Abstract
We propose a new surgical technique for superior cavopulmonary anastomosis in patients with functionally univentricular heart and bilateral superior caval veins. One of the reasons for failure of bidirectional Glenn shunts in patients with bilateral superior caval veins is the small caliber of one or both veins, with limited flow through each cavopulmonary anastomosis that can easily result in torsion, blockage, or clot formation. The conversion of two small superior caval veins into a single confluence which is large enough to connect with the pulmonary artery (PA) can resolve this problem. We present our experience with two cases in which a rolled pericardial graft was used to create a single caval vein to provide balanced pulmonary blood flow and yield growth of the central PA as well as reducing the likelihood of thrombus formation.
Keywords: bidirectional Glenn operation, unifocalization of bilateral superior vena cavae
Background
Systemic venous anomalies are no longer considered a major risk factor for bidirectional Glenn operation and Fontan procedure. However, the presence of persistent left superior vena cava (PLSVC) is still a challenge for safe and effective completion of superior cavopulmonary amalgamation. From the anatomical perspective, in the presence of bilateral superior caval veins, the small diameters of each vein will reduce the blood flow at the level of each anastomosis. If one superior vena cava (SVC) is considerably smaller than the other, the resultant asymmetrical distribution of pulmonary blood flow may contribute to increased risk of thrombus formation and/or the unfavorable growth of pulmonary arteries.1–3
In order to improve the SVC blood flow, many ideas have been proposed: innominate vein formation by Gore-Tex prosthetic graft,4 inferior anastomosis of two SVCs to form a venous confluence,3 or other means of converting two SVCs into a single vessel,2 including the use of an aortic homograft conduit.5
In this study, we present a technical modification for bidirectional Glenn operation in patients with bilateral superior caval veins that can possibly optimize the growth of pulmonary arteries and reduce the risk of thrombus formation. In addition, we also review the literature about modifications of bilateral cavopulmonary anastomosis.
Participants and Method
Two patients diagnosed with functionally univentricular heart defects with bilateral superior caval veins had the indications for bidirectional Glenn operation, treated at E Hospital. The study was reviewed and approved by the ethics committee of E Hospital. All study procedures complied with the ethical principles of biomedical research. Participants consented to take part in the study and were told that they could withdraw at any time. Participants’ information was kept secure and confidential.
Results
Case 1
An 11-month-old boy weighing 7.4 kg, cyanotic, with SpO2 67% on room air had undergone Blalock-Taussig shunt operation at the age of 3 months. At the time of the current assessment, complete blood count and biochemistry test results were within the normal range.
Echocardiographic findings were transposition of the great arteries, aorta arising from the right ventricle (RV), atresia of the main pulmonary artery (PA), a large ventricular septal defect (functionally univentricular physiology), cor triatriatum, patent arteriosus ductus, enlarged coronary sinus due to PLSVC, and patent left Blalock-Taussig shunt. Cardiac catheterization showed a large ventricular septal defect, aorta arising anteriorly from the RV, pulmonary atresia, patent Blalock shunt, left PA: 8.3 mm, right PA: 7.8 mm, and stenosis at the level of pulmonary bifurcation.
We decided to pursue a strategy of staged univentricular palliation. The patient underwent surgery. Median sternotomy was performed and anterior pericardium was harvested. The aorta arose from RV and was positioned anteriorly with respect to the small PA. There was a small right superior vena cava (RSVC), a large left superior vena cava (LSVC; left dominance), and right inferior vena cava (IVC). There was a small ductus arteriosus (5 mm) and a patent left Blalock-Taussig shunt. Preoperative PA pressure was 16 mm Hg.
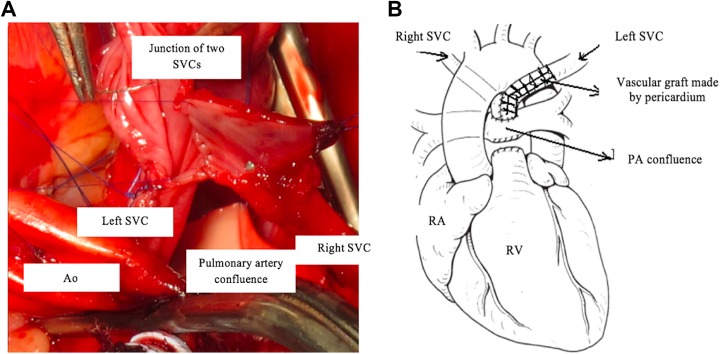
Cardiopulmonary bypass was established with cannulation of the aorta, each SVC, and the right atrium (RA). Under mild hypothermia, the aorta was clamped. Myocardial protection was achieved by antegrade infusion of Custodiol solution. After opening the RA, we removed the left atrial membrane after partially excising the interatrial septum. The pulmonary arterial trunk was separated from the RV and ligated at the proximal end. We then augmented the PA confluence. Each SVC was separated from the RA and dissected over as much length as possible. The length of the LSVC was not sufficient to allow it to be anastomosed directly to the RSVC. We created a tubular vascular graft using rolled pericardium on a Hegar dilator (number 12) using 6.0 Prolene suture (Figure 1). We used fresh pericardium to perform this technique. The pericardial tube graft was anastomosed in an end-to-end fashion to the LSVC, effectively lengthening it. The other end of the pericardial tube graft was anastomosed in a side-to-side fashion to the divided RSVC, over a length of approximately 2 cm, creating a Y-shaped confluence of the RSVC and LSVC and enabling anastomosis of an effectively enlarged “common SVC” to PA confluence using continuous 7.0 Prolene suture line (Figure 2).
Figure 1.
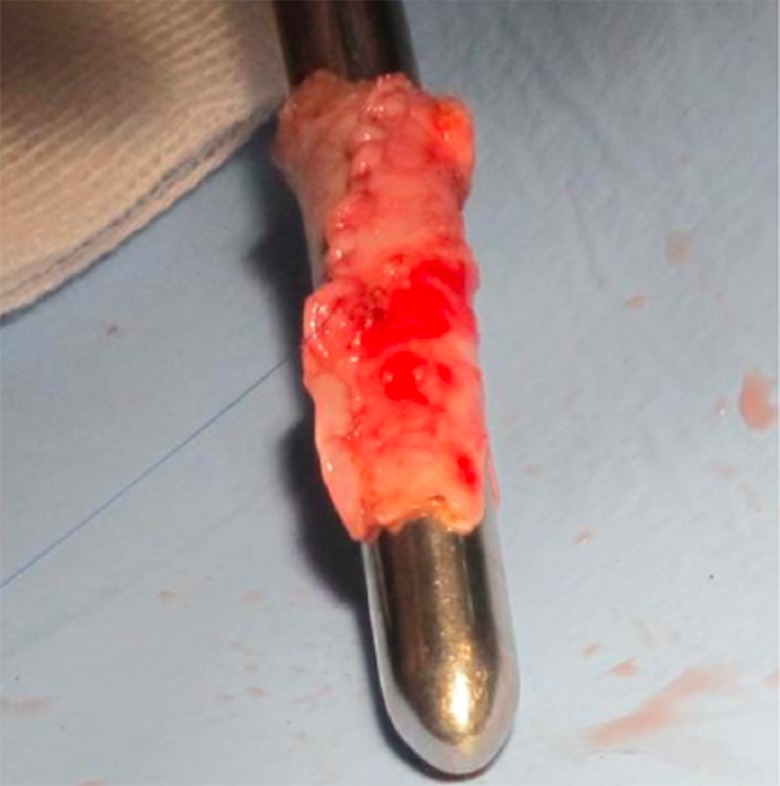
Vascular graft formation by rolled pericardium.
Figure 2.
A, Anastomosis between superior vena cava (SVC) confluence and pericardium. B, Diagram of the completed reconstruction.
The patient had stable hemodynamics during the early postoperative period, and the satisfactory status of the cavopulmonary anastomosis was confirmed by echocardiography, showing no pressure gradient through the anastomoses and good filling of both PA branches. The arterial blood pressure was 95/50, central venous pressure 15 mm Hg, SpO2 85% at fractional inspired oxygen (Fio 2) of 0.8, heart rate 118 b/m, sinus rhythm. Unfortunately, the patient had left phrenic nerve palsy resulting in inability to wean from the ventilator and we decided to perform diaphragmatic plication. Despite this intervention, the patient died due to respiratory infections after 20 days.
Case 2
A three-year-old male patient weighing 12 kg was admitted to our hospital due to cyanosis. On physical examination, the patient had cleft lip and cleft palate, cyanosis of the lips and the nail beds, and clubbed fingernails.
Echocardiography revealed transposition of the great arteries, double outlet right ventricle (DORV), complete atrioventricular canal defect, and severe stenosis of the pulmonary valve. Cardiac catheterization confirmed transposition of the great arteries, atrioventricular canal defect, valvar and subvalvar pulmonary stenosis, and dextroposition of the heart.
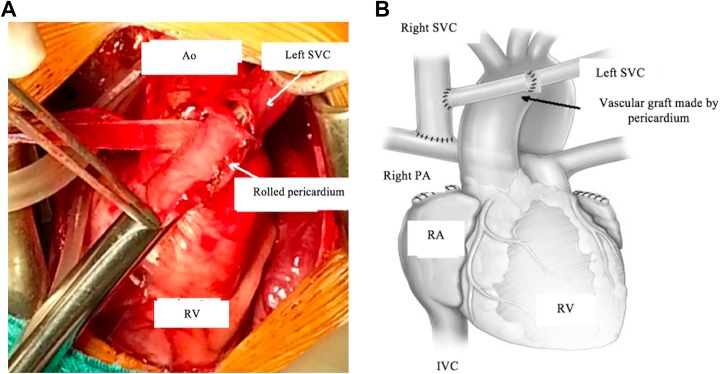
Observations at surgery included large aorta, aortic arch, equal-sized RSVC and LSVC, right-sided IVC, well-developed right and left branch pulmonary arteries, and a small PA trunk. Heparin was administered (1 mg/kg). The operation was performed without cardiopulmonary bypass support. The right SVC was divided just above its entrance to the RA, and it was anastomosed to the right PA in an end-to-side fashion, as in a conventional bidirectional Glenn operation. The LSVC was divided just above its entrance to the left atrium. A rolled pericardial tube graft was used to elongate the LSVC, making it possible to anastomose this lengthened LSVC to the RSVC in an end-to-side fashion, with the vascular graft of rolled pericardium situated in front of the aorta (Figure 3A and B).
Figure 3.
A, The rolled pericardium is used to effectively elongate the left SVC. B, Diagram of anastomoses. Ao indicates aorta; IVC, inferior vena cava; PA, pulmonary artery; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
The patient had an uneventful postoperative course. A well-functioning modified bidirectional cavopulmonary anastomosis was demonstrated in this patient by two-dimensional echocardiographic evaluation at 3, 6, and 12 months after surgery. Cardiac catheterization was performed in anticipation of Fontan completion and showed no gradient across the anastomosis. He underwent a completion Fontan procedure at 14 months after the modified bidirectional Glenn shunt.
Discussion
The presence of bilateral superior caval veins is commonly seen in different forms of congenital heart diseases, but the precise pathophysiology of this systemic venous abnormality is still unknown. Anatomical studies showed that when bilateral superior caval veins are present, the different diameters of two veins may result in imbalanced distribution of blood flow after bilateral bidirectional Glenn operation and can lead to obstruction and/or thrombus formation within the SVC or at the anastomosis to the ipsilateral PA.2,3 In addition, there is a concern that in the presence of unequal flow from the left and right SVC to the pulmonary arteries, blood flow from IVC may not symmetrically distribute to the right and left PA after Fontan procedure. The asymmetrical distribution of pulmonary blood flow can result in pulmonary arteriovenous malformations, which may increase the risk of thrombus formation and may affect the growth of the pulmonary arteries.3 In 2002, Amodeo and Di Donato studied the energetics and the distribution of pulmonary blood flow after Glenn operation and Fontan procedure using models based on magnetic resonance imaging and cardiac catheterization images from 110 patients who underwent Fontan procedure.6
In order to optimize blood flow from bilateral superior caval veins to the pulmonary arteries, many techniques for making anastomosis have been proposed in the literature. Vida et al in 2006 reported the case of a 14-month-old in whom a 5-mm Gore-Tex conduit was interposed between two SVCs in bidirectional Glenn operation. Despite the good reported results, there is a concern about the possibility of conduit occlusion as well as the fixed size of the prosthesis in relation to subsequent growth of the patient.4
Many studies have shown that disorders in pulmonary blood flow from SVCs and the distribution of blood flow from the IVC are major factors in the prognosis of the results of Glenn operation and Fontan procedure. In 2007, Amodeo and Di Donato proposed a novel method that can possibly resolve the problems in patients with bilateral SVCs. The purpose of this method is to convert two superior caval veins into a unifocalized confluence in order to increase the blood flow at the bidirectional anastomosis between SVC and pulmonary arteries. A single unifocalized connection at the level of the PA confluence is considered to be more effective than two small bidirectional peripheral anastomoses with competing blood flow, a situation which may lead to asymmetrical pulmonary blood flow.3 Technically speaking, this modification of bidirectional cavopulmonary anastomosis may be affected by the aortic arch. The large aorta can lead to stretching, compression, or even blockage of the confluence of two SVCs. This is a potential disadvantage of this technique. The advantage of this method is that the location of two SVCs can be adjusted more to the right than to the left, to allow enough compensation for the anticipated location of the extracardiac conduit placed between IVC and PA at the time of a future completion Fontan procedure.3
With the efforts to resolve the problems of unequal anatomical diameters of right and left SVC leading to reduced blood flow, which may cause blood retention, thrombus formation, and unfavorable growth of two PAs, in 2014 Nakanishi proposed a new surgical technique named “unifocalization of bilateral SVCs.”2
Nakanishi proposed the hypothesis that in unifocalization of bilateral superior caval veins, the blood flow from the joined SVCs can be distributed to two lungs in a manner similar to unilateral Glenn operation. This new technique was only applied on patients with the IVC and the larger SVC on the same side. If the smaller SVC and IVC were on the same side, the conventional bidirectional Glenn operation was performed. If the RSVC and LSVC were equal in diameters, the author chose the SVC on the same side as the IVC to be the main vessel. The disadvantage of this technique is the length of SVC may still be too short to make end-to-side cavo-caval anastomosis.2
Our first patient had the smaller SVC on the same side as the IVC with dominant PLSVC, and pulmonary atresia diagnosed preoperatively. The goal of achieving cavopulmonary anastomosis and concomitant enlargement of the PA confluence led us to choose the method of creating the single confluence of the right and left superior caval veins at the level of the cavopulmonary anastomosis with the PA confluence. However, during dissection, we found that the LSVC was not long enough to make the confluence with the RSVC. To overcome this problem, we used the rolled pericardium technique. This method not only formed a long enough vascular graft but also provided enough material to augment the PA confluence without using artificial materials.
Our second patient had equally sized LSVC and RSVC and right-sided IVC. The very large left-sided aorta covered over the LSVC. To dissect the LSVC, we had to retract the aorta to the right. We did not use cardiopulmonary bypass support, since this patient only needed cavopulmonary anastomosis. After dividing and mobilizing the LSVC, we found that it was not long enough to make the end-to-side anastomosis with right SVC. The rolled pericardium graft technique was chosen to resolve this problem.
The use of different materials with the aim to lengthen a SVC to facilitate the cavopulmonary anastomosis has been reported by many authors.4,5 Artificial conduits or rolled xenograft pericardium can be used. In our study, the application of rolled autologous pericardium to increase the length of LSVC seemed to be the most reasonable choice. It can be used to augment the PA confluence.
Conclusion
It is necessary to have more time to investigate the effectiveness of methods for cavopulmonary anastomosis in bidirectional Glenn operations for patients with bilateral superior caval veins before being widely applied to many patients. However, at least theoretically, the surgical solution to convert two SVC anastomoses into one venous confluence appears to have substantial advantages over other reported techniques. We hypothesize that such techniques may promote symmetrical distribution of pulmonary blood flow which could contribute to favorable growth of the branch pulmonary arteries and minimize the chance of thrombus formation. The lack of adequate length of SVC for direct anastomosis can be solved by using a graft made by rolled pericardium.
Acknowledgments
The authors would like to express their deepest gratitude to the Cardiovascular Center, E Hospital for supporting them in the data collection process.
Abbreviations and Acronyms
- IVC
inferior vena cava
- LSVC
left superior vena cava
- PA
pulmonary artery
- PLSVC
persistent left superior vena cava
- RA
right atrium
- RSVC
right superior vena cava
- RV
right ventricle
- SVC
superior vena cava
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tran-Thuy Nguyen, MD  http://orcid.org/0000-0001-7129-0525
http://orcid.org/0000-0001-7129-0525
References
- 1. Iyer GK, Van Arsdell GS, Dicke FP, McCrindle BW, Coles JG, Williams WG. Are bilateral superior vena cavae a risk factor for single ventricle palliation? Ann Thorac Surg. 2000;70(3):711–716. [DOI] [PubMed] [Google Scholar]
- 2. Nakanishi K, Kawasaki S, Takahashi K, Amano A. A new technique for venous unifocalization of the bilateral superior vena cava with the Glenn procedure. J Thorac Cardiovasc Surg. 2014;148(1):356–358 [DOI] [PubMed] [Google Scholar]
- 3. Amodeo A, Di Donato R. The unifocal bilateral bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2007;84(6):2134–2135. [DOI] [PubMed] [Google Scholar]
- 4. Vida VL, Leon-Wyss J, Garcia F, Castañeda AR. A Gore-Tex “new-innominate” vein: a surgical option for complicated bilateral cavopulmonary shunts. Eur J Cardiothorac Surg. 2006;29(1):112–113. [DOI] [PubMed] [Google Scholar]
- 5. Chakraborty B, Talwar S, Choudhary SK, Kothari SS, Airan B. Use of aortic homograft conduit in bidirectional Glenn shunt. Heart Lung Circ. 2007;16:52–54. [DOI] [PubMed] [Google Scholar]
- 6. Amodeo A, Grigioni M, Oppido G, et al. The beneficial vortex and best spatial arrangement in total cavopulmonary connection. J Thorac Cardiovasc Surg. 2002;124(3):471–478. [DOI] [PubMed] [Google Scholar]