Short abstract
Objectives
To develop a logic model for pharmaceutical care that can be used by stakeholders as a tool to support innovation and to monitor the performance of the pharmaceutical care system in the Netherlands and abroad. The ultimate aim of such a system is the responsible provision of drug therapy to improve patients’ quality of life.
Methods
The logic model for pharmaceutical care was created following a process consisting of four steps: (1) a literature review to identify what pharmaceutical care is and what elements it consists of; (2) separate interviews with 10 stakeholder organizations to discuss the results of the literature review; (3) construction of the logic model based on the findings from steps 1 and 2; and (4) separate interviews with three stakeholder organizations to discuss and fine-tune the model. This project was carried out by the National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu) in the Netherlands.
Results
According to the proposed logic model, pharmaceutical care is care defined as: (1) patient-centred; (2) effective and safe; (3) efficient and affordable; (4) in physical, financial and timely ways; and (5) with minimal environmental impact.
Conclusion
The proposed logic model provides stakeholders with a common framework for the innovation or further development of pharmaceutical care.
Keywords: logic model, patient-centred care, pharmacy
Introduction
Medication is the most frequent intervention within health-care systems worldwide.1 Many people regularly visit their community pharmacy and use the pharmaceutical health services that pharmacies provide. In the Netherlands, polypharmacy is a common phenomenon among the elderly – a large group of elderly people use five or more medicines on a daily basis,2 – and pharmaceutical health services have become the focus of public attention. Recent health-care reforms have put more emphasis on the role of local communities in health care, where community pharmacists will increasingly need to adopt a different approach towards pharmaceutical care.3
Hepler and Strand4 defined pharmaceutical care as the responsible provision of drug therapy for the purpose of achieving definite outcomes that improve a patient’s quality of life4 by (a) curing a disease; (b) eliminating or reducing a patient’s symptomatology; (c) arresting or slowing down a disease process; or (d) preventing a disease or symptomatology. Pharmaceutical care should also contribute to the patient’s ‘ability to cope’ and to his or her resilience and self-management.5
In her letter to the Parliament dated 8 April 2014, the Dutch Minister of Health stated that she expected community pharmacists to shift their focus from product-oriented care (i.e. preparing and distributing medicines) towards patient-centred care (i.e. care that puts the interests of the patient first). The community pharmacist should become an integral part of a multidisciplinary chain of primary and secondary health-care professionals and function as expert advisor and supporter for both health-care professionals and patients in the (daily) use of medicinal products.6 In response to this call, the Royal Dutch Pharmacists' Association (Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie, KNMP) presented its vision on the future of pharmaceutical care, in which pharmaceutical care is both product- as well as patient-centered, safe and effective, personalized, multidisciplinary and supporting of self-management.7 Furthermore, other Dutch stakeholders such as the National Association of General Practitioners (Landelijke Huisartsen Vereniging, LHV) and the Dutch Healthcare Authority (Nederlandse Zorgautoriteit, NZa) presented their vision on primary pharmaceutical care in policy reports. According to the LHV view, by 2022, general practitioners will provide a wide range of general medical care in close collaboration with pharmacists, physiotherapists, district nurses, midwives and psychologists and deliver such services near to the patient’s home.8 In 2013, the NZa market scan of primary pharmaceutical care was sent to the Parliament, together with a special exploration of primary pharmaceutical care by two designated advisers of the Minister.3,9,10 Both documents concluded that the performance of primary pharmaceutical care was overall positive. In the Netherlands, community pharmacy has a high performance score compared to other European countries and its accessibility is very good. The costs of primary pharmaceutical care are relatively low, waiting times, are less than the maximum acceptable waiting times, and patient satisfaction is high.3,9 However, the advisers also warned that the future of pharmaceutical care is inextricably linked with further integration into general primary care.
The aim of shifting the focus of pharmaceutical health services into a more patient-centered pharmaceutical care has a long history, both in the Netherlands and elsewhere. There is an abundant amount of literature on pharmaceutical care innovation and practices. Yet, it is not clear how fast and to what extent innovations are implemented, what the potential barriers for implementation are and what implications these developments have for the quality, safety, accessibility, costs and efficacy of pharmaceutical health care. If progress is to be made, it is important that policy-makers put pharmaceutical care high on their political agenda, and that the performance of (new) activities for pharmaceutical care is measured and monitored with the use of quality indicators.1
The aim of the project described here was to develop a logic model for pharmaceutical care that can be used by stakeholders as a tool for innovating and implementing pharmaceutical care into integrated primary care in the Netherlands or abroad and to design quality indicators for performance monitoring.
Methods
The logic model for pharmaceutical care was created following a process consisting of four steps: (1) a literature review to identify what pharmaceutical care is and what elements it consists of; (2) interviews with stakeholders involved in pharmaceutical care to discuss the results of the literature review; (3) construction of the logic model based on the findings from steps 1 and 2; and (4) interviews with potential users of the model to discuss and fine-tune the model.
Step 1: literature review
We performed a systematic literature search on PubMed on articles about trends and developments in community pharmacy practices, theoretical reflections with respect to pharmaceutical care, relevant quality indicators and theoretical frameworks. The scientific articles had to be published from 2003 to August 2015 and written in English, German, French, Dutch, or Spanish. The search keywords were: (Pharmacist: organization & administration) AND/OR (Pharmacist: standards) AND/OR (Pharmacist: trends) AND/OR (Patient care team) AND/OR (Professional Role) NOT (Pharmacy service: hospital).
Figure 1 shows the flowchart of the literature search. After removing the duplicates, the search resulted in 194 records. The resulting articles were then independently scored for relevance on a scale from 1 to 5 by two researchers, based on the abstract, with each score meaning: 1 = very low relevance, 2 = low relevance, 3 = medium relevance, 4 = high relevance and 5 = very high relevance. Articles of high or very high relevance were those on the organization of primary pharmaceutical care, monitoring of primary pharmaceutical care or the role of the pharmacist in integrated care and interdisciplinary cooperation, while articles of medium, low, or very low relevance were those on hospital pharmacy exclusively or on specific therapeutic interventions. When the scores of the two researchers differed by three or more points (this happened in only four cases), discussion took place to understand the reason for such a difference. For each article, the two scores given by the two reviewers were finally added up to give a final score in the range from 2 to 10. The 157 records that gained a final score equal to or of less than 8 were excluded, while the articles with a score of 9 or higher (n = 37) were selected for full-text assessment (online Appendix 1). The two researchers independently read the selected articles in descending order of score and extracted from them the elements of pharmaceutical care that were mentioned.
Figure 1.
Flowchart literature search.
Step 2: first round of interviews with stakeholders
Selection of stakeholders
Potential candidates for interview were those that had an important role or insight into one or more of the phases of pharmaceutical care in a primary care setting, namely prescription, dispensing and pharmaceutical care, use, health insurance and health-care financing, supervision/policy and supervision/health systems monitoring. Table 1 provides an overview of the selected stakeholders. All invited interviewees agreed to participate.
Table 1.
Selected stakeholders for interview involved in the different phases of pharmaceutical care in a primary care setting.
| Phase of pharmaceutical care | Stakeholder | Organization | First or second round of interviews | Description of the organization |
|---|---|---|---|---|
| Prescription | General practitioners | Dutch Society of General Practitioners (NHG) | First round (step 2) | Scientific association of general practitioners |
| National Association of General Practitioners (LHV) | First round (step 2) | Professional association and advocacy group of general practitioners | ||
|
| ||||
| Dispensing and pharmaceutical care | Pharmacists | Royal Dutch Pharmacists Association (KNMP) | Second round (step 4) | Professional association and advocacy group of pharmacists |
| Pharmacist assistants | Optima Farma | First round (step 2) | Organization of pharmacist’ assistants | |
|
| ||||
| Use | Patient organizations | De Hart&Vaatgroep (DHV) | First round (step 2)a | Organization for patients with cardiovascular disease |
| Longfonds | First round (step 2)a | Organization for patients with lung/pulmonary conditions | ||
| Diabetesvereniging Nederland (DVN) | First round (step 2)a | Organization for patients with diabetes | ||
| Landelijk Platform GGZ | First round (step 2)a | Organization for patients with mental disorders | ||
| Nederlandse Patiënten en Consumentenfederatie (NPCF) | First round (step 2)a | Branch organization for patient interests | ||
|
| ||||
| Health insurance and health-care financing | Health insurers | Health Insurers Netherlands (ZN) | First round (step 2) | Branch organization of health insurers |
| Package control | Dutch Health Care Institute (ZINL) | First round (step 2) | Government body responsible for HTA and Package control of standard health insurance | |
|
| ||||
| Supervision/policy | Policy-makers | Ministry of Health, Welfare and Sports (VWS) | Second round (step 4) | |
|
| ||||
| Supervision/health systems monitoring | Scientists | National Institute for Public Health and the Environment (RIVM) | Second round (step 4) | |
RIVM: Rijksinstituut voor Volksgezondheid en Milieu; NHG: Nederlands Huisartsen Genootschap; LHV: Landelijke Huisartsenvereniging; KNMP: Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie; GGZ: Geestelijke Gezondheidszorg; ZN: Zorgverzekeraars Nederland; ZiNL: Zorginstituut Nederland; HTA: Health Technology Assessment.
aInformation collected via interviews in the context of another RIVM research project11 was used. See further details in the methods section, step 2.
At the time of carrying out this study, another project was being performed at the Rijksinstituut voor Volksgezondheid en Milieu (RIVM) about the patient perspective around pharmaceutical care.11 In the context of that research, five patient organizations were interviewed via face-to-face semi-structured interviews. Since the nature of the questions asked was comparable across both studies and we had obtained good quality information, it was decided not to burden these organizations with new interviews but to use instead the information already available.
Performing the interviews
The resulting list of elements of pharmaceutical care derived from the international literature was discussed with the selected stakeholders through a face-to-face, semi-structured interview. The topics covered in the interview (online Appendix 2) were selected based on the literature review from step 1. Stakeholders were asked to reflect on the findings and prioritize the elements of pharmaceutical care according to their organization’s perspective on pharmaceutical care.
Each interview lasted 1–2 hours and took place with one or two representatives of each organization. All interviews were done by two of the researchers involved in this study; one was mainly responsible for asking the questions and the other for taking notes. In addition, all interviews were audio recorded. The audio recording and the field notes were used to write a summary of each interview. This summary was sent to all interviewees for verification and additions. The data analysis phase involved an inductive content analysis of the interviews, starting with the summary and developing a conceptual coding scheme, completing it with inductive codes. While coding, researchers paid special attention to similarities and differences in opinion and perception between the experts. The results were then clustered into various themes.12
Step 3: construction of the logic model
Logic models are often used in the design, planning, implementation, monitoring and evaluation of complex programs, systems, or services in the field of public health and primary care.13,14 The American Centres for Disease Control and Prevention (CDC), for example, uses logic models to evaluate its programs concerning Heart Disease and Stroke Prevention.15 Following the CDC’s example, we organized the core elements of pharmaceutical care, identified in the literature review and the first round of interviews, in the form of a logic model.
Logic models provide a systematic, simplified (visual) picture of the various components of a program or system, how the different components relate to one another and to the whole, and what the intended results of the program, system or service are.15 Logic models in general deal with the ‘big picture’, setting out important resources and activities needed to achieve a certain goal without going into the level of detail such as which actor should perform a certain task.16 The components of a logic model are summarized in Figure 2. According to the literature, logic modelling is best done with a small group of stakeholders to complement systems thinking as a tool and technique for achieving a valid but simplified representation of a complex system.16
Figure 2.
Components of a logic model.15
Step 4: second round of interviews with stakeholders
In order to fine tune the logic model, the draft was then discussed in three separate meetings with three stakeholders involved in the organization and supervision of pharmaceutical care (Table 1). Stakeholders were asked to reflect on the proposed logic model, its relevance, potential and aspects for improvement according to their organization’s perspective on pharmaceutical care. Each interview lasted 1 hour and took place with one or two representatives of the stakeholder organization and two of the researchers involved in this study. These interviews were not audio recorded but the researchers took notes during the interview. The input provided by these experts was used to make, if needed, final corrections in the model.
Results
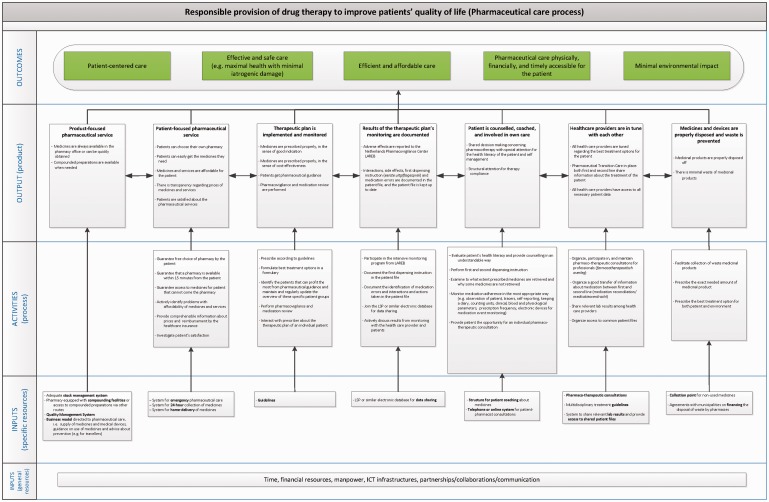
The proposed logic model is shown in Figure 3. The interviewed stakeholders endorsed the findings in the literature that pharmaceutical care should ultimately contribute to the patient’s physical health and wellbeing. These two elements were incorporated into the model as the outcomes ‘Patient-centred care’ and ‘Effective and safe care: maximal health with minimal iatrogenic damage’. Both the literature and stakeholders also pointed out the importance of high quality and highly accessible health care. Stakeholders added the need for sustainable financing of health care,17 and the recent awareness that pharmaceutical care should have minimal impact on the environment (a healthy environment contributes to better health).18 These goals appeared in the model via the outcomes ‘Efficient and affordable care’, ‘Pharmaceutical care accessible in physical, financial and timely ways’ and ‘Minimal environmental impact’.
Figure 3.
Proposed logic model for pharmaceutical care.
While the ultimate outcomes are generic in the sense that most of them could apply to different health-care services, the outputs and activities are more specific for pharmaceutical care. The outputs contribute to achieving the outcomes, and the activities contribute to achieving the outputs.
The proposed model includes the outputs ‘product-focused pharmaceutical service’ and ‘patient-focused pharmaceutical service’. Traditionally, community pharmacies have focused on the former, i.e. making or dispensing medicinal products. The experts’ opinion matched the finding from the literature in that product-focused care is still key: pharmaceutical services should strive to a situation where medicines are always available for the patient. This means that pre-packed medicines should be available at the pharmacy or be quickly obtainable, and the pharmacy should have the facilities to make (or the means to order) compounded preparations quickly. Therefore, pharmacies should make sure they have the necessary inputs to achieve this, namely adequate stock management and quality management systems, as well as compounding facilities and the expertise to use them adequately. At the same time, experts agreed that the focus shift from product-oriented towards patient-centered pharmaceutical care asks for the output ‘patient-focused pharmaceutical service’. Here, the aim is to increase patient satisfaction, with services such as free choice of pharmacy, home-delivery, 24 hour collection points and/or emergency pharmaceutical care.19
Four outputs related to the therapeutic plan were identified: (1) a therapeutic plan is implemented and monitored; (2) results of the therapeutic plan’s monitoring are documented; (3) a patient is counselled, coached and involved in their own care and (4) health-care providers are in tune with each other. Core elements of these outputs and necessary activities are for instance timely reporting of adverse effects, interactions and side effects; providing good instruction at the moment of dispensing; and keeping the patient file up-to-date. The pharmacist alone cannot achieve these goals; patient-centred care also requires pharmacists to work more closely with other health-care providers and with the patient to develop a therapeutic plan and to monitor its effects. It requires effort at the very start of the pharmaceutical care process: prescribers have the responsibilities to give patients the right product that fits the indication, is cost-effective and is in accordance – if possible – with national and international guidelines. Yet, it also requires effort in the area of pharmacovigilance, both at the national level and at the level of individual pharmacies. Prescribers and pharmacists have to share lab results, use the same patient file and regularly review medication use of high-risk patients. Lastly, they need to engage in ‘shared decision making’ with the patient. The literature shows that providing support and information to patients, involving them more closely in decisions regarding the choice of medicinal products and, perhaps most importantly, listening to patients to understand why they take or do not take a medicinal product, has a high impact on therapy compliance.20–22
The seventh output in the model is ‘medicines and devices are properly disposed and waste is prevented’. Preventing waste and making sure that unused or returned medicines are disposed properly minimizes the environmental impact of the use of medicinal products. This can be achieved by pharmacies facilitating the collection of medicinal waste and by prescribers by prescribing the exact needed amount of medicines. Yet, this also requires an agreement with municipalities on financing the disposal of waste by pharmacies.18,23–25
The general resources identified (i.e. time, funding, skilled people, routes of communication, information technology and partnerships) are common to many other kind of services. Next to those general resources, we identified specific resources such as an adequate quality management system, a home-delivery system, an electronic database sharing system, guidelines and collection points for unused medicines.
Discussion
As mentioned earlier in this article, the European Directorate for the Quality of Medicines states in its ‘Policies and Practices for a Safer, More Responsible and Cost-effective Health System’ that the implementation of pharmaceutical care needs to be put high on the health and social political agenda by policy-makers.1 In the Netherlands, achieving such a system is now indeed one of the objectives of the Ministry of Health, and concrete actions are called for in order to make this happen.26 This study presents a logic model for pharmaceutical care that aims to serve as a guide to develop, implement and monitor a product- and patient-centred pharmaceutical care system in the Netherlands or abroad.
Logic models have been successfully developed and used in other studies as a tool to plan, implement, monitor and evaluate performance in numerous fields, including health care.13,27,28 As mentioned elsewhere, a logic model ‘will enable diverse stakeholders to work from a shared conceptual foundation (and lexicon) of the main inputs, activities, outputs and outcomes of a sector’. In addition ‘it will lay the foundation for the development of performance indicators’.28 A potential limitation of the research presented here is that the proposed model shows what needs to happen, but does not set out which stakeholders should be involved and how activities should be organized. At the same time, this is also a strength of this research, since the model objectively describes what needs to happen and is minimally influenced by vested interests.
The elements of pharmaceutical care that have been included in the model are extracted from the international literature (online Appendix 1). It is interesting that most of the articles reviewed, although from different countries, reached similar conclusions regarding the elements that are currently missing in health systems to achieve pharmaceutical care that is centred in the product and the patient. Missing resources often mentioned are time and remuneration,29–40 as well as a centralized patient record system or proper access to it.33,35,37,38,41–44 In addition, it is often mentioned that a change of mentality of both health-care professionals and patients is needed.36 Health-care professionals will need to work more and more in multidisciplinary teams;29,30,32,33,40–42,45–52 therefore, communication needs to improve,31,38,44,53–56 roles and competencies need to be clarified, understood and respected,34,39,43,44,54,57 and proper training needs to be given in order to fulfil those roles and competencies.31,32,36,37,43,44,47,54,58,59 The patient, in turn, needs to understand the (new) role of the pharmacist and be open to building a closer relationship with this health-care provider.33,54,60
The logic model presented here is expected to fit particularly well in the Dutch health system, since the importance of each element has been discussed (via interviews) with potential users of the model in the Netherlands. To arrive at our final model, we removed elements that did not fit the Dutch reality. For example, vaccination delivery or patient screening (e.g. for pre-diabetes, hypertension, or depression) was mentioned in the international literature as a potential task for the pharmacist (e.g. Patwardhan et al.,19 Farris and Johnson,24 de Bittner et al.,36 George et al.38 and American Society of Health-System Pharmacists61). However, our interviewees pointed out that, within the Dutch health-care system, other health-care providers have those responsibilities and that changing these roles would not substantially lead to better pharmaceutical care.
We expect this model to be also applicable in other countries. Our recommendation is to discuss the model with key stakeholders and potential users in order to confirm the relevance and feasibility of each element within a given country and customize the model when necessary.
For monitoring pharmaceutical care, the next step would be the identification of feasible indicators for outputs and activities, which can provide useful information on the state of pharmaceutical care. We expect that this knowledge will support both policy-makers and pharmacists in their efforts to achieve the optimal balance between traditional product-focused and modern patient-focused pharmaceutical services.
Conclusion
Pharmaceutical care requires product as well as patient care. The pharmacist in the community pharmacy needs to become more and more part of a multidisciplinary team of health-care providers. Therefore, good communication and cooperation between stakeholders (policy-makers, health-care providers, health-care insurers and patients) are key to achieve success. The model presented here can serve as a guide to identify what actions are needed to further develop, implement and monitor pharmaceutical care in the Netherlands and (after customization) also abroad. Having an agreed upon logic model can also provide a strong basis for future evaluation of innovations in pharmaceutical care and community pharmacy practice.
Supplemental Material
Supplemental material for A logic model for pharmaceutical care by Carolina Moltó-Puigmartí, Robert AA Vonk, Gerlise L van Ommeren and Ingrid Hegger in Journal of Health Services Research & Policy
Acknowledgements
We thank the experts from KNMP, NHG, LHV, Rijksuniversiteit Groningen, Zorginstituut Nederland, Zorgverzekeraars Nederland, IGJ i.o., VWS and RIVM for their valuable contribution to the development of the logic model.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Strategic Research Program RIVM 2014–2018 of the National Institute for Public Health and the Environment in the Netherlands (project number S/132009).
References
- 1.EDQM. Pharmaceutical care – policies and practices for a safer, more responsible and cost-effective health system. Strasbourg: EDQM, 2012. [Google Scholar]
- 2.Lemmens LC, Weda M. Improving medication safety for the elderly: some bottlenecks. Bilthoven: RIVM, 2013. [Google Scholar]
- 3.Reibestein R, Rinnooij Kan A. Letter to the Minister of Health by ‘verkenners extramurale farmaceutische Zorg’. The Hague: VWS, 2013. [Google Scholar]
- 4.Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 1990; 47: 533–543. [PubMed] [Google Scholar]
- 5.Huber M, van Vliet M, Giezenberg M, et al. Towards a 'patient-centred' operationalisation of the new dynamic concept of health: a mixed methods study. BMJ Open 2016; 6: e010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VWS. Letter of the Minister of Health to Parliament on the results of her policy consultation pharmacy (in dutch). The Hague: VWS, 2014. [Google Scholar]
- 7.KNMP. Outlines for a vision pharmaceutical care 2020. The Hague: KNMP, 2013. [Google Scholar]
- 8.LHV-NHG. Vision for the future of general practitioner care 2022 (in dutch: Toekomstvisie huisartsenzorg 2022). Utrecht: Landelijke Huisartsen Vereniging; Nederlands Huisartsen Genootschap, 2012. [Google Scholar]
- 9.Nederlandse Zorgautoriteit. Marktscan en beleidsbrief farmacie 2010-2014. Utrecht: Nederlandse Zorgautoriteit, 2014. [Google Scholar]
- 10.VWS. Letter of the Minister of Health to Parliament regarding medicines policy. (In dutch). The Hague: VWS, 2013.
- 11.Lemmens LCWM. Patiëntenperspectief op veilige zorg rondom medicijnen. RIVM rapport 2016-0078, 2016.
- 12.Krippendorff KH. Content analysis – 3rd edition: an introduction to its methodology. Thousand Oaks: SAGE Publications, 2013. [Google Scholar]
- 13.Hayes H, Parchman ML, Howard R. A logic model framework for evaluation and planning in a primary care practice-based research network (PBRN). JABFM 2011; 24: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro IC, Torres A, Parra DC, et al. Using logic models as iterative tools for planning and evaluating physical activity promotion programs in Curitiba, Brazil. J Phys Activ Health 2010; 7: S155–S162. [DOI] [PubMed] [Google Scholar]
- 15.CDC. CDC-Division for heart disease and stroke prevention evaluation guide: developing and using a logic model. Atlanta, GA: CDC, 2006, www.cdc.gov/dhdsp/programs/spha/evaluation_guides/logic_model.htm (accessed 3 March 2016).
- 16.WK Kellogg Foundation. Logic model development guide. Michigan: WK Kellogg Foundation, 2004. [Google Scholar]
- 17.EC. Council conclusions on common values and principles in European Union Health Systems (2006/C 146/01), http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2006:146:0001:0003:EN:PDF (2006, accessed 10 January 2016).
- 18.van der Grinten E, Breure AM, Lambooij MS, et al. Towards balancing the benefits of pharmaceutical care and minimizing its environmental harm: identification of potential levers in the medicinal product chain. RIVM Rapport 2015–0145. Bilthoven: RIVM, 2016: 100 [Google Scholar]
- 19.Patwardhan A, Duncan I, Murphy P, et al. The value of pharmacists in health care. Popul Health Manag 2012; 15: 157–162. [DOI] [PubMed] [Google Scholar]
- 20.Koster ES, Blom L, Overbeeke MR, et al. Quality of pharmaceutical care at the pharmacy counter: patients' experiences versus video observation. Patient Pref Adher 2016; 10: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaae S, Traulsen JM, Norgaard LS. Customer interest in and experience with various types of pharmacy counselling – a qualitative study. Health Expect 2014; 17: 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collum JL, Marcy TR, Stevens EL, et al. Exploring patient expectations for pharmacist-provided literacy-sensitive communication. RSAP 2013; 9: 626–632. [DOI] [PubMed] [Google Scholar]
- 23.Bush J, Langley CA, Wilson KA. The corporatization of community pharmacy: implications for service provision, the public health function, and pharmacy's claims to professional status in the United Kingdom. RSAP 2009; 5: 305–318. [DOI] [PubMed] [Google Scholar]
- 24.Farris KB, Johnson KA. Pharmacists in public health: it's a good start! JAPhA 2010; 50: 128–130. [DOI] [PubMed] [Google Scholar]
- 25.American Pharmacists Association and the National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). JAPhA 2008; 48: 341–353. [DOI] [PubMed] [Google Scholar]
- 26.VWS. Letter of the Minister of Health to Parliament on her vision on medicines, Annex 1: agenda vision medicines. The Hague: VWS, 2016. [Google Scholar]
- 27.Watson DE, Broemeling AM, Wong ST. A results-based logic model for primary healthcare: a conceptual foundation for population-based information systems. Healthc Policy 2009; 5: 33–46. [PMC free article] [PubMed] [Google Scholar]
- 28.Watson D, Broemeling AM, Reid RJ, et al. A results-based logic model for primary health care: laying an evidence-based foundation to guide performance measurement, monitoring and evaluation. Vancouver: University of British Columbia, 2004. [Google Scholar]
- 29.Bluml BM, Watson LL, Skelton JB, et al. Improving outcomes for diverse populations disproportionately affected by diabetes: final results of Project IMPACT: diabetes. JAPhA 2014; 54: 477–485. [DOI] [PubMed] [Google Scholar]
- 30.Tan EC, Stewart K, Elliott RA, et al. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. RSAP 2014; 10: 608–622. [DOI] [PubMed] [Google Scholar]
- 31.Tan EC, Stewart K, Elliott RA, et al. Pharmacist consultations in general practice clinics: the Pharmacists in Practice Study (PIPS). RSAP 2014; 10: 623–632. [DOI] [PubMed] [Google Scholar]
- 32.Jorgenson D, Laubscher T, Lyons B, et al. Integrating pharmacists into primary care teams: barriers and facilitators. Int J Pharm Pract 2014; 22: 292–299. [DOI] [PubMed] [Google Scholar]
- 33.Smith M, Cannon-Breland ML, Spiggle S. Consumer, physician, and payer perspectives on primary care medication management services with a shared resource pharmacists network. RSAP 2014; 10: 539–553. [DOI] [PubMed] [Google Scholar]
- 34.Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood) 2013; 32: 1963–1970. [DOI] [PubMed] [Google Scholar]
- 35.Dolovich L. Ontario pharmacists practicing in family health teams and the patient-centered medical home. Ann Pharmacother 2012; 46: S33–S39. [DOI] [PubMed] [Google Scholar]
- 36.de Bittner MR, Adams AJ, Burns AL, et al. Report of the 2010-2011 Professional Affairs Committee: Effective partnerships to implement pharmacists' services in team-based, patient-centered healthcare. Am J Pharm Educ 2011; 75: S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong FY, Chan FW, You JH, et al. Patient self-management and pharmacist-led patient self-management in Hong Kong: a focus group study from different healthcare professionals' perspectives. BMC Health Serv Res 2011; 11: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George PP, Molina JA, Cheah J, et al. The evolving role of the community pharmacist in chronic disease management – a literature review. Ann Acad Med Singap 2010; 39: 861–867. [PubMed] [Google Scholar]
- 39.Kolodziejak L, Remillard A, Neubauer S. Integration of a primary healthcare pharmacist. J Interprof Care 2010; 24: 274–284. [DOI] [PubMed] [Google Scholar]
- 40.Trygstad T. The role of the pharmacist in CCNC. N C Med J 2009; 70: 274–276. [PubMed] [Google Scholar]
- 41.Traynor K. Pharmacist fits on high-intensity transitional care team. Am J Health Syst Pharm 2014; 71: 1066–1068. [DOI] [PubMed] [Google Scholar]
- 42.Schoenhaus R. 2013 Prescott lecture: embracing our roles as medication navigators. J Am Pharm Assoc 2013; 53: 362. [DOI] [PubMed] [Google Scholar]
- 43.American Pharmacists Association Foundation and American Pharmacists Association. Consortium recommendations for advancing pharmacists' patient care services and collaborative practice agreements. J Am Pharm Assoc 2013; 53: e132–e141. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher RM, Gallagher HC. Improving the working relationship between doctors and pharmacists: is inter-professional education the answer? Adv Health Sci Educ 2012; 17: 247–257. [DOI] [PubMed] [Google Scholar]
- 45.Sminkey PV. Pharmacists on the team: tapping invaluable expertise to foster the triple aim. Prof Case Manag 2014; 19: 148–150. [DOI] [PubMed] [Google Scholar]
- 46.Freeman C, Cottrell WN, Kyle G, et al. Does a primary care practice pharmacist improve the timeliness and completion of medication management reviews? Int J Pharm Pract 2012; 20: 395–401. [DOI] [PubMed] [Google Scholar]
- 47.Novak CJ, Hastanan S, Moradi M, et al. Reducing unnecessary hospital readmissions: the pharmacist's role in care transitions. Consult Pharm 2012; 27: 174–179. [DOI] [PubMed] [Google Scholar]
- 48.Ross LA. Pharmacists as mid-level practitioners/providers. Ann Pharmacother 2011; 45: 810–812. [DOI] [PubMed] [Google Scholar]
- 49.Chisholm-Burns MA, Kim L J, Spivey CA, et al. US pharmacists' effect as team members on patient care: systematic review and meta-analyses. Med Care 2010; 48: 923–933. [DOI] [PubMed] [Google Scholar]
- 50.Dobson RT, Taylor JG, Henry CJ, et al. Taking the lead: community pharmacists' perception of their role potential within the primary care team. RSAP 2009; 5: 327–336. [DOI] [PubMed] [Google Scholar]
- 51.Pottie K, Haydt S, Farrell B, et al. Pharmacist's identity development within multidisciplinary primary health care teams in Ontario: qualitative results from the IMPACT project. RSAP 2009; 5: 319–326. [DOI] [PubMed] [Google Scholar]
- 52.Gurney MK. Health care at a crossroad: what will the pharmacists' role be? RSAP 2009; 5: 302–304. [DOI] [PubMed] [Google Scholar]
- 53.Twigg MJ, Desborough JA, Bhattacharya D, et al. An audit of prescribing for type 2 diabetes in primary care: optimising the role of the community pharmacist in the primary healthcare team. Prim Health Care Res Dev 2013; 14: 315–319. [DOI] [PubMed] [Google Scholar]
- 54.Farrell B, Ward N, Dore N, et al. Working in interprofessional primary health care teams: what do pharmacists do? RSAP 2013; 9: 288–301. [DOI] [PubMed] [Google Scholar]
- 55.Tarn DM, Paterniti DA, Wenger NS, et al. Older patient, physician and pharmacist perspectives about community pharmacists' roles. Int J Pharm Pract 2012; 20: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raju A, Sorge LA, Lounsbery J, et al. The expanding role of Minnesota pharmacists in primary care. Minn Med 2011; 94: 49–51. [PubMed] [Google Scholar]
- 57.Bush J, Langley CA, Wilson KA. The corporatization of community pharmacy: implications for service provision, the public health function, and pharmacy's claims to professional status in the United Kingdom. RSAP 2009; 5: 305–318. [DOI] [PubMed] [Google Scholar]
- 58.Sinnett MJ. You have the tools…now create that PPMI model! J Pharm Pract 2013; 26: 158–159. [DOI] [PubMed] [Google Scholar]
- 59.Albanese NP, Rouse MJ, Schlaifer M. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of pharmacists and pharmacy technicians. J Am Pharm Assoc 2010; 50: e35–e69. [DOI] [PubMed] [Google Scholar]
- 60.Cameron KA, Pinkowitz J. Connecting with assisted living consumers. Consult Pharm 2009; 24: 16–18, 21–28. [DOI] [PubMed] [Google Scholar]
- 61.American Society of Health-System Pharmacists. ASHP statement on the role of health-system pharmacists in public health. Am J Health Syst Pharm 2008; 65: 462–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for A logic model for pharmaceutical care by Carolina Moltó-Puigmartí, Robert AA Vonk, Gerlise L van Ommeren and Ingrid Hegger in Journal of Health Services Research & Policy