Abstract
Background
Bovine lactoferrin (bLf) reduces Staphylococcus aureus infection in premature infants and promotes the growth of Bifidobacterium infantis, a predominant infant gut species. We hypothesized that bLf in combination with B. infantis would reduce the severity of systemic S. aureus infection.
Objective
The aim was to determine the effects of oral administration of bLf and B. infantis on the course of systemic S. aureus infection.
Methods
Colostrum-deprived piglets were fed formulas containing 4 g whey/L (CON group) or bLf (LF group). One-half of the piglets in each group were gavaged with B. infantis (109 colony-forming units/d), resulting in 2 additional groups (BI or COMB, respectively). On day 7, piglets were intravenously injected with S. aureus. Blood samples were collected preinfection and every 12 h postinfection for immune analyses. Tissue samples were collected on day 12 for analysis of bacterial abundance and gene expression.
Results
Preinfection, LF piglets had lower serum interleukin 10 (IL-10), a higher percentage of lymphocytes, and a lower percentage of neutrophils than BI or COMB piglets. After infection, dietary bLf increased piglet weight gain, reduced staphylococcal counts in the kidneys, and tended to lower staphylococcal counts in the lungs and heart. Dietary bLf also decreased kidney IL-10 and increased lung interferon γ (IFN-γ) mRNA. B. infantis increased splenic IFN-γ expression. Renal Toll-like receptor 2 was upregulated in BI piglets but not in COMB piglets. Postinfection, BI piglets had increased serum IL-10 and decreased memory T cell populations. LF and COMB piglets had fewer circulating monocytes and B cells than CON or BI piglets.
Conclusions
Dietary bLf and B. infantis produced independent and tissue-specific effects. Piglets fed bLf alone or in combination with B. infantis mounted a more effective immune response and exhibited lower bacterial abundance. This study provides biological underpinnings to the clinical benefits of bLf observed in preterm infants but does not support B. infantis administration during S. aureus infection.
Keywords: lactoferrin, infection, probiotic, immune system, sepsis, neonate, Staphylococcus aureus
Introduction
Staphylococcus aureus infection is the most common fatal bacterial infection in neonates worldwide (1). The infection can result in endocarditis, pneumonia, osteomyelitis, and septic shock (2). In recent years, antibiotic-resistant strains of S. aureus have emerged, limiting treatment options for life-threatening S. aureus infections in infants (2). A clinical trial (3) and subsequent meta-analysis (4) showed that orally administered bovine lactoferrin (bLf) reduced the incidence of late-onset sepsis in very-low-birth-weight infants colonized with gram-positive bacteria, including Staphylococcus species (3).
The role of lactoferrin in the immune response and infection has recently been reviewed (5, 6). These effects likely occur in the intestinal lumen as well as through direct effects on immune cells because bLf is resistant to proteolytic digestion (7, 8) and because both piglet and human intestinal epithelial cells express lactoferrin receptors (9–11). Although the mechanism of action whereby dietary bLf reduces S. aureus infection is unknown, several modes of action are plausible. First, lactoferrin is an iron-binding glycoprotein, which may exert antimicrobial effects by preventing microbes from accessing adequate iron. Lactoferrin is also released by neutrophils at the sites of injury or infection. The release of lactoferrin inhibits infiltration of inflammatory neutrophils while attracting monocytes, suggesting that lactoferrin plays an important role in regulating inflammation, thereby preventing sepsis without inhibiting the original immune response (12). In addition to having effects on the early immune response, reports in the literature suggest that lactoferrin is important in generating a T-helper 1 (Th1) adaptive immune response in mice (13, 14) and piglets (15). A Th1 adaptive response would favor a cellular-immune response to intracellular S. aureus that would increase bacterial clearance from tissues. Lactoferrin also promotes the growth of beneficial commensal bacteria of the Bifidobacteria spp., especially Bifidobacterium infantis (16). Forkhead box protein P3 (Foxp3)-expressing intestinal and splenic T-regulatory cells were increased in B. infantis–fed mice (17) and humans (18). This evidence suggests that lactoferrin and B. infantis may act synergistically to influence regulatory immune function in addition to having independent effects on the innate and adaptive immune responses, resulting in an improved immune response to a pathogenic challenge.
This study was designed to analyze the immune response to a systemic S. aureus infection in piglets fed bLf alone, B. infantis alone, or bLf and B. infantis in combination. Because bLf and B. infantis promote key innate and adaptive immune responses, we hypothesized that bLf in combination with B. infantis would be more efficacious at improving the antibacterial immune response and bacterial clearance during a blood-borne infection than either bLf or B. infantis alone.
Methods
Animal protocol and diets
All of the animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois. Pregnant sows (n = 15) at the University of Illinois Swine Research Center were monitored for farrowing beginning on day 110 of gestation, and female piglets (n = 49) were removed before ingestion of colostrum. Due to the close proximity of the umbilicus and urethral opening in male piglets, females were selected to avoid soaking of bandages and urethral catheters with urine. Piglets were randomly assigned to treatment group on the basis of birth weight and litter. All pigs weighed ∼1.5 kg at birth. To provide passive immunity, sow serum was administered to the piglets via oral gavage at a volume of 5 mL/kg body weight (BW) at birth and at 12, 24, and 36 h postpartum. Piglets were individually housed in cages in environmentally controlled rooms (25°C). Plastic heating pads in each enclosure were used to maintain an ambient temperature of 30°C. Piglets were fed a nonmedicated, sow-milk replacer formula (Liqui-Wean Advance; Milk Specialties Global Animal Nutrition) with either 4 g supplemental protein/L as whey protein [control (CON) group; Provon 192; Glanbia] or as bLf (LF group; Bioferrin 2000; Glanbia). This dose of bLf was chosen on the basis of our previous research (15, 19), and whey was added to the CON diet to keep the diets isonitrogenous. Piglets were fed 20 times daily, totaling 360 mL ⋅ kg BW−1 ⋅ d−1. Half of the piglets in each group were further randomly assigned to receive B. infantis (3 × 109 CFUs/d)—whey + B. infantis (BI group) and bLf + B. infantis (COMB group)—resulting in 4 treatment groups: LF, CON, BI, and COMB.
B. infantis preparation, storage, and administration
B. infantis ATCC 15697 (American Type Culture Collection) was grown from a frozen stock in deMan, Rogosa, Sharpe broth (Difco) supplemented with cysteine (0.05%) and incubated anaerobically as previously described (20). Midexponential phase B. infantis was harvested by centrifugation, resuspended in sterile PBS, and cryogenically preserved in a 1:1 cell to glycerol (25%) suspension. Each batch of B. infantis inoculum was validated for viability on Reinforced Clostridial Agar. On average, each stock contained 5 × 108 CFUs/mL. Before administration, B. infantis was washed in PBS, and the bacterial pellet was resuspended in PBS for administration. Piglets in the BI and COMB groups were orally gavaged with 2 mL washed B. infantis 3 times daily for a total dosage of ∼3 × 109 CFUs/d. This dose was selected on the basis of typical probiotic dosing regimens (21–23).
Umbilical catheterization
Piglets underwent a surgical procedure within 12 h of birth to place 2 umbilical catheters with the use of established methods (24). One catheter was used for administering the S. aureus and the other for blood sampling. Briefly, piglets were lightly sedated with 2% isoflurane (IsoFlo; Abbott Laboratories). The abdomen was washed and an iodine disinfectant applied to the umbilicus and surrounding region. To numb the umbilicus, lidocaine (Henry Schein) was injected subcutaneously into multiple sites surrounding the umbilical stump. One catheter (3.5-F polyvinyl chloride catheter; Tyco Healthcare Group) was inserted 22 cm into the dorsal aorta to a position near the heart. A second catheter for blood sampling was inserted and advanced 20 cm. Catheters were sutured to the umbilical stump and secured to the body with suture and elastic tape. Piglets were fitted into a jacket to protect the catheterization site while allowing free movement within the cages. Catheter patency was maintained by flushing twice daily with heparinized saline (10 IU heparin/mL in 0.9% NaCl). Piglets received 1 systemic dose of Enrofloxacin (2.5 mg/kg; Bayer) immediately after surgery.
S. aureus infection
S. aureus strain S54F9 was a gift from Dr. Bent Aalbaek, (University of Copenhagen, Denmark) (25). S. aureus was cultured in Brain Heart Infusion broth aerobically at 37°C. Before administration, stationary-phase S. aureus cells were harvested by centrifugation, resuspended in 0.9% sterile isotonic saline, and diluted to 105 CFUs/mL. On day 7, all piglets were administered S. aureus at 1 mL/kg BW via the umbilical catheter. This dose was chosen on the basis of a pilot study in which the temporal clinical signs and immune responses to 2 S. aureus doses (103 and 105 CFUs) were evaluated and compared with noninfected piglets (26).
Sample collection
BW and formula intake were assessed daily. Rectal temperature and activity were assessed every 12 h postinfection. Blood samples were collected into nonheparinized vacuum tubes via the sampling umbilical catheter before infection and every 12 h postinfection for cytokine analyses. Heparinized blood was collected at 72 and 120 h postinfection for peripheral blood mononuclear cell (PBMC) isolation. Before infection and at 120 h postinfection, fresh blood smears were collected for complete blood counts (Clinical Pathology Laboratory, Hematology Department, University of Illinois College of Veterinary Medicine, Urbana, Illinois). At 120 h postinfection, piglets were killed by using an intravenous injection of sodium pentobarbital (72 mg/kg BW; Fatal Plus; Vortech Pharmaceuticals).
Measuring B. infantis in ascending colon contents and feces
DNA extraction
Total genomic DNA was isolated from ascending colon (AC) contents and feces by using a combination of bead beating and the QIAamp Fast DNA Stool Mini Kit (Qiagen). Approximately 200 mg AC or feces were combined with 1 mL InhibitEx buffer in a 2-mL Lysing Matrix B tube. Tubes were shaken at 6 m/s for 30 s with the use of the Fastprep 24 System (MP Biomedicals). Samples were incubated at 95°C for 5 min and centrifuged at 20,800 × g for 1 min at RT. DNA was purified from 200 μL supernatant by using the QIAmp Fast DNA Stool Mini Kit according to the manufacturer's instructions. Isolated DNA was quantified with a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific).
RT-qPCR
The absolute abundance of B. infantis in AC contents and feces was quantified by qPCR with the use of the primers BiINF-1 and BiINF-2 (27). PCR was performed with an Applied Biosystems 7900HT Fast Real-Time PCR System with the use of SYBR Green assays. PCR was run in triplicate with a reaction volume of 10 µL:5 µL of 2× Power SYBR Green PCR Master mix (Applied Biosystems), 1 µL BSA (1mg/mL; New England Biolabs), 0.5 µM of each primer, and 10 ng template DNA. The cycling conditions were 50°C for 2 min, 95˚C for 10 min, followed by 40 cycles of 95˚C for 15 s, 5°C for 20 s, and 72°C for 60 s. After amplification, a dissociation step was included to analyze the melting profile of the amplified products. Standard curve [102–107 16S ribosomal RNA (rRNA) gene copies/reaction] was generated by using purified pCR 4 TOPO-TA plasmids (ThermoFisher Scientific) containing the 16S rRNA genes of B. infantis. Data were processed with SDS version 2.3 software (ThermoFisher Scientific).
Flow cytometry to identify PBMC subpopulations
PBMCs were obtained by Ficoll-Hypaque centrifugation of heparinized blood. Cells were resuspended in flow staining buffer (PBS, 1% BSA, 0.1% sodium azide). Cell populations were assessed by flow cytometry with the use of fluorescently labeled antibodies as described (28). Cell staining antibody cocktails are presented in Supplemental Table 1.
T lymphocyte populations were expressed as a percentage of CD3+ events for CD3+CD4+CD8− (T-helper cells), CD3+CD4−CD8+ (cytotoxic T cells), and CD3+CD4+CD8+ (memory T cells). NK cells were identified as CD3−CD4−CD8+ events and expressed as a percentage of CD3− events. Monocytes were identified as CD14+CD163+CD172a+ events and expressed as a percentage of CD172a+ events. B cells were identified as CD21+MHCII+ events and expressed as a percentage of total lymphocytes.
Serum cytokines
Serum was obtained by centrifugation of blood samples and was analyzed by using porcine-specific ELISA kits for IFN-γ [limit of detection (LOD): 62.5 pg/mL], IL-6 (LOD: 125 pg/mL), and IL-10 (LOD: 23.4 pg/mL) (R&D Systems). IL-10 was measured in blood samples taken preinfection and every 12 h postinfection. IFN-γ was measured in blood samples taken preinfection and 24, 36, and 72 h postinfection. IL-6 was measured in blood samples taken preinfection and 72 h postinfection. For samples with concentrations below the LOD of the assay (the lowest point on the standard curve), the value was set to one-half the LOD (29).
Detecting S. aureus in blood and tissue samples
Immediately after killing, blood was collected by cardiac puncture into heparin-laced vials. Five grams of whole-organ cross-sections for the kidneys (each section included cortex and medulla), lungs (each section included caudal lobe), heart (each section included apex, left and right ventricles), and spleen were collected. Tissues were homogenized at a 1:5 dilution in sterile PBS by using a stomacher (Stomacher 80 Biomaster; Seward Laboratory Systems, Inc.). For each tissue sample, 3 cultures were started on mannitol salt agar (BD), a selective and differential media for S. aureus: 200 µL was hand-plated at 1:5, 50 µL was spiral-plated (Neu-Tec Group, Inc.) at 1:5, and 50 µL was spiral-plated at 1:10. Blood samples were plated undiluted on mannitol salt agar. All tissue samples were plated in triplicates at each dilution. Plates were incubated for 48 h aerobically at 37°C before being counted. Colony counts were averaged for each set of triplicates and corrected for the dilution factor. Final results for S. aureus load are expressed as CFUs per gram of tissue.
Tissue cytokine analysis
RNA extraction
Frozen kidney, lung, and spleen samples (100 mg) were homogenized with 1 mL TRIzol reagent per the manufacturer's instructions (ThermoFisher Scientific). RNA was dissolved in 20 µL nuclease-free water (ThermoFisher Scientific) and quantified by using a NanoDrop 1000 instrument (ThermoFisher Scientific). Samples were diluted to an RNA concentration of ≤500 ng/µL, and RNA quality was determined by using a 2100 Bioanalyzer (Agilent Technologies, Inc.).
RT-qPCR
Samples with an RNA integrity number >6 were transformed into cDNA by using a high-capacity cDNA reverse-transcription kit (ThermoFisher Scientific). PCR was performed by using TaqMan gene expression assays for interferon γ (IFNG; Ss03391053_g1), IL10 (Ss03382372_u1), and Toll-like receptor 2 (TLR2; Ss03381278_u1). The expression of the 60S ribosomal protein L19 (RPL19; Ss03375624_g1) gene was used as an endogenous control. The relative standard curve method was used for quantitation. Standard curves consisted of dilutions of cDNA created from spleen mRNA pooled from piglets in all of the treatment groups. Normalized values for each target were calculated by dividing the target quantity mean by the ribosomal protein L19 (RPL-19) quantity mean. A fold-difference was calculated for each measurement by dividing the normalized target values by the normalized calibrator sample. Piglets fed whey (CON) were used as the calibrator in each instance.
Statistical analysis
Statistical analyses were performed by using SAS version 9.2 (SAS Institute). BW, rectal temperatures, serum cytokines, and immune cells were compared between groups by repeated-measures ANOVA with the use of PROC MIXED with treatment (bLf or no bLf), probiotic (received or did not receive B. infantis), and time as fixed effects. The model also included all interactions of treatment by time, treatment by probiotic, and probiotic by time. Because some preinfection measurements were missing, complete blood count or differential data were analyzed by 1-factor ANOVA (treatment) by using PROC GLM. RT-qPCR data were analyzed by 1-factor ANOVA (treatment) with the use of PROC GLM. The statistical model for these data included fixed effects for treatment (bLf or no bLf), probiotic (received or did not receive B. infantis), and the interaction between treatment and probiotic. Staphylococcal load was analyzed by using a nonparametric Wilcoxon’s rank-sum test on CFUs per gram, where the one-sided Z-statistic was used due to a sample size >10. In the event of a significant main effect, a post hoc least significant difference test was used. The abundances of B. infantis are presented as means (log10 copies per gram of content) ± SDs. The number of 16S rRNA gene copies was log10-transformed before analysis. To compare B. infantis abundance, statistical analysis was performed by using the PROC MIXED procedure of SAS version 9.2 (SAS Institute) with Tukey’s post hoc tests. Fixed effects included diet, probiotic, and the interaction of diet and probiotic. Replicate was included as a random effect. When the abundance of B. infantis was below the LOD (2 × 105 copies of 16S rRNA genes/g content), one-half the value of the LOD was used. The occurrence of B. infantis among treatments was analyzed by Fisher's exact test. Significance was defined as P < 0.05, and trends are reported as P < 0.10. Unless otherwise stated, data are presented as means ± SEMs.
Results
Formula intake, BW, and survival
All of the piglets were fed an additional 4 g protein/L. CON and BI pigs were fed whey protein. LF and COMB pigs were fed bLf. Formula intake was unaffected by treatment or infection and averaged 793 ± 30 mL/d on days 1–7 and 1092 ± 27 mL/d after infection between days 7 and 12 postpartum. Thus, mean supplemental protein intake was 4 g/d at day 7 and 4.8 g/d at day 12. Before infection, all of the piglets had similar BWs. The addition of the probiotic B. infantis to either of the diets had no effect on BW; therefore, values were pooled by bLf supplement: bLf-supplemented (LF and COMB) and non–bLf-supplemented (CON and BI) (Figure 1). After infection, bLf-supplemented piglets weighed more than non–bLf-supplemented piglets (treatment × time, P < 0.0001). At the end of the study, bLf-supplemented piglets weighed 3.7 ± 0.1 kg and non–bLf-supplemented piglets weighed 3.4 ± 0.1 kg (Figure 1). Four piglets died during the experiment (1 LF, 2 BI, and 1 COMB) and were not included in the analyses. The LF piglet showed failure to thrive throughout the experiment. The other 3 piglets died 1, 2, or 3 d postinfection. Although these postinfection deaths could be attributed to the S. aureus infection, the piglets’ growth and rectal temperatures postinfection did not differ from those of piglets that survived the study.
FIGURE 1.
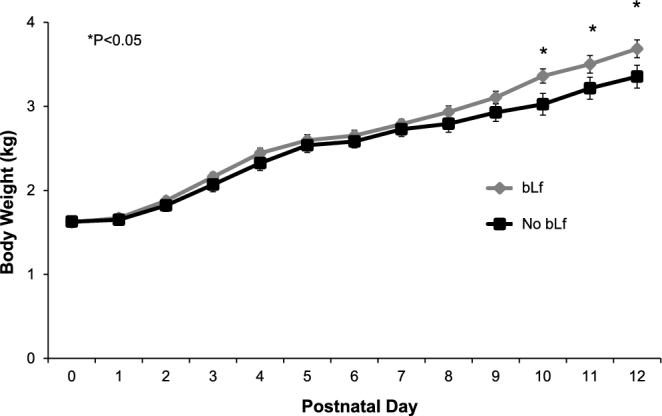
Body weight of piglets fed formula from birth and infected with Staphyloccus aureus on day 7 postpartum. Before infection all piglets had similar body weights. The addition of the probiotic Bifido infantis had no effect on body weight; therefore, groups were pooled by bLf supplement: bLf-supplemented (bLf; LF and COMB) and non–bLf-supplemented (no bLf; CON and BI) piglets. After infection, on days 10, 11, and 12, bLf-supplemented piglets weighed more than non–bLf-supplemented piglets (treatment × time, P < 0.0001). Values are means ± SEMs. *P < 0.05 between no bLf and bLf. bLf, bovine lactoferrin; BI, group receiving B. infantis; COMB, piglets fed bovine lactoferrin and receiving B. infantis; CON, control formula group; LF, bovine lactoferrin formula group.
Rectal temperature
Rectal temperatures peaked 36 h postinfection and remained elevated until killing at 120 h postinfection. B. infantis had no effect on rectal temperatures; therefore, temperatures were pooled by bLf supplement: bLf-supplemented (LF and COMB) and non–bLf-supplemented (CON and BI). Postinfection, there was a significant overall effect in which bLf-supplemented piglets had greater rectal temperatures than non–bLf-supplemented piglets (P = 0.01), with no interaction between protein supplement and hour. On average, postinfection rectal temperatures in bLf-supplemented piglets were 0.21°C higher than those in non–bLf-supplemented piglets. Rectal temperatures differed by time (P < 0.0001). All of the piglets had elevated rectal temperatures at 36 h postinfection compared with baseline and 12 or 24 h postinfection. All of the piglets continued to have elevated rectal temperatures until being killed at 120 h postinfection. At baseline, just before infection, piglets had rectal temperatures of 39.2° ± 0.2°, 39.1° ± 0.2°, 39.0° ± 0.1°, and 39.1° ± 0.1°C for CON, BI, LF, and COMB diets, respectively. At 36 h postinfection, piglets had rectal temperatures of 39.3° ± 0.1°, 39.5° ± 0.2°, 40.0° ± 0.2°, and 39.7° ± 0.2°C for CON, BI, LF, and COMB diets, respectively.
Detection of B. infantis in AC and feces
B. infantis was detected more frequently in the AC and feces from piglets inoculated with B. infantis than in noninoculated pigs (94.4% compared with 4.2% in AC; 100% compared with 5% in feces; P < 0.0001) (Supplemental Table 2). The occurrences of B. infantis were similar in BI and COMB (the treatment groups that included probiotic) pigs. The occurrences B. infantis were significantly higher in BI and COMB piglets than in CON and LF piglets (the treatment groups without probiotic) in both sampling sites. bLf had no effect on the abundance of B. infantis. B. infantis abundance was greater in both AC and feces of BI (7.0 ± 1.1 and 7.2 ± 1.0 log10 copies/g, respectively) and COMB (7.5 ± 1.4 and 7.4 ± 1.0 log10 copies/g, respectively) piglets than in CON and LF (B. infantis was detected in only 1 LF AC sample and 1 CON fecal sample) piglets. In both AC and fecal samples, the abundance of B. infantis did not differ between BI and COMB groups.
Peripheral blood cell populations
Blood cell populations were assessed pre- and 120 h postinfection by using cell counts from whole-blood smears. B. infantis administration had no effect on whole-blood cell populations. However, bLf influenced circulating cell populations (Supplemental Table 3). Before infection, the peripheral blood from bLf-supplemented piglets (LF and COMB) had more lymphocytes and tended to have fewer neutrophils than the blood from non–bLf-supplemented piglets (CON and BI). Postinfection, only 1 pig (in the BI group) met the immature-to-total neutrophil ratio cutoff (>0.25) indicative of sepsis (30). On average, bLf-supplemented piglets had 43% ± 3.7% and non–bLf-supplemented piglets had 31% ± 3.0% blood lymphocytes. bLf-supplemented piglets had 53% ± 3.8% and non–bLf-supplemented piglets had 62% ± 3.4% blood neutrophils. After infection, nucleated RBC (NRBC) populations were lower (P = 0.047) in bLf-supplemented piglets than in non–bLf-supplemented piglets. On average, NRBCs were 3.5% ± 0.9% and 7.3% ± 1.7% of total blood cells in bLf-supplemented and non–bLf-supplemented piglets, respectively. With the use of PBMCs isolated by density gradient centrifugation, B cell, T cell, NK cell, and monocyte and macrophage cell populations were assessed at 72 and 120 h postinfection.
B cell populations were similar for all piglets within each time point (Table 1). The percentage increase in PBMC B cells from 72 to 120 h postinfection differed by protein supplement group, with bLf-supplemented piglets experiencing a smaller increase in B cell population size than CON piglets (P = 0.04).
TABLE 1.
Immune cells in isolated PBMCs at 72 and 120 h after Staphylococcus (strain S54F9) aureus infection in piglets fed control formula, control formula with Bifidobacterium infantis administration, or formula with 4 g bLf/L alone or with B. infantis administration1
| B cells (CD21+MHCII+), % of total PBMCs | Monocytes (CD14+CD163+CD172a+), % of CD172+ cells | Memory T cells (CD4+CD8+CD3+), % of CD3+ cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 72 h | 120 h | Difference,2 % | 72 h | 120 h | Difference,2 % | 72 h | 120 h | Difference,2 % | |
| CON | 4.2 ± 0.8 | 10.0 ± 2.9 | 240 ± 59 | 18.5 ± 5.2 | 24.9 ± 7.5 | 192 ± 74 | 9.6 ± 1.2 | 10.7 ± 1.2 | 119 ± 15 |
| BI | 6.1 ± 1.3 | 11.2 ± 2.6 | 177 ± 20 | 20.3 ± 4.2 | 23.3 ± 5.2 | 201 ± 71 | 9.5 ± 0.9 | 8.3 ± 0.6* | 92 ± 9* |
| LF | 6.0 ± 1.1 | 8.4 ± 1.8 | 151 ± 25* | 23.5 ± 4.3 | 18.6 ± 4.6 | 102 ± 25† | 9.2 ± 1.1 | 10.8 ± 1.2 | 124 ± 17 |
| COMB | 5.3 ± 1.0 | 6.6 ± 2.0 | 112 ± 18* | 22.3 ± 4.6 | 18.7 ± 5.1 | 104 ± 31† | 10.2 ± 1.4 | 9.0 ± 0.8* | 91 ± 9* |
Values are means ± SEMs. *Different by treatment, P < 0.05; †trend to differ by treatment, P = 0.07. BI, group receiving B. infantis (3 × 109 CFUs/d); bLf, bovine lactoferrin; COMB, piglets receiving B. infantis and fed bovine lactoferrin; CON, control formula group; LF, bovine lactoferrin formula group; PMBC, peripheral blood mononuclear cell.
Immune cell percentage at 120 h divided by the immune cell percentage at 72 h × 100.
Monocyte populations were similar for piglets in all 4 treatment groups at both 72 and 120 h postinfection (Table 1). The percentage difference in PBMC monocytes from 72 to 120 h postinfection in bLf-supplemented piglets tended (P = 0.07) to be smaller than that in non–bLf-supplemented piglets (Table 1).
Neither T-helper (70.2% ± 8.4%) nor cytotoxic T cell (5.3% ± 0.6%) populations differed between treatment groups at either time point. However, in an analysis measuring only the effect of treatment at 120 h postinfection, memory T cell populations were smaller (P = 0.03) in piglets that received B. infantis than in piglets that did not receive the probiotic (Table 1). The percentage difference in PBMC memory T cell populations from 72 to 120 h postinfection in probiotic supplemented piglets was lower (P = 0.03) than the percentage difference in memory T cell populations from 72 to 120 h postinfection in non–probiotic-supplemented piglets.
NK cell populations were similar for all piglets at each time point, but NK cell populations were larger at 120 h postinfection (5.8% ± 1.0%) than at 72 h postinfection (2.6% ± 0.4%).
Serum cytokines
Of the 3 serum cytokines analyzed, only IL-10 was detected in the serum of piglets at multiple time points. Neither IFN-γ nor IL-6 was detectable in piglets before infection nor were these 2 cytokines detectable in the majority of piglets at 72 h postinfection. Before infection on postpartum day 7, there was no effect of probiotic; therefore, data were pooled by bLf-supplemented (LF and COMB) compared with non–bLf-supplemented (CON and BI) piglets. Piglets fed formula with bLf had 3-fold lower (P = 0.01) serum IL-10 than non–bLf-supplemented piglets (Figure 2A). After infection, serum IL-10 concentrations changed significantly over time (P = 0.03). The highest concentrations of IL-10 were observed at 96 h (58.2 ± 24.0 pg/mL) and 108 h (45.4 ± 16.7 pg/mL) postinfection (Figure 2B). There was no effect of bLf on serum IL-10 postinfection. Therefore, data were pooled by probiotic treatment. Probiotic-treated piglets (BI and COMB) had higher serum IL-10 (P = 0.03) concentrations than piglets that did not receive B. infantis (Figure 2B).
FIGURE 2.
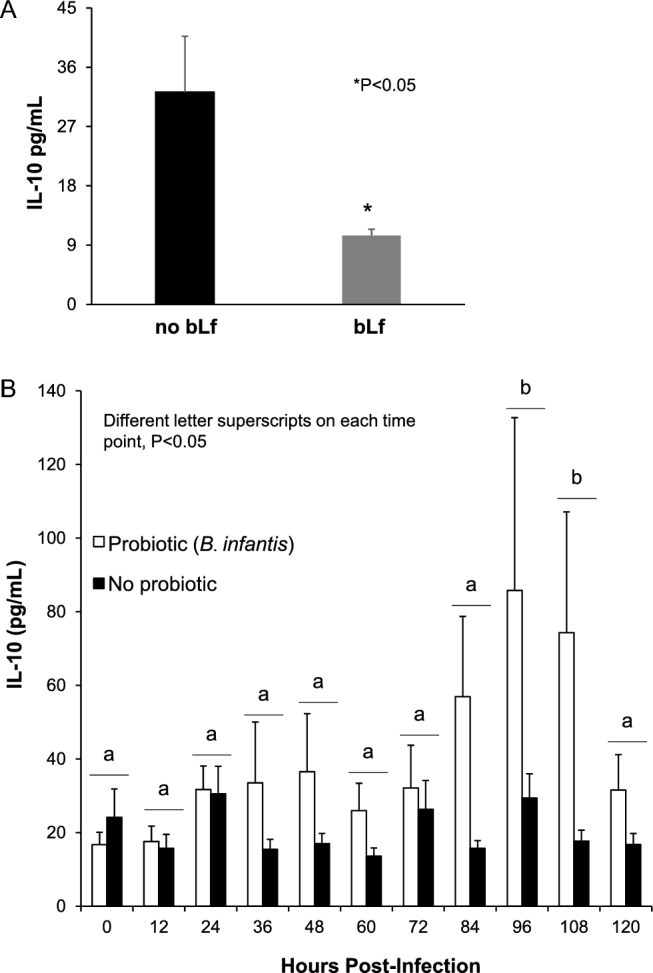
Serum IL-10 concentrations measured before Staphylococcus aureus infection and every 12 h postinfection. Preinfection, bLf, but not Bifidobacterium infantis, significantly affected serum IL-10; therefore, groups were pooled by bLf (LF and COMB; n = 26) or non-bLf (CON and BI; n = 23). Before infection, bLf-supplemented piglets had lower (P = 0.01) serum IL-10 concentrations than non–bLf-supplemented piglets (A). After S. aureus infection, there was a main effect of time (P = 0.03), in which piglets produced the highest IL-10 concentrations at 96 and 108 h postinfection (B). There was also a main effect of probiotic (P = 0.03), but not bLf; therefore, values were pooled for probiotic (BI and COMB; n = 26) and no probiotic (CON and LF; n = 23). Probiotic-treated piglets had greater serum IL-10 than non–probiotic-treated piglets. Values are means ± SEMs, n = 11–13/group. *P < 0.05. Different letters on each time point, P < 0.05. BI, group receiving B. infantis; bLf, bovine lactoferrin; COMB, piglets fed bovine lactoferrin and receiving B. infantis; CON, control formula group; LF, bovine lactoferrin group.
S. aureus in blood and tissues
S. aureus was detected in the kidneys, lungs, heart, and spleen, but not in the blood. There was no effect of B. infantis on S. aureus content in blood or tissues; therefore, data were pooled by bLf supplement (Figure 3). bLf-supplemented pigs had lower numbers (CFUs) of S. aureus in the kidneys (P = 0.02) and tended to have lower numbers in the lungs (P = 0.07) and heart (P = 0.06) than piglets fed diets without bLf. There was no effect of bLf on S. aureus numbers in the spleen. Overall, dietary bLf decreased kidney, lung, and heart S. aureus load by 7.3-, 4-, and 1.8-fold, respectively.
FIGURE 3.
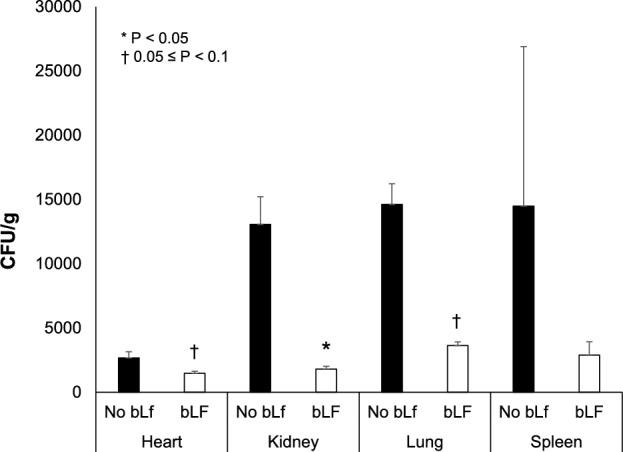
Staphylococcus aureus counts (CFUs per gram) in the heart, kidneys, lungs, and spleen 120 h after S. aureus infection in 12-d-old piglets fed control formula, control formula with Bifidobacterium infantis administration (109 CFU/d), or formula with 4 g bLf/L alone or with B. infantis administration. There was no effect of B. infantis; therefore, values were pooled by bLf supplement: bLf-supplemented (LF and COMB, bLf; n = 26) and non–bLf-supplemented (CON and BI, no bLf; n = 23). No differences were detected in the spleen. Piglets fed diets containing bLf had decreased S. aureus counts in the kidneys and tended to have decreased CFUs per gram in the lungs and heart. Values are means ± SEMs. *P < 0.05 and †0.05 > P < 0.1 between no bLf and bLf. BI, group receiving B. infantis; bLf, bovine lactoferrin; COMB, piglets fed bovine lactoferrin and receiving B. infantis; CON, control formula group; LF, bovine lactoferrin formula group.
Immune gene expression in the tissues
Kidney
Piglets exposed to B. infantis in the context of a whey protein diet (BI) had the highest (P = 0.01) kidney TLR2 mRNA expression. However, piglets exposed to B. infantis in the context of a bLf diet (COMB) had renal TLR2 mRNA expression similar to that of CON or LF piglets (Table 2). Pigs fed diets with bLf (LF and COMB) showed decreased renal IL-10 expression (P = 0.03). Piglets administered B. infantis in the absence of dietary bLf (BI) tended (P = 0.09) to have the highest renal IL-10 expression. Renal IFN-γ expression was similar in all piglets.
TABLE 2.
Immune-related gene expression in kidneys, lungs, and spleen at 120 h after Staphylococcus aureus (strain S54F9) infection in piglets fed control formula, control formula with Bifidobacterium infantis administration, or formula with 4 g bLf/L alone or with B. infantis administration1
| Kidneys | Lungs | Spleen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ | TLR2 | IL-10 | IFN-γ | TLR2 | IL-10 | IFN-γ | TLR2 | IL-10 | |
| CON | 1.0 ± 1.0 | 1.0 ± 0.16 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| BI | 1.2 ± 1.1 | 2.5 ± 0.6* | 2.3 ± 1.0 | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.3 | 2.5 ± 2.1* | 1.2 ± 0.1 | 1.0 ± 0.1 |
| LF | 0.6 ± 0.5 | 1.1 ± 0.1 | 0.4 ± 0.1* | 2.0 ± 0.5* | 0.9 ± 0.1 | 1.6 ± 0.5 | 1.1 ± 0.3 | 1.4 ± 0.2 | 1.1 ± 0.2 |
| COMB | 1.2 ± 1.3 | 1.3 ± 0.2 | 0.7 ± 0.2* | 1.5 ± 0.4* | 1.2 ± 0.3 | 1.4 ± 0.6 | 2.7 ± 0.8* | 1.2 ± 0.3 | 1.0 ± 0.2 |
A fold-difference was calculated for each measurement by dividing the normalized target values (calculated by dividing the target quantity mean by the RPL-19 quantity mean) by the normalized calibrator values. For samples from the same tissue, values for CON piglets were used as the calibrator. *Different by treatment, P < 0.05. BI, group receiving B. infantis (3 × 109 CFUs/d); bLf, bovine lactoferrin; COMB, piglets receiving B. infantis and fed bovine lactoferrin; CON, control formula group; LF, bovine lactoferrin formula group; RPL-19, ribosomal protein L19; TLR2, Toll-like receptor 2.
Lungs
IFN-γ expression was significantly higher in LF and COMB piglets than in CON and BI piglets (Table 2). No significant differences in TLR2 or IL-10 mRNA expression in the lungs were observed.
Spleen
Piglets exposed to B. infantis showed higher (P = 0.02) splenic IFN-γ mRNA expression (Table 2) than piglets that were not given the probiotic. Splenic TLR2 and IL-10 mRNA expression was similar in all piglets.
Discussion
The goal of this study was to investigate the individual and combined effects of bLf and B. infantis on the clinical course of a systemic S. aureus infection in neonatal piglets. This research was predicated on previous clinical trials that showed that orally administered bLf reduced the incidence of late-onset sepsis in very-low-birth-weight infants colonized with gram-positive bacteria, including Staphylococcus species (3). Furthermore, B. infantis has been shown to be immunomodulatory (17, 31) and bLf may promote the growth of B. infantis (16), suggesting the potential for synergistic effects. On the basis of our previous research (15, 19), by the seventh day of life bLf improved gastrointestinal development and immune system development compared with piglets fed formula alone. Thus, these early effects on the gastrointestinal and immune systems likely give the bLf-fed piglets an advantage over their formula-fed peers upon systemic challenge with S. aureus.
Despite promising clinical outcomes, the immune response is difficult to assess in human infants; thus, the systemic and tissue immune responses over the course of S. aureus infection were assessed by using newborn piglets. The piglet is an excellent preclinical model for this research for the following reasons: S. aureus is a dominant cause of widespread septicemia in pigs and humans (32); swine physiology, immune response; and anatomy are highly similar to those of humans (33); and most importantly, pigs are capable of reproducing the gradual pathophysiologic changes and clinical characteristics of neonatal sepsis (34), including BW and temperature, which are easily monitored in piglets. The use of bLf in this appropriate preclinical model for human infants was deliberate because this is the same compound that can be used in human infant formulas. In the current study, dietary bLf and B. infantis each influenced the response to S. aureus infection in the neonatal piglet, but the combination of bLf and B. infantis did not exert synergistic effects. Overall, the severity of S. aureus infection was reduced in piglets fed dietary bLf, with limited beneficial effects of B. infantis.
Dietary bLf improved weight gain after S. aureus infection. The beneficial effect of bLf on growth cannot be attributed to the presence of additional protein, because the CON and BI diets were supplemented with 4 g whey protein/L to maintain a protein content similar to the LF and COMB diets (supplemented with 4g bLf/L). Previous studies have shown that bLf improves weight gain in piglets (35) and human infants (36). The increase in BW has been partially attributed to its proposed role in regulating the immune system and providing protection against microbial infection, wherein animals that more effectively respond to infection have a reduced period of cachexia and can apportion more energy toward growth instead of directing that energy toward a sustained immune response.
A key finding of this study was that dietary bLf significantly reduced bacterial abundance in the kidneys and tended to lower the S. aureus counts in the lungs and hearts of infected piglets. The improved bacterial clearance in the presence of bLf was likely due, in part, to an enhanced Th1 immune response in bLf-supplemented pigs. In our study, bLf supplementation increased IFN-γ mRNA expression in the lungs after infection, indicating a Th1 response. Furthermore, bLf-supplemented pigs had less serum IL-10 on day 7 postpartum just before S. aureus infection. A high concentration of IL-10 is known to inhibit Th1 immune responses (37). Others have shown that lactoferrin-transgenic mice showed an enhanced Th1 response to S. aureus infection. These mice showed a greater IFN-γ response and increased ability to clear the bacterial infection (13).
Accordingly, S. aureus persistence was affected by dietary treatment. As in previous studies (25, 38), S. aureus was rapidly cleared from the blood of infected piglets but persisted in other tissues. Compared with supplementation with whey protein (CON, BI), supplementation with bLf (COMB, LF) significantly reduced S. aureus load. This was expected on the basis of previous reports in rodents in which bLf supplementation reduced S. aureus load in the kidneys of S. aureus–challenged mice (14) and where Lf-transgenic mice showed an increased ability to clear S. aureus after a challenge (13). Importantly, bLf supplementation decreased S. aureus counts in the kidneys and tended to decrease counts in the lungs and heart.
In contrast, the probiotic B. infantis had no effect on bacterial clearance when administered alone. This may be related to increased TLR2 message and IL-10 message as well as IL-10 protein in these piglets. In vitro work has shown that S. aureus downregulates the inflammatory T cell response by triggering IL-10 production by monocytes via TLR2 activation (39, 40). The increased IL-10 dampens the immune response, enabling S. aureus to colonize the host (40). Consistent with the IL-10 serum cytokine data, probiotic-treated piglets also had fewer circulating memory T cells, with significantly fewer memory T cells compared with non–probiotic-treated piglets at 120 h postinfection. In the kidneys, the enhanced TLR2 and IL-10 mRNA expression in response to probiotic treatment was decreased when bLf was present. In fact, the presence of bLf also decreased the renal IL-10 message compared with the CON diet. However, because the effects were not consistent across tissues (kidneys, lungs, spleen, blood), other mechanisms may be at work. TLR2 signaling regulates additional inflammatory cytokine responses (41, 42), and S. aureus stimulates additional immune receptors so effects of bLf and B. infantis on other cytokines are also important. For instance, bLf supplementation increased IFN-γ expression in the lungs. It is important to note that bLf may not only have decreased bacterial load but also may have impaired S. aureus viability, leading to these lower tissue counts.
We hypothesized that bLf would stimulate a robust Th1 immune response. Because B. infantis is known to downregulate inflammatory responses (17), we hypothesized that B. infantis would provide protection in the later phase of infection. In addition, bLf has been shown to promote growth of B. infantis in vitro (16), suggesting a potential mechanism by which these dietary components could synergize. In this experiment, bLf did not support the growth of B. infantis over that seen in piglets exposed to B. infantis in the absence of bLf. However, we observed that bLf supplementation was most important for S. aureus bacterial clearance. Postinfection, B. infantis had an overall effect of increased serum IL-10, which is consistent with what has been observed in mice and humans supplemented with B. infantis (17, 18). High concentrations of IL-10 have consistently been shown to be a strong indicator of septic shock and a predictor of mortality during infection due to its broadly immunosuppressive function (43). Consistent with this, the only piglet to have reached the diagnostic immature-to-mature neutrophil ratio for sepsis (44) was in the BI group. Furthermore, probiotic-treated pigs had higher postinfection NRBC counts. NRBCs are another cell population that has been associated with septicemia (45). It has been shown that, in addition to promoting a Th1 response through increased IFN-γ production, bLf can also increase the IL-12-to-IL-10 ratio in LPS-stimulated splenocytes to promote the Th1 response (46). The presence of IL-12, a cytokine that promotes the Th1 response, in addition to IFN-γ, leads to a significantly decreased production of IL-10 (46). However, in our studies, we did not measure IL-12 and no IFN-γ was detected in the serum. One possible explanation for this could be that IFN-γ is an intracellular cytokine and its presence may be too low in the serum to detect. Previously, we found that spleen cells isolated from pigs fed bLf produced more IFN-γ and TNF-α than cells isolated from pigs fed a control diet (15). Although NK cells are the most likely potential sources of IFN-γ, it may be that peripheral memory T cells contain intracellular IFN-γ; both of these cells could release IFN-γ when needed during an immune response. Future experiments should use intracellular cytokine staining in combination with flow cytometry or ELISpot assays to determine the IFN-γ production potential of cells isolated from areas local to the infection.
Despite the novelty of the model, the study described herein has several limitations. Neither iron status nor amounts of bLf in stool and serum were measured. Therefore, the current study could not assess the fate of ingested bLf nor determine if the effects of bLf on S. aureus infection were due to bLf sequestration of iron from S. aureus. This study was designed to test the regulatory and adaptive immune response to S. aureus infection, based on the hypothesis that those would be the stages of the immune response during which the effects of combining probiotic and bLf treatment would be most efficacious at reducing the severity of S. aureus infection. Therefore, most details about the effects of the dietary treatments on the early immune response to S. aureus infection are unknown. However, blood was collected preinfection and every 12 h postinfection for some analyses. IFN-γ was measured preinfection and at 24, 36, and 72 h postinfection. IL-6 was measured preinfection and at 72 h postinfection. These time points were chosen on the basis of a pilot study. Because a limited volume of blood could be sampled (due to piglet size and the repeated sampling design), blood was used for cytokine analyses rather than S. aureus quantification at the early time points postinfection. Thus, conclusions about the effects of the dietary treatment on S. aureus counts in the blood at early time points postinfection cannot be drawn. Finally, although the presence of B. infantis was determined in feces and AC content; no attempt was made to test for B. infantis in the blood or other tissues. Therefore, any effects that the potential translocation of the probiotic from the gut to the bloodstream could have on the immune response to infection are unknown. Future studies should measure bLf in fecal matter, serum, and urine and should carefully assess the iron status (ferritin, transferrin, free iron) of subjects. Additional studies should also examine the effects of probiotic and bLf on early inflammatory and innate immune responses to infection.
This preliminary animal study provides mechanistic insight supporting clinical studies that show the effectiveness of orally administered bLf in the prevention of systemic S. aureus infections and suggests that future studies investigating lactoferrin's role in treating S. aureus are warranted. IL-10 is a critically important predictor of mortality during infection. Therefore, the observation that bLf lowers serum IL-10 preinfection and B. infantis increases serum IL-10 postinfection provides evidence that modulation of IL-10 is one potential mechanism by which bLf reduces tissue bacterial abundance in this model of systemic S. aureus infection. Furthermore, another indicator of sepsis, NRBC counts, were lower in pigs fed bLf. Although NRBC counts were not affected by the presence of B. infantis, exposure to the probiotic increased serum IL-10 concentrations postinfection, potentially indicating that this probiotic should be avoided in neonates at risk of systemic S. aureus infection. However, due to its ability to induce IL-10, B. infantis may be a useful therapy in already septic neonates. In conclusion, this study suggests that colostrum and mother's milk, 2 liquids with abundant lactoferrin concentrations (47, 48), may protect neonates from S. aureus infections and from the complications caused by such infections.
Supplementary Material
Acknowledgments
We thank Gianna Vella for assistance with conducting the RT-qPCR analyses. We thank Barbara Pilas at the Roy J Carver Biotechnology Center Flow Cytometry Core at the University of Illinois for her guidance. The authors’ responsibilities were as follows—EAR: designed and conducted the research, analyzed data, performed statistical analyses, and wrote the manuscript; SSC: designed the research, analyzed data, performed statistical analyses, and wrote the manuscript; JLH and MW: provided technical assistance; MJM: provided essential reagents and materials as well as technical assistance; SMD: directed the research and has primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by USDA Hatch Project ILLU-698-311.
Author disclosures: EAR, SSC, JLH, MW, MJM, and SMD, no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- AC
ascending colon
- BI
group receiving Bifidobacterium infantis
- bLf
bovine lactoferrin
- BW
body weight
- COMB
piglets fed bLf and receiving B. infantis
- CON
control formula
- LF
bLf formula group
- LOD
limit of detection
- NRBC
nucleated RBC
- PBMC
peripheral blood mononuclear cell
- RPL-19
ribosomal protein L19
- rRNA
ribosomal RNA
- Th1
T-helper 1
- TLR2
Toll-like receptor 2
References
- 1. Park DA, Lee SM, Peck KR, Joo EJ, Oh EG. Impact of methicillin-resistance on mortality in children and neonates with Staphylococcus aureus bacteremia: a meta-analysis. Infect Chemother 2013;45:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maraqa NF, Aigbivbalu L, Masnita-Iusan C, Wludyka P, Shareef Z, Bailey C, Rathore MH. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization and infection among infants at a level III neonatal intensive care unit. Am J Infect Control 2011;39:35–41. [DOI] [PubMed] [Google Scholar]
- 3. Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, Stolfi I, Decembrino L, Laforgia N, Vagnarelli F, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009;302:1421–8. [DOI] [PubMed] [Google Scholar]
- 4. Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2017;6:CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manzoni P. Clinical benefits of lactoferrin for infants and children. J Pediat 2016;173(Suppl):S43–52. [DOI] [PubMed] [Google Scholar]
- 6. Drago-Serrano ME, Campos-Rodriguez R, Carrero JC, de la Garza M. Lactoferrin: balancing ups and downs of inflammation due to microbial infections. Intl J Molec Sci 2017;18:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidson LA, Lönnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand 1987;76:733–40. [DOI] [PubMed] [Google Scholar]
- 8. Hutchens TW, Henry JF, Yip TT. Structurally intact (78-kDa) forms of maternal lactoferrin purified from urine of preterm infants fed human milk: identification of a trypsin-like proteolytic cleavage event in vivo that does not result in fragment dissociation. Proc Natl Acad Sci USA 1991;88:2994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harada E, Itoh Y, Sitizyo K, Takeuchi T, Araki Y, Kitagawa H. Characteristic transport of lactoferrin from the intestinal lumen into the bile via the blood in piglets. Comp Biochem Physiol A Mol Integr Physiol 1999;124:321–7. [DOI] [PubMed] [Google Scholar]
- 10. Kawakami H, Lonnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol 1991;261:G841–6. [DOI] [PubMed] [Google Scholar]
- 11. Liao Y, Lopez V, Shafizadeh TB, Halsted CH, Lonnerdal B. Cloning of a pig homologue of the human lactoferrin receptor: expression and localization during intestinal maturation in piglets. Comp Biochem Physiol A Mol Integr Physiol 2007;148:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol 2008;180:6868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guillen C, McInnes IB, Vaughan DM, Kommajosyula S, Van Berkel PH, Leung BP, Aguila A, Brock JH. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J Immunol 2002;168:3950–7. [DOI] [PubMed] [Google Scholar]
- 14. Bhimani RS, Vendrov Y, Furmanski P. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J Appl Microbiol 1999;86:135–44. [DOI] [PubMed] [Google Scholar]
- 15. Comstock SS, Reznikov EA, Contractor N, Donovan SM. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J Nutr 2014;144:525–32. [DOI] [PubMed] [Google Scholar]
- 16. Rahman MM, Kim WS, Ito T, Kumura H, Shimazaki K. Examination of bovine lactoferrin binding to bifidobacteria. Prikl Biokhim Mikrobiol 2008;44:529–32. [PubMed] [Google Scholar]
- 17. O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathogens 2008;4:e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, Kiely B, Akdis CA, O'Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 2012;61:354–66. [DOI] [PubMed] [Google Scholar]
- 19. Reznikov EA, Comstock SS, Yi C, Contractor N, Donovan SM. Dietary bovine lactoferrin increases intestinal cell proliferation in neonatal piglets. J Nutr 2014;144:1401–8. [DOI] [PubMed] [Google Scholar]
- 20. Francl AL, Hoeflinger JL, Miller MJ. Identification of lactose phosphotransferase systems in Lactobacillus gasseri ATCC 33323 required for lactose utilization. Microbiology 2012;158:944–52. [DOI] [PubMed] [Google Scholar]
- 21. Athalye-Jape G, Deshpande G, Rao S, Patole S. Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am J Clin Nutr 2014;100:1508–19. [DOI] [PubMed] [Google Scholar]
- 22. Lyseng-Williamson K. Bifidobacterium infantis 35624 as a probiotic dietary supplement: a profile of its use. Drugs Ther Perspect 2017;33:368–74. [Google Scholar]
- 23. Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes 2017;8:143–51. [DOI] [PubMed] [Google Scholar]
- 24. Donovan SM, Chao JC, Zijlstra RT, Odle J. Orally administered iodinated recombinant human insulin-like growth factor-I (125I-rhIGF-I) is poorly absorbed by the newborn piglet. J Pediatr Gastroenterol Nutr 1997;24:174–82. [DOI] [PubMed] [Google Scholar]
- 25. Leifsson PS, Iburg T, Jensen HE, Agerholm JS, Kjelgaard-Hansen M, Wiinberg B, Heegaard PM, Astrup LB, Olsson AE, Skov MG, et al. Intravenous inoculation of Staphylococcus aureus in pigs induces severe sepsis as indicated by increased hypercoagulability and hepatic dysfunction. FEMS Microbiol Lett 2010;309:208–16. [DOI] [PubMed] [Google Scholar]
- 26. Reznikov EA, Hoeflinger JL, Monaco MH, Miller MJ, Donovan SM. Development of a piglet model of neonatal systemic Staphylococcus aureus infection. FASEB J 2013;27(Suppl 1):1083.2. [Google Scholar]
- 27. Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl Environ Microbiol 1999;65:4506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Comstock SS, Wang M, Hester SN, Li M, Donovan SM. Select human milk oligosaccharides directly modulate peripheral blood mononuclear cells isolated from 10-d-old pigs. Br J Nutr 2014;111:819–28. [DOI] [PubMed] [Google Scholar]
- 29. Chung MK, Riby J, Li H, Iavarone AT, Williams ER, Zheng Y, Rappaport SM. A sandwich enzyme-linked immunosorbent assay for adducts of polycyclic aromatic hydrocarbons with human serum albumin. Anal Biochem 2010;400:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caserta MT. Infections in neonates. Neonatal sepsis. The Merck Manual Online Medical Library Database Merck Manual Professional Pediatrics [Internet]. 2015 [cited 2017 Jul 20]. Available from: http://www.merckmanuals.com/professional/pediatrics/infections-in-neonates/neonatal-sepsis.
- 31. Underwood MA, Arriola J, Gerber CW, Kaveti A, Kalanetra KM, Kananurak A, Bevins CL, Mills DA, Dvorak B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res 2014;76:326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen HE, Nielsen OL, Agerholm JS, Iburg T, Johansen LK, Johannesson E, Moller M, Jahn L, Munk L, Aalbaek B, et al. A non-traumatic Staphylococcus aureus osteomyelitis model in pigs. In Vivo 2010;24:257–64. [PubMed] [Google Scholar]
- 33. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 2012;20:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kato T, Hussein MH, Sugiura T, Suzuki S, Fukuda S, Tanaka T, Kato I, Togari H. Development and characterization of a novel porcine model of neonatal sepsis. Shock 2004;21:329–35. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Shan T, Xu Z, Liu J, Feng J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J Anim Sci 2006;84:2636–41. [DOI] [PubMed] [Google Scholar]
- 36. Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr 2002;76:858–64. [DOI] [PubMed] [Google Scholar]
- 37. Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991;146:3444–51. [PubMed] [Google Scholar]
- 38. Nielsen OL, Iburg T, Aalbaek B, Leifsson PS, Agerholm JS, Heegaard P, Boye M, Simon S, Jensen KB, Christensen S, et al. A pig model of acute Staphylococcus aureus induced pyemia. Acta Vet Scand 2009;51:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000;165:5392–6. [DOI] [PubMed] [Google Scholar]
- 40. Chau TA, McCully ML, Brintnell W, An G, Kasper KJ, Vines ED, Kubes P, Haeryfar SM, McCormick JK, Cairns E, et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nature Med 2009;15:641–8. [DOI] [PubMed] [Google Scholar]
- 41. Yimin, Kohanawa M, Zhao S, Ozaki M, Haga S, Nan G, Kuge Y, Tamaki N. Contribution of toll-like receptor 2 to the innate response against Staphylococcus aureus infection in mice. PloS One 2013;8:e74287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nandi A, Dey S, Biswas J, Jaiswal P, Naaz S, Yasmin T, Bishayi B. Differential induction of inflammatory cytokines and reactive oxygen species in murine peritoneal macrophages and resident fresh bone marrow cells by acute Staphylococcus aureus infection: contribution of Toll-like receptor 2 (TLR2). Inflammation 2015;38:224–44. [DOI] [PubMed] [Google Scholar]
- 43. Heper Y, Akalin EH, Mistik R, Akgoz S, Tore O, Goral G, Oral B, Budak F, Helvaci S. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis 2006;25:481–91. [DOI] [PubMed] [Google Scholar]
- 44. Engle WD, Rosenfeld CR, Mouzinho A, Risser RC, Zeray F, Sanchez PJ. Circulating neutrophils in septic preterm neonates: comparison of two reference ranges. Pediatrics 1997;99:E10. [DOI] [PubMed] [Google Scholar]
- 45. Cremer M, Roll S, Graf C, Weimann A, Buhrer C, Dame C. Nucleated red blood cells as marker for an increased risk of unfavorable outcome and mortality in very low birth weight infants. Early Hum Dev 2015;91:559–63. [DOI] [PubMed] [Google Scholar]
- 46. Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol 2007;196:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masson PL, Heremans JF. Lactoferrin in milk from different species. Comp Biochem and Physiol B 1971;39:119–29. [DOI] [PubMed] [Google Scholar]
- 48. Hirai Y, Kawakata N, Satoh K, Ikeda Y, Hisayasu S, Orimo H, Yoshino Y. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J Nutr Sci Vitaminol 1990;36:531–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


