Abstract
In many domains, including cognition and personality, greater variability is observed in males than in females in humans. However, little is known about how variability differences between sexes are represented in the brain. The present study tested whether there is a sex difference in variance in brain structure using a cohort of 643 males and 591 females aged between 3 and 21 years. The broad age-range of the sample allowed us to test if variance differences in the brain differ across age. We observed significantly greater male than female variance for several key brain structures, including cerebral white matter and cortex, hippocampus, pallidum, putamen, and cerebellar cortex volumes. The differences were observed at both upper and lower extremities of the distributions and appeared stable across development. These findings move beyond mean levels by showing that sex differences were pronounced for variability, thereby providing a novel perspective on sex differences in the developing brain.
Keywords: brain structure, development, sex differences, variability, X-chromosome
Introduction
Many prior studies have reported sex differences in brain structure, but the regional patterns observed are not consistent across studies. In addition, it is unclear how regional sex differences relate to global brain size differences or how this pattern may change with development (Sacher et al. 2012; Koolschijn and Crone 2013; Ruigrok et al. 2014; Marwha et al. 2017). Therefore, sex differences in brain structure are currently not well understood. One possible shortcoming of most previous studies is that the focus has been on mean group differences, whereas much less is known about variance differences between males and females. It has, however, repeatedly been observed that variability differs between sexes across a variety of other domains, including cognitive abilities and personality traits, and also physical properties including body weight and height, even in the absence of mean differences, with males consistently showing greater variability than females (Arden and Plomin 2006; Johnson et al. 2008; Lehre et al. 2009; Borkenau et al. 2013; Hyde 2014; Baye and Monseur 2016). That is to say, males may in these cases be over-represented at both ends of the distribution. A pertinent question concerns whether this is also the case for the human brain, but this has not yet been empirically addressed.
Prior studies have provided important, but inconclusive evidence for sex differences in brain structure. For example, a meta-analysis showed that males have an average of 8–13% larger volume for a range of brain measures (e.g., total brain volume and white matter volume) than females (Ruigrok et al. 2014). However, the reported size and directionality of regional sex differences in brain volumes are inconsistent across studies, likely partly explained by how the difference in overall brain size is accounted for (Giedd et al. 2015; Mills et al. 2016). Also, it should be noted that even raw volume sex differences are relatively small compared with the interindividual variability in brain morphology, for example, ~30% for total brain volume (Allen et al. 2002). Based on this and the observation that the magnitude of sex differences in mean volumes differs substantially between regions of the brain, it has been highly debated to what degree the male and female brain can be distinguished (Del Giudice et al. 2016; Glezerman 2016; Rosenblatt 2016) or whether they are more alike than different (Joel et al. 2015).
These conclusions appear to stand in sharp contrast with epidemiological studies that show large sex differences in the prevalence of many neurodevelopmental disorders, for example, Tourette syndrome (90% males), autism spectrum disorder (80% males), attention deficit hyperactivity disorder (ADHD) (80% males), schizophrenia (73% males), depression (63% females), anxiety disorder (67% females), and anorexia nervosa (93% females) (Bao and Swaab 2010). In general, these male-biased disorders are characterized by an early onset, while the female-biased disorders more often show age of onset in adolescence and show lower heritability estimates than the male-biased disorders (Costello et al. 2003). Moreover, there is evidence that females are protected against some mutations that are related to male-biased disorders. For example, females need a larger number and more severe mutations to show clinical symptoms of autism spectrum disorder (Jacquemont et al. 2014) or ADHD (Taylor et al. 2016). This is also in line with the observation that not only the prevalence, but also symptoms and the course of several mental disorders are more severe in males. Males with schizophrenia, for example, have been found to show poorer premorbid functioning, earlier onset, and more cognitive deficits, in addition to more severe structural brain abnormalities than females with schizophrenia (Goldstein et al. 2002). The mechanisms involved in these sex differences in vulnerability and protective effects remain unknown, but we suggest that assessing brain morphology beyond mean differences is an essential step in a better understanding of underlying mechanisms related to sex differences in the brain.
Several prior studies have speculated about possible genetic underpinnings for the emergence of sex differences in variability. It has for instance been suggested that the lack of 2 parental X-chromosomal copies in males may directly relate to greater variability and vulnerability in males compared with females (see for review (Arnold 2012)). If this is true one could theoretically expect that any trait related to X-linked genes would express greater diversity in females than males. While 100% of cells in males would express a X-linked trait, female brain tissue would show 2 variants of the trait. We question whether this can be observed in the brain, by comparing inter-regional anatomical correlations in males and females.
Only a limited number of studies have focused on sex-related variability in brain structure in humans of which samples were small (Lange et al. 1997) or included adults (Ritchie et al. 2017). A large population-based study including developing individuals is currently lacking. Hence, the present study compared sex differences in variability in regional brain volumes estimated by magnetic resonance imaging (MRI) scans from 1234 individuals aged 3–21 years (52% males) recruited from the general population at 9 different sites across the United States of America. This large population-based sample provided us with sufficient power to test variance differences in the brain, and also provided a possibility to test for emergence of variance difference over development. Even though prior studies suggested a potential genetic determinacy, it remains an open question whether variance differences would already be observed in early childhood or would emerge when children develop into teenagers and adults. Another important question concerns the nature of these variability differences, for example, whether variability differences would be observed at both extremities of the distribution. To assess sex differences in tail distributions in brain volumes we used quantile distance functions, a nonparametric method that estimates the distance between male and female distributions (Lehre et al. 2013). Last, we compared inter-regional correlation between males and females and hypothesize stronger correlations in males.
We examined global brain volumes in addition to regions of interest (ROIs) that have been indicated to show mean or developmental differences between sexes (Lenroot et al. 2007; Ruigrok et al. 2014) and/or have been associated with male-biased developmental disorders (Giedd et al. 2015). These ROIs included the volumes of cerebral cortex and white matter, cerebellar cortex and white matter, accumbens, caudate, pallidum, putamen, amygdala, hippocampus, and thalamus. In a follow-up analysis, cortical surface area and thickness were examined separately, as these components of cortical volume are influenced by different genes and develop differently (Wierenga et al. 2014; Vijayakumar et al. 2016; Tamnes et al. 2017).
Materials and Methods
Participants
Data used in the preparation of this article were obtained from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study database (http://ping.chd.ucsd.edu/). PING was launched in 2009 by the National Institute on Drug Abuse (NIDA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The primary goal of PING has been to create a data resource of highly standardized and carefully curated MRI data, comprehensive genotyping data, and developmental and neuropsychological assessments for a large cohort of developing children. The scientific aim of the project is, by openly sharing these data, to amplify the power and productivity of investigations of healthy and disordered development in children, and to increase understanding of the origins of variability in neurobehavioral phenotypes.
Initially over 1700 participants were enrolled in the PING study, collected at 1 of 9 sites and 13 different scanners in the United States of America (for details see (Jernigan et al. 2016)). Each data collection site’s Office of Protection of Research Subjects and Institutional Review Board approved the study. Written parental informed consent was obtained for all participants, in addition child assent was obtained for all participants older than 7 years. Participants had no diagnosis of neurological disorders; history of head trauma; preterm birth (less than 36 weeks); diagnosis of autism spectrum disorder, bipolar disorder, schizophrenia, mental retardation, or contraindications for MRI (such as dental braces, metallic, or electronic implants, or claustrophobia). For a detailed description of the data collection, we refer the reader to (Jernigan et al. 2016).
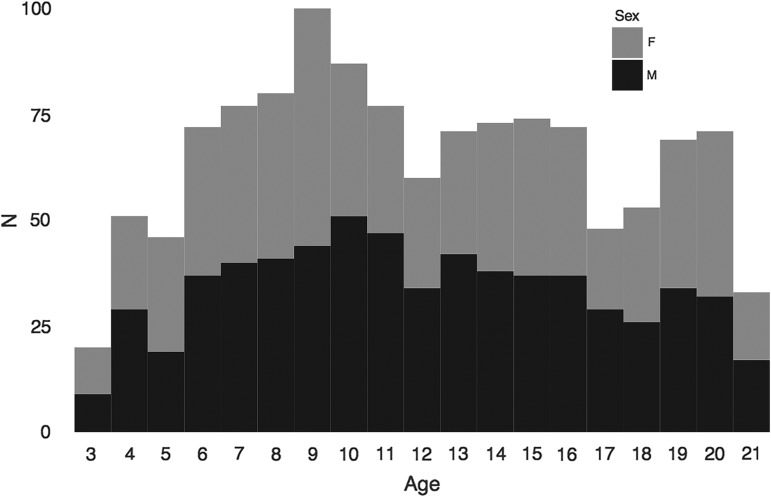
The sample for the current study was limited to 1234 participants aged between 3 and 21 years (52% males) with complete acceptable data on imaging measures (see Table 1 for demographics). There was no significant difference in variability of age between males and females (P = 0.9491). The imaging acquisition protocol, structural preprocessing, quality control, and analysis protocols were developed specifically to meet the challenges associated with multisite imaging and imaging of children. Similar proportions of males and females participated across the entire age-range (Fig. 1).
Table 1.
Demographic variables PING data set
| Site | Sex | N | Mean age | (SD) | Age-range | |
|---|---|---|---|---|---|---|
| a | F | 57 | 15.394 | (4.354) | 4.080–21.000 | |
| M | 57 | 14.963 | (4.028) | 7.420–20.920 | n.s. | |
| b | F | 11 | 13.682 | (2.913) | 7.670–17.670 | |
| M | 17 | 10.612 | (3.603) | 5.580–15.920 | * | |
| c | F | 48 | 14.632 | (4.631) | 3.750–20.920 | |
| M | 62 | 14.712 | (3.735) | 5.420–21.000 | n.s. | |
| d | F | 59 | 12.453 | (5.426) | 3.170–20.750 | |
| M | 59 | 12.185 | (4.956) | 3.420–21.000 | n.s. | |
| e | F | 19 | 12.744 | (3.629) | 6.580–17.580 | |
| M | 25 | 12.567 | (3.131) | 6.330–16.830 | n.s. | |
| f | F | 57 | 13.296 | (6.149) | 3.670–20.920 | |
| M | 69 | 10.760 | (5.969) | 3.170–20.750 | * | |
| g | F | 115 | 10.360 | (4.984) | 3.080–20.500 | |
| M | 127 | 10.793 | (4.943) | 3.000–20.670 | n.s. | |
| h | F | 30 | 13.944 | (3.809) | 6.750–20.920 | |
| M | 35 | 13.155 | (4.875) | 4.170–20.000 | n.s. | |
| i | F | 68 | 8.459 | (3.401) | 3.250–17.830 | |
| M | 67 | 8.922 | (3.658) | 3.580–20.250 | n.s. | |
| j | F | 55 | 10.068 | (3.705) | 3.420–19.830 | |
| M | 65 | 11.224 | (3.927) | 3.420–21.000 | n.s. | |
| k | F | 9 | 7.730 | (1.49) | 6.080–11.000 | |
| M | 4 | 10.373 | (0.494) | 9.830–10.830 | ** | |
| l | F | 8 | 14.679 | (3.132) | 8.920–18.500 | |
| M | 6 | 14.360 | (4.832) | 8.830–20.750 | n.s. | |
| m | F | 55 | 13.549 | (4.271) | 6.080–20.670 | |
| M | 50 | 14.698 | (3.533) | 6.670–20.580 | n.s. | |
| Total | F | 591 | 12.074 | (5.046) | 3.080–21.000 | |
| M | 643 | 12.040 | (4.826) | 3.000–21.000 | n.s. |
F, females; M, males; SD, standard deviation; *P < 0.05; **P < 0.01; n.s., not significant.
Figure 1.
Equal distributions of males and females across the age-range. The x-axis shows age from 3–21 years old for males (black) and females (grey) in a stacked histogram. In total, 643 males and 591 females between 3 and 21 years old were included in the sample.
Imaging Data Acquisition and Processing
For complete details of the image acquisition and processing methods used in the creation of the PING data set, see Jernigan et al. (2016). In brief, at 9 sites and 13 3T scanners, a standardized multiple-modality MRI protocol was implemented. The protocol included a high-resolution sagittal 3D inversion recovery spoiled echo T1- weighted volume optimized for maximum gray/white matter contrast (flip angle = 8°; receiver bandwidth = ±31.25 kHz, freq = 256, phase = 192, slice thickness = 1.2 mm, FoV = 24 cm; TE = 3.5 ms; TR = 8.1 ms; TI = 640 ms). These volumes were acquired using prospective motion correction (PROMO), as described in White et al. (2010). This procedure has been showed to effectively reduce effects of subject motion (Brown et al. 2010; Kuperman et al. 2011). Descriptions of the specific scanner models used at each site can be found in Fjell et al. (2012).
Tissue classification and anatomical labeling was performed on the basis of the T1- weighted MR image using the well-validated and well-documented Freesurfer v5.3.0 software (http://surfer.nmr.mgh.harvard.edu/). This software encompasses tools for cortical surface reconstruction, subcortical segmentation, cortical parcellation, and estimation of various measures of brain morphometry. Technical details of the automated reconstruction scheme are described elsewhere (Dale et al. 1999; Fischl et al. 1999, 2002). In addition to the standard processing pipeline, extensions made at the UCSD MultiModal Imaging Laboratory (MMIL) were implemented. These include maps of relative cortical surface area changes and genetically informed cortical parcellations (Jernigan et al. 2016). For the present study, volumes of the cerebral cortex and white matter, cerebellar cortex and white matter, accumbens, caudate, pallidum, putamen, amygdala, hippocampus, and thalamus were included as ROIs. All ROIs were averaged across hemisphere within subject. For follow-up analyses, cerebral cortex total surface area and average thickness were assessed.
Quality Control
An important part of the data processing is the quality control procedure, which is critical for imaging data in developmental samples (Mills and Tamnes 2014). Details of this procedure can be found in Jernigan et al. (2016). In short, raw images of each scan session were automatically checked for completeness and protocol compliance. Next, T1-weighted images were examined for evidence of excessive motion and rated as either acceptable and processed in Freesurfer or recommended for rescan. Processed scans were also examined by checking subcortical volumetric segmentations, cortical areal parcellations, and white and pial surface reconstructions. The proportion of individuals that failed to pass quality control are described in detail by Brown et al. (2012) for a subset of the sample used in the current study. The final sample of the current study included 1234 scans.
Statistical Analysis
Variance Ratio
Differences in variance between males and females were examined by first controlling for age and scan site. This was done by using a random forest regression model (Breiman 2001), which is implemented in R-package randomForest, and can accommodate models with interactions and nonlinear effects. Letting denote the observed outcome for observation number i and its predicted outcome, the residuals were then formed:
The standard deviations and were computed separately for males and females, and used to form the test statistic
For each outcome, a permutation test of the hypothesis that the sex-specific standard deviations were equal was performed. This was done by random permutation of the sex variable among the residuals. Using B permutations, the P-value for the k-th outcome (ROI) was computed as
where is an indicator function that is 1 when , and 0 otherwise. Thus, the P-value is the proportion of permuted test statistics () that were greater than the observed value T of the test statistic above. Here, B was set to 10 000.
A combined test of difference in variance across the different outcomes was performed, using the test statistic
with the permutation distribution of T constructed as described previously (Pesarin and Salmaso 2010).
Quantile Distance Function
In order to assess the nature of the variability difference between males and females quantile distance functions were estimated for each ROI using quantile regression forests (Meinshausen 2006), implemented in the quantreg Forest R library (see Lehre et al. 2013). As a first step quantile distribution functions are estimated for males and females separately. Let q be a probability between 0 and 1. The quantile function specifies the values at which the volume of a ROI will be at or below any given q. The quantile function for males is given as and for girls as . The quantile distance function is then defined as:
For illustration purposes, we show these quantile distance functions as shift functions for each 10th quantile, that is, decile, where mean differences between males and females were removed (Rousselet et al. 2017). This function describes how the distribution of females should be re-arranged to match the distribution of males. If the shift function is a straight line parallel to the x-axis, this would indicate a stable difference between the sexes across the distribution and thus no difference in variability. A positive slope on the other hand would indicate greater male variance. More specifically this would show that the largest males are relatively larger than the largest females, and the smallest males are relatively smaller than the smallest females. A negative slope of the shift function would indicate larger variability in females at both ends of the distribution.
Variance Change with Age
To study the age effects on variability we used the residuals of the predicted outcome of the random forest model described earlier:
The absolute value of these was then used as the response in a linear regression model with an age by sex interaction.
Anatomical Correlation Analysis
Anatomical correlation analysis assesses the inter-regional anatomical associations by defining the statistical similarity between 2 ROIs. The Pearson correlation coefficient between any 2 regions i and j was assessed for males and females separately. This produces 2 group correlation matrices and where , and is the number of brain regions, here N = 11.
Sex-specific means and standard deviations were removed, by performing sex-specific standardization. The significance of the differences between and was assessed by the difference in their Fisher’s z-transformed values, and P-values were computed using permutations as above.
Results
Sex Differences in Mean and Variability of Brain Volumes
As a background analysis, we first assessed whether the investigated brain regions showed mean volume differences between males and females, by using 10 000 random permutations and accounting for scan site and age using random forest analysis. All ROIs showed significantly larger volume in males than females (P < 0.001), effects sizes (Cohen’s d) range from 0.033 (caudate volume) to 0.083 (cerebral white matter volume). The effects also remained significant after accounting for intracranial volume, with exception of the caudate (P = 0.539).
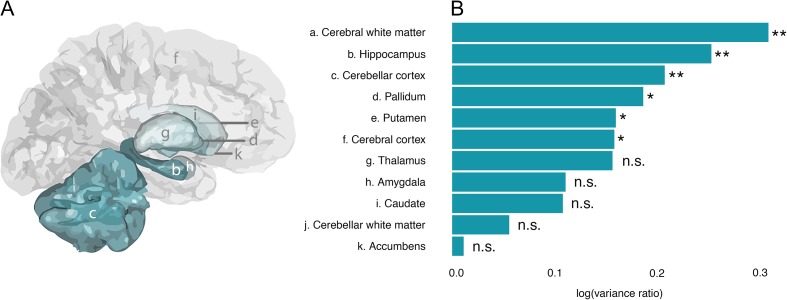
Our first research question was whether there are sex differences in variance of brain structure. In order to test whether variability in brain volumes differed between males and females we estimated the log transformed variance ratios. A positive variance ratio is indicative of greater variability in males than females. We accounted for mean sex difference, scan site and age using random forest analysis. First, a combined test of sex difference in variance across all the included volumetric outcomes was performed using permutation testing (10 000 permutations). This analysis confirmed a general greater variance in boys than girls as indicated by a significant combined P-value (P = 0.0034). The next step was to follow-up on this effect with post hoc analyses for each brain region separately, following the same method described above. The results showed that all ROIs had greater variance in males than females as indicated by positive log transformed variance ratios (Fig. 2). The ROIs for which boys show significantly greater variance in volume than girls were cerebral white matter (P < 0.0001), hippocampus (P = 0.0017), pallidum (P = 0.0202), cerebellar cortex (P = 0.0011), putamen (P = 0.0335), and cerebral cortex (P = 0.0414). Follow-up analyses revealed that the variance difference in cerebral cortex volume was reflected in cortical surface area (P = 0.0035) but not thickness (P = 0.9688). Together, these results indicate that there is greater male variability in brain structure, beyond mean differences.
Figure 2.
Greater brain volume variance in males than females. (A) Gray matter ROI are indicated in a, note that cerebral white matter (a) and cerebellar white matter (j) are not displayed. (B) Log transformed variance ratio (x-axis) for all investigated brain regions averaged across hemispheres (y-axis). Variance ratio is estimated as the difference in variance between males and females after adjusting for mean sex difference, scan site, and age. Positive values are indicative of larger variance in males than females, vice versa for negative values. All investigated brain regions showed positive values, that is, larger variance in males. Permutation test (10 000 permutations) showed significant effects for volumes of cerebral white matter, hippocampus, cerebellar cortex, pallidum, putamen, and cerebral cortex. **P < 0.05; *P < 0.01; n.s., non significant.
Greater Variance in Boys at Both Extremities
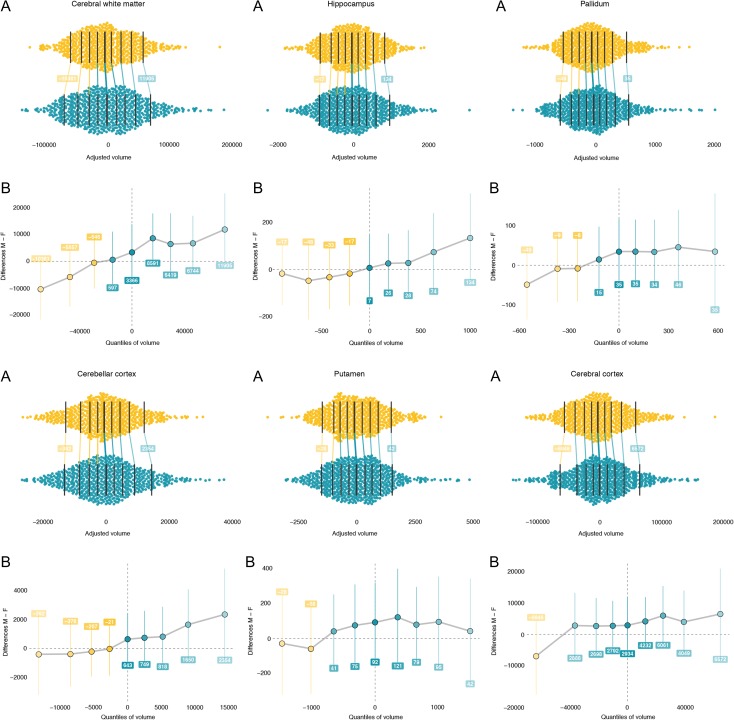
To further explore the nature of the significant variability differences between the sexes, we examined whether they were expressed at both upper and lower extremities of the volume distributions for cerebral white matter, hippocampus, pallidum, cerebellar cortex, putamen, and cerebral cortex. To assess this effect, cumulative distribution functions were estimated using the quantile function. This function estimates the value at which a given volume will be at or below a given probability between 0 and 1. We estimated quantile functions separately for boys and girls. Next, the quantile distance function was assessed as the difference between these functions (Fig. 3). The functions were adjusted for scan site and age using the quantile regression forest model.
Figure 3.
Greater brain volume variability for males than females in both upper and lower extremities of distributions for volumes adjusted for age, scan site, and mean sex difference for cerebral white matter, hippocampus, pallidum, cerebellar cortex, putamen, and cerebral cortex. (A) Panels shows spread difference in volume (in mm3, x-axis) distributions of females (yellow) and males (green) are illustrated using jittered scatterplots. The vertical lines mark the deciles for each sex with the median indicated by the thicker middle line. Because of the difference in spread the first decile of each volume is lower for males than females (indicated with a yellow line) while the nineth decile is larger for males than females (indicated with a green line). The values of the differences (adjusted volumes) for deciles 1 and 9 are indicated in the superimposed labels. Panels B focus on the proportion of the A panels marked by the gray shaded box. Shift functions (95% bootstrap CI) x-axis values match male volumes for each decile in panel A. The y-axis shows the difference compared with females for each decile. The superimposed labels indicate how much each decile should be shifted for females to match males. Dotted lines indicate difference between the medians of males and females (distance at quantile 0.5). All curves show greater male variability at both extremities as indicated by the positive values on the right and negative values on the left.
Boys on average had larger volumes than girls for all ROIs that showed significant variance differences between males and females, which can be observed as positive values at quantile 0.5 (median) in each distance function in Figure 3. Furthermore, the upward deflections in these functions indicate larger variability in boys at both upper and lower extreme ends of the distributions. The right part of any given quantile distance function show that boys with large volumes have relatively even larger volume than girls with large volumes (compared with the median volume difference, dotted line). While the left part of the plot shows that boys with small volumes have relatively smaller volume compared to girls with small volumes, that is, at the distribution ends boys have relatively larger and smaller volumes, respectively.
Variance Difference Between Sexes is Stable Across Development
Next, we explored whether the differences in variance of brain volumes between boys and girls differed across the age-range 3–21 years. To do so, we used a linear regression model to test for age by sex interaction effects on the residual ROI volume values after accounting for mean sex difference, age, and scan site. None of the ROIs showed significant interaction effects between age and sex on variance. This suggests that the variability difference between males and females is stable across age from childhood to young adulthood.
Sex Differences in Anatomical Correlations
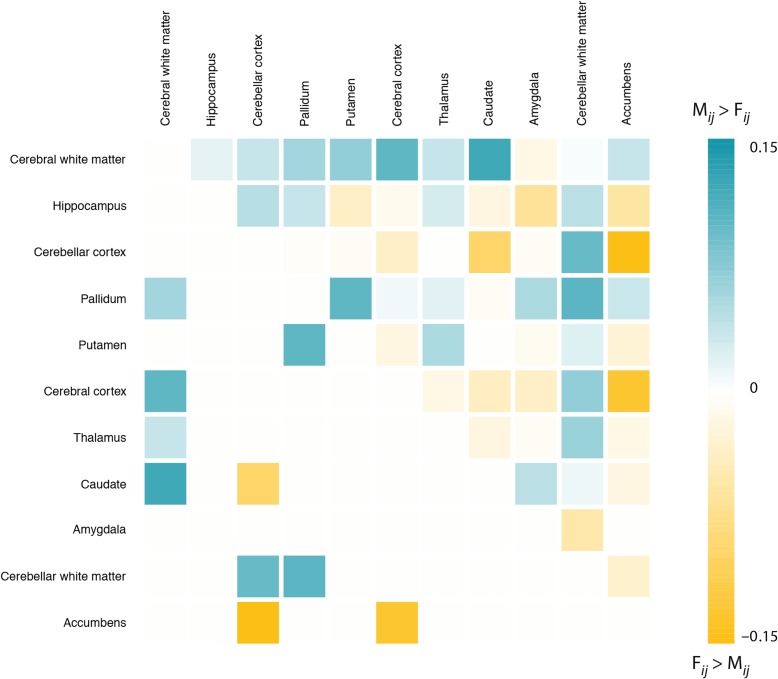
Finally, we investigated whether females showed greater diversity than males in regional volumes across the brain. To do so anatomical inter-regional anatomical associations were compared between males and females. First, anatomical correlation matrices were estimated as previously applied in several structural MRI studies for males and females separately (see e.g. Baaré et al. 2001; Lerch et al. 2006). Pearson correlations were calculated for each ROI combination. Next, the anatomical correlation matrix for females was subtracted from the anatomical correlation matrix for males, yielding a difference matrix (Fig. 4). Stronger anatomical correlations for males than females are indicated in blue (indicative of larger homogeneity across regions in males and greater diversity in females), while stronger correlations for females are displayed in yellow (indicative of larger homogeneity in females and greater diversity in males). There were 45 unique correlations coefficients of which 55% showed stronger correlations in males than females. This percentage was larger for anatomical correlations between ROIs that showed significantly greater male volume variance (cerebral white matter, hippocampus, cerebellar cortex, pallidum, putamen, and cerebral cortex): 60% of these ROI pairs showed stronger anatomical correlation in males than females.
Figure 4.
Overall stronger anatomical correlation between brain regions in males than females. Anatomical correlation sex difference matrix between all pairs of brain regions (i and j). Blue colors indicate stronger covariance in males (), and yellow colors indicate stronger covariance in females (). Deeper colors indicate stronger differences in covariance between sexes (up to r = ±0.15). Post hoc analysis revealed that 10 brain region pairs showed significant differences between the sexes (10 000 permutations). These are displayed on the left lower side of the matrix.
We further explored significant differences for each ROI pair using 10 000 permutations, where sex was permuted. Ten pairs showed significant difference in anatomical correlation between males and females (shown on the left lower side of the difference matrix in Fig. 4), of which 7 were male biased. These results indicate that there is, in line with our hypothesis, somewhat larger homogeneity across regions in brain volumes in the male brain and greater diversity in the female brain.
Discussion
The present study shows that looking beyond mean effects in the brain provides new insights into differences between males and females. We observed greater brain structure variability in males compared to females in a large sample of children and adolescents. This observation is in line with findings in adults (Ritchie et al. 2017) and supports the greater male variability hypothesis. More specifically, 4 key findings in this study support this hypothesis. First, a combined significant effect indicated larger variance in males than females across investigated brain volumes, and region-specific analyses showed the same effect for multiple brain structures. Second, the quantile distance models indicated that males show relatively more extreme values at both the upper and lower ends of the distributions. Third, we observed that the greater male variance does not show a significant interaction effect with age. This suggests that the sex differences in brain structure variability are stable across the age-range 3–21 years. Last, stronger structural anatomical correlations were observed for males compared to females, especially between brain regions that showed significant difference in variance between the sexes, indicating larger homogeneity of brain volumes in males than females. The methods and results provide new avenues for linking sex differences in the human brain with behavior, mental disorders, and genetic influences.
This study showed global, as well as regional-specific findings for greater male variance in brain structure. Among the brain regions investigated, the largest difference in variance between sexes was observed for cerebral white matter volume. The region with the second largest sex variance effects was the hippocampus, followed by the cerebellar cortex, pallidum, and putamen. Interestingly, several of these structures have been linked to male-biased disorders, including schizophrenia; for example, the cerebellar vermis showed more severe abnormalities in males than females (Frazier et al. 2007; Womer et al. 2016). Hence, this study may provide new strategies to identify brain regions involved in these disorders. Furthermore, cerebral cortex volume also showed a male biased variability effect. Follow-up analysis indicated that this difference was reflected in cortical surface area but not thickness; separate determinants of cortical volume that have previously been shown to have distinct sources of genetic influences (Panizzon et al. 2009; Kremen et al. 2013). The current study concurs with the previous findings of moderate to high and distinct genetic contributions to individual differences in these brain structures (see review [Gu and Kanai 2014]), although it cannot be ruled out that early socialization effects may have contributed to the greater male variability observed. Future studies could further investigate this by investigating a larger and younger sample.
The results from this study may have important implications for understanding mental disorders, several of which are more prevalent in males than in females. Specifically, the finding that males are over-represented in both upper and lower extremities of volume distributions complements studies that investigate traits as quantitative distributions rather than dichotomous variables. For example, a growing number of studies investigate not only negative but also positive tails of normally distributed traits, such as extreme low and extreme high hyperactivity (Greven et al. 2016). Additionally, genetic epidemiology studies indicated that ADHD reflects the extreme ends of one or more traits that are continuously distributed throughout the population (Chen, Zhou, et al. 2008). Despite the high heritability rates observed for many developmental disorders, linking single genes to qualitative measures of psychiatric disorders has shown to be extremely challenging (Uher 2009). Rather, the inheritance of multiple genes (polygenic liabilities) has been hypothesized to quantitatively contribute to the expression of traits associated with these disorders (Plomin et al. 2009). Herewith, both low and high polygenic liabilities may be associated with adaptive or desired traits, however, the mid-range may represent a favorable trade-off between advantages and disadvantages (Nettle 2006). For example, the opposite end of the hyperactivity trait, that is, extreme low hyperactivity, may be associated with behavioral rigidity.
In the same way both very large and very small brain volumes may be associated with extreme ends of quantitative traits. And if males at both ends of the distribution show relatively more extreme brain volumes, they may be at higher risk. Perhaps, it is this higher representation of males in both extremities, rather than mean differences in brain structures, that may be associated with higher prevalence of several developmental disorders in males than females. A better understanding of variability and extreme ends of distributions in brain structures may help to better understand risk factors and identify neurobiological phenotypes associated with developmental disorders.
An important step in unraveling the nature of greater male variability has been to identify whether differences in variability are already present at birth (Arden and Plomin 2006). The present paper observed that the variance difference between sexes in brain volumes is stable across the range of 3–21 years of age, even after controlling for age-related mean changes in brain structure. This suggests that variability differences in brain structure are already present early in development. The early presence of a sex difference in variability supports a genetic contribution to larger male variability rather than social cultural effects (Hyde 2014). This is in contrast with a variety of observed mean differences between males and females; for example, sex differences in math performance have been shown to be highly associated with cultural variation in opportunity structures for girls (Else-Quest et al. 2010), although this has recently been debated (Stoet and Geary 2015). In sum, our findings are consistent with the notion that genetic mechanisms moderate greater male variability. However, it cannot be ruled out that alternative explanations are at play, as our sample for example did not include newborns and infants, and our study may have been underpowered to study age effects on variability differences between sexes.
The finding of larger homogeneity in regional volumes across the male brain, indicated by stronger anatomical correlations in males than females, indirectly supports a specific role for the X-chromosome in greater male variability. Foremost, the pattern of brain correlations observed is in line with the mosaic pattern of X-inactivation in females, in comparison to the single X-chromosome in males. This finding is supported by twin studies that observed that dizygotic male twin pairs (inheriting a single maternal X-chromosomes at random) showed a much lower within twin correlation than female dizygotic twin pairs (sharing at least 1 paternal X-chromosome) for a number of global brain volumes (Baaré et al. 2001). Another line of evidence supporting the notion that the number of X-chromosomes is related to sex differences in variability comes from studies that show that in species where females are the heterogametic sex (e.g. birds and butterflies), females have significant higher variability in for example body size than males (Reinhold and Engqvist 2013). Further evidence that supports that the number of X-chromosomes relate to sex differences comes from disorders that are observed to have increased lethality levels in males, for example, Rett syndrome, Aicardi syndrome, and neural tube deficits (Ryan et al. 1997; Chen, Watkins, et al. 2008). These studies indicate a direct link between vulnerability and the presence of a single X-chromosome, independent of Y-chromosomal or gonadal effects, as these X-linked disorders were also present in the absence of Y-chromosomes. It is thought that in females the effect of a (lethal) mutation on 1 of the X-chromosomes could be compensated by the second parental copy. In females, both copies of X-chromosomes have been shown to be expressed in the brain, through a process called X-chromosome silencing. This process is indicated to occur at random, such that neighboring nerve cells may have different expressions of the two X-chromosomes (Wu et al. 2014). Hence, mutations in X-linked genes would be expressed in 50% of the cells in females, while expression rates would be 100% in males. Interestingly, skewed X-inactivation patterns have been observed in some of the syndromes mentioned above. For example, in females with Rett syndrome the X-chromosome that contains the affected gene is inactivated in 80% of the cells (Plenge et al. 2002; Amos-Landgraf et al. 2006). In a similar fashion, (nonlethal) X-linked traits may result in increased representation at the extreme ends of a distribution and herewith increased variability, as males are fully dependent on their maternal X-chromosome copy, and all X-linked genes will be fully expressed. Although not yet tested in the human brain, this line of research suggests that there may be a genetic influence for why there could potentially be more variance in the brain of males compared to females.
There are several other lines of evidence that support the notion that X-chromosome linked genes play a substantial role in the brain and herewith may directly influence sex variability differences in cognition and behavior, independent of e.g. social factors or sex steroids. For example, a disproportionately large number of genes related to “mental retardation” have been linked to protein-coding genes on the X-chromosome (Zechner et al. 2001). In addition, X-linked genes show high expression rates in brain tissue compared to rates in somatic tissue (Graves et al. 2002; Nguyen and Disteche 2005). These findings suggest that X-linked genes play a disproportionate large role in shaping the human brain. We speculate that brain regions observed to show male biased variability might have particularly high expression rates of X-linked genes in the developing human brain in vivo.
It should be noted that this study is inherently limited by several factors. First, our study design may have been limited in detecting developmental effects as we used a cross-sectional data set of participants 3–21 years old. Hence, future large studies including longitudinal data are warranted to further look into when variability differences between the sexes occur and how these develop. Second, head motion during MRI acquisition can affect morphometry estimates (Reuter et al. 2015; Ducharme et al. 2016). In addition, individual differences in psychological traits have been linked to head motion (Kong et al. 2014), and some of these traits, such as impulsivity, are more prevalent in boys than girls. This stresses the importance of the quality control procedures applied in this study in addition to the use of prospective motion correction procedures during acquisition. We acknowledge that our data may nevertheless be influenced by motion effects. However, the quantile distance functions show that at both ends of the distributions males showed more extreme values. As head motion typically results in underestimations of tissue volumes (Reuter et al. 2015), we believe it is unlikely that motion effects could account for our findings. Third, we did not directly address the influence of genetic factors in sex variability differences. Future studies including twin models could address the hypothesized genetic mechanisms involved.
This study provides new avenues for studies of sex differences in the human brain. Our results show greater variance in brain volumes in males compared to females. This observation is in line with previous findings for behavioral traits, such as greater male variability in cognition and general intelligence (Arden and Plomin 2006; Baye and Monseur 2016). Furthermore, sex differences in brain volume variability were observed to be stable across development, and males also showed greater homogeneity across regional brain volumes than females. Our findings indirectly support the hypothesis that the X-chromosome might play an essential role in shaping brain structure and sexual dimorphisms in the brain. We encourage future studies to investigate sex differences in brain variability, including studies of young children and clinical groups, as well as studies of brain microstructure and functional activation patterns. Our study provides a novel perspective that looks beyond mean sex differences in order to better understand brain—and eventually behavioral—differences between males and females and how these emerge and develop.
Notes
We would like to thank Prof. Dr. E.A. Crone and M.G.N. Bos PhD, for their consistent support, valuable comments and efforts in facilitating this study. Conflict of Interest: None declared.
Funding
European Research Council (ERC CoG PROSOCIAL 681632 to E.A. C.) and Research Council of Norway and the University of Oslo (FRIMEDBIO 230345 to C.K.T.) and the KNAW ter Meulen grant (to L.M.W.). Data collection and sharing for this project was funded by the Pediatric Imaging, Neurocognition, and Genetics Study (PING) (National Institutes of Health Grant RC2DA029475). PING is funded by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. PING data are disseminated by the PING Coordinating Center at the Center for Human Development, University of California, San Diego.
References
- Allen JS, Damasio H, Grabowski TJ. 2002. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol. 118:341–358. [DOI] [PubMed] [Google Scholar]
- Amos-Landgraf JM, Cottle A, Plenge RM, Friez M, Schwartz CE, Longshore J, Willard HF. 2006. X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet. 79:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden R, Plomin R. 2006. Sex differences in variance of intelligence across childhood. Pers Individ Dif. 41:39–48. [Google Scholar]
- Arnold AP. 2012. The end of gonad-centric sex determination in mammals. Trends Genet. 28:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaré W, Pol H, Boomsma DI, Posthuma D. 2001. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 111:816–824. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. 2010. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 16:550–565. [DOI] [PubMed] [Google Scholar]
- Baye A, Monseur C. 2016. Gender differences in variability and extreme scores in an international context. Large-scale Assess Educ. 4:541. [Google Scholar]
- Borkenau P, McCrae RR, Terracciano A. 2013. Do men vary more than women in personality? A study in 51 cultures. J Res Pers. 47:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. 2001. Random forests. Mach Learn. 45:5–32. [Google Scholar]
- Brown TT, Kuperman JM, Erhart M, White NS, Roddey JC, Shankaranarayanan A, Han ET, Rettmann D, Dale AM. 2010. Prospective motion correction of high-resolution magnetic resonance imaging data in children. NeuroImage. 53:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, et al. 2012. Neuroanatomical assessment of biological maturity. Curr Biol, 22(18):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, Fleischman K, Knight J, Andreou P, Arnold R, et al. 2008. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet. 147B:1450–1460. [DOI] [PubMed] [Google Scholar]
- Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP. 2008. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 68:265–273. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. 2003. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 60:837. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Lippa RA, Puts DA, Bailey DH, Bailey JM, Schmitt DP. 2016. Joel et al.’s method systematically fails to detect large, consistent sex differences. Proc Natl Acad Sci USA. 113(14):E1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen T-V, Hudziak JJ, Mateos-Pérez JM, Labbe A, Evans AC, Karama S, Brain Development Cooperative Group . 2016. Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures. NeuroImage. 125:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Linn MC. 2010. Cross-national patterns of gender differences in mathematics: a meta-analysis. Psychol Bull. 136(1):103–127. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. NeuroImage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Venkatraman V, Roddey JC, Erhart M, McCabe C, et al. Pediatric Imaging, Neurocognition and Genetics Study . 2012. Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci USA. 109:19620–19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Hodge SM, Breeze JL, Giuliano AJ, Terry JE, Moore CM, Kennedy DN, Lopez-Larson MP, Caviness VS, Seidman LJ, et al. 2007. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and Schizophrenia. Schizophr Bull. 34:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. 2015. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 40:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezerman M. 2016. Yes, there is a female and a male brain: morphology versus functionality. Proc Natl Acad Sci. 113(14):E1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’brien LM. 2002. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 59:154–164. [DOI] [PubMed] [Google Scholar]
- Graves JAM, Gécz J, Hameister H. 2002. Evolution of the human X—a smart and sexy chromosome that controls speciation and development. Cytogenet Genome Res. 99:141–145. [DOI] [PubMed] [Google Scholar]
- Greven CU, Merwood A, van der Meer JMJ, Haworth CMA, Rommelse N, Buitelaar JK. 2016. The opposite end of the attention deficit hyperactivity disorder continuum: genetic and environmental aetiologies of extremely low ADHD traits. J Child Psychol Psychiatry. 57:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Kanai R. 2014. What contributes to individual differences in brain structure? Front Hum Neurosci. 8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS. 2014. Gender similarities and differences. Annu Rev Psychol. 65:373–398. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, Beckmann JS, Rosenfeld JA, Eichler EE. 2014. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 94:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ, Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Pediatric Imaging, Neurocognition and Genetics Study . 2016. The pediatric imaging, neurocognition, and genetics (PING) data repository. NeuroImage. 124:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, Shefi N, Pool J, Urchs S, Margulies DS, et al. 2015. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci. 112:15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Carothers A, Deary IJ. 2008. Sex differences in variability in general intelligence: a new look at the old question. Perspect Psychol Sci. 3(6):518–531. [DOI] [PubMed] [Google Scholar]
- Kong X-Z, Zhen Z, Li X, Lu H-H, Wang R, Liu L, He Y, Zang Y, Liu J. 2014. Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS One. 9:e104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, Crone EA. 2013. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 5:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Fennema-Notestine C, Eyler LT, Panizzon MS, Chen C-H, Franz CE, Lyons MJ, Thompson WK, Dale AM. 2013. Genetics of brain structure: contributions from the Vietnam Era Twin Study of Aging. Am J Med Genet B Neuropsychiatr Genet. 162B:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman JM, Brown TT, Ahmadi ME, Erhart MJ. 2011. Prospective motion correction improves diagnostic utility of pediatric MRI scans. Pediatric Raiol. 41:1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Giedd JN, Castellanos FX, Vaituzis AC. 1997. Variability of human brain structure size: ages 4–20 years. Psychiatry Res. 74:1–12. [DOI] [PubMed] [Google Scholar]
- Lehre A-C, Laake P, Sexton JA. 2013. Differences in birth weight by sex using adjusted quantile distance functions. Stat Med. 32:2962–2970. [DOI] [PubMed] [Google Scholar]
- Lehre A-C, Lehre KP, Laake P, Danbolt NC. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev Psychobiol. 51:198–206. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. 2006. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 31:993–1003. [DOI] [PubMed] [Google Scholar]
- Marwha D, Halari M, Eliot L. 2017. Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume. Neuroimage. 147:282–294. [DOI] [PubMed] [Google Scholar]
- Meinshausen N. 2006. Quantile regression forests. J Mach Learn Res. 7:983–999. [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, et al. 2016. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage. 141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Tamnes CK. 2014. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci. 9:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D. 2006. The evolution of personality variation in humans and other animals. Am Psychol. 61:622–631. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. 2005. Dosage compensation of the active X chromosome in mammals. Nat Genet. 38:47–53. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesarin F, Salmaso L. 2010. Permutation testing for repeated measurements. Chichester, UK: John Wiley & Sons, Ltd; p. 225–266. [Google Scholar]
- Plenge RM, Stevenson RA, Lubs HA, Schwartz CE, Willard HF. 2002. Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet. 71:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Haworth CMA, Davis OSP. 2009. Common disorders are quantitative traits. Nat Rev Genet. 10:872–878. [DOI] [PubMed] [Google Scholar]
- Reinhold K, Engqvist L. 2013. The variability is in the sex chromosomes. Evolution. 67:3662–3668. [DOI] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. 2015. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 107:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM (2017). Sex differences in the adult human brain: Evidence from 5,216 UK Biobank participants. bioRxiv Available from: URL http://biorxiv.org/. [DOI] [PMC free article] [PubMed]
- Rosenblatt JD. 2016. Multivariate revisit to “sex beyond the genitalia”. Proc Natl Acad Sci. 113(14):E1966–E1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet GA, Pernet CR, Wilcox RR (2017). Beyond differences in means: robust graphical methods to compare two groups in neuroscience. bioRxiv Available from: URL http://biorxiv.org/. [DOI] [PubMed]
- Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SG, Chance PF, Zou CH, Spinner NB, Golden JA, Smietana S. 1997. Epilepsy and mental retardation limited to females: an X-linked dominant disorder with male sparing. Nat Genet. 17:92–95. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H. 2012. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 31:366–375. [DOI] [PubMed] [Google Scholar]
- Stoet G, Geary DC. 2015. Sex differences in academic achievement are not related to political, economic, or social equality. Intelligence. 48:137–151. [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, et al. 2017. Development of the cerebral cortex across adolescence: A multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. J Neurosci. 37:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Lichtenstein P, Larsson H, Anckarsäter H, Greven CU, Ronald A. 2016. Is there a female protective effect against attention-deficit/hyperactivity disorder? Evidence from two representative twin samples. J Am Acad Child Adolesc Psychiatry. 55:504–512.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. 2009. The role of genetic variation in the causation of mental illness: an evolution-informed framework. Mol Psychiatry. 14:1072–1082. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, Whittle S. 2016. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 37:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, Dale A. 2010. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 63:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Womer FY, Tang Y, Harms MP, Bai C, Chang M, Jiang X, Wei S, Wang F, Barch DM. 2016. Sexual dimorphism of the cerebellar vermis in schizophrenia. Schizophr Res. 176:164–170. [DOI] [PubMed] [Google Scholar]
- Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, Smallwood PM, Erlanger B, Wheelan SJ, Nathans J. 2014. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron. 81:103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner U, Wilda M, Kehrer-Sawatzki H, Vogel W, Fundele R, Hameister H. 2001. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution? Trends Genet. 17:697–701. [DOI] [PubMed] [Google Scholar]