–153. [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto S, Batra S, Saito K, Yasui H, Choudhuri R, Gadisetti C, Subramanian S, Devasahayam N, Munasinghe JP, Mitchell JB. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71(20):6350–6359. doi: 10.1158/0008-5472.CAN-11-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergers G. Hanahan. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JD, Ahluwalia D, Liu Q, Li W, Wang Y, Meng F, Bhupathi D, Matteucci MD, Hart CP. Combination treatment with hypoxia-activated prodrug evofosfamide (TH-302) and mTOR inhibitors results in enhanced antitumor efficacy in preclinical renal cell carcinoma models. Am J Cancer Res. 2015;5(7):2139–2155. [PMC free article] [PubMed] [Google Scholar]
- 34.Badar T, Handisides DR, Benito JM, Richie MA, Borthakur G, Jabbour E, Harutyunyan K, Konoplev S, Faderl S, Kroll S. Phase I study of evofosfamide, an investigational hypoxia-activated prodrug, in patients with advanced leukemia. Am J Hematol. 2016;91(8):800–805. doi: 10.1002/ajh.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duran R, Mirpour S, Pekurovsky V, Ganapathy-Kanniappan S, Brayton CF, Cornish TC, Gorodetski B, Reyes J, Chapiro J, Schernthaner RE. Preclinical benefit of hypoxia-activated intra-arterial therapy with evofosfamide in liver cancer. Clin Cancer Res. 2017;23(2):536–548. doi: 10.1158/1078-0432.CCR-16-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]